Ketamina como coadyuvante de los opiáceos para el dolor por cáncer
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003351.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 28 junio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
RFB: assessed the updated search results for trials for inclusion in the review, undertook quality and validity evaluation of the included studies, extracted data and wrote the update.
EK: assessed the results of the updated search, undertook quality and validity evaluation of the included studies, and contributed to the writing of the update.
CE: undertook quality and validity evaluation of the included studies, and contributed to the writing of the update.
Sources of support
Internal sources
-
Regional Centre of Excellence in Palliative Care, Haukeland University Hospital; Centre for Clinical Research, Haukeland University Hospital, Norway.
For the original review and all updates RF Bell has received funding for a 20% research position
External sources
-
Norwegian Research Council, Norway.
For the original review RF Bell received a grant to fund a 30% research position
Declarations of interest
RFB: none known. RFB is a retired specialist pain physician who has worked with patients having acute, chronic or cancer pain, including palliative care patients.
CE: none known. Since CE is an author as well as the PaPaS Co‐ordinating Editor at the time of writing, we acknowledge the input of Phil Wiffen who acted as Sign Off Editor for this review. CE had no input into the editorial decisions or processes for this review.
EK: none known. EK is a specialist pain physician who has worked with patients having acute, chronic or cancer pain, including palliative care patients.
Acknowledgements
The updated review was supported by the Regional Centre of Excellence for Palliative Care at Haukeland University Hospital, Bergen, Norway which funds a part‐time research position for RFB.
Searches for the current update were performed by Joanne Abbott. Anna Erskine and Phil Wiffen assisted on the update.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We are grateful to Dr. Augusto Caraceni, Italian Ministry of Health, Rome, who provided rapid peer review for the 2017 update.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jun 28 | Ketamine as an adjuvant to opioids for cancer pain | Review | Rae Frances Bell, Christopher Eccleston, Eija A Kalso | |
| 2012 Nov 14 | Ketamine as an adjuvant to opioids for cancer pain | Review | Rae F Bell, Christopher Eccleston, Eija A Kalso | |
| 2003 Jan 20 | Ketamine as an adjuvant to opioids for cancer pain | Review | Rae F Bell, Christopher Eccleston, Eija A Kalso | |
Differences between protocol and review
-
We did not search CancerLit as we judged it redundant against current databases. We did not search the PaPaS specialised register as it is no longer updated. For this update we searched trial registries.
-
In this 2017 update, we excluded studies having a group size of fewer than 10 participants who completed the study, and unpublished studies.
-
We excluded studies that were not double‐blind. We added the secondary outcomes of total opioid consumption and rescue medication.
-
We also reinstated the secondary outcomes distress and function, which were omitted in previous versions of this review.
-
We updated the methods sections to conform to current Cochrane standards. We included 'Risk of bias' and GRADE assessments.
-
We reported participant withdrawal (dropouts) from the study but no longer narrate it as a secondary outcome but as a feature of the methods.
-
We no longer refer to pain relief as a primary outcome.
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, following discussion with the authors and editors, this review has now been stabilised until 2022, at which point we will assess the review for updating. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Analgesics [adverse effects, *therapeutic use];
- Analgesics, Opioid [*therapeutic use];
- Cancer Pain [*drug therapy];
- Chemotherapy, Adjuvant;
- Hallucinations [chemically induced];
- Ketamine [adverse effects, *therapeutic use];
- Morphine [adverse effects, *therapeutic use];
- Palliative Care;
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Adult; Aged; Female; Humans; Male; Middle Aged;
PICO
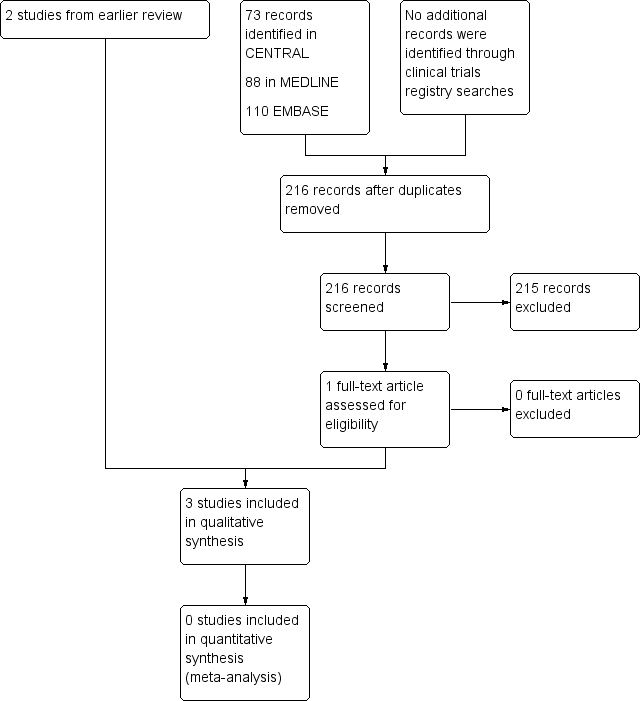
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
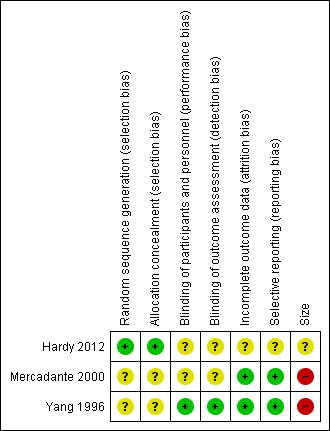
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

