Cloroquina como agente economizador de esteroides para el asma
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | DESIGN Randomised controlled cross‐over trial | |
| Participants | Enrolled into study N = 9 Age: Mean (SD) 44 (10.3) | |
| Interventions | Hydroxychloroquine = 400 mg/day | |
| Outcomes | Reduction in oral prednisolone | |
| Notes | TAPERING OF STEROID USE: Classified B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Open trial | |
| Cohort study | |
| Case study. | |
| Double‐blind, parallel group study. Randomisation not mention. Authors contacted, but no response forthcoming | |
| Review article. | |
| Double‐blind quasi experiment (alternate allocation) investigating the efficacy of Aralen versus placebo rather than as a steroid sparing agent for non‐steroid dependent asthmatics. | |
| Open trial |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral steroid consumption (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 1 Oral steroid consumption (change from baseline). | ||||
| 2 Symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 2 Symptoms. | ||||
| 3 Peak flow (am) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 3 Peak flow (am). | ||||
| 4 Peak flow (pm) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 4 Peak flow (pm). | ||||
| 5 FEV1 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 5 FEV1. | ||||
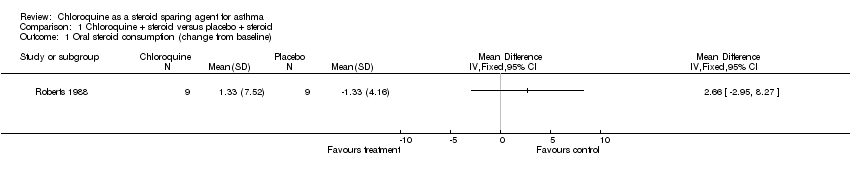
Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 1 Oral steroid consumption (change from baseline).
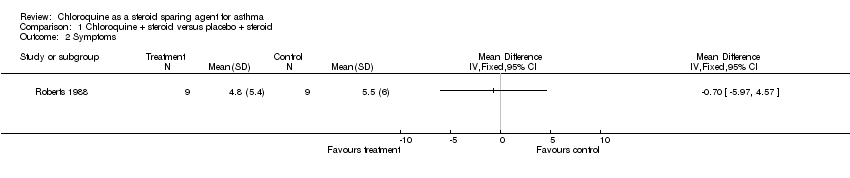
Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 2 Symptoms.

Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 3 Peak flow (am).

Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 4 Peak flow (pm).
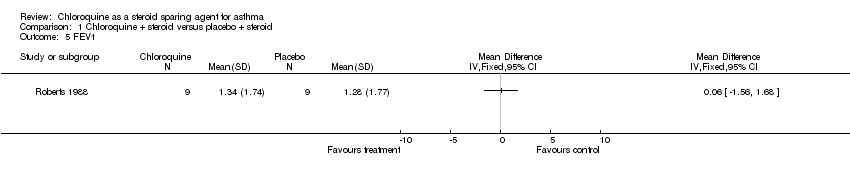
Comparison 1 Chloroquine + steroid versus placebo + steroid, Outcome 5 FEV1.
| Trials | N | Duration | SEs noted |
| Isomaki 1968 | 19 (no withdrawals) | 28 weeks | Nausea (n = 1). No other SEs related to treatment were noted. No participants withdrew as a result of SEs. |
| Stern 1960 | 13 completed out of 25 | Described as 'long‐term'. Treatment given ranged from 9 weeks to 1 year | No serious toxic effect noted. Chloroquine: Nausea n = 1. Three participants switched from chloroquine to placebo due to adverse effects |
| Tennenbaum 1966 | 4 (all completed) | 6 months | Early keratopathy in one patient. No other SEs reported. Mild nausea (n = 1) |
| Charous 1991 | 14 | 12 months | Non reported. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral steroid consumption (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Peak flow (am) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Peak flow (pm) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 FEV1 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

