Transfusión de sangre para la prevención del accidente cerebrovascular primario y secundario en pacientes con anemia de células falciformes
Resumen
Antecedentes
La anemia de células falciformes es uno de los trastornos monogénicos graves más frecuentes en el mundo, debido a la herencia de dos genes de hemoglobina (globina beta) anormales. La anemia de células falciformes puede causar dolor intenso, daño significativo a un órgano diana, complicaciones pulmonares y muerte prematura. El accidente cerebrovascular afecta a alrededor del 10% de los niños con anemia de células falciformes (HbSS). Las transfusiones de sangre crónicas pueden reducir el riesgo de vasoclusión y accidente cerebrovascular al diluir la proporción de células falciformes en la circulación.
Esta es una actualización de una revisión Cochrane publicada por primera vez en 2002 y actualizada por última vez en 2017.
Objetivos
Evaluar los riesgos y los efectos beneficiosos de los regímenes de transfusión sanguínea crónica en pacientes con anemia de células falciformes para la prevención primaria y secundaria de los accidentes cerebrovasculares (excepto los infartos cerebrales silentes).
Métodos de búsqueda
Se hicieron búsquedas de ensayos relevantes en la Biblioteca Cochrane, MEDLINE (desde 1946), Embase (desde 1974), en la Transfusion Evidence Library (desde 1980), y en bases de datos de ensayos en curso; todas las búsquedas se actualizaron hasta el 8 de octubre de 2019.
Se realizaron búsquedas en el Registro de Ensayos de Hemoglobinopatías del Grupo Cochrane de Fibrosis Quística y Trastornos Genéticos (Cochrane Cystic Fibrosis and Genetic Disorders Group): 19 setiembre 2019.
Criterios de selección
Ensayos controlados aleatorizados que compararon las transfusiones de glóbulos rojos como profilaxis del accidente cerebrovascular en pacientes con anemia de células falciformes, con un tratamiento alternativo o estándar. No hubo restricciones con respecto a los resultados examinados, el idioma ni el estado de publicación.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente evaluaron la elegibilidad de los ensayos y el riesgo de sesgo y extrajeron los datos.
Resultados principales
Se incluyeron cinco ensayos (660 participantes) publicados entre 1998 y 2016. Cuatro de estos ensayos terminaron antes de tiempo. La gran mayoría de los participantes presentaban la forma de hemoglobina (Hb)SS de la enfermedad de células falciformes.
Tres ensayos compararon las transfusiones regulares de glóbulos rojos con la atención estándar en la prevención primaria del accidente cerebrovascular, dos en niños sin transfusiones previas a largo plazo, y uno en niños y adolescentes con transfusiones a largo plazo.
Dos ensayos compararon el fármaco hidroxiurea (hidroxicarbamida) y la flebotomía con las transfusiones a largo plazo y el tratamiento de quelación del hierro, uno en la prevención primaria (niños), y otro en la prevención secundaria (niños y adolescentes).
La calidad de la evidencia fue muy baja a moderada en los diferentes resultados según la metodología GRADE. Este hecho se debió a que los ensayos tuvieron alto riesgo de sesgo debido a la falta de cegamiento, la falta de direccionalidad y las estimaciones imprecisas de los resultados.
Transfusiones de glóbulos rojos versus atención estándar
Niños sin transfusiones previas a largo plazo
Las transfusiones a largo plazo probablemente reducen la incidencia de accidentes cerebrovasculares clínicos en los niños con un mayor riesgo de accidente cerebrovascular (velocidades transcraneales anormales del doppler o antecedentes de infarto cerebral silente), riesgo relativo 0,12 (intervalo de confianza del 95%: 0,03 a 0,49; dos ensayos, 326 participantes), evidencia de calidad moderada.
Las transfusiones a largo plazo pueden reducir la incidencia de otras complicaciones relacionadas con la enfermedad de células falciformes (síndrome torácico agudo, riesgo relativo 0,24 [intervalo de confianza del 95%: 0,12 a 0,48]), (dos ensayos, 326 participantes); aumentar la calidad de vida (estimación de la diferencia ‐0,54, intervalo de confianza del 95%: ‐0,92 a ‐0,17) (un ensayo, 166 participantes); pero hay poca o ninguna diferencia en cuanto a las puntuaciones del coeficiente intelectual (media cuadrática mínima: 1,7; intervalo de confianza del 95% del error estándar: ‐1,1 a 4,4) (un ensayo, 166 participantes), evidencia de calidad baja.
No se conoce con certeza si las transfusiones a largo plazo reducen el riesgo de ataques isquémicos transitorios, odds‐ratio de Peto 0,13 (intervalo de confianza del 95%: 0,01 a 2,11) (dos ensayos, 323 participantes); tienen algún efecto sobre la mortalidad por todas las causas, no se informaron muertes (dos ensayos, 326 participantes); o aumentan el riesgo de aloinmunización, riesgo relativo 3,16 (intervalo de confianza del 95%: 0,18 a 57,17) (un ensayo, 121 participantes), evidencia de calidad muy baja.
Niños y adolescentes con transfusiones previas a largo plazo (un ensayo, 79 participantes)
No se conoce con certeza si la continuación de las transfusiones a largo plazo reduce la incidencia de accidente cerebrovascular, riesgo relativo 0,22 (intervalo de confianza del 95%: 0,01 a 4,35); o la mortalidad por todas las causas, odds ratio de Peto 8,00 (intervalo de confianza del 95%: 0,16 a 404,12), evidencia de calidad muy baja.
Varios resultados de la revisión solo se informaron en un brazo del ensayo (complicaciones relacionadas con la enfermedad de células falciformes, aloinmunización, ataques isquémicos transitorios).
El ensayo no informó sobre el deterioro neurológico o la calidad de vida.
Hidroxiurea y flebotomía versus transfusiones de glóbulos rojos y quelación
Ninguno de los ensayos informó sobre el deterioro neurológico, la aloinmunización o la calidad de vida.
Prevención primaria, niños (un ensayo, 121 participantes)
El cambio a la hidroxiurea y la flebotomía puede tener poco o ningún efecto en las concentraciones de hierro en el hígado, diferencia de medias ‐1,80 mg Fe/g de hígado seco (intervalo de confianza del 95%: ‐5,16 a 1,56), evidencia de calidad baja.
No se conoce con certeza si el cambio a la hidroxiurea y la flebotomía tiene algún efecto sobre el riesgo de accidente cerebrovascular (ningún accidente cerebrovascular); la mortalidad por todas las causas (ninguna muerte); los ataques isquémicos transitorios, riesgo relativo 1,02 (intervalo de confianza del 95%: 0,21 a 4,84); u otras complicaciones relacionadas con la enfermedad de células falciformes (síndrome torácico agudo, riesgo relativo 2,03 [intervalo de confianza del 95%: 0,39 a 10,69]), evidencia de calidad muy baja.
Prevención secundaria, niños y adolescentes (un ensayo, 133 participantes)
El cambio a la hidroxiurea y la flebotomía puede aumentar el riesgo de eventos adversos graves relacionados con la enfermedad de células falciformes, riesgo relativo 3,10 (intervalo de confianza del 95%: 1,42 a 6,75); pero puede tener poco o ningún efecto sobre las concentraciones medias de hierro en el hígado (hidroxiurea, 17,3 mg Fe/g de hígado seco [rango intercuartil 10,0 a 30,6]); la transfusión 17,3 mg Fe/g de hígado seco (rango intercuartil 8,8 a 30,7), evidencia de calidad baja.
No se conoce con certeza si el cambio a la hidroxiurea y la flebotomía aumenta el riesgo de accidente cerebrovascular, riesgo relativo 14,78 (intervalo de confianza del 95%: 0,86 a 253,66); o tiene algún efecto sobre la mortalidad por todas las causas, odds ratio de Peto 0,98 (intervalo de confianza del 95%: 0,06 a 15,92); o los ataques isquémicos transitorios, riesgo relativo 0,66 (intervalo de confianza del 95%: 0,25 a 1,74), evidencia de calidad muy baja.
Conclusiones de los autores
No hay evidencia para el tratamiento de los adultos o los niños que no presentan anemia de células falciformes HbSS.
En los niños con un mayor riesgo de sufrir un accidente cerebrovascular y que no han recibido transfusiones previas a largo plazo, existe evidencia de calidad moderada de que las transfusiones de glóbulos rojos a largo plazo reducen el riesgo de sufrir un accidente cerebrovascular, y evidencia de calidad baja de que también reducen el riesgo de otras complicaciones relacionadas con la anemia de células falciformes.
En la prevención primaria y secundaria de los accidentes cerebrovasculares hay evidencia de calidad baja de que el cambio a la hidroxiurea con flebotomía tiene poco o ningún efecto sobre la concentración de hierro en el hígado.
En la prevención secundaria de los accidentes cerebrovasculares hay evidencia de calidad baja de que el cambio a la hidroxiurea con la flebotomía aumenta el riesgo de eventos relacionados con la enfermedad de células falciformes.
El resto de la evidencia de esta revisión es de calidad muy baja.
PICO
Resumen en términos sencillos
Transfusiones de sangre a largo plazo para prevenir el accidente cerebrovascular en pacientes con anemia de células falciformes
Pregunta de la revisión
Se deseaba determinar si las transfusiones de sangre a largo plazo administradas a pacientes con anemia de células falciformes que tienen un mayor riesgo de sufrir un accidente cerebrovascular (prevención primaria) o que han sufrido un accidente cerebrovascular anterior (prevención secundaria) disminuyen su riesgo de sufrir un accidente cerebrovascular posterior, sin causar efectos secundarios graves. Se compararon las transfusiones de sangre a largo plazo con el tratamiento estándar u otras formas de prevenir un accidente cerebrovascular. Ésta es una actualización de una revisión Cochrane publicada anteriormente.
Las intervenciones para el accidente cerebrovascular silente se abordan en otra revisión Cochrane.
Antecedentes
La anemia de células falciformes es un trastorno sanguíneo hereditario grave en el que los eritrocitos, que transportan el oxígeno en el cuerpo, se desarrollan de forma anormal.
Los eritrocitos normales son flexibles y tienen forma de disco, pero en la anemia de células falciformes se pueden volver rígidos y con forma de semicírculo. Este hecho puede provocar el bloqueo de los vasos sanguíneos y dar lugar a daño tisular y orgánico, así como episodios de dolor intenso. Los glóbulos anormales son más frágiles y se rompen, lo que provoca una menor cantidad de eritrocitos, lo que se conoce como anemia.
Los eritrocitos falciformes pueden bloquear el flujo en los vasos sanguíneos del cerebro, lo cual da lugar al accidente cerebrovascular.
Los accidentes cerebrovasculares se producen en hasta el 10% de los niños con anemia de células falciformes (HbSS) y pueden causar debilidad en las extremidades, dificultad para hablar, convulsiones y deterioro cognitivo.
Se han utilizado dos pruebas para identificar a los niños con mayor riesgo de sufrir un primer accidente cerebrovascular. Una (ultrasonografía Doppler transcraneal) mide la velocidad de la sangre que fluye por las arterias del cerebro, y los niños con un flujo sanguíneo elevado corren un mayor riesgo de sufrir un accidente cerebrovascular. El otro (imágenes por resonancia magnética) toma imágenes del cerebro para ver si hay pequeñas áreas de daño (accidentes cerebrovasculares silentes), y los niños con evidencia de daño tienen un mayor riesgo de sufrir un accidente cerebrovascular.
Las transfusiones de sangre pueden ayudar a prevenir un accidente cerebrovascular al reducir el nivel de anemia, diluir los glóbulos rojos falciformes y aumentar el nivel de oxígeno en la sangre.
Las transfusiones de sangre se pueden asociar a eventos adversos, p.ej. desarrollo de anticuerpos contra las proteínas de los glóbulos rojos del donante (aloinmunización), la acumulación de demasiado hierro en el cuerpo a causa de las transfusiones repetidas, el aumento del riesgo de infección y la prolongación de la estancia hospitalaria.
Fecha de la búsqueda
La evidencia está actualizada hasta: 8 de octubre de 2019.
Características de los estudios
Se encontraron cinco ensayos controlados aleatorizados que incluyeron a 660 participantes. Tres ensayos compararon las transfusiones de sangre con ninguna transfusión de sangre y dos ensayos compararon la transfusión de sangre con la hidroxiurea. Los ensayos se publicaron entre 1998 y 2016 e incluyeron niños y en ocasiones adolescentes; la mayoría presentaba una forma de anemia de células falciformes (HbSS).
Todos los ensayos recibieron financiamiento del gobierno.
Resultados clave
En los niños con un mayor riesgo de sufrir un accidente cerebrovascular y que no han recibido transfusiones de sangre anteriormente, un régimen de transfusiones de sangre a largo plazo probablemente reduce los accidentes cerebrovasculares clínicos, y también puede reducir otras complicaciones relacionadas con la anemia de células falciformes.
No existe certeza acerca de que interrumpir las transfusiones de sangre en niños y adolescentes que reciben transfusiones durante mucho tiempo (más de 12 meses) aumente el riesgo de sufrir un accidente cerebrovascular.
No existe certeza acerca de que el cambio de las transfusiones a largo plazo con quelación de hierro a la hidroxiurea con flebotomía tenga algún efecto sobre el accidente cerebrovascular, la mortalidad o las complicaciones relacionadas con la anemia de células falciformes en los niños que no han sufrido un accidente cerebrovascular. La hidroxiurea puede tener poco o ningún efecto sobre los niveles de hierro en el hígado.
No se conoce con certeza si el cambio de las transfusiones a largo plazo con quelación del hierro a la hidroxiurea con flebotomía aumenta el riesgo de accidente cerebrovascular o de mortalidad en niños y adolescentes que han sufrido un accidente cerebrovascular y que anteriormente recibían transfusiones regulares. El cambio de las transfusiones a largo plazo a la hidroxiurea puede aumentar algunos eventos adversos graves relacionados con la anemia de células falciformes, como las crisis dolorosas.
Calidad de la evidencia
En los niños con mayor riesgo de accidente cerebrovascular que no han recibido transfusiones a largo plazo anteriores, hay evidencia de calidad moderada de que las transfusiones de eritrocitos a largo plazo reducen el riesgo de accidente cerebrovascular. La calidad de la evidencia se consideró baja a muy baja para el resto de los resultados, incluido el riesgo de infartos cerebrales silentes, debido a que los ensayos tuvieron alto riesgo de sesgo y hubo un número reducido de ensayos y de participantes incluidos en los ensayos.
Authors' conclusions
Summary of findings
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have not had previous long‐term red cell transfusions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard care | Risk with Blood transfusion | |||||
| Clinical stroke | Trial population | RR 0.12 | 326 | ⊕⊕⊕⊝ | ||
| 110 per 1000 | 13 per 1000 (3 to 54) | |||||
| All‐cause mortality | No deaths occurred in either trial arm | ‐ | 326 | ⊕⊝⊝⊝ | ||
| Adverse events associated with transfusion | Moderatea | RR 3.16 | 121 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 32 per 1000 (2 to 572) | |||||
| TIA | Trial population | Peto OR 0.13 (0.01 to 2.11) | 323 (2 RCTs) | ⊕⊝⊝⊝ | ||
| 21 per 1000 | 5 per 1000 (0 to 43) | |||||
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 0.24 | 326 | ⊕⊕⊝⊝ | ||
| 232 per 1,000 | 56 per 1000 (28 to 111) | |||||
| Moderate | ||||||
| 230 per 1000 | 55 per 1000 (28 to 110) | |||||
| Measures of neurological impairment assessed with: WASI IQ score | Least square mean 1.7 (SE 95% CI ‐1.1 to 4.4) | ‐ | 166 (1 RCT) | ⊕⊕⊝⊝ | Author reported data from SIT 2014 | |
| Quality of life assessed with: Child Health Questionnaire Parent Form 50 | Difference estimate ‐0.54 (‐0.92 to ‐0.17) | ‐ | 196 (1 RCT) | ⊕⊕⊝⊝ | Author reported data from SIT 2014 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by 1 due to imprecision. Rare event. No deaths occurred. 2 We downgraded the quality of the evidence by 1 due to risk of bias. Unblinded trial and cross‐overs, and imbalance between loss to follow‐up between trial arms 3 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 4 We downgraded the quality of evidence by 2 due to imprecision. The estimate has very wide CIs a Based on Chou 2013 | ||||||
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have had long‐term red cell transfusions to prevent a stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard care | Risk with blood transfusion | |||||
| Clinical stroke | Trial population | RR 0.22 (0.01 to 4.35) | 79 | ⊕⊝⊝⊝ | ||
| 49 per 1000 | 11 per 1000 (0 to 212) | |||||
| All‐cause mortality | Moderatea | Peto OR 8.00 (0.16 to 404.12) | 79 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 75 per 1000 (2 to 803) | |||||
| Adverse events associated with transfusion | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| TIA | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| Serious adverse events as a result of sickle cell‐related complications assessed with: ACS | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We did not downgrade the evidence due to risk of bias because the evidence was already very low grade evidence. There was attrition bias. Imbalance between loss to follow‐up between trial arms 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of evidence by 2 due to imprecision. The estimate has very wide CIs a Assuming a mortality rate of 1% | ||||||
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have had long‐term red cell transfusions to prevent a stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with hydroxyurea and phlebotomy | Risk with Blood transfusion | |||||
| Clinical stroke | No strokes occurred in either trial arm | ‐ | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| All‐cause mortality | No deaths occurred in either trial arm | ‐ | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse events associated with transfusion | The mean liver iron concentration was 9.5 mg Fe/g dry weight | MD 1.8 mg Fe/g dry weight lower (5.16 lower to 1.56 higher) | ‐ | 121 (1 RCT) | ⊕⊕⊝⊝ | Switching to hydroxyurea and phlebotomy may reduce serum ferritin levels compared to continuing to receive red cell transfusions and chelation (MD) ‐1398 μg/L, 95% CI ‐1929 to ‐867; one trial, 121 participants) |
| Incidence of TIA | 49 per 1000 | 50 per 1,000 (10 to 238) | RR 1.02 (0.21 to 4.84) | 121 (1 RCT) | ⊕⊝⊝⊝ | |
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 2.03 (0.39 to 10.69) | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| 33 per 1000 | 67 per 1,000 (13 to 350) | |||||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of the evidence by 2 due to imprecision. Rare event. No deaths or stroke occurred. 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of the evidence by 1 due to risk of bias.Trial was not blinded and stopped early 4 We downgraded the quality of the evidence by 1 due to imprecision. The estimate has very wide CIs | ||||||
| Secondary prevention | ||||||
| Patient or population: individuals with sickle cell disease who have had a stroke who have had long‐term red cell transfusions to prevent another stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with hydroxyurea and phlebotomy | Risk with Blood transfusion | |||||
| Clinical stroke | Trial population | RR 14.78 | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| All‐cause mortality | 15 per 1000 | 15 per 1000 (1 to 198) | Peto OR 0.98 (0.06 to 15.92) | 133 (1 RCT) | ⊕⊝⊝⊝ | |
| Transfusion‐related adverse events ‐ assessed with liver iron concentration mg Fe/g dry weight liver | Hydroxyurea arm: median 17.2 mg IQR 10.0 to 30.6 Transfusion arm: median 17.3 mg IQR 8.8 to 30.7 | 56 (1 RCT) | ⊕⊕⊝⊝ | P = 0.7920a Switching to hydroxyurea and phlebotomy may reduce serum ferritin levels compared to continuing to receive red cell transfusions and chelation 1994 μg/L, interquartile range (IQR) 998 to 3475, in the hydroxyurea arm and 4064 μg/L, IQR 2330 to 7126, in the transfusion arm; one trial, 133 participants; P < 0.001 a | ||
| Incidence of TIA | Trial population | RR 0.66 | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 136 per 1000 | 90 per 1000 (34 to 237) | |||||
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 0.33 (0.04 to 3.08) | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 45 per 1000 | 15 per 1000 (2 to 140) | |||||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of the evidence by 1 due to risk of bias. Trial was not blinded and stopped early 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of the evidence by 1 due to imprecision. The estimate has very wide CIs a Analysis performed by the trial authors | ||||||
Background
Description of the condition
Sickle cell disease (SCD) is a genetic haemoglobin disorder which can cause severe pain crises and dysfunction of virtually every organ system in the body, ultimately causing premature death. Populations originating from sub‐Saharan Africa, South and Central America, the Caribbean, the Middle East, India and parts of the Mediterranean are predominantly affected. Reductions in infant and child mortality and increasing migration from highly affected countries have made this a worldwide problem (Piel 2012). Over 12,500 people in the UK and 100,000 in the USA suffer from the disease (NICE 2010; Pleasants 2014). A recent study estimated that approximately 305,800 babies were born with SCD in 2010, of which two thirds were born in Africa, and this could increase to approximately 404,200 by 2050 (Piel 2012). In high‐income countries, people with SCD are expected to live into their 40's, 50's and beyond, whereas in low‐income countries including some African nations it is estimated that between 50% to 90% of children born with HbSS die before their fifth birthday (Gravitz 2014; Grosse 2011).
The term 'sickle cell disease' refers to all genotypes that cause the clinical syndrome. There are three main types of SCD. Sickle cell anaemia is the most common form of the disease (up to 70% of cases of SCD in people of African origin) and is due to the inheritance of two beta globin S (S) alleles (haemoglobin (Hb)SS). The second most common genotype (up to 30% of cases in people of African origin) is haemoglobin SC disease (HbSC disease) it is due to the co‐inheritance of the HbS and HbC alleles and tends to be a more moderate form of the disease (Nagel 2003).The third major type of SCD occurs when HbS is inherited with a β‐thalassaemia allele, causing HbS/β‐thalassaemia (Rees 2010). People who have inherited a β‐thalassaemia null mutation along with HbS (HbSβº) have a disease that is clinically indistinguishable from sickle cell anaemia, whereas people with HbSβ⁺ thalassaemia have a milder disorder.
In SCD, under certain conditions in the absence of oxygen, the haemoglobin molecules within the red blood cells can associate as polymers, making the cells rigid and distorted into a variety of shapes, some resembling a sickle. The red blood cells have a shortened life span, resulting in anaemia. They also demonstrate increased adherence to endothelial cells lining the blood vessels, contributing to vaso‐occlusion. In later life, chronic damage to poorly perfused organs becomes apparent (Steinberg 1999). Individual heterogeneity among persons with sickle cell disease make the symptoms highly variable in frequency and severity, but the most common clinical manifestation is the acute sickle pain crisis which occurs when small vessels are blocked, depriving the tissues of oxygen and causing ischaemic damage and pain. Vaso‐occlusion can also occur in some large vessels, such as those in the brain, causing or contributing to stroke.
Stroke, usually ischaemic, occurs in up to 10% of children with sickle cell anaemia (HbSS) (Cohen 1996) and can cause weakness in the limbs, slurring of speech, seizures, coma and cognitive impairment. Recurrent (secondary) strokes occur in a half to two thirds of untreated individuals and are associated with increasing morbidity and mortality (Cohen 1996). 'Silent' cerebral infarctions (SCIs) often go unnoticed but can also cause significant neurological damage and cognitive disability and are present in a further 17% to 27% of children with sickle cell anaemia (Kinney 1999; Kwiatkowski 2009). Transcranial Doppler (TCD) velocities (tests that measure the speed of blood flow through the brain's blood vessels (either the internal carotid artery or the middle cerebral artery) by ultrasound) are used to identify children at high risk of stroke. TCD velocities are classed as normal (less than 170 cm per second); conditional (170 to 199 cm per second); or abnormal (at least 200 cm per second) in (Adams 1998b).
Description of the intervention
The focus in the past has largely been on secondary prevention with long‐term transfusion, as risk factors for first stroke were not well established. However, with the technological breakthrough of the use of TCD cerebral blood flow velocity measurement, screening has become feasible and is currently the standard of care. Abnormally high blood flow in one or more major arteries is associated with vascular narrowing and predicts an increased risk of stroke, allowing preventative treatment (i.e. long‐term red cell transfusion programme) prior to the first stroke (Adams 1998a). The fetal haemoglobin (HbF) stimulating drug hydroxyurea has been substituted successfully for long‐term red cell transfusion for the prevention of secondary stroke in a limited number of cases (Ware 2010). Serial phlebotomy may be highly effective in the reduction of iron overload if transfusions are no longer necessary (Ware 2004).
As well as the direct and indirect costs, long‐term red cell transfusions can have adverse side effects. Iron overload is a problem and requires daily oral iron chelation with deferasirox or deferiprone (or daily subcutaneous or intravenous infusions with desferrioxamine) to avoid the toxic effects of excess iron (Inati 2011). However, compliance with the chelation programmes is often poor, and therefore problems of iron overload are potentially serious. Alloimmunisation occurs when the individual develops antibodies to the foreign red cells (Smith‐Whitley 2012), which is a major problem for future transfusion. Blood products can be contaminated with infective agents such as hepatitis C and HIV, and while this now occurs only rarely in developed countries, the risk is much higher in the developing countries where sickle cell disease is most prevalent. Other problems with transfusions include hyperviscosity of the blood due to over‐transfusion, and haemolytic transfusion reactions, both potentially serious side effects. The regimen is often complex and time‐consuming, requiring monthly transfusions to maintain the HbS at approximately 20% to 30%. In short, blood transfusion is a lengthy and costly process which is not without risks, and these must be balanced against the possible benefits prior to embarking on a long‐term regimen.
How the intervention might work
Red cell transfusions are undertaken in many people with SCD to dilute the circulating sickle cells, thus reducing the risk of vaso‐occlusive episodes and anaemia (Serjeant 1992) and increasing tissue oxygen delivery. Transfusions can be given acutely, in emergency treatment of complications such as acute splenic sequestration, aplastic crisis, and acute chest syndrome (ACS), and are also frequently used in preparation for surgery. In addition, many people with SCD receive chronic transfusion regimens in an attempt to prevent severe vaso‐occlusion and stroke (Smith‐Whitley 2012).
The mechanisms for the reduction in stroke risk from long‐term red cell transfusion are not known (DeBaun 2006). However, a reduction in cells containing high amounts of HbS or an increase in Hb level could have beneficial effects on cerebral blood vessels or interactions between red blood cells and endothelial cells (Adams 1998b). Transfusion does have an immediate haemodynamic effect measured by reduction of middle cerebral artery velocity (Venkatesubramanian 1994).
Hydroxyurea is currently the only approved therapeutic drug for the treatment of sickle cell anaemia (for adults with severe vaso‐occlusive episodes of pain or acute chest syndrome) and its use has become widespread in both children and adults with this condition. In preliminary studies it was substituted successfully for long‐term transfusion in the prevention of secondary strokes, leading to its consideration for use in the phase III SWiTCH trial (SWiTCH 2012).
For the past three decades the standard treatment for iron overload related to long‐term red cell transfusion has been the use of iron chelating agents, including desferrioxamine, deferiprone and deferasirox. Although serial phlebotomy has long been utilized for conditions such as polycythaemia, it has recently been found to be highly effective in the reduction of iron overload from chronic red blood cell transfusion in people who are no longer requiring that treatment (Ware 2004). Pilot information on the combination use of hydroxyurea and phlebotomy led to the development of the SWiTCH trial (SWiTCH 2012).
Why it is important to do this review
In the USA the National Institutes of Health (NIH) guidelines recommend a long‐term red cell transfusion programme for prevention of stroke in people with SCD who have had a prior stroke, or who have an abnormal TCD reading (blood flow velocity equal to or greater than 200 cm per second in the internal carotid artery or the middle cerebral artery). While many studies support the efficacy of this treatment (Adams 1998a; Bernaudin 2011), the optimum regimen and duration of treatment for primary stroke prophylaxis have not been widely agreed upon. This review aims to assess the relative risks and benefits of red cell transfusion regimens for preventing primary strokes in people with SCD, with or without SCIs. Furthermore, older data indicated the need for indefinite continuation of long‐term red cell transfusion and iron removal in the prevention of secondary strokes and its treatment consequences (Wang 1991). The potential substitution of an oral drug (hydroxyurea) for long‐term red cell transfusion and of periodic phlebotomy for oral or subcutaneous iron chelation offered less demanding and potentially less expensive secondary stroke preventative management.
The publication is an update of a Cochrane Review first published in 2002 and most recently updated in 2017 (Hirst 2002; Wang 2013).
Objectives
In this review we aimed to determine whether long‐term red cell transfusion regimens in people with SCD:
-
reduce occurrence of stroke (primary prevention);
-
reduce recurrence of stroke (secondary prevention);
-
reduce mortality;
-
reduce other complications of SCD including pain crises, ACS and splenic sequestration;
-
are associated with unacceptable adverse events or costs.
This version of the review does not address the question of whether the risk of silent cerebral infarction is affected by blood transfusion because this is the subject of a separate Cochrane Review (Estcourt 2016).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with no limits on language or publication status.
Types of participants
People with SCD (HbSS, SC, Sβ⁺ and Sβ⁰ proven by electrophoresis, with family studies or DNA tests as appropriate) of all ages and both sexes, whether or not they have a history of prior stroke or transient ischaemic attack (TIA).
Types of interventions
Long‐term red cell transfusion regimens compared to other transfusion regimens, no treatment or the use of hydroxyurea to reduce the incidence of stroke in people with SCD.
Types of outcome measures
We grouped outcome data, where appropriate, into those measured prior to transfusion regimen, one month, one year, five years and more than five years after initiation of transfusion, and one year, five years and more than five years after stopping transfusion.
Primary outcomes
-
Incidence of clinical diagnosis of any type of stroke (by clinical symptoms and signs, magnetic resonance imaging (MRI) scan, computed tomography imaging (CT) scan or autopsy)
-
Deaths from any cause in each treatment group
-
Transfusion‐related complications (e.g. alloimmunisation, infection from blood product, procedural complications, transfusion reactions, reduced immunocompetency, iron overload (measured by serum ferritin, liver iron or quantitative MRI))
Secondary outcomes
-
Incidence of TIA
-
Measures of neurological impairment, and measures of neuropsychiatric performance (e.g. full scale intelligence quotient (FSIQ))
-
Incidence of other sickle cell complications (e.g. pain crises, acute chest syndrome, splenic sequestration)
-
Quality of life (measured on a validated scale)
-
Measures of organ damage (e.g. renal function, liver function, and lung function tests)
-
Haemoglobin level and HbS percentage (mean, pre‐ and post‐transfusion, and at time of event)
Search methods for identification of studies
Electronic searches
We identified relevant trials from the Cochrane Cystic Fibrosis and Genetic Disorders Group’s Haemoglobinopathies Trials Register using the terms: sickle cell OR (haemoglobinopathies AND general) AND stroke AND blood transfusion.
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (Clinical Trials) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Group's Haemoglobinopathies Trials Register: 19 September 2019.
We also searched the following databases for RCTs and SRs on 8 October 2019 without language, publication year or publication status restrictions:
-
the Cochrane Library (CENTRAL) (Issue 10, 2019) (http://www.cochranelibrary.com/);
-
MEDLINE (OvidSP, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, 1946 to 8 October 2019);
-
Embase (OvidSP, 1974 to 8 October 2019);
-
CINAHL (EBSCOHost, 1937 to 8 October 2019);
-
PubMed (for epublications ahead of print, in‐process & other non‐indexed citations only on 8 October 2019) (https://www.ncbi.nlm.nih.gov/pubmed);
-
Transfusion Evidence Library (1950 to 8 October 2019) (http://www.transfusionevidencelibrary.com/);
-
LILACS (1982 to 8 October 2019) (LILACS);
-
IndMed (1986 to 8 October 2019) (IndMed);
-
KoreaMed (1997 to 8 October 2019) (KoreaMed);
-
Web of Science (Conference Proceedings Citation Index‐ Science (CPCI‐S) ‐ 1990 to 8 October 2019).
We also searched the following trial databases for ongoing trials on 8 October 2019:
-
ClinicalTrials.gov (https://clinicaltrials.gov/);
-
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/);
The full search strategies for each database are available in Appendix 1.
Searching other resources
We augmented database searching with the following.
Handsearching of reference lists
We checked references of all included trials, relevant review articles and current treatment guidelines for further literature. These searches were limited to the ’first generation’ reference lists.
Personal contacts
We contacted authors of relevant trials for unpublished material or further information.
Data collection and analysis
Selection of studies
Two independent review authors (LE, PF) screened all electronically‐derived citations and abstracts of papers identified in the search for relevance. We excluded trials that were clearly irrelevant at this stage based on a review of the abstract. Two independent review authors (LE, PF) formally assessed the full texts of all potentially‐relevant trials for eligibility against the criteria outlined above. We resolved all disagreements by discussion without the need for a third review author. We sought further information from trial authors if the article contained insufficient data to make a decision about eligibility. We used Covidence to screen all abstracts and full‐text articles (Covidence 2015). We recorded the reasons why potentially‐relevant trials failed to meet the eligibility criteria.
Data extraction and management
Since the previous versions of this review, we have updated the data extraction and risk of bias assessment for all included trials (Hirst 2002; Wang 2013). Two review authors (LE, PF) conducted the data extraction according to Cochrane guidelines (Higgins 2011a). We resolved disagreements between the review authors by consensus. The review authors were not blinded to names of authors, institutions, journals, or the outcomes of the trials. We used Covidence (Covidence 2015) to extract data for the two new trials and to assess the risk of bias for all included trials. Two authors (LE, PF) extracted data independently for all the trials. We used the available tables in Review Manager 5 to present extracted data on trial characteristics (Review Manager 5.3).
We extracted the following data.
General information
Review author’s name, date of data extraction, study ID, first author of trial, author’s contact address (if available), citation of paper, objectives of the trial.
Trial details
Trial design, location, setting, sample size, power calculation, treatment allocation, inclusion and exclusion criteria, reasons for exclusion, comparability of groups, length of follow‐up, stratification, stopping rules described, statistical analysis, results, conclusion, and funding.
Characteristics of participants
Age, gender, total number recruited, total number randomised, total number analysed, types of underlying disease, lost to follow‐up numbers, dropouts (percentage in each arm) with reasons, protocol violations, previous treatments, current treatment, prognostic factors, haemoglobin S levels.
Interventions
Experimental and control interventions, method of red cell transfusion (top‐up, partial or full exchange transfusion).
Assessment of bias
Sequence generation, allocation concealment, blinding (participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, other sources of bias.
Outcomes measured
-
Incidence of clinical diagnosis of any type of stroke (by clinical symptoms and signs, MRI scan, computed tomography imaging (CT) scan or autopsy)
-
Deaths from any cause in each treatment group
-
Transfusion‐related complications (e.g. alloimmunisation, infection from blood product, procedural complications, transfusion reactions, reduced immunocompetency, iron overload (measured by serum ferritin, liver iron or quantitative MRI)
-
Incidence of TIA
-
Measures of neurological impairment, and measures of neuropsychiatric performance (e.g. full scale intelligence quotient (FSIQ))
-
Incidence of other sickle cell complications (e.g. pain crises, ACS, splenic sequestration)
-
Quality of life (measured on a validated scale)
-
Measures of organ damage (e.g. renal function, liver function, and lung function tests)
-
Haemoglobin level and HbS percentage (mean, pre‐ and post‐transfusion, and at time of event
We used both full‐text versions and abstracts to retrieve the data. We used one data extraction form per trial, regardless of number of publications relating to that trial. Where these sources did not provide sufficient information, we contacted authors and trial groups for additional details. One review author entered data into Review Manager 5 and a second review author checked these for accuracy Review Manager 5.3) .
Assessment of risk of bias in included studies
We updated the risk of bias assessments from those in the previous versions of this review (Hirst 2002; Wang 2013). Two review authors (LE, PF) assessed all included trials for possible risk of bias (as described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011b)). The assessment included information about the design, conduct and analysis of the trial. We evaluated each criterion using the Cochrane three‐point scale (low, high, or unclear risk of bias) in the following areas.
-
Selection bias (random sequence generation and allocation concealment)
-
Performance bias (blinding of participants and personnel)
-
Detection bias (blinding of outcome assessment)
-
Attrition bias (incomplete outcome data)
-
Reporting bias (selective reporting)
-
Other bias
If disagreement arose on the assessment of quality of an included trial, we reached a consensus by discussion, without the need for a third review author.
Measures of treatment effect
For continuous outcomes we recorded the mean, standard deviation (SD) and total number of participants in both the treatment and control groups. For those continuous outcomes using the same scale, we performed analyses using the mean difference (MD) with 95% confidence intervals (CIs). There were no continuous outcomes measured using different scales (when we would have used the standardised MD).
For dichotomous outcomes we recorded the number of events and the total number of participants in both the treatment and control groups. We reported the pooled risk ratio (RR) with a 95% CI. Where the number of observed events was small (less than 5% of sample per group), and where trials have balanced treatment groups, we reported the Peto odds ratio (OR) with 95% CI (Deeks 2011).
If data allowed, we undertook quantitative assessments using Review Manager 5 (Review Manager 5.3). If we could not report the available data in any of the formats described above, we performed a narrative report, and if appropriate we presented the data in tables.
Unit of analysis issues
We did not pre‐specify in the protocol how we would deal with unit of analysis issues. We did not expect to encounter unit of analysis issues as cluster randomised trials, cross‐over trials, and multiple observations for the same outcome were not included in this review. Should we have found any trials with these designs we would have treated these in accordance with the advice given in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
We dealt with missing data according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). Where information was missing or unclear, we contacted the primary investigator or where applicable the funding source. In order to allow an intention‐to‐treat (ITT) analysis, irrespective of later exclusion (regardless of cause) or loss to follow‐up, we collected data by allocated treatment groups.
Assessment of heterogeneity
We conducted a meta‐analysis and assessed the statistical heterogeneity if trials were sufficiently homogenous in their design (Deeks 2011). We assessed statistical heterogeneity of treatment effects between trials using a Chi² test with a significance level at P < 0.1 and used the I² statistic to quantify possible heterogeneity (I² value greater than 50% moderate heterogeneity, I² value greater than 75% considerable heterogeneity). If statistical heterogeneity was considerable, we did not report the overall summary statistic. We could not assess potential causes of heterogeneity by sensitivity analyses due to the lack of data (Deeks 2011).
Assessment of reporting biases
We did not perform a formal assessment of potential publication bias (small trial bias) by generating a funnel plot and statistically test using a linear regression test (Sterne 2011) as no meta‐analysis contained 10 or more trials.
Data synthesis
We performed analyses according to Cochrane recommendations (Deeks 2011). We used aggregated data for analysis. For statistical analysis, we entered data into the Review Manager software (Review Manager 5.3). Where meta‐analysis was feasible, we used the fixed‐effect model for pooling the data. We used the Mantel‐Haenszel method for dichotomous outcomes or Peto method as necessary, and the inverse variance method for continuous outcomes. There was no statistical heterogeneity, but if statistical heterogeneity was found to be above 75%, we would identify a reason for clinical heterogeneity and not perform a meta‐analysis but comment instead on the results as a narrative. Even in the absence of statistical heterogeneity, we planned to explore the robustness of any summary measures, particularly with respect to trial methodological quality, but we were unable to perform sensitivity analyses due to inadequate data.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on the following characteristics:
-
TCD velocities (normal (less than 170 cm/s), conditional (170 to 199 cm/s), abnormal (at least 200 cm/s));
-
presence or absence of previous SCI on MRI.
We did not perform subgroup analyses on the following characteristics, due to a lack of data:
-
trials comparing transfusion therapy with no treatment or other treatments separately from those comparing different transfusion regimens;
-
severity of the disease;
-
age of the participant (paediatric, adults, older adults (over 60 years)).
If, in future updates, we identify moderate heterogeneity between trials, we will examine subgroups to help explain the reasons for this.
Sensitivity analysis
We planned to use the random‐effects model for sensitivity analyses as part of the exploration of heterogeneity.
-
Including only those trials with a 'low risk of bias' (e.g. RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation)
-
Including only those trials with less than a 20% dropout rate
We could not do sensitivity analyses due to inadequate data.
Summary of findings table
We used the GRADE approach to create a 'Summary of findings' table, as suggested in chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b). We used the GRADE approach to rate the quality of the evidence as 'high', 'moderate', 'low', or 'very low' using the five GRADE considerations as follows.
-
Risk of Bias: serious or very serious
-
Inconsistency: serious or very serious
-
Indirectness: serious or very serious
-
Imprecision: serious or very serious
-
Publication bias: likely or very likely
We presented summary of findings table on the following outcomes for each intervention comparison.
-
Incidence of clinical diagnosis of any type of stroke
-
Deaths due to any cause
-
Transfusion‐related complications
-
Incidence of TIA
-
Measures of neurological impairment,
-
SCD‐related complications
-
Quality of life (measured on a validated scale)
We have also rated the quality of evidence with summary of findings table for risk of stroke associated with SCI and TCD velocities above or below the transfusion threshold in primary and secondary prevention of stroke.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See PRISMA flow diagram (Figure 1).
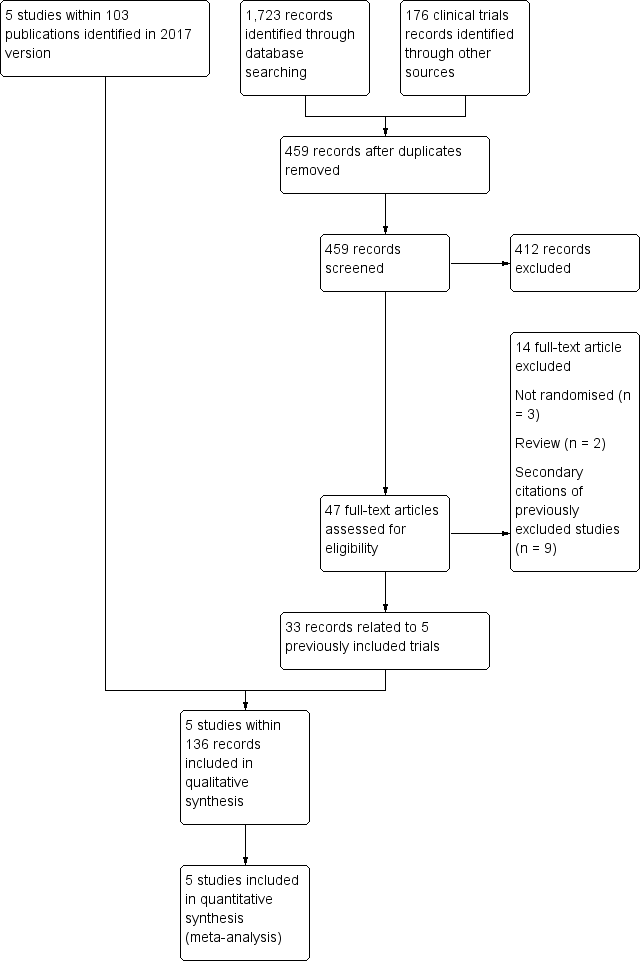
Study flow diagram.
The original review (Hirst 2002) identified two eligible trials (STOP 1998; STOP 2 2005). The first update of the review (Wang 2013) identified one additional completed trial (SWiTCH 2012) and three potentially eligible ongoing trials (SIT 2014; TWiTCH 2016; SCATE 2015).
In the previous version of this review (Estcourt 2017) we assessed 83 full text articles for relevance. We identified two completed trials within 22 publications which were classified as ongoing trials in Wang 2013 (SIT 2014,TWiTCH 2016). We identified 60 additional citations related to previously included trials (STOP 1998; STOP 2 2005, SWiTCH 2012). Therefore there were five included trials (SIT 2014; STOP 1998; STOP 2 2005;SWiTCH 2012; TWiTCH 2016), and no ongoing trials.
The updated searches (conducted 19 September 2019 and 8 October 2019) identified a total of 1899 potentially relevant records (1723 articles and 176 clinical trials). There were 459 records after duplicates were removed. Two review authors (LE, RK) excluded 412 records on the basis of the abstract, and two authors (LE, RK) reviewed 47 full text articles for relevance. We identified no new completed trials. We identified 33 citations related to previously included trials (SIT 2014; STOP 1998; STOP 2 2005, SWiTCH 2012; TWiTCH 2016). We excluded five new trials (Bernaudin 2016; EXTEND 2016; Gwam 2016; SACRED 2018; SPIN 2017). In this update we included the same five trials identified in Estcourt 2017 (SIT 2014; STOP 1998; STOP 2 2005;SWiTCH 2012; TWiTCH 2016). We found no ongoing trials.
Included studies
See Characteristics of included studies for full details of each trial.
Five trials, including 660 participants, met the predefined inclusion criteria (SIT 2014; STOP 1998; STOP 2 2005; SWiTCH 2012;TWiTCH 2016).
Trial design
All five trials were multicentre randomised trials, ranging from 12 centres (STOP 1998; STOP 2 2005) to 29 centres (SIT 2014).
Four trials were terminated early (STOP 1998; STOP 2 2005; SWiTCH 2012; TWiTCH 2016):
-
STOP 1998 was terminated 16 months early by the trial's data monitoring board when a 92% reduction in incidence of stroke in the transfused group was seen.
-
STOP 2 2005 was terminated two years early due to safety concerns.
-
SWiTCH 2012 was stopped early due to futility for the composite primary endpoint.
-
TWiTCH 2016, a non‐inferiority trial, was stopped after the first scheduled interim analysis because non‐inferiority had been demonstrated.
The SIT trial was the only trial that was not stopped before the planned end of recruitment and follow‐up (SIT 2014).
Trial size
The number of participants enrolled in the five trials ranged from 79 (STOP 2 2005) to 196 (SIT 2014). Power calculations were reported in four trials (SIT 2014; STOP 1998; STOP 2 2005; TWiTCH 2016), three of these studies were stopped early (STOP 1998; STOP 2 2005; TWiTCH 2016). The STOP 2 trial planned to recruit 100 children and the TWiTCH trial planned to recruit 148 children (STOP 2 2005; TWiTCH 2016).
Settings
The trials were published between 1998 (STOP 1998) and 2016 (TWiTCH 2016). All were multicentre trials (12 to 29 recruitment centres). One trial was conducted in the USA (SWiTCH 2012), three trials were conducted in the USA and Canada (STOP 1998; STOP 2 2005; TWiTCH 2016); and one trial was conducted in the USA, Canada, France and the UK (SIT 2014).
Participants
All trials included participants with HbSS disease and HbSβ⁰ thalassaemia. Two trials also included participants with HbS/OArab disease (SWiTCH 2012; TWiTCH 2016). Three trials did not specify the distribution of phenotypes (SIT 2014; STOP 1998; STOP 2 2005). In the SWiTCH trial, 100% of participants in the transfusion arm and 99% in the no transfusion arm had the HbSS phenotype, and in the TWiTCH trial, 97% in the transfusion arm and 100% in hydroxyurea arm had the HbSS phenotype (SWiTCH 2012; TWiTCH 2016).
All participants in the trials were children and adolescents aged from two to 20 years. Two trials included participants over 16 years (STOP 2 2005; SWiTCH 2012). Mean (SD) ages in the trials ranged from the lowest age of eight (three) years in the SIT and STOP trials (SIT 2014;STOP 1998) to a high of 13 (four) years in the SWiTCH trial (SWiTCH 2012). Participants tended to be equally divided between males and females with the highest participation of males (57%) in the SIT trial (SIT 2014) and the lowest (39%) in the TWiTCH trial (TWiTCH 2016).
All trials excluded females who were pregnant or people who had HIV. Other inclusion and exclusion criteria varied depending on the objectives of the trial. In the SWiTCH trial, individuals were included if they had an overt clinical stroke after the age 12 months (SWiTCH 2012), whereas in the other four trials individuals were excluded if they had a clinical history of stroke (SIT 2014; STOP 1998; STOP 2 2005; TWiTCH 2016).
In the SIT trial, children had to have evidence of at least one silent cerebral infarct confirmed on MRI and normal TCD velocities (SIT 2014). In the STOP, STOP 2 and the TWiTCH trials, individuals had to have abnormal TCD velocities prior to any transfusion therapy (greater than or equal to 200 cm/s) (STOP 1998; STOP 2 2005; TWiTCH 2016), and in the STOP 2 trial these abnormal TCD velocities had to have normalised with transfusion therapy (STOP 2 2005). Three trials excluded individuals with a history of seizures (SIT 2014; STOP 1998; STOP 2 2005); two trials excluded individuals with a severe vasculopathy (STOP 2 2005; TWiTCH 2016); and one trial excluded children with a previous TIA (TWiTCH 2016).
Intervention
All trials had a transfusion arm with the aim of keeping HbS to 30% or less with local discretion as to the type of red blood cell transfusion administered (simple, manual exchange or automated exchange). Three trials reported using leucocyte‐depleted red cells and blood matched for C, D, E and Kell antigen (SIT 2014; STOP 1998; STOP 2 2005), the remaining two trials did not report the type of blood component used (SWiTCH 2012; TWiTCH 2016). In the SWiTCH and TWiTCH trials, participants in the transfusion arm also received iron chelation (SWiTCH 2012; TWiTCH 2016), in the SIT and STOP 2 trials, participants received iron chelation if required (SIT 2014; STOP 2 2005) and in the STOP trial, participants did not receive iron chelation (STOP 1998).
Three trials compared long‐term transfusion therapy to standard care with no hydroxyurea (SIT 2014; STOP 1998; STOP 2 2005). In two of these trials participants had not had previous long‐term transfusions (SIT 2014; STOP 1998), and in one trial all participants had previous long‐term transfusions to prevent primary stroke (STOP 2 2005). In the STOP 2 trial, the transfusion halted arm could receive transfusions to treat SCD complications but if hydroxyurea or regular transfusions were initiated it was considered a cross‐over and data were censored at treatment initiation (STOP 2 2005).
Hydroxyurea was the comparator in the SWiTCH and TWiTCH trials, which was initiated at 20 mg/kg/day with escalation to a maximum tolerated dose (MTD) with transfusion overlap for four to nine months until MTD was reached. Once MTD was reached, phlebotomy was commenced with a target of 10 mL/kg of blood removed monthly to reduce iron burden (maximum 500 mL) (SWiTCH 2012; TWiTCH 2016)..
Outcomes
Outcomes varied across trials depending on the objectives.
The primary outcome in SIT was the recurrence of infarct or haemorrhage as determined by neuroimaging. Secondary outcomes were TIAs and changes in cognition (SIT 2014).
In both STOP trials, the primary outcomes were cerebral infarction or intracranial haemorrhage and STOP 2 also included reversion to abnormal velocity on TCD (STOP 1998; STOP 2 2005). In both trials secondary outcomes reported were death, and transfusion‐ and SCD‐related adverse events.
In the SWiTCH trial the primary outcome was a composite primary endpoint of secondary stroke recurrence rate and quantitative liver iron concentration, while non‐stroke neurological events, non‐neurological sickle cell clinical events, quality of life evaluation, and measures of organ function were all secondary outcomes (SWiTCH 2012).
In the TWiTCH trial the primary outcome was TCD time‐averaged mean velocity on the index side, defined as the cerebral hemisphere with the higher mean arterial velocity at baseline assessment (TWiTCH 2016). TCD velocity on the non‐index side, new stroke or non‐stroke neurological events, new brain MRI or MRA lesions, hepatic iron overload, sickle‐related events, neuropsychological status, quality of life, growth, and treatment‐related complications were secondary outcomes.
Funding source
All five trials received government funding.
Ongoing studies
We did not identify any ongoing studies for the update of the review.
Excluded studies
We excluded seven studies or reviews within 17 full text records:
-
four were not randomised trials (DREPAGREFFE 2017; EXTEND 2016; SACRED 2018; SPIN 2017).
-
two were reviews (Bernaudin 2016; Gwam 2016)
-
one trial did not include a transfusion arm (SCATE 2015)
Risk of bias in included studies
Refer to the figures section of the review for visual representations of the assessments of risk of bias across all trials and for each item in the included trials (Figure 2; Figure 3). See the risk of bias section in the Characteristics of included studies section for further information about the bias identified within the individual trials.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation (selection bias)
We considered four trials to be at low risk of bias, as randomisation was done centrally by the statistical data coordinating centre or was randomly generated or both (SIT 2014; STOP 1998; STOP 2 2005; TWiTCH 2016). We judged the SWiTCH trial to have an unclear risk of bias as the method of randomisation was not adequately reported (SWiTCH 2012).
Allocation concealment (selection bias)
We considered three trials to be at low risk of bias for allocation concealment as assignment was done by a central statistical data centre or allocation was statistically determined or both (SIT 2014; STOP 1998; TWiTCH 2016). We considered two trials to be at unclear risk of bias because no description of allocation concealment was provided (STOP 2 2005; SWiTCH 2012).
Blinding
Blinding of participants and personnel (performance bias)
We considered all five trials to be at high risk of performance bias as it is impractical to mask a blood transfusion intervention so all participants and personnel were unblinded.
Blinding of outcome assessment (detection bias)
We considered all five trials to be at low risk of bias for the outcome assessment of stroke or TIA as these outcomes were adjudicated by experts masked to treatment assignments.
We judged all five trials to be at high risk of bias for all other outcomes except mortality as all trials were unblinded.
Incomplete outcome data
We considered four trials to be at low risk for attrition bias as they all used an ITT analysis and all participants were accounted for in the trials (SIT 2014; STOP 1998; SWiTCH 2012; TWiTCH 2016). We judged the STOP 2 trial to be at high risk for attrition bias as it was not stated if an ITT analysis was used and 17% of participants discontinued or data were censored (STOP 2 2005).
Selective reporting
We considered one trial to be at low risk of reporting bias as a protocol was provided and all planned outcomes were reported (SIT 2014). We rated three trials as unclear risk for reporting bias (STOP 1998; STOP 2 2005; TWiTCH 2016): one trial had no protocol and no prospective trial registration (STOP 1998); one trial did not report any secondary outcomes and it was not clear if all adverse events were reported as some participant data were censored (STOP 2 2005); one trial did not report some secondary outcomes and it was unclear if these outcomes will be reported in future publications (TWiTCH 2016).
We judged one trial to be at high risk for selective reporting bias as several secondary outcomes were not reported (i.e. quality of life, growth and development, organ damage, transfusion‐related, chelation‐related and phlebotomy related complications) (SWiTCH 2012).
Other potential sources of bias
We considered all trials to be at unclear risk for other sources of bias. We judged four trials to have unclear risk due to early termination of these trials (STOP 1998; STOP 2 2005; SWiTCH 2012; TWiTCH 2016). As well, in the TWiTCH trial, children with severe vasculopathy were excluded during screening, so these children might not be suitable candidates for hydroxyurea and longer follow‐up is required to determine if findings are maintained over time (TWiTCH 2016). The SIT trial was considered to have unclear risk because there was a 20% cross‐over rate to either transfusion or hydroxyurea treatment and also because hydroxyurea was started in 17% of participants due to disease severity even though it was part of the exclusion criteria (SIT 2014). In the SWiTCH trial, more participants had moya‐moya in the hydroxyurea arm (11 participants) than the transfusion arm (five participants), it was not known if there was a difference between treatment arms in the number of participants with severe vasculopathy (SWiTCH 2012).
Effects of interventions
See: Summary of findings 1 Long‐term red cell transfusion versus no transfusion in people who are at risk of a primary stroke who have not had previous long‐term red cell transfusions; Summary of findings 2 Long‐term red cell transfusion versus no transfusion in people who are at risk of a primary stroke who have had previous long‐term red cell transfusions; Summary of findings 3 Long‐term red cell transfusion versus hydroxyurea and phlebotomy in people who are at risk of a primary stroke who have had previous long‐term red cell transfusions; Summary of findings 4 Long‐term red cell transfusion versus hydroxyurea and phlebotomy in people who are at risk of a secondary stroke who have had previous long‐term red cell transfusions
Red cell transfusion versus standard care
Three trials evaluated this comparison (SIT 2014; STOP 1998; STOP 2 2005), all trials assessed the primary prevention of stroke.
Two trials included children who were at higher risk of a primary stroke who had not had previous long‐term red cell transfusions to prevent stroke (SIT 2014; STOP 1998). Children in the SIT trial had evidence of previous silent cerebral infarcts on MRI but normal or conditional TCD velocities (SIT 2014). Children in the STOP trial had abnormal TCD velocities and 35% of these children also had SCIs on MRI (STOP 1998).
One trial included children and adolescents who were at higher risk of a primary stroke (previous history of abnormal TCD velocities) who had previous long‐term red cell transfusions (at least 30 months) to prevent stroke (STOP 2 2005). The majority of participants in the STOP 2 trial had also participated in the earlier STOP trial, participants were included if their TCD velocities had normalised (STOP 1998; STOP 2 2005).
Throughout this review results from the two trials in which participants had not been transfused were not combined with the results from the trial when participants had received previous long‐term transfusions.
Primary outcomes
1. Incidence of clinical stroke (any type)
No previous long‐term red cell transfusion
Long‐term red cell transfusions probably reduce the incidence of clinical stroke in children with a higher risk of stroke (abnormal TCD velocities or previous history of SCIs), compared to children receiving standard care, RR 0.12 (95% CI 0.03 to 0.49) (two trials, 326 participants, moderate quality evidence) (Analysis 1.1; summary of findings Table 1).
Previous long‐term red cell transfusion
We are very uncertain whether continuing long‐term red cell transfusions reduces the incidence of clinical stroke in children and adolescents whose TCD velocities have normalised, compared to those receiving standard care, RR 0.22 (95% CI 0.01 to 4.35) (one trial, 79 participants, very low quality evidence) (Analysis 1.1;summary of findings Table 2).
TCD velocity subgroup analysis
Normal TCD velocities ‐ we very uncertain whether red blood cell transfusions reduce the incidence of clinical stroke in children with normal TCD velocities compared to children receiving standard care, RR 0.14 (95% CI 0.02 to 1.12) (one trial, 196 participants, very low quality evidence) (Analysis 1.2). Quality of the evidence was very low due to imprecision of the effect estimate and indirectness (findings only apply to children with HbSS).
Normalised TCD velocities ‐ we are very uncertain whether continuing long‐term red cell transfusions reduces the incidence of stroke in children and adolescents with normalised TCD velocities compared to children and adolescents receiving standard care, RR 0.22 (95% CI 0.01 to 4.35) (one trial, 79 participants, very low quality evidence) (Analysis 1.2). Quality of the evidence was very low due to imprecision of the effect estimate; risk of bias; and indirectness (findings only apply to children with HbSS).
Abnormal TCD velocities ‐ red cell transfusions may reduce the incidence of clinical stroke in children with abnormal TCD velocities compared to children receiving standard care, RR 0.10 (95% CI 0.01 to 0.73) (one trial, 130 participants, low quality evidence) (Analysis 1.2). Quality of the evidence was low due to risk of bias (imbalance in follow‐up between treatment arms and imbalance in number of participants with alpha thalassaemia trait between treatment arms) and indirectness (findings only apply to children with HbSS).
Since the STOP trial was terminated early due to safety concerns, the groups had different periods of follow‐up, a total of 1229 months in the standard care group, and 1321 in the transfusion group (STOP 1998). The trial authors reported that the rate of stroke per person year of follow‐up was 0.107 in the standard care group, and 0.009 in the transfusion group. Although in the transfusion group the target HbS percentage of 30% was occasionally not met, none of these participants had a stroke.
There was no evidence of subgroup differences (test for subgroup differences: Chi² = 0.20, df = 2 (P = 0.91), I² = 0%).
Presence or absence of SCI on MRI subgroup analysis
Previous lesions (SCIs) on MRI: long‐term red cell transfusions may reduce the incidence of clinical stroke in children with previous SCIs on MRI compared to children receiving standard care, RR 0.11 (95% CI 0.02 to 0.59) (two trials, 243 participants, low quality evidence) (Analysis 1.3). Quality of the evidence was low due to risk of bias (this analysis includes data from a cohort subset who had an MRI in the STOP trial (STOP 1998)) and indirectness (findings only apply to children with HbSS).
No previous lesions (SCIs) on MRI: we are very uncertain whether red cell transfusions reduce the incidence of stroke in children with no previous SCIs on MRI compared to children receiving standard care, RR 0.27 (95% CI 0.03 to 2.31) (one trial, 79 participants, very low quality evidence) (Analysis 1.3). Quality of the evidence was very low due to: risk of bias (this analysis is derived from data from a cohort subset who had an MRI in the STOP trial (STOP 1998)); imprecision of the effect estimate; and indirectness (findings only apply to children with HbSS).
There was no evidence of subgroup differences (test for subgroup differences: Chi² = 0.41, df = 1 (P = 0.52), I² = 0%).
2. Mortality
No previous long‐term red cell transfusion
No participants died in the SIT and STOP trials (two trials, 326 participants), therefore we have no evidence of any difference in mortality rates between the treatments (very low‐quality evidence) (SIT 2014; STOP 1998; summary of findings Table 1)
Previous long‐term red cell transfusion
We are very uncertain whether continuing long‐term red cell transfusions reduces mortality in children and adolescents compared to children and adolescents receiving standard care, Peto OR 8.00 (95% CI 0.16 to 404.12) (one trial, 79 participants, very low quality evidence) (Analysis 1.4; summary of findings Table 2). One participant who was assigned to continue transfusion, died in the STOP 2 trial due to complications of ACS (STOP 2 2005).
We did not perform any subgroup analyses for this outcome because deaths occurred in only one trial (STOP 2 2005).
3. Transfusion related complications
Alloimmunisation
Three trials reported this outcome (SIT 2014; STOP 1998; STOP 2 2005).
No previous long‐term red cell transfusion
From data on transfused participants in the SIT trial, we are very uncertain whether children receiving regular long‐term transfusions have a higher risk for developing alloimmunisations compared to children receiving standard care, RR 3.16 (95% CI 0.18 to 57.17) (one trial, 121 participants, very low quality evidence) (SIT 2014) (Analysis 1.5; summary of findings Table 1).
In the STOP trial, 10 participants in the transfusion arm developed an alloimmunisation, despite a more rigorous matching protocol than usual (STOP 1998). It was not reported if any participants in the standard care developed any alloimmunisations.
Previous long‐term red cell transfusion
In the STOP 2 trial, one participant who was in the continuing transfusion arm developed an alloimmunisation (STOP 2 2005). It was not reported if any participants in the halted transfusion arm developed any alloimmunisations.
Infection from blood component
Two trials reported this outcome (STOP 1998; STOP 2 2005).
No previous long‐term red cell transfusion
In the STOP trial, no participant developed evidence of hepatitis C infection, and all 100 children who were tested were negative for antibodies against HIV and HTLV‐I (STOP 1998).
Previous long‐term red cell transfusion
In the STOP 2 trial, no cases of hepatitis C were identified among the 68 participants who had serologic testing at the end of the trial (STOP 2 2005).
Procedural complications
One trial reported this outcome and in this trial, three participants in the transfusion arm had procedural complications (SIT 2014). A catheter infection developed in one participants, and complications requiring catheter replacement developed in two participants.
Transfusion reactions
Three trials reported this outcome (SIT 2014; STOP 1998; STOP 2 2005).
No previous long‐term red cell transfusion
From data from transfused participants in the SIT trial, we are very uncertain whether children receiving regular long‐term transfusions have a higher risk of developing a transfusion reaction than children not receiving regular transfusions, RR 5.17 (95% CI 0.71 to 37.52) (one trial, 121 participants, very low quality evidence) (SIT 2014) (Analysis 1.5). Quality of the evidence was very low due to imprecision of the effect estimate and indirectness (findings only apply to children with HbSS).
In the STOP trial, 12 participants in the transfusion arm had 16 mild reactions to blood products or transfusion procedures (STOP 1998). This was not reported for the standard treatment arm.
Previous long‐term red cell transfusion
In the STOP 2 trial, seven participants in the continuing transfusion arm had nine reactions to transfusions (STOP 2 2005). One of the reactions was serious and required hospitalisation. This was not reported for the halted transfusion arm.
Reduced immunocompetence
None of the trials reported this outcome.
Iron overload
No previous long‐term red cell transfusion
In the SIT trial, the trial authors reported that there was an increased risk of iron overload (measured by serum ferritin greater than 1500 μg/L) in children receiving long‐term red cell transfusions, incidence rate ratio, 14.42 (95% CI 5.41 to 875.17) (one trial, 121 participants) ‐ this analysis was reported in the SIT trial (SIT 2014) (Table 1).
| Outcomes | Trials | Number of participants with at least one event | Adverse events/100 person‐years | Incidence rate ratioc (95% CI) | ||
|---|---|---|---|---|---|---|
| Transfusion | Standard | Transfusion | Standard | |||
| Transfusion reactions | 15 out of 90a | 1 out of 31b | 8.85 | 1.66 | 5.33 (1.67 to 23.52) | |
| Ferritin > 1500 μg/L | 76 out of 90a | 3 out of 31b | 534.70 | 37.07 | 14.42 (5.41 to 875.17) | |
aNine participants who declined transfusion were excluded from the analysis.
b31 participants assigned to observation received one or more transfusions.
cThe incidence ratio was calculated as the rate of adverse events per 100 person‐years in the transfusion group divided by the rate of adverse events per 100 person‐years in the observation group. The 95% confidence intervals were calculated with the use of the bootstrap method with 10,000 replications.
Abbreviations: CI: confidence interval
In the STOP trial, iron overload developed faster than anticipated in children receiving transfusion, with mean (SD) serum ferritin rising to 1804 (773) μg/L at 12 months and 2509 (974) μg/L at 24 months (Adams 1998b; STOP 1998). This outcome was not reported in the children receiving standard care who had transfusions.
Previous long‐term red cell transfusion
In the STOP 2 trial, mean (SD) baseline serum ferritin levels were similar in both treatment arms (continued transfusion group 3274 (1718) μg/L; halted transfusion group mean 3005 (1504) μg/L) (STOP 2 2005). The mean (SD) serum ferritin levels decreased in the halted transfusion group after 12 months (continued transfusion group 3562 (1536) μg/L; halted transfusion group mean (SD) 1832 (912) μg/L). According to an analysis performed by the trial authors this difference was statistically significant (P = 0.002) (STOP 2 2005).
Secondary outcomes
1. Incidence of transient ischaemic attack (TIA)
No previous long‐term red cell transfusion
Two trials recruiting 323 participants reported this outcome; however, in one trial recruiting 127 participants (STOP 1998), no TIAs occurred in either treatment group so this trial does not contribute to the analysis in this review. In children with SCI we are very uncertain whether long‐term red cell transfusions reduce the incidence of TIAs, compared to children receiving standard care, Peto OR 0.13 (95% CI 0.01 to 2.11) (very low quality evidence) (Analysis 1.6; summary of findings Table 1).
Previous long‐term red cell transfusion
One TIA was reported in one participant in the halted transfusion arm in the STOP 2 trial (STOP 2 2005). It was not reported if there were any TIAs in the group who continued transfusions.
2. Measures of neurological impairment
Two trials reported this outcome, in both trials participants had not had previous long‐term red cell transfusions (SIT 2014; STOP 1998).
In the STOP trial, at the time of discharge, of the 11 children in the standard care group diagnosed with cerebral infarction, two had a major disability, five had mild to moderate disability, two had symptoms but no disability and one was asymptomatic (STOP 1998). Measures of neurological impairment in other participants were not reported in this trial.
The SIT trial reported the difference between trial arms in the Weschler Abbreviated Scale of Intelligence (WASI) in performance, verbal, and full IQ scores (data from baseline to interim and to exit visits were analysed by the trial authors who reported estimated least squares means (LSM) and standard error (SE) based on two‐way repeated measures analysis of variance) (SIT 2014).
The SIT trial authors reported that long‐term red cell transfusion may make little or no difference to WASI performance, verbal or full IQ scores in children with SCIs compared to children receiving standard care (LSM: 1.7, SE 95% CI ‐1.1 to 4.4) (one trial, 166 participants, low quality evidence) (SIT 2014; summary of findings Table 1).
3. Incidence of other sickle cell complications
Acute chest syndrome
Children with no previous long‐term red cell transfusion
Long‐term red cell transfusions may reduce the incidence of ACS in children compared to children receiving standard care, RR 0.24 (95% CI 0.12 to 0.48) (two trials, 326 participants, low quality evidence) (Analysis 1.7; summary of findings Table 1).
Children with previous long‐term red cell transfusion
In the STOP 2 trial, the authors state that there were fewer incidences of ACS in participants continuing transfusions (STOP 2 2005). In the halted transfusion group, 18 of 41 participants had at least one episode of ACS. Comparative numbers for the transfusion group were not reported.
Painful crisis
No previous long‐term red cell transfusion
Long‐term red cell transfusions may reduce the incidence of painful crisis in children compared to children receiving standard care, RR 0.62 (95% CI 0.46 to 0.84), two trials, 326 participants (Analysis 1.7). Quality of the evidence was low due to risk of bias and indirectness (findings only apply to children with HbSS).
Previous long‐term red cell transfusion
In the STOP 2 trial, the authors state that there were fewer painful crises in the continuing transfusion arm (STOP 2 2005). Comparative numbers were not reported.
Splenic sequestration
No trials looked at this outcome.
Other sickle cell‐related complications
One trial reported other sickle cell‐related complications (SIT 2014) (Table 2 and Analysis 1.7). In this trial participants had not had previous long‐term red cell transfusions.
| Outcomes | Trials | Number of participants with at least one event | Adverse events/100 person‐years | Incidence rate ratioa (95% CI) | ||
|---|---|---|---|---|---|---|
| Transfusion | Standard | Transfusion | Standard | |||
| Acute chest syndrome | 4 out of 63 | 14 out of 67 | 4.8b | 15.3b | ‐ | |
| 5 out of 99 | 24 out of 97 | 1.81b | 14.35b | 0.41 (0.20 to 0.75) | ||
| Painful crisis | 11 out of 63 | 13 out of 67 | 16.2 | 27.6 | ‐ | |
| 32 out of 99 | 56 out of 97 | 41.58 | 102.21 | 0.13 (0.04 to 0.28) | ||
| Priapism | 1 out of 59 | 7 out of 52 | 0.84 | 6.65 | 0.13 (0.03 to 0.55) | |
| Symptomatic avascular necrosis of the hip | 1 out of 99 | 6 out of 97 | 0.4 | 2.25 | 0.22 (0.05 to 0.85) | |
a The incidence ratio was calculated as the rate of adverse events per 100 person‐years in the transfusion group divided by the rate of adverse events per 100 person‐years in the observation group. The 95% confidence intervals were calculated with the use of the bootstrap method with 10,000 replications.
b One child from the standard care group was excluded from these analyses due to a stroke on day 16 of the trial.
Abbreviation: CI: confidence interval
4. Quality of life, inpatient stay, immobility and disability
Quality of life
One trial reported this outcome (SIT 2014). The trial reported quality of life measured on the Child Health Questionnaire Parent Form 50 (CHQ_PF50) completed at baseline and at the occurrence of an overt stroke or at trial exit at 36 months. It is not known what the minimal clinically important differences are for the CHQ_PF50.
The SIT trial authors stated that quality of life may improve slightly in children receiving long‐term red cell transfusions compared to children receiving standard care, (difference estimate ‐0.54, 95% CI ‐0.92 to ‐0.17) (one trial, 196 participants, low quality evidence; analysis performed by the SIT trial authors) (SIT 2014; summary of findings Table 1).
Inpatient stay
No trials looked at this outcome.
Immobility and disability
The STOP trial noted that after 24 months of transfusion, children receiving transfusion had improved growth (height, weight and body mass index (BMI)) which approached normal, whereas there was no improvement in growth rate in children receiving standard care (STOP 1998; Wang 2005).
5. Measures of organ damage
No trials looked at this outcome.
6. Haemoglobin level and haemoglobin S percentage (mean, pre‐ and post‐transfusion and at time of the event)
Haemoglobin level
One of the trials reported the mean haemoglobin at six months after randomisation (STOP 2 2005). The mean haemoglobin was higher in the group that continued red cell transfusions (Table 3).
| Trial | Intervention | Baseline | 6 to 12 months | 12 to 18 months | 18 to 24 months | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | ||
| No previous long‐term transfusions | |||||||||
| Transfusion | Median 77 IQR (72 to 84) | Median 85 90% CI (51 to 95) | ‐ | Median 30 90% CI (17 to 43) | ‐ | Median 29 90% CI (16 to 43) | ‐ | Median 30 90% CI (16 to 43) | |
| Standard | Median 79 IQR (74 to 89) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Transfusion | Mean (SD) 72 (8) | Mean (SD) 87 (10) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Standard | Mean (SD) 76 (7) | Mean (SD) 87 (7) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Previous long‐term transfusions | |||||||||
| Transfusion | Mean (SD) 93 (9) | Mean (SD) 21 (8.6) | Mean (SD) 94 (9) | Mean (SD) 25.4 (10.9) | ‐ | ‐ | ‐ | ‐ | |
| Standard | Mean (SD) 98 (12) | Mean (SD) 19 (11) | Mean (SD) 77 (8) | Mean (SD) 81.0 (8.6) | ‐ | ‐ | ‐ | ‐ | |
Abbreviations: CI: confidence interval; IQR: interquartile range; SD: standard deviation
None of the trials reported the haemoglobin level pre‐transfusion, post‐transfusion or at the time of the event.
Haemoglobin S level
One of the trials reported the mean haemoglobin S level at six months after randomisation in both trial arms (STOP 2 2005). The mean Hb S levels were lower in the group that continued red cell transfusions (Table 3).
Two trials reported the number of pre‐transfusion HbS level measurements that were above the target HbS level of 30% (STOP 1998; STOP 2 2005). Only a small number of transfusions had a HbS level above the target threshold (Table 4).
| Trial | Number of transfusions | Number of HbS levels measured | HbS less than 30% | HbS 30 to 40% | HbS greater than 40% |
|---|---|---|---|---|---|
| No previous long‐term transfusions | |||||
| 1521 | ‐ | ‐ | 101 | 42 | |
| Previous long‐term transfusions | |||||
| 1070 | 988 | 748 (76%) | 192 (19%) | 48 (5%) | |
None of the trials reported the haemoglobin S level post transfusion or at the time of the event.
Hydroxyurea and phlebotomy versus transfusions and chelation
Two trials are included in this comparison (254 participants) (TWiTCH 2016; SWiTCH 2012). One trial assessed primary prevention of stroke (TWiTCH 2016) and one trial assessed secondary prevention of stroke (SWiTCH 2012)
Throughout this review results from the two trials assessing primary prevention and secondary prevention will not be combined.
Primary outcomes
1. Incidence of clinical stroke
Primary prevention
No clinical strokes occurred in the TWiTCH trial (TWiTCH 2016); summary of findings Table 3
In the TWiTCH trial, general stroke adjudications were done for 29 possible new neurological events and no child had a positive adjudication for stroke (TWiTCH 2016).
Secondary prevention
We are very uncertain whether switching to hydroxyurea and phlebotomy increase the risk of stroke compared to red cell transfusions and chelation in children and adolescents with a history of stroke, RR 14.78 (95% CI 0.86 to 253.66) (one trial, 133 participants, very low quality evidence) (Analysis 2.1; summary of findings Table 4).
Seven participants, all in the hydroxyurea and phlebotomy group, experienced a stroke and all had baseline MRA exams which showed severe vasculopathy, including two with moya‐moya. There were more participants with moya‐moya in the hydroxyurea arm (11 participants) compared to the transfusion arm (five participants), the number of participants with severe vasculopathy in both treatment arms was not reported.
2. Mortality
Primary prevention
No deaths occurred in the TWiTCH trial (TWiTCH 2016); summary of findings Table 3
Secondary prevention
We are very uncertain whether switching to hydroxyurea and phlebotomy reduces mortality compared to red cell transfusions and chelation in children and adolescents with a history of stroke, Peto OR 0.98 (95% CI 0.06 to 15.92) (one trial,133 participants, very low quality evidence) (Analysis 2.2; summary of findings Table 4).
3. Transfusion related complications
Alloimunisation
None of the trials reported this outcome.
Infection from blood component
None of the trials reported this outcome.
Procedural complications
None of the trials reported this outcome.
Transfusion reactions
None of the trials reported this outcome.
Reduced immunocompetence
None of the trials reported this outcome.
Iron overload
Primary prevention
Switching to hydroxyurea and phlebotomy may reduce serum ferritin levels compared to continuing to receive red cell transfusions and chelation in children who have a previous history of abnormal TCD velocities and at least 12 months of transfusions, MD ‐1398.00 μg/L (95% CI ‐1929.23 to ‐866.77) (one trial, 121 participants, low quality evidence) (Analysis 2.3). Quality of the evidence was low due to risk of bias and indirectness.
Switching to hydroxyurea and phlebotomy may have little or no effect on liver iron concentrations compared to continuing to receive red cell transfusions and chelation in children who have a previous history of abnormal TCD velocities and at least 12 months of transfusions, MD ‐1.80 mg Fe/g dry weight liver (95% CI ‐5.16 to 1.56) (one trial, 121 participants, low quality evidence) (Analysis 2.4; summary of findings Table 3).
Secondary prevention
Switching to hydroxyurea and phlebotomy may reduce serum ferritin levels compared to continuing to receive red cell transfusions and chelation in children and adolescents with a history of stroke (at the final assessment, median serum ferritin was 1994 μg/L, interquartile range (IQR) 998 to 3475, in the hydroxyurea arm and 4064 μg/L, IQR 2330 to 7126, in the transfusion arm; one trial, 133 participants; P < 0.001 analysis performed by the trial authors) (SWiTCH 2012). Quality of the evidence was low due to risk of bias and indirectness.
Switching to hydroxyurea and phlebotomy may have little or no effect on liver iron concentrations compared to continuing to receive red cell transfusions and chelation in children and adolescents with a history of stroke (at the final assessment, median liver iron concentration was 17.3 mg Fe/g dry weight iron, IQR 10.0 to 30.6 in the hydroxyurea arm and 17.3 mg Fe/g dry weight iron, IQR 8.8 to 30.7 in the transfusion arm (one trial,133 participants, low quality evidence, P = 0.79 analysis performed by the trial authors) (SWiTCH 2012; summary of findings Table 4).
Secondary outcomes
1. Incidence of TIA
Primary prevention
We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on the incidence of TIA compared to continuing to receive red cell transfusions and chelation in children who have a previous history of abnormal TCD velocities and at least 12 months of transfusions, RR 1.02 (95% CI 0.21 to 4.84) (one trial, 121 participants, very low quality evidence) (Analysis 2.5; summary of findings Table 3).
Secondary prevention
We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on the incidence of TIA compared to continuing to receive red cell transfusions and chelation in children and adolescents who have a previous history of stroke, RR 0.66 (95% CI 0.25 to 1.74) (one trial, 133 participants, very low quality evidence) (Analysis 2.5; summary of findings Table 4).
2. Measures of neurological impairment
Primary prevention
Neurological status is not yet reported in the TWiTCH trial (TWiTCH 2016).
Secondary prevention
Measures of neurological function are not reported in the SWiTCH trial (SWiTCH 2012).
3. Incidence of other sickle cell complications
Total SCD‐related serious adverse events
The SWiTCH trial reported the number of non‐neurological SCD‐related adverse events (SWiTCH 2012).
Secondary prevention
Switching to hydroxyurea and phlebotomy may increase the risk of SCD‐related serious adverse events compared to continuing to receive red cell transfusions and chelation in children and adolescents with a history of stroke, RR 3.10 (95% CI 1.42 to 6.75) (one trial, 133 participants, low quality evidence) (Analysis 2.6). Quality of the evidence was low due to risk of bias and indirectness.
Acute chest syndrome (ACS)
Primary prevention
We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on the risk of ACS compared to continuing to receive red cell transfusions and chelation in children who have a previous history of abnormal TCD velocities and at least 12 months of transfusions, RR 2.03 (95% CI 0.39 to 10.69) (one trial, 121 participants, very low quality evidence) (Analysis 2.6; summary of findings Table 3).
Secondary prevention
We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on the risk of ACS compared to continuing to receive red cell transfusions and chelation in children and adolescents who have a previous history of stroke, RR 0.33 (95% CI 0.04 to 3.08) (one trial, 133 participants, very low quality evidence) (Analysis 2.6; summary of findings Table 4).
Painful crisis
Primary prevention
We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on the risk of painful crisis compared to continuing to receive red cell transfusions and chelation in children who have a previous history of abnormal TCD velocities and at least 12 months of transfusions, RR 5.08 (95% CI 0.61 to 42.23) (one trial, 121 participants, very low quality evidence) (Analysis 2.6). Quality of the evidence was very low due to imprecision of the effect estimate, risk of bias, and indirectness.
Secondary prevention
Switching to hydroxyurea and phlebotomy may increase the risk of painful crisis compared to continuing to receive red cell transfusions and chelation in children and adolescents who have a previous history of stroke, RR 3.15 (95% CI 1.23 to 8.11), one trial, 133 participants, low quality evidence (Analysis 2.6). Quality of the evidence was low due to risk of bias, and indirectness.
Splenic sequestration
Secondary prevention
No cases of splenic sequestration occurred in the SWiTCH trial (SWiTCH 2012).
4. Quality of life, inpatient stay, immobility and disability
Quality of life has not yet been reported in either trial.
5. Measures of organ damage
Not reported in either trial.
6. Haemoglobin level and haemoglobin S percentage (mean, pre and post transfusion and at time of the event)
Haemoglobin level
Primary prevention
Switching to hydroxyurea and phlebotomy may have little or no effect on haemoglobin levels compared to continuing to receive red cell transfusions and chelation in children who have a previous history of abnormal TCD velocities and at least 12 months of transfusions, MD ‐1.00 g/L (95% CI ‐4.29 to 2.29) (one trial, 121 participants, low‐quality evidence) (Analysis 2.7). Quality of the evidence was low due to imprecision, and indirectness.
Secondary prevention
Switching to hydroxyurea and phlebotomy may have little or no effect on haemoglobin levels compared to continuing to receive red cell transfusions and chelation in children and adolescents with a history of stroke (at the final assessment median Hb level was 90 g/L, IQR 84 to 96 for the hydroxyurea arm and 90 g/L, IQR 87 to 96 for the transfusion arm) (one trial, 133 participants, P = 0.93 analysis performed by the trial authors) (SWiTCH 2012).
None of the trials reported the haemoglobin level pre‐transfusion, post‐transfusion or at the time of the event.
Haemoglobin S level
Primary prevention
As expected, switching to hydroxyurea and phlebotomy increased the haemoglobin S levels compared to continuing to receive red cell transfusions and chelation in children who have a previous history of abnormal TCD velocities and at least 12 months of transfusions, MD 43.10% (95% CI 39.59 to 46.61) (one trial, 121 participants) (Analysis 2.8).
Secondary prevention
As expected, switching to hydroxyurea and phlebotomy increased the haemoglobin S levels compared to continuing to receive red cell transfusions and chelation in children and adolescents with a history of stroke (At the final assessment median Hb S level was 64.1%, IQR 52.6 to 76.4 for the hydroxyurea arm and 32.3%, IQR 25.0 to 38.3 for the transfusion arm (one trial, 133 participants, P < 0.001 analysis performed by the trial authors) (SWiTCH 2012).
None of the trials reported the haemoglobin S level pre transfusion, post transfusion or at the time of the event.
Discussion
Summary of main results
Stroke is a potentially devastating event which affects up to 10% of children with sickle cell anaemia. Recurrences are common after the first stroke, and result in progressively more severe neurological dysfunction. The use of TCD screening has made it possible to identify a subset of children who are at higher risk for primary stroke (Adams 1992).
This Cochrane Review aimed to evaluate the literature on the effectiveness and safety of red cell transfusions for primary and secondary prevention of stroke.
We identified five randomised controlled trials that met our inclusion criteria and included a total of 660 participants. The trials were published between 1998 and 2016. Three trials compared red cell transfusions to standard care and two trials compared hydroxyurea with phlebotomy to red cell transfusions with chelation. The majority of participants had HbSS SCD, all trials included children and two trials also included adolescents. Four trials were for primary stroke prevention and one trial dealt with secondary stroke prevention. No trials included adults for primary or secondary prevention of stroke.
Red cell transfusions versus standard care
Primary prevention
Three randomised trials compared red cell transfusions to standard care for primary prevention of stroke. Two of these trials included children with no previous long‐term transfusions; one of these included children with abnormal TCD velocities, and the other included children with silent cerebral infarcts on magnetic resonance imaging (MRI) but normal TCD velocities. The third trial included children and adolescents on long‐term transfusion whose TCD velocities had normalised. Two of the three trials were terminated early due to safety concerns.
The findings of the review led to the following main conclusions regarding red cell transfusions versus standard care.
Children with no previous long‐term red cell transfusions
-
Long‐term transfusions probably reduce the incidence of clinical stroke in children with a higher risk of stroke (abnormal TCD velocities or previous history of SCIs).
-
Long‐term transfusions may: reduce the incidence of other SCD‐related complications (ACS and painful crisis); make little or no difference to IQ scores in children with SCIs; and may increase quality of life.
-
We are very uncertain whether long‐term transfusions: reduce all‐cause mortality (no deaths in either trial); reduce the risk of TIAs; or increase the risk for developing alloimmunisation.
Children and adolescents with previous long‐term red cell transfusions and normalised TCD velocities
-
We are very uncertain whether continuing red cell transfusions reduces the incidence of clinical stroke or all‐cause mortality.
Several review outcomes were only reported in one of the trial arms (SCD‐related complications, alloimmunisation, incidence of TIAs), and the trial did not report neurological impairment or quality of life.
Hydroxyurea and phlebotomy versus red cell transfusions and chelation
Primary prevention in children
-
There were no deaths or clinical strokes in either arm of the trial
-
Switching to hydroxyurea and phlebotomy may have little or no effect on liver iron concentrations, but may reduce serum ferritin levels.
-
We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on the incidence of TIA, or the risk of other SCD‐related complications (ACS or painful crisis)
Secondary prevention in children and adolescents
-
Switching to hydroxyurea and phlebotomy may increase the risk of SCD‐related serious adverse events
-
Switching to hydroxyurea and phlebotomy may have little or no effect on liver iron concentrations, but may reduce serum ferritin levels.
-
We are very uncertain whether switching to hydroxyurea and phlebotomy increases the risk of stroke.
-
We are very uncertain whether switching to hydroxyurea and phlebotomy has any effect on: all‐cause mortality, or the risk of a TIA.
Neither trial reported on neurological impairment, alloimmunisation, or quality of life.
Overall completeness and applicability of evidence
This review provides the most up‐to‐date assessment of the effectiveness and safety of red cell transfusions for the prevention of primary and secondary stroke. In this 2016 updated review we include two new trials and we have not identified any ongoing trials.
The results of this review can only be interpreted in consideration of the following factors.
-
The findings in this review can only be generalised to children with HbSS disease. Two trials also included adolescents (STOP 2 2005; SWiTCH 2012).
-
Only one of the trials assessed secondary prevention of stroke (SWiTCH 2012).
-
All of the trials were conducted in high‐income countries (USA, Canada, France and the UK). The potential risks associated with transfusion therapy are increased in low‐income countries due to a lack of trained staff, modern equipment, sanitary conditions and clean, infection‐free blood products (Ansong 2013; Ohene‐Frempong 1999). Therefore, the risk‐benefit ratio will be different in low‐income countries to those in the included trials, and the results discussed in this review may not be generalisable to that setting.
-
Co‐inheritance of alpha thalassaemia may reduce the frequency of complications in individuals with HbSS (Rumaney 2014; Steinberg 2012). Only one trial reported co‐existence of alpha thalassaemia (STOP 1998). In the Stop trial, alpha thalassaemia (at least one alpha globin gene missing) occurred in 22% in the transfusion arm and 9% in the standard treatment arm (STOP 1998).
-
The chances of having a stroke must be weighed carefully against the burden of regular blood transfusion. In the STOP trial, of the 192 children eligible to take part in the trial, 52 declined to undergo randomisation due to either reluctance of the child or their parents, or due to concern of the physician about compliance with the regimen (STOP 1998). In the SIT trial, of the 99 randomised to transfusion, 15 crossed over to observation, nine of which declined transfusion immediately after randomisation (SIT 2014). These figures may illustrate a relatively poor level of acceptance of this therapy.
-
The trials did not answer the question of how long red cell transfusion needs to be continued in this population, once commenced. The STOP 2 trial attempted to determine a safe age, or period, for discontinuation of chronic transfusion therapy for primary stroke prevention (STOP 2 2005). However, because of the high proportion (39%) of participants who reverted to abnormal TCD high stroke risk or had a stroke after transfusion was ceased, the trial was terminated two years early with the implication that transfusion therapy should be continued indefinitely in indicated participants. Although eight participants (20%) of those who discontinued transfusion had no abnormal TCD over 25 months of observation, there is currently no way of predicting which individuals will require continuance of transfusion and which will not. Extended follow‐up analysis of the STOP trial also failed to identify predictors for lower‐risk groups (Lee 2006).
-
The optimal level of sensitivity and specificity for TCD screening remains unclear. The STOP trial included high‐risk children with abnormal TCD velocities, the rate of stroke in the standard care group was approximately 10% per year (STOP 1998). The number of participants needed to treat (NNT) was approximately 11 participants per year to prevent one stroke. In a lower risk group this NNT would be higher. However, if the specificity of the screening, or the 'cut‐off point' for a high‐risk classification, was increased, only the very highest risk children would be treated and children at risk of stroke may be missed. It must therefore be judged at what level of risk it is justifiable to enrol a child to a potentially life‐long regimen of transfusion. Despite these barriers, the routine use of TCD screening and appropriate prophylactic transfusion has greatly lowered the risk of stroke in people who are managed in contemporary sickle cell programmes (Enninful‐Eghan 2010; Fullerton 2004; McCarville 2008; Telfer 2007). At present, the majority of transfusions provided by large sickle cell centres are for the provision of primary rather than secondary stroke prevention.
-
Four of the five trials were stopped early. The STOP trial was terminated 16 months before the planned end date because of the high rate of stroke in the standard care arm (STOP 1998). The STOP 2 trial was terminated early over safety concerns because high‐risk TCD results developed in 14 participants and two participants had a stroke in the transfusion halted arm (STOP 2 2005). The SWiTCH trial was stopped early due to futility when hydroxyurea and phlebotomy showed no advantage in iron removal compared with long‐term transfusion and iron chelation (SWiTCH 2012). The TWiTCH trial was stopped early because after the first interim analysis, when 33% of participants had completed the trial, non‐inferiority was found, and non‐inferiority was confirmed again after 50% of participants had exited the trial (TWiTCH 2016).
-
Children with severe vasculopathy were excluded from the TWiTCH trial, and only individuals with severe vasculopathy had a stroke in the SWiTCH trial (SWiTCH 2012; TWiTCH 2016). So individuals with severe vasculopathy may not be candidates for hydroxyurea. As well, it is not known if the effectiveness of hydroxyurea with phlebotomy is sustained over the long term.
-
In the SIT trial there was a 20% cross‐over rate to either transfusion or hydroxyurea therapy due to increasing disease severity in children receiving standard care (SIT 2014). Six per cent of children receiving standard care crossed over to regular monthly transfusions and 14% began receiving hydroxyurea treatment. We do not know how this may have influenced outcomes over the long term, but this increases uncertainty as to the efficacy of standard care compared to transfusions in these children.
Quality of the evidence
Overall the quality of the evidence was rated moderate to very low across different outcomes according to GRADE methodology (summary of findings Table 1; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4). This was due to trials being at serious risk of bias; outcome estimates being imprecise (wide confidence intervals); and a serious risk of indirectness (no direct evidence for adults or children without HbSS) since the trials only included children and adolescents primarily with HbSS disease. The HbSS and HbSβº thalassaemia genotypes included within the trials account for approximately 70% of people with SCD. Individuals with these genotypes have a higher prevalence of stroke that presents earlier than individuals with HbSC and other milder forms of SCD. Nevertheless, the participant inclusion criteria for this review is broad and although the prevalence of stroke is estimated to be rare in children with milder forms of SCD a recent trial in a cohort of 96 children with HbSC disease, estimated the prevalence for SCIs to be 13.5% (Guilliams 2015). The outcome for clinical stroke in primary prevention in children with no previous long‐term transfusions and at higher risk of stroke (abnormal TCD velocities and presence of SCIs) was the only outcome rated as moderate quality evidence.
Red cell transfusions versus standard care
No previous long‐term red cell transfusions
We considered one outcome, clinical stroke in children with higher risk of stroke (abnormal TCD velocities and SCIs), as moderate quality evidence due to indirectness.
We considered three outcomes as low quality evidence due to serious risk of bias and indirectness. These were:
-
SCD‐related adverse events (acute chest syndrome and painful crises);
-
neurological impairment (IQ);
-
quality of life.
We considered three outcomes to be very low quality evidence due to serious risk of bias, indirectness and imprecision. These were:
-
all‐cause mortality;
-
TIAs;
-
transfusion‐related adverse events (alloimmunisation).
Previous long‐term red cell transfusions
We considered two outcomes as very low quality evidence due to serious risk of bias, indirectness and imprecision. These were:
-
clinical stroke;
-
all‐cause mortality.
Several review outcomes were only reported in one of the trial arms (SCD‐related complications, alloimmunisation, incidence of TIAs).
The trial did not report neurological impairment or quality of life.
Hydroxyurea and phlebotomy versus red cell transfusions and iron chelation
Primary prevention
We considered one outcome (transfusion‐related adverse events ‐ iron overload) as low quality evidence due to serious risk of bias and indirectness
We considered four outcomes as very low quality evidence due to serious risk of bias, indirectness and imprecision. These were:
-
clinical stroke;
-
all‐cause mortality;
-
TIAs;
-
SCD‐related adverse events (ACS, painful crises).
The trial did not report neurological impairment or quality of life.
Secondary prevention
We considered one outcome as low quality evidence (transfusion‐related adverse events ‐ iron overload) as low quality evidence due to serious risk of bias and indirectness.
We considered four outcomes to be very low quality evidence due to serious risk of bias, indirectness, and imprecision. These were:
-
clinical stroke;
-
all‐cause mortality;
-
TIAs;
-
SCD‐related adverse events.
The trial did not report neurological impairment or quality of life.
Potential biases in the review process
To our knowledge, our review process is free from bias. We conducted a comprehensive search; searching data sources (including multiple databases, and clinical trial registries) to ensure that all relevant trials would be captured. There were no restrictions for the language in which the paper was originally published. The relevance of each paper was carefully assessed and all screening and data extractions were performed in duplicate. We pre‐specified all outcomes and subgroups prior to analysis. There were insufficient numbers of included trials within the meta‐analyses for us to use a funnel plot to examine the risk of publication bias.
Agreements and disagreements with other studies or reviews
With regard to the conclusions of the STOP and STOP 2 trials, multiple reports have supported the efficacy of chronic transfusion in preventing primary stroke in those at high risk based on abnormal TCD velocities (Enninful‐Eghan 2010; Fullerton 2004; McCarville 2008); this has become standard of care practice in almost all sickle cell centres and has led to more subjects being transfused for primary than for secondary stroke prevention in those centres. The findings of the SWiTCH trial were somewhat unexpected because of previously published smaller single institution studies which suggested greater efficacy from phlebotomy in reducing iron overload and not as great a degree of protection from chronic transfusion in secondary stroke prophylaxis (SWiTCH 2012).
Several observational studies for secondary stroke prevention have also attempted to define whether transfusion may be safely stopped. One review suggested that transfusion may be safely stopped at age 18 years (Powars 2000), and no recurrences occurred in a small series of seven adults with SCD followed up for three years to 18 years (Rana 1997). However, a study of 10 participants who discontinued transfusion after one to two years showed a 70% recurrence rate after only 11 months (Wilimas 1980). In a subsequent study, five out of 10 people with SCD who had been receiving regular transfusions for five to 12 years experienced an ischaemic event in the 12‐month period after transfusions were stopped (Wang 1991). These findings suggest that the risk of secondary stroke may be almost as high after discontinuing transfusion therapy 'prematurely' as before starting it. Evaluation of risk factors related to the initial stroke might be an important consideration in the design of any further studies. For example, in a retrospective analysis of 137 stroke participants, those who had an identified medical or concurrent event associated with their initial stroke did not have a recurrent stroke two or more years after the initial event (Scothorn 2002).
This systematic review noted a lack of prospective randomised controlled trials of blood transfusion for prevention of secondary stroke (following an initial stroke) in SCD. However, the practice of long‐term transfusion therapy is supported by evidence from several observational studies (Sarnaik 1979; Styles 1994). It was demonstrated in a small study of 15 participants that a target HbS level of 30% to 50% retains a high protective value against stroke, but reduces transfusion requirements and the associated risks (Cohen 1992). However, in a study which observed 60 persons with SCD for 192 person years, HbS was greater than 30% in seven of the 16 reported transient ischaemic events and five of six recurrent infarctions (Pegelow 1995). A recent survey has indicated that an acceptable "community standard" level of pre‐transfusion HbS is less than 45% rather than less than 30% (Aygun 2009).
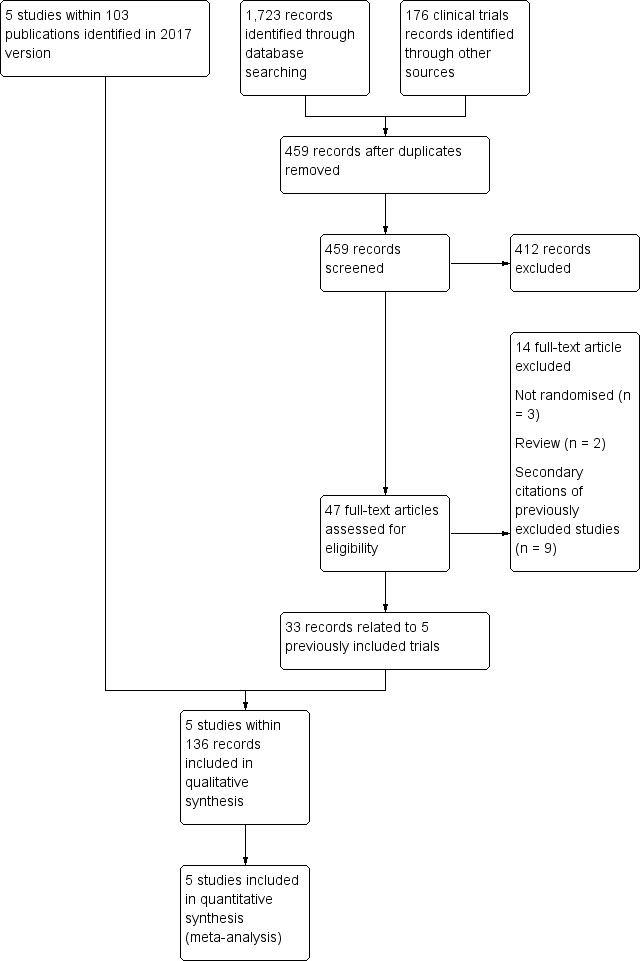
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Blood transfusion versus standard care, Outcome 1: Clinical stroke

Comparison 1: Blood transfusion versus standard care, Outcome 2: Clinical stroke ‐ velocity

Comparison 1: Blood transfusion versus standard care, Outcome 3: Clinical stroke ‐ SCI

Comparison 1: Blood transfusion versus standard care, Outcome 4: Mortality

Comparison 1: Blood transfusion versus standard care, Outcome 5: Transfusion‐related adverse events

Comparison 1: Blood transfusion versus standard care, Outcome 6: TIA

Comparison 1: Blood transfusion versus standard care, Outcome 7: Other sickle cell related complications

Comparison 2: Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 1: Clinical stroke ‐ Secondary prevention

Comparison 2: Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 2: Mortality

Comparison 2: Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 3: Transfusion‐related complications ‐ Serum ferritin; Primary prevention

Comparison 2: Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 4: Transfusion related complications ‐ Liver iron concentration ‐ Primary prevention

Comparison 2: Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 5: Other neurological event

Comparison 2: Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 6: Other sickle cell related complications

Comparison 2: Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 7: Haemoglobin levels

Comparison 2: Hydroxyurea and phlebotomy versus standard treatment (transfusions/chelation), Outcome 8: Haemoglobin S levels
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have not had previous long‐term red cell transfusions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard care | Risk with Blood transfusion | |||||
| Clinical stroke | Trial population | RR 0.12 | 326 | ⊕⊕⊕⊝ | ||
| 110 per 1000 | 13 per 1000 (3 to 54) | |||||
| All‐cause mortality | No deaths occurred in either trial arm | ‐ | 326 | ⊕⊝⊝⊝ | ||
| Adverse events associated with transfusion | Moderatea | RR 3.16 | 121 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 32 per 1000 (2 to 572) | |||||
| TIA | Trial population | Peto OR 0.13 (0.01 to 2.11) | 323 (2 RCTs) | ⊕⊝⊝⊝ | ||
| 21 per 1000 | 5 per 1000 (0 to 43) | |||||
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 0.24 | 326 | ⊕⊕⊝⊝ | ||
| 232 per 1,000 | 56 per 1000 (28 to 111) | |||||
| Moderate | ||||||
| 230 per 1000 | 55 per 1000 (28 to 110) | |||||
| Measures of neurological impairment assessed with: WASI IQ score | Least square mean 1.7 (SE 95% CI ‐1.1 to 4.4) | ‐ | 166 (1 RCT) | ⊕⊕⊝⊝ | Author reported data from SIT 2014 | |
| Quality of life assessed with: Child Health Questionnaire Parent Form 50 | Difference estimate ‐0.54 (‐0.92 to ‐0.17) | ‐ | 196 (1 RCT) | ⊕⊕⊝⊝ | Author reported data from SIT 2014 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by 1 due to imprecision. Rare event. No deaths occurred. 2 We downgraded the quality of the evidence by 1 due to risk of bias. Unblinded trial and cross‐overs, and imbalance between loss to follow‐up between trial arms 3 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 4 We downgraded the quality of evidence by 2 due to imprecision. The estimate has very wide CIs a Based on Chou 2013 | ||||||
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have had long‐term red cell transfusions to prevent a stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard care | Risk with blood transfusion | |||||
| Clinical stroke | Trial population | RR 0.22 (0.01 to 4.35) | 79 | ⊕⊝⊝⊝ | ||
| 49 per 1000 | 11 per 1000 (0 to 212) | |||||
| All‐cause mortality | Moderatea | Peto OR 8.00 (0.16 to 404.12) | 79 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 75 per 1000 (2 to 803) | |||||
| Adverse events associated with transfusion | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| TIA | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| Serious adverse events as a result of sickle cell‐related complications assessed with: ACS | See comment | 79 (1 RCT) | ‐ | No comparative numbers reported | ||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We did not downgrade the evidence due to risk of bias because the evidence was already very low grade evidence. There was attrition bias. Imbalance between loss to follow‐up between trial arms 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of evidence by 2 due to imprecision. The estimate has very wide CIs a Assuming a mortality rate of 1% | ||||||
| Primary prevention | ||||||
| Patient or population: individuals with sickle cell disease who are at risk of a primary stroke who have had long‐term red cell transfusions to prevent a stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with hydroxyurea and phlebotomy | Risk with Blood transfusion | |||||
| Clinical stroke | No strokes occurred in either trial arm | ‐ | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| All‐cause mortality | No deaths occurred in either trial arm | ‐ | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse events associated with transfusion | The mean liver iron concentration was 9.5 mg Fe/g dry weight | MD 1.8 mg Fe/g dry weight lower (5.16 lower to 1.56 higher) | ‐ | 121 (1 RCT) | ⊕⊕⊝⊝ | Switching to hydroxyurea and phlebotomy may reduce serum ferritin levels compared to continuing to receive red cell transfusions and chelation (MD) ‐1398 μg/L, 95% CI ‐1929 to ‐867; one trial, 121 participants) |
| Incidence of TIA | 49 per 1000 | 50 per 1,000 (10 to 238) | RR 1.02 (0.21 to 4.84) | 121 (1 RCT) | ⊕⊝⊝⊝ | |
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 2.03 (0.39 to 10.69) | 121 (1 RCT) | ⊕⊝⊝⊝ | ||
| 33 per 1000 | 67 per 1,000 (13 to 350) | |||||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of the evidence by 2 due to imprecision. Rare event. No deaths or stroke occurred. 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of the evidence by 1 due to risk of bias.Trial was not blinded and stopped early 4 We downgraded the quality of the evidence by 1 due to imprecision. The estimate has very wide CIs | ||||||
| Secondary prevention | ||||||
| Patient or population: individuals with sickle cell disease who have had a stroke who have had long‐term red cell transfusions to prevent another stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with hydroxyurea and phlebotomy | Risk with Blood transfusion | |||||
| Clinical stroke | Trial population | RR 14.78 | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| All‐cause mortality | 15 per 1000 | 15 per 1000 (1 to 198) | Peto OR 0.98 (0.06 to 15.92) | 133 (1 RCT) | ⊕⊝⊝⊝ | |
| Transfusion‐related adverse events ‐ assessed with liver iron concentration mg Fe/g dry weight liver | Hydroxyurea arm: median 17.2 mg IQR 10.0 to 30.6 Transfusion arm: median 17.3 mg IQR 8.8 to 30.7 | 56 (1 RCT) | ⊕⊕⊝⊝ | P = 0.7920a Switching to hydroxyurea and phlebotomy may reduce serum ferritin levels compared to continuing to receive red cell transfusions and chelation 1994 μg/L, interquartile range (IQR) 998 to 3475, in the hydroxyurea arm and 4064 μg/L, IQR 2330 to 7126, in the transfusion arm; one trial, 133 participants; P < 0.001 a | ||
| Incidence of TIA | Trial population | RR 0.66 | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 136 per 1000 | 90 per 1000 (34 to 237) | |||||
| Serious adverse events as a result of sickle cell‐related complications | Trial population | RR 0.33 (0.04 to 3.08) | 133 (1 RCT) | ⊕⊝⊝⊝ | ||
| 45 per 1000 | 15 per 1000 (2 to 140) | |||||
| Measures of neurological impairment ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| Quality of life ‐ not reported | Outcome not reported | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of the evidence by 1 due to risk of bias. Trial was not blinded and stopped early 2 We downgraded the quality of the evidence by 1 due to indirectness. Only children with HbSS or HbSβº thalassaemia included in trials 3 We downgraded the quality of the evidence by 1 due to imprecision. The estimate has very wide CIs a Analysis performed by the trial authors | ||||||
| Outcomes | Trials | Number of participants with at least one event | Adverse events/100 person‐years | Incidence rate ratioc (95% CI) | ||
|---|---|---|---|---|---|---|
| Transfusion | Standard | Transfusion | Standard | |||
| Transfusion reactions | 15 out of 90a | 1 out of 31b | 8.85 | 1.66 | 5.33 (1.67 to 23.52) | |
| Ferritin > 1500 μg/L | 76 out of 90a | 3 out of 31b | 534.70 | 37.07 | 14.42 (5.41 to 875.17) | |
| aNine participants who declined transfusion were excluded from the analysis. Abbreviations: CI: confidence interval | ||||||
| Outcomes | Trials | Number of participants with at least one event | Adverse events/100 person‐years | Incidence rate ratioa (95% CI) | ||
|---|---|---|---|---|---|---|
| Transfusion | Standard | Transfusion | Standard | |||
| Acute chest syndrome | 4 out of 63 | 14 out of 67 | 4.8b | 15.3b | ‐ | |
| 5 out of 99 | 24 out of 97 | 1.81b | 14.35b | 0.41 (0.20 to 0.75) | ||
| Painful crisis | 11 out of 63 | 13 out of 67 | 16.2 | 27.6 | ‐ | |
| 32 out of 99 | 56 out of 97 | 41.58 | 102.21 | 0.13 (0.04 to 0.28) | ||
| Priapism | 1 out of 59 | 7 out of 52 | 0.84 | 6.65 | 0.13 (0.03 to 0.55) | |
| Symptomatic avascular necrosis of the hip | 1 out of 99 | 6 out of 97 | 0.4 | 2.25 | 0.22 (0.05 to 0.85) | |
| a The incidence ratio was calculated as the rate of adverse events per 100 person‐years in the transfusion group divided by the rate of adverse events per 100 person‐years in the observation group. The 95% confidence intervals were calculated with the use of the bootstrap method with 10,000 replications. b One child from the standard care group was excluded from these analyses due to a stroke on day 16 of the trial. Abbreviation: CI: confidence interval | ||||||
| Trial | Intervention | Baseline | 6 to 12 months | 12 to 18 months | 18 to 24 months | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | Hb (g/L) | Hb S (%) | ||
| No previous long‐term transfusions | |||||||||
| Transfusion | Median 77 IQR (72 to 84) | Median 85 90% CI (51 to 95) | ‐ | Median 30 90% CI (17 to 43) | ‐ | Median 29 90% CI (16 to 43) | ‐ | Median 30 90% CI (16 to 43) | |
| Standard | Median 79 IQR (74 to 89) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Transfusion | Mean (SD) 72 (8) | Mean (SD) 87 (10) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Standard | Mean (SD) 76 (7) | Mean (SD) 87 (7) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Previous long‐term transfusions | |||||||||
| Transfusion | Mean (SD) 93 (9) | Mean (SD) 21 (8.6) | Mean (SD) 94 (9) | Mean (SD) 25.4 (10.9) | ‐ | ‐ | ‐ | ‐ | |
| Standard | Mean (SD) 98 (12) | Mean (SD) 19 (11) | Mean (SD) 77 (8) | Mean (SD) 81.0 (8.6) | ‐ | ‐ | ‐ | ‐ | |
| Abbreviations: CI: confidence interval; IQR: interquartile range; SD: standard deviation | |||||||||
| Trial | Number of transfusions | Number of HbS levels measured | HbS less than 30% | HbS 30 to 40% | HbS greater than 40% |
|---|---|---|---|---|---|
| No previous long‐term transfusions | |||||
| 1521 | ‐ | ‐ | 101 | 42 | |
| Previous long‐term transfusions | |||||
| 1070 | 988 | 748 (76%) | 192 (19%) | 48 (5%) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Clinical stroke Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 No previous long‐term red cell transfusions | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.49] |
| 1.1.2 Previous long‐term red cell transfusions | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.01, 4.35] |
| 1.2 Clinical stroke ‐ velocity Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Normal TCD velocities and no previous long‐term red cell transfusions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 Normalised TCD velocities and previous long‐term red cell transfusions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.3 Abnormal TCD velocities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Clinical stroke ‐ SCI Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Presence of previous SCI on MRI | 2 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.59] |
| 1.3.2 Absence of previous SCI on MRI | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.31] |
| 1.4 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.5 Transfusion‐related adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.1 Alloimmunisation ‐ No previous long‐term red cell transfusions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.2 Transfusion reactions ‐ No previous long‐term red cell transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 TIA Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 No previous long‐term red cell transfusions | 2 | 323 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.11] |
| 1.7 Other sickle cell related complications Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Acute chest syndrome | 2 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.12, 0.48] |
| 1.7.2 Painful crises | 2 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.46, 0.84] |
| 1.7.3 Priapism | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.99] |
| 1.7.4 Avascular necrosis of the hip | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Clinical stroke ‐ Secondary prevention Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Mortality Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 Mortality ‐ Primary prevention | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.2.2 Mortality ‐ Secondary prevention | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.3 Transfusion‐related complications ‐ Serum ferritin; Primary prevention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 Transfusion related complications ‐ Liver iron concentration ‐ Primary prevention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Other neurological event Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 TIA ‐ Primary prevention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5.2 TIA ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6 Other sickle cell related complications Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.1 Total SCD‐related SAEs ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.2 Acute chest syndrome ‐ Primary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.3 Acute chest syndrome ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.4 Painful crisis ‐ Primary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.5 Painful crisis ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.6 Infections and infestations SAEs ‐ Primary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.7 Infections and infestations SAEs ‐ Secondary prevention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.7 Haemoglobin levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.8 Haemoglobin S levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

