Переливание крови для профилактики первичного и вторичного инсульта у людей с серповидноклеточной анемией
Appendices
Appendix 1. Search Strategies
CENTRAL (CENTRAL & DARE) (issue 4, 2016; issue 2, 2015 respectively)
#1 MeSH descriptor: [Anemia, Sickle Cell] explode all trees
#2 MeSH descriptor: [Hemoglobin, Sickle] explode all trees
#3 ("hemoglobin S" or "haemoglobin S" or "hemoglobin SC" or "haemoglobin SC" or "hemoglobin SE" or "haemoglobin SE" or "hemoglobin SS" or "haemoglobin SS" or "hemoglobin C disease" or "hemoglobin D disease" or "hemoglobin E disease" or "haemoglobin C disease" or "haemoglobin D disease" or "haemoglobin E disease" or "Hb SC" or HbSC or HbAS or HbSS or HbAC or "Hb SE" or "Hb SS" or "Hb C disease" or "Hb D disease" or "Hb E disease" or "SC disease" or "SC diseases")
#4 (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*)
#5 (sickle and SCD)
#6 ((Hb S or HbS or sickle) near/3 (disease* or thalassemi* or thalassaemi*))
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Cerebrovascular Disorders] explode all trees
#9 ((brain* or cerebr* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or anterior circulation or basilar artery or vertebral artery or subcortical or cortical or choroidal) near/5 (ischemi* or ischaemi* or infarct* or thrombo* or phlebothrombo* or stenosis or emboli* or occlus* or vaso‐occlus* or vasocclus* or vasooclus* or obstruction* or hypoxi* or accident* or abnormalit* or vasculopath* or vasospasm* or disorder* or damage* or disease* or lesion* or insufficien*))
#10 ((ischemi* or ischaemi*) near/5 (apoplex* or attack* or accident* or seizure* or arrest* or injur* or failure* or lesion* or insult*))
#11 (stroke* or CVA or silent infarct* or incomplete infarct*)
#12 MeSH descriptor: [Cerebrovascular Circulation] explode all trees
#13 MeSH descriptor: [Ultrasonography, Doppler, Transcranial] this term only
#14 (transcranial* near/3 (doppler or ultrasound or sonograph* or ultrasonograph*))
#15 ((brain* or cerebr* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or anterior circulation or basilar artery or vertebral artery or subcortical or cortical or choroidal) near/3 (hemodynamics or haemodynamics or blood flow or blood velocit* or volume))
#16 MeSH descriptor: [Cerebral Arteries] explode all trees
#17 MeSH descriptor: [Blood Volume Determination] this term only
#18 MeSH descriptor: [Blood Flow Velocity] this term only
#19 (TCD near/3 (flow* or velocit*))
#20 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19
#21 #7 and #20
MEDLINE (OvidSP, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, 1946 to 4 April 2016)
1. exp Anemia, Sickle Cell/
2. Hemoglobin, Sickle/
3. (h?emoglobin S or h?emoglobin SC or h?emoglobin SE or h?emoglobin SS or h?emoglobin C disease or h?emoglobin D disease or h?emoglobin E disease or Hb SC or HbSC or HbAS or HbSS or HbAC or Hb SE or Hb SS or Hb C disease or Hb D disease or Hb E disease or SC disease*).tw,kf.
4. (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*).tw,kf.
5. (sickle and SCD).tw,kf.
6. ((Hb S or HbS or sickle) adj3 (disease* or thalass?emi*)).tw,kf.
7. or/1‐6
8. exp Cerebrovascular Disorders/
9. ((brain* or cerebr* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or anterior circulation or basilar artery or vertebral artery or subcortical or cortical or choroidal) adj5 (isch?emi* or infarct* or thrombo* or phlebothrombo* or stenosis or emboli* or occlus* or vaso‐occlus* or vasocclus* or vasooclus* or obstruction* or hypoxi* or accident* or abnormalit* or vasculopath* or vasospasm* or disorder* or damage* or disease* or lesion* or insufficien*)).tw,kf.
10. (isch?emi* adj5 (apoplex* or attack* or accident* or seizure* or arrest* or injur* or failure* or lesion* or insult*)).tw,kf.
11. (stroke* or CVA or silent infarct* or incomplete infarct*).tw,kf.
12. or/8‐11
13. exp Cerebrovascular Circulation/
14. Ultrasonography, Doppler, Transcranial/
15. (transcranial adj3 (doppler or ultrasound or sonograph* or ultrasonograph*)).tw,kf.
16. ((brain* or cerebr* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or anterior circulation or basilar artery or vertebral artery or subcortical or cortical or choroidal) adj3 (h?emodynamics or blood flow or blood velocit* or volume)).tw,kf.
17. exp Cerebral Arteries/us
18. Blood Volume Determination/
19. Blood Flow Velocity/
20. (TCD adj3 (flow* or velocit*)).tw,kf.
21. or/13‐20
22. 12 or 21
23. 7 and 22
Embase (OvidSP, 1974 to 4 April 2016)
1. exp Sickle Cell Anemia/
2. Hemoglobin S/
3. (h?emoglobin S or h?emoglobin SC or h?emoglobin SE or h?emoglobin SS or h?emoglobin C disease or h?emoglobin D disease or h?emoglobin E disease or Hb SC or HbSC or HbAS or HbSS or HbAC or Hb SE or Hb SS or Hb C disease or Hb D disease or Hb E disease or SC disease*).tw.
4. (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*).tw.
5. (sickle and SCD).tw.
6. ((Hb S or HbS or sickl*) adj3 (disease* or thalass?emi*)).tw.
7. or/1‐6
8. exp Cerebrovascular Disease/
9. (isch?emi* adj5 (apoplex* or attack* or accident* or seizure* or arrest* or injur* or failure* or lesion* or insult*)).tw.
10. ((brain* or cerebr* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or anterior circulation or basilar artery or vertebral artery or subcortical or cortical or choroidal) adj5 (isch?emi* or infarct* or thrombo* or phlebothrombo* or stenosis or emboli* or occlus* or vaso‐occlus* or vasocclus* or vasooclus* or obstruction* or hypoxi* or accident* or abnormalit* or vasculopath* or vasospasm* or disorder* or damage* or disease* or lesion* or insufficien*)).tw.
11. (stroke* or CVA or silent infarct* or incomplete infarct*).tw.
12. or/8‐11
13. exp Brain Circulation/
14. Doppler Echography/
15. exp Brain Artery/
16. Brain Blood Volume/
17. Blood Flow Velocity/
18. (TCD adj3 (flow* or velocit*)).tw.
19. ((brain* or cerebr* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or anterior circulation or basilar artery or vertebral artery or subcortical or cortical or choroidal) adj3 (h?emodynamics or blood flow or blood velocit* or volume)).tw.
20. (transcranial adj3 (doppler or ultrasound or sonograph* or ultrasonograph*)).tw.
21. or/13‐20
22. 12 or 21
23. 7 and 22
CINAHL (EBSCOHost, 1937 to 4 April 2016)
S1 (MH "Anemia, Sickle Cell+")
S2 TX ("hemoglobin S" or "haemoglobin S" or "hemoglobin SC" or "haemoglobin SC" or "hemoglobin SE" or "haemoglobin SE" or "hemoglobin SS" or "haemoglobin SS" or "hemoglobin C disease" or "hemoglobin D disease" or "hemoglobin E disease" or "haemoglobin C disease" or "haemoglobin D disease" or "haemoglobin E disease" or "Hb SC" or HbSC or HbSS or HbAC or "Hb SE" or "Hb SS" or "Hb C disease" or "Hb D disease" or "Hb E disease" or "SC disease" or "SC diseases" OR sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*)
S3 TX ((Hb S or HbS or sickle) N3 (disease* or thalass?emi*))
S4 S1 OR S2 OR S3
S5 (MH "Cerebrovascular Disorders+") S6 TX ((brain* or cerebr* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or anterior circulation or basilar artery or vertebral artery or subcortical or cortical or choroidal) N5 (ischemi* or ischaemi* or infarct* or thrombo* or phlebothrombo* or stenosis or emboli* or occlus* or vaso‐occlus* or vasocclus* or vasooclus* or obstruction* or hypoxi* or accident* or abnormalit* or vasculopath* or vasospasm* or disorder* or damage* or disease* or lesion* or insufficien*))
S7 TX ((ischemi* or ischaemi*) N5 (apoplex* or attack* or accident* or seizure* or arrest* or injur* or failure* or lesion* or insult*))
S8 TX (stroke* or CVA or silent infarct* or incomplete infarct*)
S9 (MH "Cerebrovascular Circulation+")
S10 (MH "Ultrasonography, Doppler, Transcranial")
S11 TX (transcranial N3 (doppler or ultrasound or sonograph* or ultrasonograph*))
S12 TX ((brain* or cerebr* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or anterior circulation or basilar artery or vertebral artery or subcortical or cortical or choroidal) N3 (hemodynamics or haemodynamics or blood flow or blood velocit* or volume))
S13 (MH "Cerebral Arteries+/US")
S14 (MH "Blood Volume Determination") S15 (MH "Blood Flow Velocity") S16 (TCD N3 (flow* or velocit*))
S17 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16
S18 S4 AND S17
S19 (MH Clinical Trials+)
S20 PT Clinical Trial
S21 TI ((controlled trial*) or (clinical trial*)) OR AB ((controlled trial*) or (clinical trial*))
S22 TI ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) OR AB ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*))
S23 TI randomi* OR AB randomi*
S24 MH RANDOM ASSIGNMENT
S25 TI ((phase three) or (phase III) or (phase three)) or AB ((phase three) or (phase III) or (phase three))
S26 ( TI (random* N2 (assign* or allocat*)) ) OR ( AB (random* N2 (assign* or allocat*)) )
S27 MH PLACEBOS
S28 MH META ANALYSIS
S29 MH SYSTEMATIC REVIEW
S30 TI ("meta analys*" OR metaanalys* OR "systematic review" OR "systematic overview" OR "systematic search*") OR AB ("meta analys*" OR metaanalys* OR "systematic review" OR "systematic overview" OR "systematic search*")
S31 TI ("literature review" OR "literature overview" OR "literature search*") OR AB ("literature review" OR "literature overview" OR "literature search*")
S32 TI (cochrane OR embase OR cinahl OR cinhal OR lilacs OR BIDS OR science AND citation AND index OR cancerlit) OR AB (cochrane OR embase OR cinahl OR cinhal OR lilacs OR BIDS OR science AND citation AND index OR cancerlit)
S33 TI placebo* OR AB placebo*
S34 MH QUANTITATIVE STUDIES
S35 S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34
S36 S18 AND S35
PubMed (for epublications ahead of print, in‐process & other non‐indexed citations only on 4 April 2016)
#1 ("hemoglobin S" OR "haemoglobin S" OR "hemoglobin SC" OR "haemoglobin SC" OR "hemoglobin SE" OR "haemoglobin SE" OR "hemoglobin SS" OR "haemoglobin SS" OR "hemoglobin C disease" OR "hemoglobin D disease" OR "hemoglobin E disease" OR "haemoglobin C disease" OR "haemoglobin D disease" OR "haemoglobin E disease" OR "Hb SC" OR HbSC OR HbAS OR HbSS OR HbAC OR "Hb SE" OR "Hb SS" OR "Hb C disease" OR "Hb D disease" OR "Hb E disease" OR "SC disease" OR "SC diseases" OR sickle* OR sickled OR sickling OR meniscocyt* OR drepanocyt*)
#2 (("Hb S" OR HbS) AND (disease* OR thalassemi* OR thalassaemi*))
#3 #1 OR #2
#4 ((ischemi* OR ischaemi*) AND (apoplex* OR attack* OR accident* OR seizure* OR arrest* OR injur* OR failure* OR lesion* OR insult*))
#5 (stroke* OR CVA OR silent infarct* OR incomplete infarct*)
#6 (transcranial* AND (doppler OR ultrasound OR sonograph* OR ultrasonograph*))
#7 ((brain OR brains OR cerebral OR cerebrovascular OR cerebro‐vascular OR vertebrobasil* OR hemispher* OR intracran* OR intracerebral OR infratentorial OR supratentorial OR MCA OR "anterior circulation" OR "basilar artery" OR "vertebral artery" OR subcortical OR cortical OR choroidal) AND (hemodynamics OR haemodynamics OR "blood flow" OR blood velocit* OR volume))
#8 (TCD AND (flow* OR velocit*))
#9 #4 OR #5 OR #6 OR #7 OR #8
#10 #3 AND #9
TRANSFUSION EVIDENCE LIBRARY (1950 to April 4 2016)
sickle AND (ischemic OR ischaemic OR ischemia OR ischaemia OR stroke OR infarct OR doppler OR transcranial OR ultrasound OR sonography OR ultrasonography)
LILACS (1982 to April 4 2016)
tw:(sickle) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
IndMed (1986 to April 4 2016)
(sickle OR sickled OR sickling OR SC disease) AND (ischemic OR ischaemic OR ischemia OR ischaemia OR stroke OR strokes OR subcortical OR cortical OR choroidal OR infarct OR infarcts OR doppler OR transcranial OR ultrasound OR sonography OR ultrasonography) AND (random OR randomly OR randomised OR randomized OR blind OR blinded OR control group OR placebo OR controlled study OR groups OR trial OR trials OR systematic review OR meta‐analysis OR metaanalysis OR literature search OR medline OR pubmed OR cochrane OR embase)
KoreaMed (1997 to April 4 2016)
sickle [TI] OR sickle [AB] "Randomized Controlled Trial" [PT]
Web of Science CPCI‐S (Conference Proceedings Citation Index‐ Science (CPCI‐S) ‐ 1990 to to April 4 2016)
TOPIC: (sickle OR sickled OR sickling) AND
TOPIC: (ischemic OR ischaemic OR ischemia OR ischaemia OR stroke OR strokes OR infarct OR infarcts OR brain OR hemisphere* OR intracranial OR intracerebral OR cerebral OR cerebrovascular OR subcortical OR cortical OR choroidal OR doppler OR transcranial OR ultrasound OR sonography OR ultrasonography) AND
TOPIC: (randomly OR randomised OR randomized OR blind OR blinded OR control group OR placebo OR controlled study OR groups OR trial OR trials OR systematic review OR meta‐analysis OR metaanalysis OR literature search OR medline OR pubmed OR cochrane OR embase)
ClinicalTrials.gov
Search Terms: ischemia OR stroke OR infarct OR brain OR hemisphere OR intracranial OR intracerebral OR cerebral OR cerebrovascular OR subcortical OR cortical OR choroidal OR doppler OR transcranial OR ultrasound OR sonography OR ultrasonography
Condition: sickle cell anemia
Study Type: Interventional Studies
WHO ICTRP
Title/Intervention: ischemia OR stroke OR infarct OR brain OR hemisphere OR intracranial OR intracerebral OR cerebral OR cerebrovascular OR subcortical OR cortical OR choroidal OR doppler OR transcranial OR ultrasound OR sonography OR ultrasonography
Condition: sickle cell anemia
Recruitment Status: ALL

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
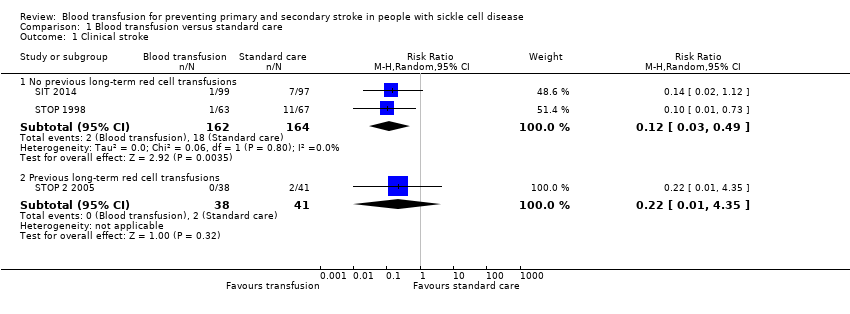
Comparison 1 Blood transfusion versus standard care, Outcome 1 Clinical stroke.
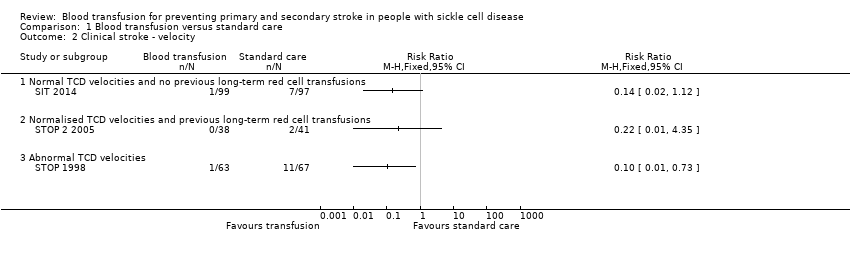
Comparison 1 Blood transfusion versus standard care, Outcome 2 Clinical stroke ‐ velocity.

Comparison 1 Blood transfusion versus standard care, Outcome 3 Clinical stroke ‐ SCI.

Comparison 1 Blood transfusion versus standard care, Outcome 4 Mortality.
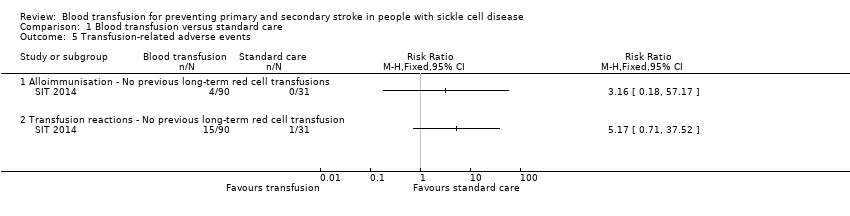
Comparison 1 Blood transfusion versus standard care, Outcome 5 Transfusion‐related adverse events.

Comparison 1 Blood transfusion versus standard care, Outcome 6 TIA.
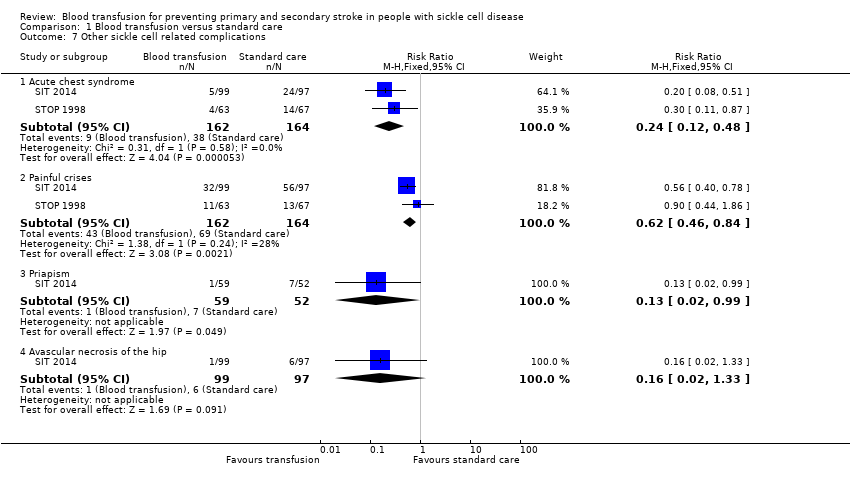
Comparison 1 Blood transfusion versus standard care, Outcome 7 Other sickle cell related complications.

Comparison 2 Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 1 Clinical stroke ‐ Secondary prevention.

Comparison 2 Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 2 Mortality.

Comparison 2 Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 3 Transfusion‐related complications ‐ Serum ferritin; Primary prevention.

Comparison 2 Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 4 Transfusion related complications ‐ Liver iron concentration ‐ Primary prevention.
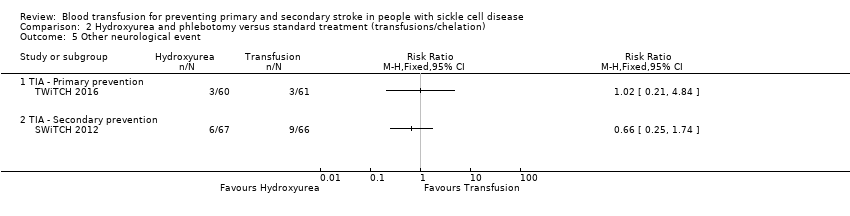
Comparison 2 Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 5 Other neurological event.

Comparison 2 Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 6 Other sickle cell related complications.

Comparison 2 Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 7 Haemoglobin levels.

Comparison 2 Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 8 Haemoglobin S levels.
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have not had previous long‐term red cell transfusions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard care | Risk with Blood transfusion | |||||
| Clinical stroke | Trial population | RR 0.12 | 326 | ⊕⊕⊕⊝ | ||
| 110 per 1000 | 13 per 1000 (3 to 54) | |||||
| All‐cause mortality | No deaths occurred in either trial arm | ‐ | 326 | ⊕⊝⊝⊝ | ||
| Adverse events associated with transfusion | Moderatea | RR 3.16 | 121 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 32 per 1000 (2 to 572) | |||||
| TIA | Trial population | Peto OR 0.13 (0.01 to 2.11) | 323 (2 RCTs) | ⊕⊝⊝⊝ | ||
| 21 per 1000 | 5 per 1000 (0 to 43) | |||||
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 0.24 | 326 | ⊕⊕⊝⊝ | ||
| 232 per 1,000 | 56 per 1000 (28 to 111) | |||||
| Moderate | ||||||
| 230 per 1000 | 55 per 1000 (28 to 110) | |||||
| Measures of neurological impairment assessed with: WASI IQ score | Least square mean 1.7 (SE 95% CI ‐1.1 to 4.4) | ‐ | 166 (1 RCT) | ⊕⊕⊝⊝ | Author reported data from SIT 2014 | |
| Quality of life assessed with: Child Health Questionnaire Parent Form 50 | Difference estimate ‐0.54 (‐0.92 to ‐0.17) | ‐ | 196 (1 RCT) | ⊕⊕⊝⊝ | Author reported data from SIT 2014 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by 1 due to imprecision. Rare event. No deaths occurred. 2 We downgraded the quality of the evidence by 1 due to risk of bias. Unblinded trial and cross‐overs, and imbalance between loss to follow‐up between trial arms 3 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 4 We downgraded the quality of evidence by 2 due to imprecision. The estimate has very wide CIs a Based on Chou 2013 | ||||||
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have had long‐term red cell transfusions to prevent a stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard care | Risk with blood transfusion | |||||
| Clinical stroke | Trial population | RR 0.22 (0.01 to 4.35) | 79 | ⊕⊝⊝⊝ | ||
| 49 per 1000 | 11 per 1000 (0 to 212) | |||||
| All‐cause mortality | Moderatea | Peto OR 8.00 (0.16 to 404.12) | 79 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 75 per 1000 (2 to 803) | |||||
| Adverse events associated with transfusion | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| TIA | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| Serious adverse events as a result of sickle cell‐related complications assessed with: ACS | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We did not downgrade the evidence due to risk of bias because the evidence was already very low grade evidence. There was attrition bias. Imbalance between loss to follow‐up between trial arms 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of evidence by 2 due to imprecision. The estimate has very wide CIs a Assuming a mortality rate of 1% | ||||||
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have had long‐term red cell transfusions to prevent a stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with hydroxyurea and phlebotomy | Risk with Blood transfusion | |||||
| Clinical stroke | No strokes occurred in either trial arm | ‐ | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| All‐cause mortality | No deaths occurred in either trial arm | ‐ | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse events associated with transfusion | The mean liver iron concentration was 9.5 mg Fe/g dry weight | MD 1.8 mg Fe/g dry weight lower (5.16 lower to 1.56 higher) | ‐ | 121 (1 RCT) | ⊕⊕⊝⊝ | Switching to hydroxyurea and phlebotomy may reduce serum ferritin levels compared to continuing to receive red cell transfusions and chelation (MD) ‐1398 μg/L, 95% CI ‐1929 to ‐867; one trial, 121 participants) |
| Incidence of TIA | 49 per 1000 | 50 per 1,000 (10 to 238) | RR 1.02 (0.21 to 4.84) | 121 (1 RCT) | ⊕⊝⊝⊝ | |
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 2.03 (0.39 to 10.69) | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| 33 per 1000 | 67 per 1,000 (13 to 350) | |||||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of the evidence by 2 due to imprecision. Rare event. No deaths or stroke occurred. 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of the evidence by 1 due to risk of bias.Trial was not blinded and stopped early 4 We downgraded the quality of the evidence by 1 due to imprecision. The estimate has very wide CIs | ||||||
| Secondary prevention | ||||||
| Patient or population: individuals with sickle cell disease who have had a stroke who have had long‐term red cell transfusions to prevent another stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with hydroxyurea and phlebotomy | Risk with Blood transfusion | |||||
| Clinical stroke | Trial population | RR 14.78 | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| All‐cause mortality | 15 per 1000 | 15 per 1000 (1 to 198) | Peto OR 0.98 (0.06 to 15.92) | 133 (1 RCT) | ⊕⊝⊝⊝ | |
| Transfusion‐related adverse events ‐ assessed with liver iron concentration mg Fe/g dry weight liver | Hydroxyurea arm: median 17.2 mg IQR 10.0 to 30.6 Transfusion arm: median 17.3 mg IQR 8.8 to 30.7 | 56 (1 RCT) | ⊕⊕⊝⊝ | P = 0.7920a Switching to hydroxyurea and phlebotomy may reduce serum ferritin levels compared to continuing to receive red cell transfusions and chelation 1994 μg/L, interquartile range (IQR) 998 to 3475, in the hydroxyurea arm and 4064 μg/L, IQR 2330 to 7126, in the transfusion arm; one trial, 133 participants; P < 0.001 a | ||
| Incidence of TIA | Trial population | RR 0.66 | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 136 per 1000 | 90 per 1000 (34 to 237) | |||||
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 0.33 (0.04 to 3.08) | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 45 per 1000 | 15 per 1000 (2 to 140) | |||||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of the evidence by 1 due to risk of bias. Trial was not blinded and stopped early 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of the evidence by 1 due to imprecision. The estimate has very wide CIs a Analysis performed by the trial authors | ||||||
| Outcomes | Trials | Number of participants with at least one event | Adverse events/100 person‐years | Incidence rate ratioc (95% CI) | ||
| Transfusion | Standard | Transfusion | Standard | |||
| Transfusion reactions | 15 out of 90a | 1 out of 31b | 8.85 | 1.66 | 5.33 (1.67 to 23.52) | |
| Ferritin > 1500 μg/L | 76 out of 90a | 3 out of 31b | 534.70 | 37.07 | 14.42 (5.41 to 85.17) | |
| aNine participants who declined transfusion were excluded from the analysis. Abbreviations: CI: confidence interval | ||||||
| Outcomes | Trials | Number of participants with at least one event | Adverse events/100 person‐years | Incidence rate ratioa (95% CI) | ||
| Transfusion | Standard | Transfusion | Standard | |||
| Acute chest syndrome | 4 out of 63 | 14 out of 67 | 4.8b | 15.3b | ‐ | |
| 5 out of 99 | 24 out of 97 | 1.81b | 14.35b | 0.41 (0.20 to 0.75) | ||
| Painful crisis | 11 out of 63 | 13 out of 67 | 16.2 | 27.6 | ‐ | |
| 32 out of 99 | 56 out of 97 | 41.58 | 102.21 | 0.13 (0.04 to 0.28) | ||
| Priapism | 1 out of 59 | 7 out of 52 | 0.84 | 6.65 | 0.13 (0.03 to 0.55) | |
| Symptomatic avascular necrosis of the hip | 1 out of 99 | 6 out of 97 | 0.4 | 2.25 | 0.22 (0.05 to 0.85) | |
| a The incidence ratio was calculated as the rate of adverse events per 100 person‐years in the transfusion group divided by the rate of adverse events per 100 person‐years in the observation group. The 95% confidence intervals were calculated with the use of the bootstrap method with 10,000 replications. b One child from the standard care group was excluded from these analyses due to a stroke on day 16 of the trial. Abbreviation: CI: confidence interval | ||||||
| Trial | Intervention | Baseline | 6 to 12 months | 12 to 18 months | 18 to 24 months | ||||
| Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | ||
| No previous long‐term transfusions | |||||||||
| Transfusion | Median 77 IQR (72 to 84) | Median 85 90% CI (51 to 95) | ‐ | Median 30 90% CI (17 to 43) | ‐ | Median 29 90% CI (16 to 43) | ‐ | Median 30 90% CI (16 to 43) | |
| Standard | Median 79 IQR (74 to 89) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Transfusion | Mean (SD) 72 (8) | Mean (SD) 87 (10) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Standard | Mean (SD) 76 (7) | Mean (SD) 87 (7) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Previous long‐term transfusions | |||||||||
| Transfusion | Mean (SD) 93 (9) | Mean (SD) 21 (8.6) | Mean (SD) 94 (9) | Mean (SD) 25.4 (10.9) | ‐ | ‐ | ‐ | ‐ | |
| Standard | Mean (SD) 98 (12) | Mean (SD) 19 (11) | Mean (SD) 77 (8) | Mean (SD) 81.0 (8.6) | ‐ | ‐ | ‐ | ‐ | |
| Abbreviations: CI: confidence interval; IQR: interquartile range; SD: standard deviation | |||||||||
| Trial | Number of transfusions | Number of HbS levels measured | HbS less than 30% | HbS 30 to 40% | HbS greater than 40% |
| No previous long‐term transfusions | |||||
| 1521 | ‐ | ‐ | 101 | 42 | |
| Previous long‐term transfusions | |||||
| 1070 | 988 | 748 (76%) | 192 (19%) | 48 (5%) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical stroke Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 No previous long‐term red cell transfusions | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.49] |
| 1.2 Previous long‐term red cell transfusions | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.01, 4.35] |
| 2 Clinical stroke ‐ velocity Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Normal TCD velocities and no previous long‐term red cell transfusions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Normalised TCD velocities and previous long‐term red cell transfusions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Abnormal TCD velocities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Clinical stroke ‐ SCI Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Presence of previous SCI on MRI | 2 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.59] |
| 3.2 Absence of previous SCI on MRI | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.31] |
| 4 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Transfusion‐related adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Alloimmunisation ‐ No previous long‐term red cell transfusions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Transfusion reactions ‐ No previous long‐term red cell transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 TIA Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 No previous long‐term red cell transfusions | 2 | 323 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.11] |
| 7 Other sickle cell related complications Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Acute chest syndrome | 2 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.12, 0.48] |
| 7.2 Painful crises | 2 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.46, 0.84] |
| 7.3 Priapism | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.99] |
| 7.4 Avascular necrosis of the hip | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical stroke ‐ Secondary prevention Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mortality Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mortality ‐ Primary prevention | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mortality ‐ Secondary prevention | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Transfusion‐related complications ‐ Serum ferritin; Primary prevention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Transfusion related complications ‐ Liver iron concentration ‐ Primary prevention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Other neurological event Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 TIA ‐ Primary prevention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 TIA ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Other sickle cell related complications Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Total SCD‐related SAEs ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Acute chest syndrome ‐ Primary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Acute chest syndrome ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Painful crisis ‐ Primary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Painful crisis ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Infections and infestations SAEs ‐ Primary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Infections and infestations SAEs ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Haemoglobin levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Haemoglobin S levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

