Espesante de alimentos para neonatos de hasta seis meses de edad con reflujo gastroesofágico
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: Randomised controlled trial. Study grouping: Parallel group. | |
| Participants | Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: Non‐breastfed infants (age 2 to 4 months) presenting with 3 or more episodes of regurgitation/vomiting per day. Exclusion criteria: Mechanical obstruction such as infantile hypertrophic pyloric stenosis and malrotation were excluded with an upper gastrointestinal barium study before inclusion. Infants with atopic symptoms such as eczema, watery rhinorrhoea, or diarrhoea suspecting of cow's milk allergy were excluded. Pretreatment: There was no difference in demographics and baseline clinical characteristics between intervention and control groups. Study period: 2 years (July 2002 to July 2004). | |
| Interventions | Intervention characteristics Control/placebo
Feed thickener
| |
| Outcomes | Outcomes were reported after 8 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
Sleep disturbance
Irritability
Cough
Diarrhoea
| |
| Notes | Sponsorship source: None declared. Country: Taiwan. Setting: Outpatients. Author name: Yvan Vandenplas. Institution: Universitair Ziekenhuis Brussel Kinderen. Email: [email protected] Address: Department of Pediatrics, Universitair Ziekenhuis Brussel Kinderen, Vrije Universiteit Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed using an envelope‐drawing system. However, there was no mention of how the random numbers were generated. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using an envelope‐drawing system. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention (cornstarch‐thickened AR formula) and control (25% strengthened regular infant formula) were unlikely. Frequency of symptoms depended on parental report. |
| Blinding of participants and personnel (performance bias) | Low risk | Not applicable. |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of intervention (cornstarch‐thickened antiregurgitation formula) and control (25% strengthened regular infant formula) were unlikely. Frequency of symptoms depended on parental report. |
| Blinding of outcome assessment (detection bias) | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) | High risk | Of 100 included infants, 81 infants completed the 2‐month clinical follow‐up. Of the 19 excluded infants, 8 developed marked diarrhoea or enteritis, 5 experienced upper respiratory infection, and 6 did not have regular follow‐up. The proportion of these infants in the feed thickener and control group was not specified. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods section of the study were reported, but the study protocol or trial registration numbers were not available. |
| Other bias | Low risk | There was no difference in the demographics and baseline clinical characteristics between the intervention and control group. |
| Methods | Study design: Randomised controlled trial. Study grouping: Parallel group. | |
| Participants | Baseline characteristics Control/placebo
Feed thickener 1
Feed thickener 2
Inclusion criteria: Healthy term‐born, formula‐fed infants (age 1 to 3 months) presenting with frequent regurgitation or vomiting, or both (4 times/day since at least 1 week before inclusion). All of the infants were exclusively fed with standard infant formula at normal concentration at baseline. Exclusion criteria:
Pretreatment: No baseline characteristics were statistically significant. Study period: Not reported. | |
| Interventions | Intervention characteristics Control/placebo
Feed thickener 1
Feed thickener 2
| |
| Outcomes | Outcomes were reported after 4 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
Sleep disturbance
Diarrhoea
| |
| Notes | Sponsorship source: None. Country: Belgium. Setting: Not specified but likely outpatient. Author name: Yvan Vandenplas. Institution: Universitair Ziekenhuis Brussel Kinderen. Email: [email protected] Address: Department of Pediatrics, Universitair Ziekenhuis Brussel Kinderen, Vrije Universiteit Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed according to an automated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) | High risk | Parents were blinded to the formulae. However, parents were likely to observe that the thickened formula had a higher viscosity, which could affect parent‐reported symptoms or signs of gastro‐oesophageal reflux and side effect. |
| Blinding of participants and personnel (performance bias) | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) | High risk | Parents were blinded to the formulae. However, parents were likely to observe that the thickened formula had a higher viscosity, which could affect parent‐reported symptoms or signs of gastro‐oesophageal reflux and side effect. |
| Blinding of outcome assessment (detection bias) | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) | Low risk | All 60 included infants completed the study and were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods section of the study were reported, but the study protocol or trial registration numbers were not available. |
| Other bias | Low risk | No statistically significant difference between intervention and control groups at baseline |
| Methods | Study design: Randomised controlled trial. Study grouping: Parallel group. | |
| Participants | Baseline characteristics Control/placebo
Intervention
Inclusion criteria: Bottle‐fed infants, under 4 months of age, who consecutively presented at the outpatient clinics of 6 paediatric centres with frequent regurgitation/vomiting due to uncomplicated GOR. Exclusion criteria: Patients over 4 months, breast‐ or mixed‐feeding, signs of “complicated” GOR, GOR secondary to food allergy. Pretreatment: No difference in regurgitation score at baseline. Study period: Not reported. | |
| Interventions | Intervention characteristics Control/placebo
Intrevention
| |
| Outcomes | Outcomes were reported after 8 weeks of intervention. Number of infants without regurgitation, posseting, or vomiting at the end of the intervention period
Diarrhoea
| |
| Notes | Comments: Published as correspondence to the editor only. We obtained further information from the author. Sponsorship source: None declared. Country: Italy. Setting: Children's outpatient clinics in 6 centres. Comments: Author name: Giuseppe Iacono. Institution: Pediatric Gastroenterology, “Di Cristina” Hospital. Email: [email protected] Address: Pediatric Gastroenterology, “Di Cristina” Hospital, Palermo, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the correspondence stated that infants were randomly assigned to 2 treatment regimens, the method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) | High risk | Method of blinding was not described. However, parents were likely to note the higher viscosity of the thickened feed, which could have affected parental report of signs and symptoms. |
| Blinding of participants and personnel (performance bias) | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) | High risk | Method of blinding was not described. However, parents were likely to note the higher viscosity of the thickened feed, which could have affected parental report of signs and symptoms. |
| Blinding of outcome assessment (detection bias) | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) | Low risk | Author did not mention the number of infants excluded prior to randomisation. However, all randomised infants were included in the final analysis. |
| Selective reporting (reporting bias) | High risk | This study was published as a correspondence to the editor only. Not all values for the measured outcome were reported. Baseline characteristics of infants pre‐intervention were not reported. Study protocol or trial registration numbers were not available. |
| Other bias | Low risk | The intergroup comparison of the data did not show any difference in regurgitation score between the group receiving the thickened formula and the control group at baseline. |
| Methods | Study design: Randomised controlled trial. Study grouping: Parallel group. | |
| Participants | Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: Paediatric patients aged between 0 and 12 months at the pretreatment assessment, with symptoms consistent with gastro‐oesophageal reflux (persistent, unmanageable vomiting/regurgitation or vomiting/regurgitation at least twice daily for the 2 days prior to the start of the study). Exclusion criteria: Infants were excluded from the study if they had known or suspected oesophageaI disease, significant gastrointestinal disease, or uncontrolled neurological, cardiac, respiratory, metabolic, hepatic disease or renal impairment; were likely to experience excessive water loss (e.g. fever, diarrhoea); had not yet completed the 37th week of development or weighed less than 2.5 kg; were receiving drugs likely to cause sodium retention; had previously participated in the present study or were currently participating in any other clinical study or had suspected or known sensitivity to alginates. Pretreatment: Patient demographics were similar with respect to age, gender, weight, and ethnic origin between the 2 study groups. 22% of infants had a pre‐existing medical condition upon entry into the study that was comparable between groups. The nature of this condition was not reported. The duration of vomiting/regurgitation was comparable between the 2 treatment groups. However, the number of vomiting/regurgitation episodes in the 24‐hour period prior to the pretreatment assessment was slightly higher in the alginate group (8.5 vs 7 episodes per day), and the number of episodes was not evenly distributed between sex and treatment groups. Study period: 18 months (April 1994 to October 1995). | |
| Interventions | Intervention characteristics Control/placebo
Feed thickener
| |
| Outcomes | Outcomes were reported after 2 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
Diarrhoea
Colic
Respiratory symptom (acute nasopharyngitis)
Constipation (bowel obstruction)
| |
| Notes | Comments: The study included infants up to 12 months of age. The author was contacted and did not have the actual age ranges of infants recruited in the study. However, the reported mean age and standard deviation of infants recruited in the study was 4 ± 0.4 months. Based upon these values, we decided to include the study, as the spread of infants was likely to be between 3 to 5 months. 2 infants were excluded from the analysis of the placebo group as they did not receive the placebo. Sponsorship source: Parexel International Ltd and Reckitt 6 Colman Products Ltd for funding the study. Country: UK. Setting: 25 general practice centres in the UK. Author name: Dr S. Miller. Institution: The New Surgery. Email: [email protected] Address: 143a Uxbridge Road, Shepherds Bush, London W12. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper stated that the study was randomised, but did not mention how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation concealment was performed |
| Blinding of participants and personnel (performance bias) | Low risk | This was a double‐blind trial with matching placebo administered. |
| Blinding of participants and personnel (performance bias) | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) | Low risk | This was a double‐blind trial with matching placebo administered. |
| Blinding of outcome assessment (detection bias) | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) | Low risk | 2 infants were excluded from the analysis of the placebo group as they did not receive the placebo. A further 20 infants withdrew from the study (7 in the alginate group and 13 in the placebo group). However, they were all included in the final analyses as per the intention‐to‐treat protocol. Results from the efficacy evaluable population were similar to those from the intention‐to‐treat protocol. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, study protocol or trial registration number, or both, were not available. |
| Other bias | High risk | The study was funded by Parexel International Ltd and Reckitt 6 Colman Products Ltd. Infants in the alginate group had a higher number of vomiting/regurgitation episodes per day at baseline as compared to the placebo group. |
| Methods | Study design: Randomised controlled trial. Study grouping: Parallel group. | |
| Participants | Baseline characteristics Control/placebo
Feed thickener 1
Feed thickener 2
Inclusion criteria: Infants under 4 months of age (1 to 4 months old) with frequent regurgitations (more than 5 regurgitations per day) without any signs of gastro‐oesophageal reflux disease were included. Exclusion criteria: Preterm, low birth weight, breastfeeding infants, infants with history of previous illness, or infants who had received any antireflux medication were excluded. Pretreatment: The baseline clinical characteristics of infants were similar between the groups, except for the age of infant at entry to the study between infants in the control group (group 1) and infants in the group receiving carob bean gum‐thickened formula (group 3). Infants in group 1 were younger at entry to the study, at 54.5 days (standard deviation 16.8 days), as compared to 82.2 days (standard deviation 24.4 days) in group 3. Study period: Not reported. | |
| Interventions | Intervention characteristics Control/placebo
Feed thickener 1
Feed thickener 2
| |
| Outcomes | Outcomes were reported after 2 weeks of intervention. Number of episodes of regurgitation, posseting, vomiting, or haematemesis per day
Diarrhoea
| |
| Notes | Comments: Published in Spanish. Sponsorship source: None mentioned. Country: Spain. Setting: Outpatients. Author name: M Juste. Institution: Universidad Miguel Hernandez. Email: [email protected] Address: Servicio de Pediatria, Hospital Universitario San Juan, Universidad Miguel Hernandez, Ctra. Nacional 332, Alicante–Valencia, s/n 03550 San Juan de Alicante. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by the hospital pharmacy, which was not involved in the trial (information from author). |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using signed, sealed envelope (information from author). |
| Blinding of participants and personnel (performance bias) | High risk | Initials 'AR' appear on the label depending on the type of formula infant received. Parents may notice the higher viscosity of the antiregurgitation formula, which could affect parental report of signs or symptoms. |
| Blinding of participants and personnel (performance bias) | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) | High risk | Parents may notice the higher viscosity of the antiregurgitation formula, which could affect parental report of signs or symptoms. |
| Blinding of outcome assessment (detection bias) | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) | Low risk | All infants were followed up until the end of the 2‐week study period (information from author). |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, the study protocol or trial registration number, or both, were not available. |
| Other bias | High risk | Infants in the control group were younger on entry to the study than those in the intervention group. This could have resulted in an overestimation of the effect of the intervention, as gastro‐oesophageal reflux normally resolves with time. |
| Methods | Study design: Quasi‐randomised controlled trial. Study grouping: Parallel group. | |
| Participants | Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: Term formula milk‐feeding infants between 1 week and 4 months old, presenting with more than 5 episodes of regurgitations a day and abnormal oesophageal pH monitoring results with percentage of time with pH < 4.0 between 10% and 30%. Exclusion criteria: Infants with secondary gastro‐oesophageal reflux caused by urinary or gastrointestinal infection and food allergy were excluded using appropriate cultures, immunoglobulin E, radioallergosorbent test (cow's milk, betalactoglobulin, lactalbumin, casein), and skin prick test for cow’s milk. Infants who were not thriving or had symptoms suggestive of peptic oesophagitis were excluded. If there was any suspicion, upper gastrointestinal endoscopy with multiple biopsies was performed. Pretreatment: There was no difference in participant characteristics between intervention and control group at baseline. Study period: Not reported. | |
| Interventions | Intervention characteristics Control/placebo
Feed thickener
| |
| Outcomes | Outcomes were reported after 1 week of intervention. Number of infants without regurgitation, posseting, or vomiting at the end of the intervention period
Reflux index
Duration of longest episode
Number of reflux episodes lasting > 5 minutes
| |
| Notes | Sponsorship source: None declared. Country: Belgium. Setting: Outpatient clinic. Author name: Yvan Vandenplas. Institution: Loeb Academic Children's Hospital, Free University of Brussels. Email: [email protected] Address: Loeb Academic Children's Hospital, Free University of Brussels, Laarbeeklaan 101, B‐1090 Brussels, Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Infants were randomised to thickened or unthickened formula in an alternate manner based on order of presentation to the clinic (information provided by author). |
| Allocation concealment (selection bias) | High risk | Infants were randomised to thickened or unthickened formula in an alternate manner based on order of presentation to the clinic (information provided by author). |
| Blinding of participants and personnel (performance bias) | High risk | Blinding was performed using anonymous milk tins. Neither parents nor physicians knew the content of the formula during the study period. Parents were informed that the study investigated a 'new' formula rather than a 'thickened' formula (information from author). However, parents could have noticed the higher viscosity of the antiregurgitation formula, which may have affected parental report of signs or symptoms. |
| Blinding of participants and personnel (performance bias) | Low risk | Potential of hydrogen probe study parameter is an objective measure of reflux and is unlikely to be affected by blinding bias. |
| Blinding of outcome assessment (detection bias) | High risk | Blinding was performed using anonymous milk tins. Neither parents nor physicians knew the content of the formula during the study period. Parents were informed that the study investigated a 'new' formula rather than a 'thickened' formula (information from author). However, parents could have noticed the higher viscosity of the antiregurgitation formula, which may have affected parental report of signs or symptoms. |
| Blinding of outcome assessment (detection bias) | Low risk | Potential of hydrogen probe study parameter is an objective measure of reflux and is unlikely to be affected by blinding bias. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data were reported and no exclusions were mentioned. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, the study protocol or trial registration number, or both, were not available. |
| Other bias | Low risk | There were no reported differences in participant characteristics at baseline. |
| Methods | Study design: Randomised controlled trial. Study grouping: Parallel group. | |
| Participants | Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: Infants with more than 5 regurgitations per day for 2 baseline days, age between 14 and 120 days, gestational age at birth > 37 weeks, birth weight > 2500 g, and maternal age > 18 years were included. Exclusion criteria: Disease or congenital anomalies interfering with normal feeding or causing repeated regurgitation, fever or infectious illness at enrolment, clinical diagnosis of milk or soy protein allergy, complicated gastro‐oesophageal reflux disease (oesophagitis, haematemesis, recurrent respiratory symptoms, failure to thrive), previous treatment with thickened formula, or treatment with prokinetic medication within 5 days before the start of the study. Pretreatment: Baseline demographics and clinical parameters were similar between infants in the intervention and control groups except for the total regurgitation volume score, which was worse in the intervention group. The total regurgitation volume score is a measure of the volume of the largest regurgitation after each bottle of the day. 6 randomised infants were excluded as they did not receive the study formula. Study period: 18 months (December 1996 to July 1998). | |
| Interventions | Intervention characteristics Control/placebo
Feed thickener
| |
| Outcomes | Outcomes were reported after 5 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
| |
| Notes | Sponsorship source: This study was supported by a grant from Mead Johnson & Co. to each of the recruitment sites. Country: USA and Canada. Setting: 6 North American paediatric centres. Comments: Author name: Jon A Vanderhoof. Institution: University of Nebraska Medical Center and Creighton University. Email: [email protected] Address: Joint Section of Pediatric Gastroenterology and Nutrition, University of Nebraska Medical Center and Creighton University, Omaha, Nebraska. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised randomisation schedule was prepared for each study site (information obtained from author). |
| Allocation concealment (selection bias) | Low risk | Each site was supplied with sealed envelopes containing the product code of the formula that the infant was to receive after verifying inclusion/exclusion criteria (information obtained from author). |
| Blinding of participants and personnel (performance bias) | High risk | This was a double‐blind trial. The study products were only identified by a randomly generated code, where each study formula group received 2 codes. The products were similar otherwise (information above obtained from author). However, parents were likely to note the higher viscosity of the thickened feed. |
| Blinding of participants and personnel (performance bias) | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) | High risk | This was a double‐blind trial. The study products were only identified by a randomly generated code, where each study formula group received 2 codes. The products were similar otherwise (information above obtained from author). However, parents were likely to note the higher viscosity of the thickened feed. |
| Blinding of outcome assessment (detection bias) | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) | High risk | 84% of infants in intervention group and 73% in the control group completed the 5‐week study. Outcome measures at the end of the study were reported in 91% and 98% of infants in the intervention and control groups, respectively. However, more than 10% of infants in the intervention and control groups did not complete the study. The data entered in the final outcome measures were based on the last data available prior to discontinuation from the study. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, study protocol or trial registration number, or both, were not available. |
| Other bias | High risk | This study was supported by a grant from Mead Johnson & Co. to each of the recruitment sites. Infants in the intervention group had a worse average total regurgitation volume score that those in the control group. |
| Methods | Study design: Randomised controlled trial. Study grouping: Parallel group. | |
| Participants | Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: All infants were term, exclusively formula‐fed, and ‘healthy’, except for excessive regurgitation and/or vomiting and abnormal pH probe study parameters of reflux index > 5%. Exclusion criteria: Infants who were very irritable or had haematemesis, passed black stools, had chronic cough, had episodes of cyanosis, or had any other medical problems were excluded. Infants on a dietetic formula (such as hydrolysed or soy‐based formula) were excluded, as the specialised formula is considered as a therapeutic intervention. Pretreatment: At baseline, infants in the feed thickener group had a higher number of episodes of vomiting per day than the control group (4.34 ± 2.42 vs 3.09 ± 1.24), but no difference in number of episodes of regurgitation per day. Study period: Not reported. | |
| Interventions | Intervention characteristics Control/placebo
Feed thickener
| |
| Outcomes | Outcomes were reported after 4 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
Reflux index
Number of reflux episodes per hour
Number of reflux episodes lasting > 5 minutes
Duration of longest episode
Side effect
| |
| Notes | Sponsorship source: United Pharmaceuticals provided funding of formula Novalac AR for the participating infants up to a maximal period of 3 months. Country: Multinational (Greece, Morocco, France, and Belgium). Setting: Outpatients. Comments: Author name: Yvan Vandenplas. Institution: Paediatric Gastroenterology, Academisch Ziekenhuis Vrije Universiteit Brussel. Email: [email protected] Address: Paediatric Gastroenterology, Academisch Ziekenhuis Vrije Universiteit Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not mentioned. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Parents were blinded to the formulae. However, they were able to observe that the thickened formula had a higher viscosity, which may have affected parent‐reported symptoms or signs of gastro‐oesophageal reflux. |
| Blinding of participants and personnel (performance bias) | Low risk | Although parents were blinded to the formulae, they were able to observe that the thickened formula had a higher viscosity. However, this should not affect objective pH probe study parameters. |
| Blinding of outcome assessment (detection bias) | High risk | Parents were blinded to the formulae. However, they were able to observe that the thickened formula had a higher viscosity, which may have affected parent‐reported symptoms or signs of gastro‐oesophageal reflux. |
| Blinding of outcome assessment (detection bias) | Low risk | Although parents were blinded to the formulae, they were able to observe that the thickened formula had a higher viscosity. However, this should not affect objective pH probe study parameters. |
| Incomplete outcome data (attrition bias) | Low risk | None of the infants dropped out. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, study protocol or trial registration number, or both, were not available. |
| Other bias | High risk | United Pharmaceuticals provided funding of formula Novalac AR for the participating infants up to a maximal period of 3 months.. At baseline, infants in the feed thickener group had a higher number episodes of vomiting per day than the control group (4.34 ± 2.42 vs 3.09 ± 1.24), but there was no difference in number of episodes of regurgitation per day. Regurgitation was defined as the effortless passage of refluxed gastric contents into the oral pharynx and the mouth with effortless drooling out of the mouth, whereas vomiting was defined as forceful expulsion of the refluxed gastric contents from the mouth. |
GOR: gastro‐oesophageal reflux
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This was a before‐after study design and not a randomised controlled trial. All preterm infants received sodium alginate with potassium bicarbonate. | |
| Participant ages ranged from 4 days to 14 months. This was a cross‐over study in which each participant received both thickened and unthickened feeds. | |
| Participant ages ranged from 5 to 11 months. All infants received either a traditional formula thickened with rice flour at a concentration of 5% or antiregurgitation formula Nutrilon AR, which contained locus bean gum as thickener. There was no control group. | |
| This was a randomised clinical trial comparing cereal‐thickened formula versus postural therapy. There was no placebo or control group. | |
| This was a cohort study where all infants received formula thickened with cornstarch. Participant ages ranged from 2 weeks to 11 months. | |
| This was a open, uncontrolled, multicentre cohort study. All infants received formula thickened with starch. | |
| This was a cross‐over study. All 5 preterm infants in the study received fortified human milk thickened with starch (70% from maize and 30% from potato). | |
| This was a cross‐over study. All 28 preterm infants in the study received Gaviscon, which contains both sodium alginate and sodium bicarbonate. | |
| This was a cross‐over study. All 32 preterm infants in the study received Gaviscon, which contains both sodium alginate and sodium bicarbonate. | |
| This was a cross‐over study. All 28 preterm infants in the study received preterm formula thickened with amylopectin. | |
| This is not a study, but rather an expert opinion piece on the topic. | |
| This was a before‐after study design and not a randomised controlled trial. All 100 recruited infants received antiregurgitation formula with baseline and postintervention outcomes measured. | |
| This is not a study, but rather an expert opinion piece on the topic. | |
| This was a cross‐over study investigating the impact of formula thickened with carob seed galactomannans on ultrasonographic evaluation of gastric emptying time. Participant ages ranged from 1 to 12 months. | |
| There was no control group in this randomised clinical trial. Infants in all 3 arms of the trial received formula thickened with carob bean gum galactomannans in varying doses or temperatures. | |
| This was a cross‐over study. All 20 preterm infants in the study received smectite and postural therapy. | |
| This was a cohort study. All 6 infants received a smaller volume of formula thickened with dry rice cereal and postural therapy. Participant ages ranged from 4 to 10 months. | |
| This was a multicentre randomised controlled trial that recruited healthy term infants without regard to signs or symptoms of gastro‐oesophageal reflux. The intervention was a formula thickened with rice starch that was also lactose free. The control was an unthickened formula with lactose. | |
| This was not a randomised controlled trial. The first part of the study investigated the impact of formula thickened with carob flour on ultrasonographic evaluation of gastric emptying time. The second part of the study was a cross‐over trial where all 27 infants received formula thickened with carob flour. | |
| This was a cross‐over study where each infant received both thickened and unthickened feeds. | |
| This was a cross‐over study where each infant received both thickened and unthickened feeds. | |
| This was a cross‐over study where each infant received both dry rice cereal‐thickened and unthickened feeds. | |
| The intervention in this randomised controlled trial was a soy protein‐based formula thickened with soy fibre, whereas the control was an unthickened whey protein‐based formula. Hence, there was co‐intervention of both thickening and substituting soy protein‐based formula. | |
| This was not a randomised controlled trial but rather a prospective case control investigating the effect of formula thickened with home‐prepared cornstarch versus antiregurgitation formula. Hence, there was no suitable control. Participant ages ranged from 0 to 12 months. | |
| This is not a study, but rather an expert opinion piece on the topic. | |
| The trial included infants up to 1 year old. | |
| The trial did not report outcome measure specified in the review. | |
| The trial included infants up to 1 year old with a mean age of 5.56 ± 2.34 months. | |
| This was a cross‐over study where each infant received both carob bean gum‐thickened and unthickened feeds. | |
| This study is not a randomised controlled trial, but rather a non‐randomised open‐label study. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of episodes per day Show forest plot | 6 | 442 | Mean Difference (IV, Fixed, 95% CI) | ‐1.97 [‐2.32, ‐1.61] |
| Analysis 1.1  Comparison 1 Regurgitation, posseting, or vomiting, Outcome 1 Number of episodes per day. | ||||
| 1.1 Rice cereal | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐3.36, 0.49] |
| 1.2 Carob bean gum | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.47 [‐3.13, 0.19] |
| 1.3 Cornstarch | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐2.35, ‐1.61] |
| 1.4 Alginate | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐6.07, ‐0.93] |
| 2 Proportion of asymptomatic infants Show forest plot | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.38, 4.51] |
| Analysis 1.2  Comparison 1 Regurgitation, posseting, or vomiting, Outcome 2 Proportion of asymptomatic infants. | ||||
| 2.1 Carob bean gum | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.38, 4.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reflux Index (percentage of time pH < 4) Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐5.08 [‐8.89, ‐1.28] |
| Analysis 2.1  Comparison 2 Oesophageal pH probe study parameters, Outcome 1 Reflux Index (percentage of time pH < 4). | ||||
| 1.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐9.36, 1.56] |
| 1.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐11.50, ‐0.90] |
| 2 Number of reflux episodes lasting > 5 minutes Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.44, ‐1.36] |
| Analysis 2.2 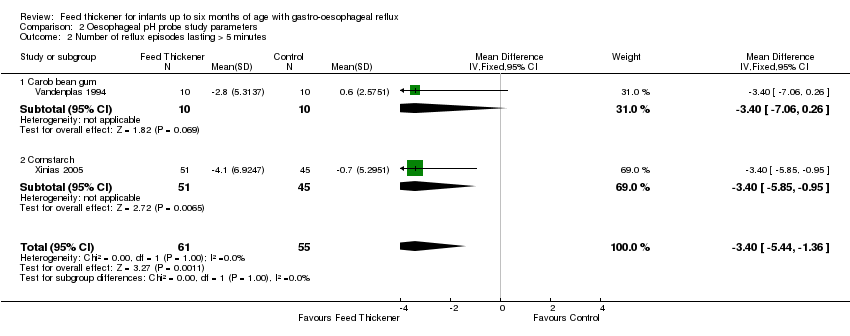 Comparison 2 Oesophageal pH probe study parameters, Outcome 2 Number of reflux episodes lasting > 5 minutes. | ||||
| 2.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.4 [‐7.06, 0.26] |
| 2.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.85, ‐0.95] |
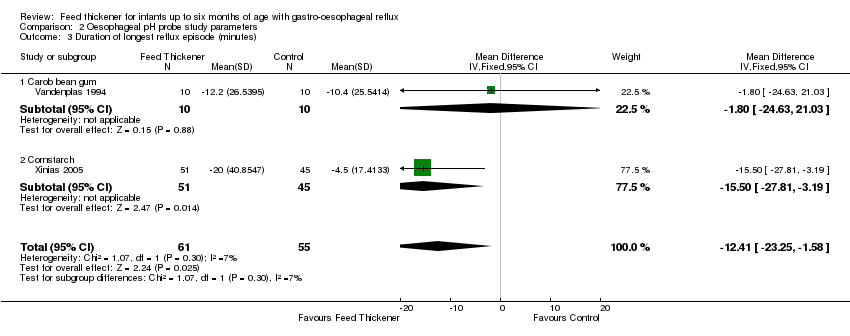
| 3 Duration of longest reflux episode (minutes) Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐12.41 [‐23.25, ‐1.58] |
| Analysis 2.3  Comparison 2 Oesophageal pH probe study parameters, Outcome 3 Duration of longest reflux episode (minutes). | ||||
| 3.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐24.63, 21.03] |
| 3.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐15.5 [‐27.81, ‐3.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint value ‐ number of episodes of regurgitation, posseting, or vomiting per day Show forest plot | 5 | 345 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐2.24, ‐1.58] |
| Analysis 3.1  Comparison 3 Sensitivity analysis, Outcome 1 Endpoint value ‐ number of episodes of regurgitation, posseting, or vomiting per day. | ||||
| 1.1 Rice cereal | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.90, 0.50] |
| 1.2 Carob bean gum | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.25, ‐0.56] |
| 1.3 Cornstarch | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐2.29, ‐1.59] |
| 1.4 Alginate | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.15, 1.15] |
| 2 Endpoint value ‐ reflux index (percentage of time pH < 4) Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐4.01 [‐6.33, ‐1.68] |
| Analysis 3.2  Comparison 3 Sensitivity analysis, Outcome 2 Endpoint value ‐ reflux index (percentage of time pH < 4). | ||||
| 2.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐6.87, 2.67] |
| 2.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.26, ‐1.94] |
| 3 Endpoint value ‐ number of reflux episodes lasting > 5 minutes Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐3.62, ‐0.85] |
| Analysis 3.3  Comparison 3 Sensitivity analysis, Outcome 3 Endpoint value ‐ number of reflux episodes lasting > 5 minutes. | ||||
| 3.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐4.30, 2.10] |
| 3.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐4.04, ‐0.96] |
| 4 Endpoint value ‐ duration of longest reflux episode (minutes) Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐8.09 [‐11.93, ‐4.25] |
| Analysis 3.4 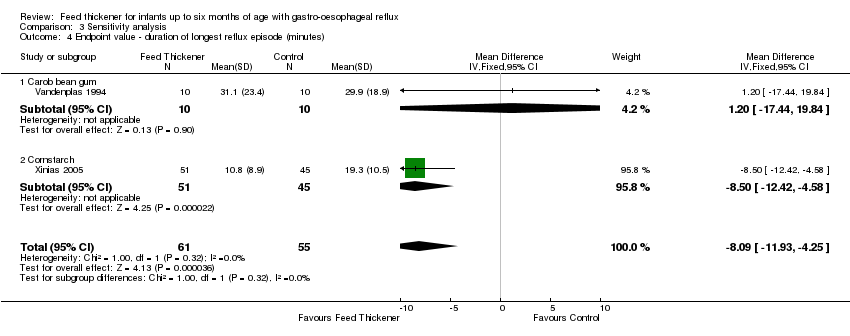 Comparison 3 Sensitivity analysis, Outcome 4 Endpoint value ‐ duration of longest reflux episode (minutes). | ||||
| 4.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐17.44, 19.84] |
| 4.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐8.5 [‐12.42, ‐4.58] |
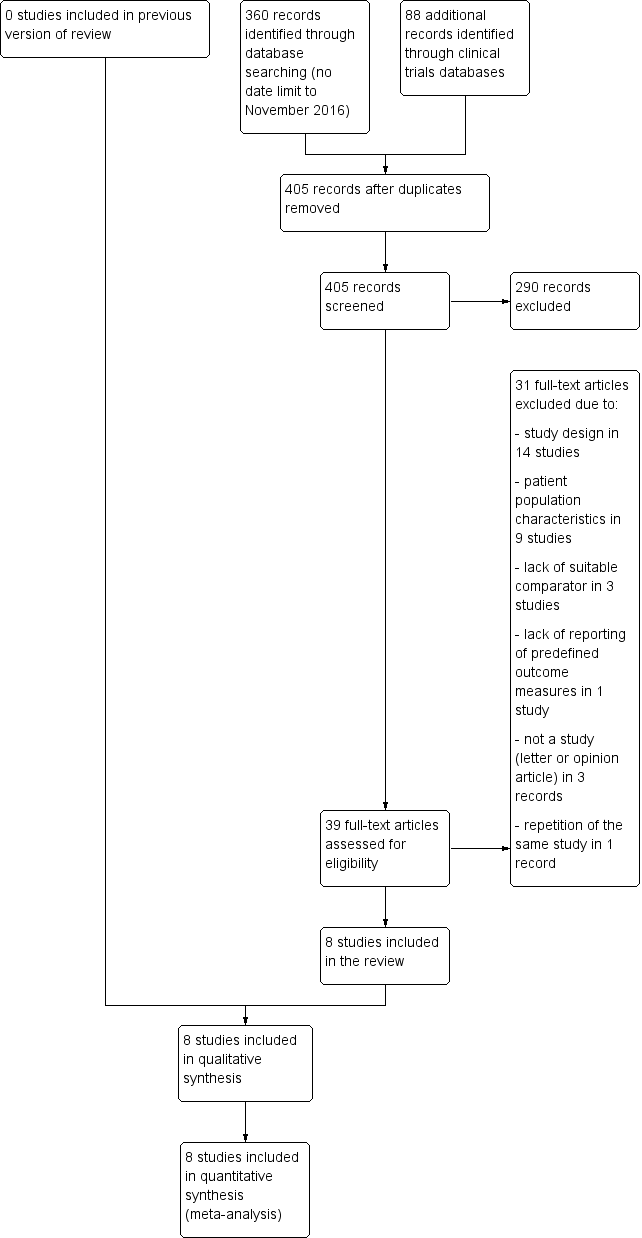
Study flow diagram.
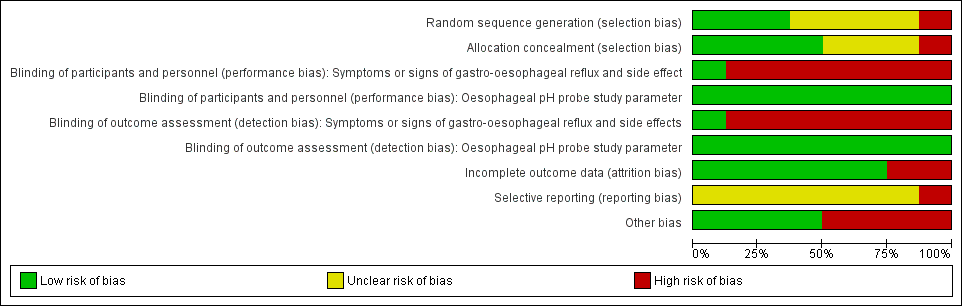
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Regurgitation, posseting, or vomiting, outcome: 1.1 Number of episodes per day.
Assumptions
1. There was insufficient information in Chao 2007 to report the change of baseline value, hence we used the endpoint data instead. Change from baseline value was used for the remaining five studies, where P value was used to determine the standard deviation for the change from baseline value.
2. Frequency of regurgitation rather than vomiting was used for the Xinias 2005 study.
3. In Miller 1999, median number of episodes of regurgitation was reported rather than the mean value. As the sample size was more than 25, it was assumed that median and mean were similar (Hozo 2005), and the standard deviation for the mean difference was obtained using the reported P value (Higgins 2011).
4. We halved control groups for Hegar 2008 and Moya 1999, as these were three‐arm trials involving one control and two intervention arms.
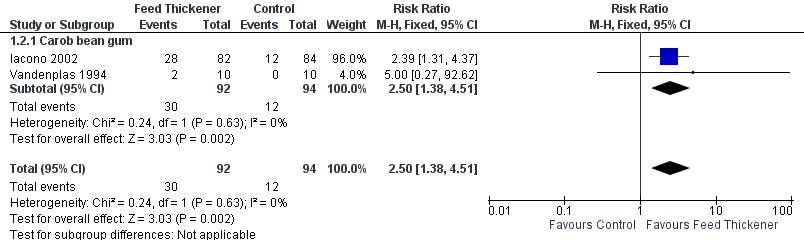
Forest plot of comparison: 1 Regurgitation, posseting, or vomiting, outcome: 1.2 Proportion of asymptomatic infants.

Forest plot of comparison: 2 Oesophageal pH probe study parameters, outcome: 2.1 Reflux index (percentage of time pH < 4).
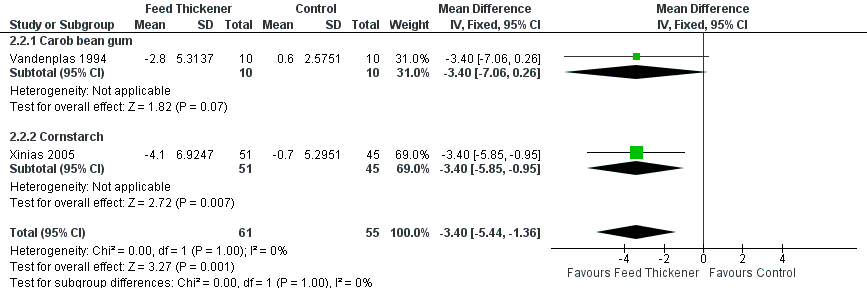
Forest plot of comparison: 2 Oesophageal pH probe study parameters, outcome: 2.2 Number of reflux episodes lasting > 5 minutes.
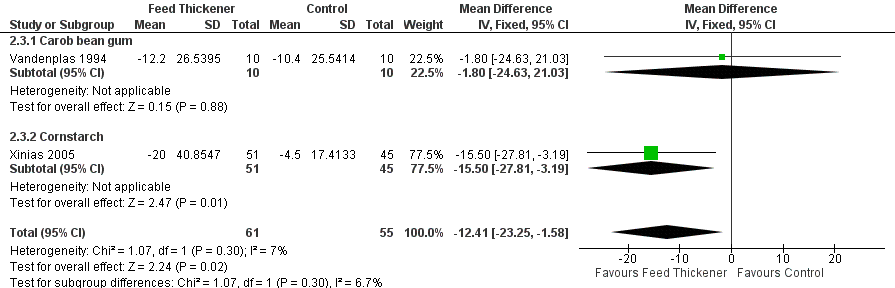
Forest plot of comparison: 2 Oesophageal pH probe study parameters, outcome: 2.3 Duration of longest reflux episode (minutes).
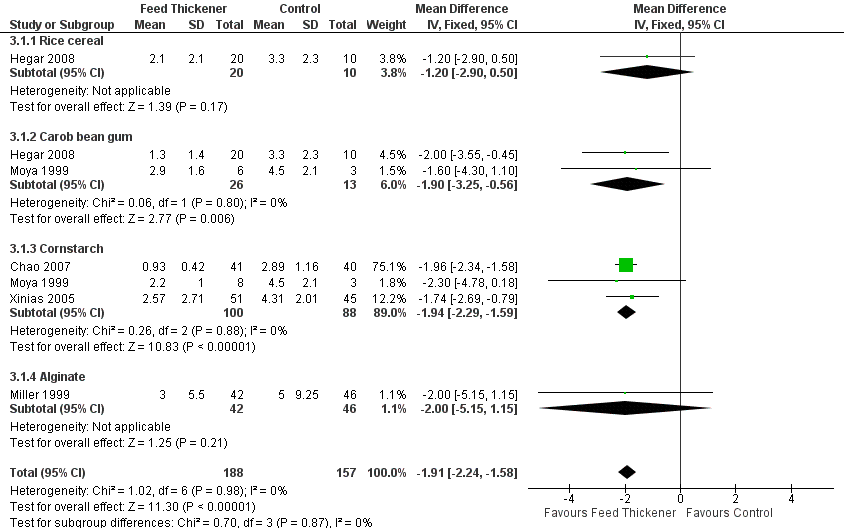
Forest plot of comparison: 3 Sensitivity analysis, outcome: 3.1 Endpoint value ‐ number of episodes of regurgitation, posseting, or vomiting per day.

Comparison 1 Regurgitation, posseting, or vomiting, Outcome 1 Number of episodes per day.

Comparison 1 Regurgitation, posseting, or vomiting, Outcome 2 Proportion of asymptomatic infants.

Comparison 2 Oesophageal pH probe study parameters, Outcome 1 Reflux Index (percentage of time pH < 4).

Comparison 2 Oesophageal pH probe study parameters, Outcome 2 Number of reflux episodes lasting > 5 minutes.
Comparison 2 Oesophageal pH probe study parameters, Outcome 3 Duration of longest reflux episode (minutes).

Comparison 3 Sensitivity analysis, Outcome 1 Endpoint value ‐ number of episodes of regurgitation, posseting, or vomiting per day.
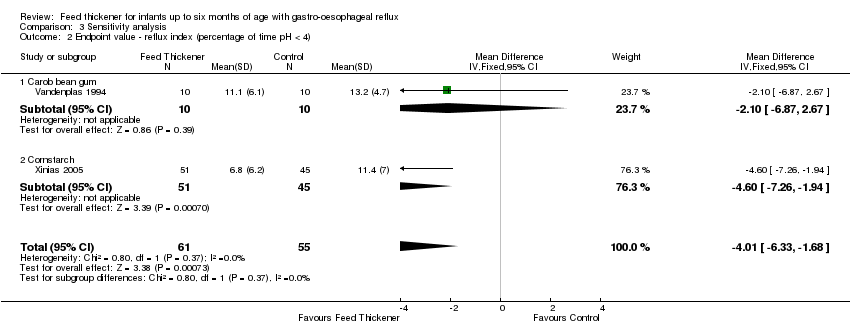
Comparison 3 Sensitivity analysis, Outcome 2 Endpoint value ‐ reflux index (percentage of time pH < 4).
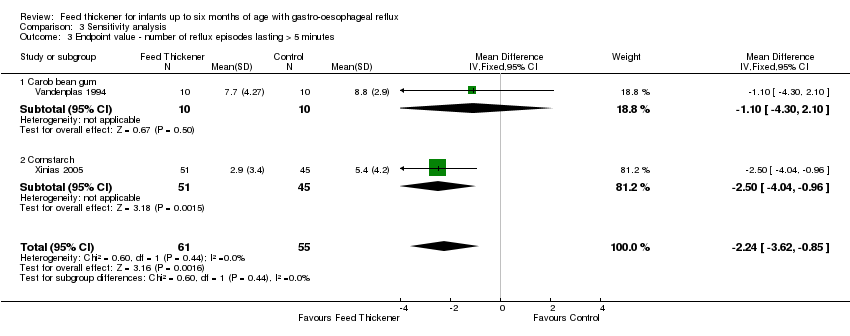
Comparison 3 Sensitivity analysis, Outcome 3 Endpoint value ‐ number of reflux episodes lasting > 5 minutes.
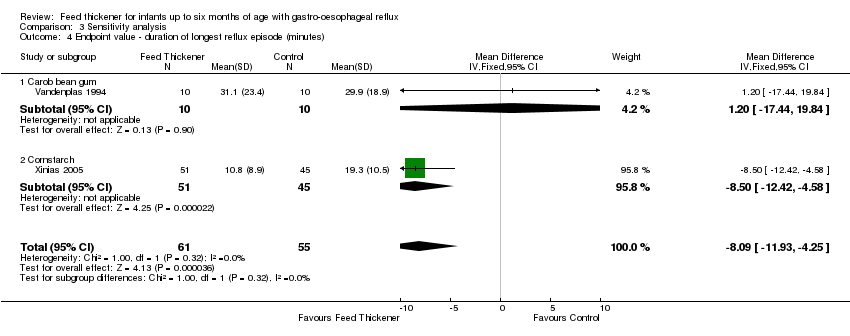
Comparison 3 Sensitivity analysis, Outcome 4 Endpoint value ‐ duration of longest reflux episode (minutes).
| Feed thickener compared to control for infants up to 6 months of age with gastro‐oesophageal reflux | ||||||
| Patient or population: Formula‐fed healthy term infants up to 6 months of age with gastro‐oesophageal reflux | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with feed thickener | |||||
| Number of episodes of regurgitation or vomiting per day | The mean number of episodes of regurgitation or vomiting per day was 3 episodes per day. | MD 1.97 episodes per day lower | ‐ | 442 | ⊕⊕⊕⊝ | Change from baseline value was used for 5 studies. Endpoint value was used for the remaining study due to insufficient data (Chao 2007). Frequency of regurgitation value was used in preference to frequency of vomiting in 1 study (Xinias 2005). |
| Proportion of infants without regurgitation or vomiting at the end of intervention period (asymptomatic infants) | Study population | RR 2.50 | 186 | ⊕⊕⊝⊝ | ||
| 128 per 1000 | 319 per 1000 | |||||
| Reflux index (percentage of time pH < 4) assessed with oesophageal pH probe study | The mean reflux index was 12%. | MD 5.08% lower | ‐ | 116 | ⊕⊕⊝⊝ | Higher reflux index indicates higher percentage of total time that oesophageal pH is less than 4. |
| Number of reflux episodes lasting > 5 minutes assessed with oesophageal pH probe study | The mean number of reflux episodes lasting > 5 minutes was 6 episodes. | MD 3.4 episodes lower | ‐ | 116 | ⊕⊕⊝⊝ | |
| Duration of longest reflux episode | The mean duration of longest reflux episode was 20 minutes. | MD 12.41 minutes lower | ‐ | 116 | ⊕⊕⊝⊝ | |
| Diarrhoea | ‐ | ‐ | 511 | ⊕⊕⊝⊝ | Insufficient data to perform analysis. No difference in diarrhoea incidence or stooling frequency in 4 studies. 17% of infants in the intervention group in Iacono 2002 and 10% of total infants in Hegar 2008 withdrew due to diarrhoea. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for serious study limitation. There was unclear risk of bias for allocation concealment and high risk of bias for blinding, as frequency of regurgitation was dependent on parental report, who were likely to note the higher viscosity of the thickened formula. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of episodes per day Show forest plot | 6 | 442 | Mean Difference (IV, Fixed, 95% CI) | ‐1.97 [‐2.32, ‐1.61] |
| 1.1 Rice cereal | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐3.36, 0.49] |
| 1.2 Carob bean gum | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.47 [‐3.13, 0.19] |
| 1.3 Cornstarch | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐2.35, ‐1.61] |
| 1.4 Alginate | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐6.07, ‐0.93] |
| 2 Proportion of asymptomatic infants Show forest plot | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.38, 4.51] |
| 2.1 Carob bean gum | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.38, 4.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reflux Index (percentage of time pH < 4) Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐5.08 [‐8.89, ‐1.28] |
| 1.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐9.36, 1.56] |
| 1.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐11.50, ‐0.90] |
| 2 Number of reflux episodes lasting > 5 minutes Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.44, ‐1.36] |
| 2.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.4 [‐7.06, 0.26] |
| 2.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.85, ‐0.95] |
| 3 Duration of longest reflux episode (minutes) Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐12.41 [‐23.25, ‐1.58] |
| 3.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐24.63, 21.03] |
| 3.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐15.5 [‐27.81, ‐3.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint value ‐ number of episodes of regurgitation, posseting, or vomiting per day Show forest plot | 5 | 345 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐2.24, ‐1.58] |
| 1.1 Rice cereal | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.90, 0.50] |
| 1.2 Carob bean gum | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.25, ‐0.56] |
| 1.3 Cornstarch | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐2.29, ‐1.59] |
| 1.4 Alginate | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.15, 1.15] |
| 2 Endpoint value ‐ reflux index (percentage of time pH < 4) Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐4.01 [‐6.33, ‐1.68] |
| 2.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐6.87, 2.67] |
| 2.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.26, ‐1.94] |
| 3 Endpoint value ‐ number of reflux episodes lasting > 5 minutes Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐3.62, ‐0.85] |
| 3.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐4.30, 2.10] |
| 3.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐4.04, ‐0.96] |
| 4 Endpoint value ‐ duration of longest reflux episode (minutes) Show forest plot | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐8.09 [‐11.93, ‐4.25] |
| 4.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐17.44, 19.84] |
| 4.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐8.5 [‐12.42, ‐4.58] |

