Vinpocetina para el deterioro cognitivo y la demencia
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre, double‐blind, placebo‐controlled, randomized, method of randomization not detailed. | |
| Participants | Degenerative and vascular dementia mild to moderate severity, fulfilling Lauter's criteria. Other types of dementia or extra/ intracranial causes excluded. The study was performed in Germany. Number of patients treated with different doses of vinpocetine: 161, and those with placebo: 56. The mean age of patients (either sex) was 74 years (range: 58‐91) | |
| Interventions | Vinpocetine or placebo 3x5 or 3x10 or 3x20 mg/day, oral route, for 12 weeks | |
| Outcomes | CGI and SKT for efficacy | |
| Notes | completers analysis | |
| Methods | Multicentre, double‐blind, placebo‐controlled, randomized, method of randomization not detailed. | |
| Participants | Degenerative and vascular dementia mild to moderate severity, fulfilling Lauter's criteria. Other types of dementia or extra/ intracranial causes excluded. The study was performed in Germany. Number of patients treated with different doses of vinpocetine: 111, and those with placebo: 53. The mean age of patients (either sex) was 72.5 years in placebo group and 73.1 years in vinpocetine group (all above 60 years). | |
| Interventions | Vinpocetine or placebo 3x20 mg/day, oral route, for 1 year | |
| Outcomes | CGI and SKT for efficacy | |
| Notes | completers analysis | |
| Methods | Multicentre, double‐blind, placebo‐controlled, randomized, method of randomization not detailed. | |
| Participants | Degenerative and vascular dementia mild to moderate severity, fulfiling Lauter's criteria. Other types of dementia or extra/ intracranial causes excluded. The study was performed in Germany. Number of patients treated with vinpocetine: 105, and those with placebo: 96. The mean age of patients (either sex) was 74.1 years in placebo group and 72.9 and 74.2 years in vinpocetine groups (range: 60‐88 years). | |
| Interventions | Vinpocetine or placebo 3x10 or 3x20 mg/day, oral route, for 16 weeks | |
| Outcomes | CGI and SKT for efficacy | |
| Notes | completers analysis | |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Described as double‐blind placebo controlled but no mention of randomization. Elderly patients with chronic cerebral dysfunction. | |
| No placebo group. | |
| Open‐label study using vinpotropil in patients with chronic “vertebrobasilar insufficiency”, ischaemic stroke in carotid territory and cerebral ischaemia after ruptured aneurism. | |
| Open study, compared vinpocetine plus idebenone vs idebenone, not placebo controlled. | |
| A review of different studies | |
| No placebo group | |
| Described as double‐blind placebo controlled but no mention of randomisation. Patients with chronic cerebral dysfunction. | |
| Not RCT. | |
| Described as double‐blind placebo controlled but no mention of randomization. Elderly patients with chronic cerebral dysfunction. | |
| Same data of Manconi 1986 | |
| Not RCT. | |
| Oben‐label pilot trial. | |
| No placebo group. | |
| Vinpocetine vs xantinol nicotinate. Not placebo controlled. Cognitive status was not evaluated. | |
| Double‐blind placebo and piracetam controlled, not randomized study. | |
| No placebo group. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CGI improvement Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 vinpocetine vs placebo, Outcome 1 CGI improvement. | ||||
| 1.1 vinpocetine 15 mg/day after 12 weeks | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.61, 3.30] |
| 1.2 vinpocetine 30 mg/day after 12 ‐ 16 weeks | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.30, 4.82] |
| 1.3 vinpocetine 60 mg/day after 12 weeks | 1 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.04, 6.81] |
| 1.4 vinpocetine 60 mg/day after 12‐16 weeks | 2 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.40, 5.46] |
| 1.5 vinpocetine 60 mg/day after 26 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.5 [2.76, 10.98] |
| 1.6 vinpocetine 60 mg/day after 52 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.5 [2.76, 10.98] |
| 2 SKT attention and memory (change from baseline) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 vinpocetine vs placebo, Outcome 2 SKT attention and memory (change from baseline). | ||||
| 2.1 vinpocetine 15 mg/day at 12 weeks | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.90, 0.10] |
| 2.2 vinpocetine 30 mg/day at 12‐16 weeks | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐1.93, ‐0.42] |
| 2.3 vinpocetine 60 mg/day at 12‐16 weeks | 3 | 418 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.50, ‐0.39] |
| 2.4 vinpocetine 60 mg at 52 weeks | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.58, ‐0.22] |
| 3 side effects Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 vinpocetine vs placebo, Outcome 3 side effects. | ||||
| 3.1 vinpocetine 15 mg/day (12 weeks of treatment) | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.43, 8.29] |
| 3.2 vinpocetine 30 mg/day (12‐16 weeks of treatment) | 3 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.04, 6.64] |
| 3.3 vinpocetine 60 mg/day (12‐16 weeks of treatment) | 2 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.84, 5.64] |
| 3.4 vinpocetine 60 mg/day (12 ‐ 52 weeks of treatment) | 3 | 419 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.71, 2.21] |
| 4 CGI Numbers who show improvement by endpoint Show forest plot | 3 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.27 [2.18, 4.91] |
| Analysis 1.4  Comparison 1 vinpocetine vs placebo, Outcome 4 CGI Numbers who show improvement by endpoint. | ||||
| 4.1 mean dose 30mg/day, endpoint 12 weeks | 1 | 217 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.00, 3.94] |
| 4.2 mean dose 45mg/day, endpoint 16 weeks | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.30, 6.55] |
| 4.3 dose 60mg/day, endpoint 26 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.5 [2.76, 10.98] |
| 5 SKT attention and memory (change from baseline) at endpoint Show forest plot | 3 | 582 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐1.73, ‐0.66] |
| Analysis 1.5  Comparison 1 vinpocetine vs placebo, Outcome 5 SKT attention and memory (change from baseline) at endpoint. | ||||
| 5.1 mean dose 35 mg/day | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.88, ‐0.32] |
| 5.2 mean dose 45 mg/day | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.15, ‐0.25] |
| 5.3 mean dose 60 mg/day | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.58, ‐0.22] |
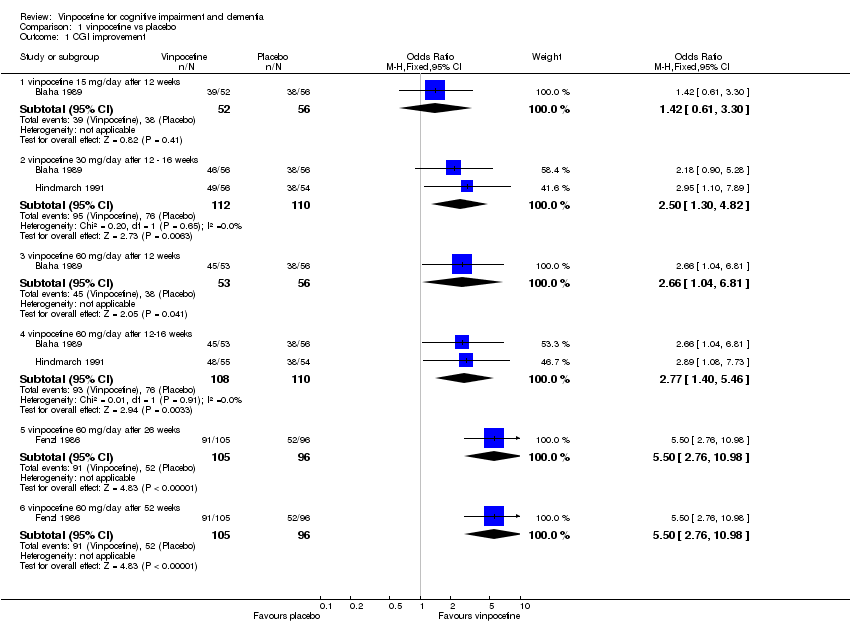
Comparison 1 vinpocetine vs placebo, Outcome 1 CGI improvement.
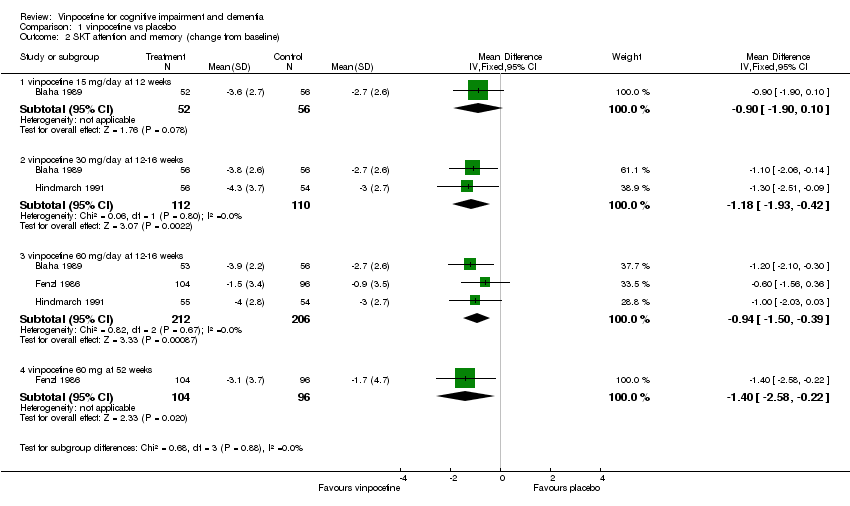
Comparison 1 vinpocetine vs placebo, Outcome 2 SKT attention and memory (change from baseline).

Comparison 1 vinpocetine vs placebo, Outcome 3 side effects.

Comparison 1 vinpocetine vs placebo, Outcome 4 CGI Numbers who show improvement by endpoint.

Comparison 1 vinpocetine vs placebo, Outcome 5 SKT attention and memory (change from baseline) at endpoint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CGI improvement Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 vinpocetine 15 mg/day after 12 weeks | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.61, 3.30] |
| 1.2 vinpocetine 30 mg/day after 12 ‐ 16 weeks | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.30, 4.82] |
| 1.3 vinpocetine 60 mg/day after 12 weeks | 1 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.04, 6.81] |
| 1.4 vinpocetine 60 mg/day after 12‐16 weeks | 2 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.40, 5.46] |
| 1.5 vinpocetine 60 mg/day after 26 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.5 [2.76, 10.98] |
| 1.6 vinpocetine 60 mg/day after 52 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.5 [2.76, 10.98] |
| 2 SKT attention and memory (change from baseline) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 vinpocetine 15 mg/day at 12 weeks | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.90, 0.10] |
| 2.2 vinpocetine 30 mg/day at 12‐16 weeks | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐1.93, ‐0.42] |
| 2.3 vinpocetine 60 mg/day at 12‐16 weeks | 3 | 418 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.50, ‐0.39] |
| 2.4 vinpocetine 60 mg at 52 weeks | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.58, ‐0.22] |
| 3 side effects Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 vinpocetine 15 mg/day (12 weeks of treatment) | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.43, 8.29] |
| 3.2 vinpocetine 30 mg/day (12‐16 weeks of treatment) | 3 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.04, 6.64] |
| 3.3 vinpocetine 60 mg/day (12‐16 weeks of treatment) | 2 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.84, 5.64] |
| 3.4 vinpocetine 60 mg/day (12 ‐ 52 weeks of treatment) | 3 | 419 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.71, 2.21] |
| 4 CGI Numbers who show improvement by endpoint Show forest plot | 3 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.27 [2.18, 4.91] |
| 4.1 mean dose 30mg/day, endpoint 12 weeks | 1 | 217 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.00, 3.94] |
| 4.2 mean dose 45mg/day, endpoint 16 weeks | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.30, 6.55] |
| 4.3 dose 60mg/day, endpoint 26 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.5 [2.76, 10.98] |
| 5 SKT attention and memory (change from baseline) at endpoint Show forest plot | 3 | 582 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐1.73, ‐0.66] |
| 5.1 mean dose 35 mg/day | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.88, ‐0.32] |
| 5.2 mean dose 45 mg/day | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.15, ‐0.25] |
| 5.3 mean dose 60 mg/day | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.58, ‐0.22] |

