Fármacos antimicrobianos para el tratamiento de la colonización por Staphylococcus aureus resistente a la meticilina
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized controlled trial. | |
| Participants | Inclusion criteria: admitted to ICU; expected stay >7 days; no antibiotics against Staphylococcal aureus given; positive surveillance cultures for MRSA (anterior nares, sputum or throat culture, skin). Number assessed: 16 of 23 eligible patients (unclear how many were randomized). Median age of 23 eligible patients: 70 years. Gender: 13 (56%) were male. Colonization: 39% had nasal colonization; 61% had throat colonization; 39% had skin colonization. Co‐morbidities: not specifically described. | |
| Interventions | (1) Fusidic acid 500 mg orally 3 times daily for 7 days. | |
| Outcomes | (1) Eradication of MRSA from all sites at days 14 to 77. | |
| Notes | Study location: Medical intensive care units in Taiwan. The study was terminated early because the fusidic acid did not appear to be effective and because of the emergence of resistance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomized controlled trial. | |
| Participants | Inclusion criteria: > 16 years of age; admitted to large tertiary care hospital; history of MRSA carriage or acquired MRSA during Exclusion criteria: pregnancy; hypersensitivity. Number randomized: 74. Mean age: 74 years. Gender: 59% were male. Co‐morbidities: mean of 3.3 co‐morbidities. Colonization: 28% had nasal colonization, 38% had groin colonization; 46% had skin colonization; and 20% had urine colonization. | |
| Interventions | (1) Calcium mupirocin (2% applied to the nares twice daily for 5 days). | |
| Outcomes | (1) Eradication of MRSA from all sites at day 26. | |
| Notes | Study location: Swiss tertiary care hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomized controlled trial. | |
| Participants | Inclusion criteria: residents of long‐term care facilities; nasal colonization with MRSA. Number randomized: 35 individuals randomized. The majority were non‐ambulatory and had one or more risk factors for MRSA colonization including decubitus ulcers and indwelling catheters. | |
| Interventions | (1) Rifampin (600 mg orally twice daily). All treatment regimens were administered for 5 days. | |
| Outcomes | (1) Eradication of MRSA from all sites at days 30 and 90. | |
| Notes | Study location: 2 Veteran's Administration long‐term care facilities in the USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomized controlled trial. | |
| Participants | Inclusion criteria: patients and healthcare workers >18 years of age from 2 ICUs and a surgical unit; at least 2 consecutive MRSA isolates from nares in a 5‐day period; no history of allergy or of intolerance to any study drugs. Exclusion criteria: pregnant women; patients with biochemical evidence of renal or hepatic dysfunction. Number of participants: 73 patients; 11 healthcare workers. Mean age: 54 years. Gender: 69% were male. Co‐morbidities: 86% had an underlying disease; 32% had an MRSA infection. Colonization: 54% had extra‐nasal colonization. | |
| Interventions | (1) Calcium mupirocin (2% applied to the nares 3 times daily). | |
| Outcomes | (1) Eradication of MRSA from all sites at days 14, 21, and 30. | |
| Notes | Study location: hospital in Spain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomized controlled trial. | |
| Participants | Inclusion criteria: all patients from a Veterans Administration Medical Center found to have MRSA in routine cultures. Exclusion criteria: active infection; history of allergy to any study medication. Number randomized: 21. MRSA needed to be susceptible to at least one of the study medications that the subject was receiving to be evaluable. | |
| Interventions | (1) Ciprofloxacin (750 mg orally twice daily) and rifampin (300 mg orally twice daily). Treatment was given for 14 days in both arms. | |
| Outcomes | (1) Eradication of MRSA from all sites at days 14 to 21, 90, and 180. | |
| Notes | Study location: a Veteran's Administration acute care hospital in the USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomized controlled trial. | |
| Participants | Inclusion criteria: patients or healthcare workers > 18 years of age from 11 acute care hospitals (80 patients and 14 healthcare providers) colonized with MRSA (wounds, nares, tracheotomy or stomal sites, respiratory secretions). Exclusion criteria: pregnant or nursing mothers; allergy to study medications; liver or renal impairment; recent or recurrent nausea; vomiting or diarrhoea; receipt of antibiotics with activity against Staphylococcus; MRSA resistant to one or study medications. Number randomized: 126. Mean age: 56 years. Gender: 83% male. Co‐morbidities: mean of 3 chronic diseases. Colonization: 49% had MRSA detected in wounds; 26% had MRSA in nares alone. | |
| Interventions | (1) Novobiocin (500 mg orally twice daily) and rifampin (300 mg orally twice daily). All medications prescribed for 7 days. | |
| Outcomes | (1) Eradication of MRSA from all sites at day 14. | |
| Notes | Study location: 9 acute care hospitals in the USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ICU: intensive care unit; MRSA: methicillin‐resistant Staphylococcus aureus
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomized controlled trial. Case‐control study with 192 cases colonized with MRSA and treated with rifampin and trimethoprim‐sulfa and topical fusidic acid or mupirocin for 5 days. | |
| Not a randomized controlled trial. Open‐sequential trial of 546 patients in an ICU setting who were treated with mupirocin for 5 days alternating with no treatment. | |
| Follow up less than 2 weeks. 30 patients with MRSA were randomized to intranasal mupirocin and triclosan body wash or intranasal tea tree oil and tea tree oil body wash for 3 days. | |
| No outcome (culture) data reported. 12 nursing home patients colonized with MRSA were randomized to ciprofloxacin or ciprofloxacin + rifampin and pharmacokinetics were assessed. | |
| Not a randomized controlled trial. 10 patients and 6 healthcare workers were treated with topical pseudomonic acid until hospital discharge or MRSA was eradicated. | |
| Not a randomized controlled trial. 11 patients in a spinal cord unit were treated with oral minocycline, rifampin, and topical mupirocin for 11 days. | |
| Not a randomized controlled trial. 8 patients with MRSA colonization were treated with lysozyme chloride in the nares and oral cavity. | |
| Not a randomized controlled trial. 65 patients in a Veteran's Administration nursing home who were colonized with MRSA were treated with topical mupirocin to nares and wounds on a schedule of tapering frequency for 3 months. | |
| Not a randomized controlled trial. 10 patients colonized with MRSA were alternately placed into a treatment group, which received oral flucloxacillin, or a control group, which was not treated. | |
| Not a randomized controlled trial. 35 children with atopic dermatitis were treated with topical nadifloxacin and bufexamac ointment or bufexamac alone for 4 weeks. | |
| Not a randomized controlled trial. 12 patients were treated with roxithromycin for the eradication of MRSA colonization and infection. | |
| Not a randomized controlled trial (quasi‐experimental). 34 patients with burns, which were colonized with MRSA, were alternately allocated 4 days therapy with flucloxacillin or no treatment. | |
| Not a randomized trial. Case‐control trial with 20 patients who were colonized with MRSA treated with ciprofloxacin for 28 days or until eradication occurred. | |
| Not a randomized controlled trial. 37 patients were treated with gentian violet topically until MRSA was eradicated. | |
| Not a randomized controlled trial. 733 patients colonized with MRSA were treated with topical mupirocin to nares and skin. | |
| Not a randomized controlled trial. 45 children with burns colonized with MRSA were treated with topical mupirocin twice daily for 5 days. | |
| Not a randomized controlled trial. 18 patients with MRSA cultured from decubitus ulcers were treated with gentian violet scrub then topical gentian violet ointment until no MRSA was cultured for 3 days. | |
| Not a randomized controlled trial. 51 patients colonized with MRSA in sputum were treated with inhaled vancomycin until MRSA was eliminated. | |
| Not a randomized controlled trial; follow up less than 2 weeks. 28 patients with MRSA colonization were treated with octenidine body wash and mupirocin for 5 days. | |
| Not a randomized controlled trial. 15 patients colonized with MRSA were treated sequentially with ciprofloxacin for 5 days, ciprofloxacin for 10 to 14 days, and ciprofloxacin and rifampin for 21 days. | |
| Not a randomized controlled trial. 17 patients treated with inhalation vancomycin therapy and 18 treated with mupirocin were followed. Treatment was continued until MRSA was eradicated. | |
| Duration of follow up unclear. 18 patients with pressure sores which were colonized with MRSA were randomized to gentian violet + dibutyryl cAMP or povidone‐iodine plus sugar. | |
| Not a randomized controlled trial. 59 nursing home residents were treated alternating with topical mupirocin for 5 days or mupirocin and chlorhexidine bath for 3 days. | |
| Duration of follow up unclear. 59 infants colonized with MRSA in the Neonatal Intensive Care Unit were randomized to nasopharyngeal disinfection with povidone‐iodine for 7 days or no treatment. | |
| Not a randomized controlled trial. 13 patients with ulcers infected with MRSA were treated with topical silver sulphadiazine daily. |
We reviewed the English abstracts of following articles, which were published in Japanese: Kono 1994, Okano 2000, Saji 1995, Shirai 1995, Soga 1999, Toba 1997, Yamada 1997, and Yoshida 1997.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
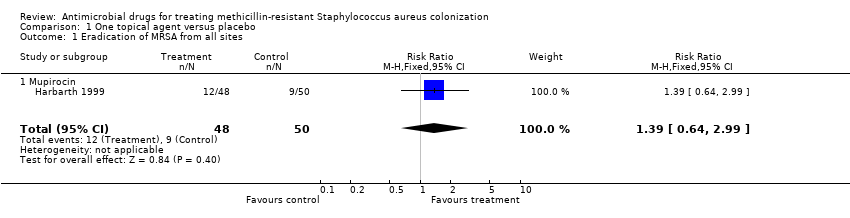
| 1 Eradication of MRSA from all sites Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.64, 2.99] |
| Analysis 1.1  Comparison 1 One topical agent versus placebo, Outcome 1 Eradication of MRSA from all sites. | ||||
| 1.1 Mupirocin | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.64, 2.99] |
| 2 Nasal MRSA eradication Show forest plot | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.96, 3.26] |
| Analysis 1.2  Comparison 1 One topical agent versus placebo, Outcome 2 Nasal MRSA eradication. | ||||
| 3 MRSA infection Show forest plot | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.70] |
| Analysis 1.3  Comparison 1 One topical agent versus placebo, Outcome 3 MRSA infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
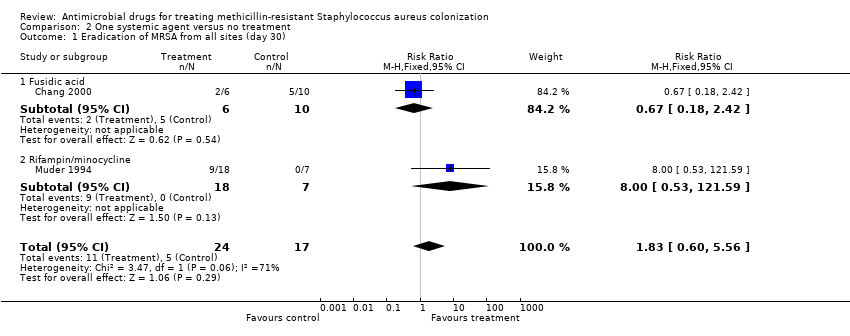
| 1 Eradication of MRSA from all sites (day 30) Show forest plot | 2 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.60, 5.56] |
| Analysis 2.1  Comparison 2 One systemic agent versus no treatment, Outcome 1 Eradication of MRSA from all sites (day 30). | ||||
| 1.1 Fusidic acid | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.42] |
| 1.2 Rifampin/minocycline | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [0.53, 121.59] |
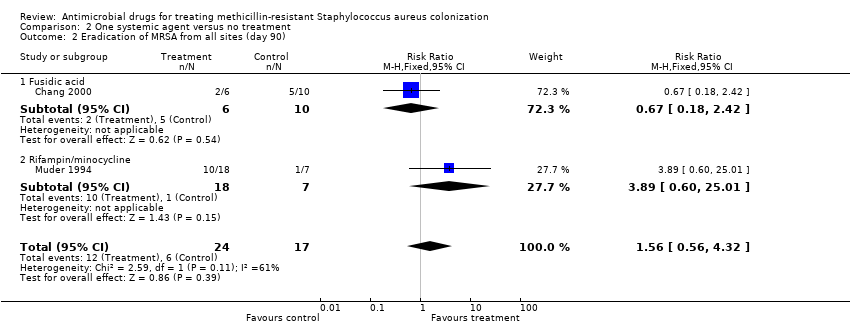
| 2 Eradication of MRSA from all sites (day 90) Show forest plot | 2 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.56, 4.32] |
| Analysis 2.2  Comparison 2 One systemic agent versus no treatment, Outcome 2 Eradication of MRSA from all sites (day 90). | ||||
| 2.1 Fusidic acid | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.42] |
| 2.2 Rifampin/minocycline | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.89 [0.60, 25.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of MRSA from all sites (day 30) Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.45 [0.62, 144.74] |
| Analysis 3.1  Comparison 3 Two systemic agents versus no treatment, Outcome 1 Eradication of MRSA from all sites (day 30). | ||||
| 1.1 Rifampin and minocycline | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.45 [0.62, 144.74] |
| 2 Eradication of MRSA from all sites (day 90) Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.51, 23.81] |
| Analysis 3.2  Comparison 3 Two systemic agents versus no treatment, Outcome 2 Eradication of MRSA from all sites (day 90). | ||||
| 2.1 Rifampin and minocycline | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.51, 23.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of nasal MRSA Show forest plot | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.97, 1.14] |
| Analysis 4.1  Comparison 4 One topical versus one topical and one systemic agent, Outcome 1 Eradication of nasal MRSA. | ||||
| 1.1 Day 14 | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.15] |
| 1.2 Day 21 | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 1.3 Day 28 | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.16] |
| 1.4 Day 90 | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.64, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of MRSA from all sites (day 30) Show forest plot | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.00] |
| Analysis 5.1  Comparison 5 One systemic agent versus one systemic agent, Outcome 1 Eradication of MRSA from all sites (day 30). | ||||
| 1.1 Minocycline versus rifampin | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.00] |
| 2 Eradication of MRSA from all sites (day 90) Show forest plot | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.43] |
| Analysis 5.2  Comparison 5 One systemic agent versus one systemic agent, Outcome 2 Eradication of MRSA from all sites (day 90). | ||||
| 2.1 Minocycline versus rifampin | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of MRSA from all sites (day 30) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.60, 2.38] |
| Analysis 6.1  Comparison 6 Two systemic agents versus one systemic agent, Outcome 1 Eradication of MRSA from all sites (day 30). | ||||
| 1.1 Rifampin and minocycline versus combined rifampin and minocycline | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.60, 2.38] |
| 2 Eradication of MRSA from all sites (day 90) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.43, 1.90] |
| Analysis 6.2  Comparison 6 Two systemic agents versus one systemic agent, Outcome 2 Eradication of MRSA from all sites (day 90). | ||||
| 2.1 Rifampin and minocycline or ciprofloxacin and rifampin versus combination | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.43, 1.90] |
| 3 Eradication of MRSA from all sites (day 14) Show forest plot | 2 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.85, 1.60] |
| Analysis 6.3  Comparison 6 Two systemic agents versus one systemic agent, Outcome 3 Eradication of MRSA from all sites (day 14). | ||||
| 3.1 Ciprofloxicin and rifampin versus trimethoprim‐sulfamethoxazole | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.27, 1.97] |
| 3.2 Novobiacin and rifampin versus trimethoprim‐sulfamethoxazole | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.90, 1.76] |
Comparison 1 One topical agent versus placebo, Outcome 1 Eradication of MRSA from all sites.

Comparison 1 One topical agent versus placebo, Outcome 2 Nasal MRSA eradication.

Comparison 1 One topical agent versus placebo, Outcome 3 MRSA infection.
Comparison 2 One systemic agent versus no treatment, Outcome 1 Eradication of MRSA from all sites (day 30).
Comparison 2 One systemic agent versus no treatment, Outcome 2 Eradication of MRSA from all sites (day 90).

Comparison 3 Two systemic agents versus no treatment, Outcome 1 Eradication of MRSA from all sites (day 30).

Comparison 3 Two systemic agents versus no treatment, Outcome 2 Eradication of MRSA from all sites (day 90).

Comparison 4 One topical versus one topical and one systemic agent, Outcome 1 Eradication of nasal MRSA.

Comparison 5 One systemic agent versus one systemic agent, Outcome 1 Eradication of MRSA from all sites (day 30).
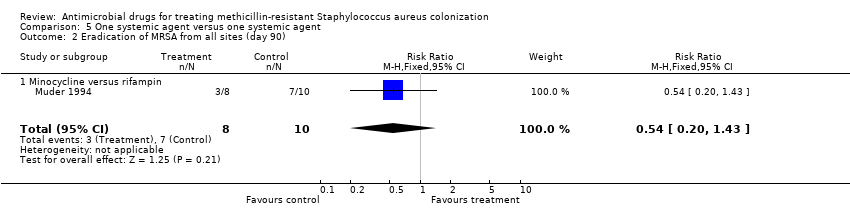
Comparison 5 One systemic agent versus one systemic agent, Outcome 2 Eradication of MRSA from all sites (day 90).

Comparison 6 Two systemic agents versus one systemic agent, Outcome 1 Eradication of MRSA from all sites (day 30).

Comparison 6 Two systemic agents versus one systemic agent, Outcome 2 Eradication of MRSA from all sites (day 90).

Comparison 6 Two systemic agents versus one systemic agent, Outcome 3 Eradication of MRSA from all sites (day 14).
| Search number | MEDLINE (on OVID) | EMBASE (on OVID) |
| (1) | Staphylococcal infections/ | Staphylococcal infection/ |
| (2) | Staphylococcal skin infections/ | Staphylococcus aureus/ |
| (3) | Staphylococcus aureus/ | Staphylococcus aureus[tw] |
| (4) | Staphylococcus aureus[tw] | Methicillin resistant Staphylococcus aureus/ |
| (5) | Methicillin resistance/ | methicillin‐resistant staphylococcus aureus[tw] |
| (6) | Methicillin resistance ointments/ | methicillin resistant staphylococcus aureus[tw] |
| (7) | methicillin‐resistant staphylococcus aureus[tw] | MRSA[tw] |
| (8) | methicillin resistant staphylococcus aureus[tw] | 1or 2 or 3 or 4 or 5 or 6 or 7 |
| (9) | MRSA[tw] | limit 8 to human |
| (10) | 1or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 | |
| (11) | limit 10 to human | |
| / = MeSH term; tw = text word | / = EMTREE term; tw = text word |
| Study | Allocation sequence | Concealment | Blinding | Follow up |
| Harbath 1999 | Unclear | Unclear | Met | Met |
| Chang 2000 | Unclear | Unclear | Unclear | Not met |
| Parras 1995 | Met | Unclear | Unclear | Not met |
| Muder 1994 | Unclear | Unclear | Unclear | Met |
| Walsh 1993 | Met | Met | Met | Not met |
| Peterson 1990 | Unclear | Unclear | Unclear | Met |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of MRSA from all sites Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.64, 2.99] |
| 1.1 Mupirocin | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.64, 2.99] |
| 2 Nasal MRSA eradication Show forest plot | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.96, 3.26] |
| 3 MRSA infection Show forest plot | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of MRSA from all sites (day 30) Show forest plot | 2 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.60, 5.56] |
| 1.1 Fusidic acid | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.42] |
| 1.2 Rifampin/minocycline | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [0.53, 121.59] |
| 2 Eradication of MRSA from all sites (day 90) Show forest plot | 2 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.56, 4.32] |
| 2.1 Fusidic acid | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.42] |
| 2.2 Rifampin/minocycline | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.89 [0.60, 25.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of MRSA from all sites (day 30) Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.45 [0.62, 144.74] |
| 1.1 Rifampin and minocycline | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.45 [0.62, 144.74] |
| 2 Eradication of MRSA from all sites (day 90) Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.51, 23.81] |
| 2.1 Rifampin and minocycline | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.51, 23.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of nasal MRSA Show forest plot | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.97, 1.14] |
| 1.1 Day 14 | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.15] |
| 1.2 Day 21 | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 1.3 Day 28 | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.16] |
| 1.4 Day 90 | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.64, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of MRSA from all sites (day 30) Show forest plot | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.00] |
| 1.1 Minocycline versus rifampin | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.00] |
| 2 Eradication of MRSA from all sites (day 90) Show forest plot | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.43] |
| 2.1 Minocycline versus rifampin | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication of MRSA from all sites (day 30) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.60, 2.38] |
| 1.1 Rifampin and minocycline versus combined rifampin and minocycline | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.60, 2.38] |
| 2 Eradication of MRSA from all sites (day 90) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.43, 1.90] |
| 2.1 Rifampin and minocycline or ciprofloxacin and rifampin versus combination | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.43, 1.90] |
| 3 Eradication of MRSA from all sites (day 14) Show forest plot | 2 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.85, 1.60] |
| 3.1 Ciprofloxicin and rifampin versus trimethoprim‐sulfamethoxazole | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.27, 1.97] |
| 3.2 Novobiacin and rifampin versus trimethoprim‐sulfamethoxazole | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.90, 1.76] |

