Opiáceos transdérmicos u orales para la artrosis de rodilla o cadera
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003115.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 17 septiembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud musculoesquelética
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Protocol completion: Nüesch, Rutjes, Husni, Jüni.
Acquisition of data: Nüesch, da Costa, Kasteler, Rutjes.
Analysis and interpretation of data: Nüesch, da Costa, Kasteler, Husni, Welch, Rutjes, Jüni.
Manuscript preparation: Nüesch, da Costa, Kasteler, Husni, Welch, Rutjes, Jüni.
Statistical analysis: Nüesch, da Costa, Rutjes, Jüni.
Drs. Nüesch and da Costa contributed equally to this review.
Sources of support
Internal sources
-
Institute of Social and Preventive Medicine, University or Bern, Switzerland.
Intramural grants
External sources
-
Swiss National Science Foundation, Switzerland.
National Research Program 53 on musculoskeletal health (grant numbers 4053‐40‐104762/3 and 3200‐066378)
-
Marie Curie Intra‐European Fellowship, Other.
Dr Nüesch was recipient of a Marie Curie Intra‐European Fellowship for Career Development (grant number FP7‐PEOPLE‐2010‐IEF‐273673)
Declarations of interest
None.
Acknowledgements
We thank the Cochrane Musculoskeletal editorial team for valuable comments and Malcolm Sturdy for database support. The authors are grateful to Hans Quiding for providing us with additional information concerning design and outcome data.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Sep 17 | Oral or transdermal opioids for osteoarthritis of the knee or hip | Review | Bruno R da Costa, Eveline Nüesch, Rahel Kasteler, Elaine Husni, Vivian Welch, Anne WS Rutjes, Peter Jüni | |
| 2009 Oct 07 | Oral or transdermal opioids for osteoarthritis of the knee or hip | Review | Eveline Nüesch, Anne WS Rutjes, Elaine Husni, Vivian Welch, Peter Jüni | |
| 2009 Jul 08 | Oral or transdermal opioids for osteoarthritis of the knee or hip | Protocol | Eveline Nüesch, Anne WS Rutjes, Elaine Husni, Vivian Welch, Peter Jüni | |
| 2001 Apr 23 | Opioid therapy for treating osteoarthritis pain | Protocol | Elaine Husni, V Welch, Lee Simon, Beverley Shea, Joan Peterson, Peter Tugwell, George A Wells, Jessie L McGowan, Vivian Robinson | |
Differences between protocol and review
The cut‐off to distinguish between short‐term and long‐term trials was changed from 26 weeks to one month. Six months was considered to be rather long as the cut‐off for an agent that is not considered to be a structure‐modifying drug. In the absence of definitions for short‐term treatment in osteoarthritis treatment guidelines, we used the median follow‐up duration in the trials included in the first review (four weeks) as a cut‐off to discriminate between trials of shorter and longer duration.
We did not include the electronic database CINAHL in our search update since, in our previous search, this database did not identify any additional hits. Finally, we did not include the OARSI database in our search update, as we no longer had access to this database. We added analyses stratified by type of osteoarthritis (hip only versus knee only versus mixed) upon request of peer reviewers.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow chart.

Methodological characteristics of included trials. (+) indicates low risk of bias, (?) unclear, and (‐) a high risk of bias on a specific item.

Forest plot of 22 trials comparing the effects of any type of opioids and control (placebo or no intervention) on knee or hip pain. Values on x‐axis denote standardised mean differences. The plot is stratified according to type of opioids. Matsumoto 2005, Hartrick 2009, Afilalo 2010, Etropolski 2011, and NCT00486811 contributed with two comparisons and the standard error was inflated and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis. Data relating to the 3, 3, 3, 2, 2, and 2 active intervention arms in Caldwell 2002, Chindalore 2005, Kivitz 2006, Matsumoto 2005, Hartrick 2009, and Etropolski 2011, respectively, were pooled.

Funnel plot for effects on knee or hip pain.
Numbers on x axis refer to standardised mean differences (SMDs), on y axis to standard errors of SMDs.

Standardised mean differences of knee or hip pain (y axis) are plotted against total daily dose of morphine equivalents (x axis). The size of the circles is proportional to the random‐effects weights that were used in the meta‐regression. The dotted line indicates predicted treatment effects (regression line) from univariable meta‐regression by using daily morphine equivalence doses the explanatory variable, and dashed lines represent the 95% confidence intervals.
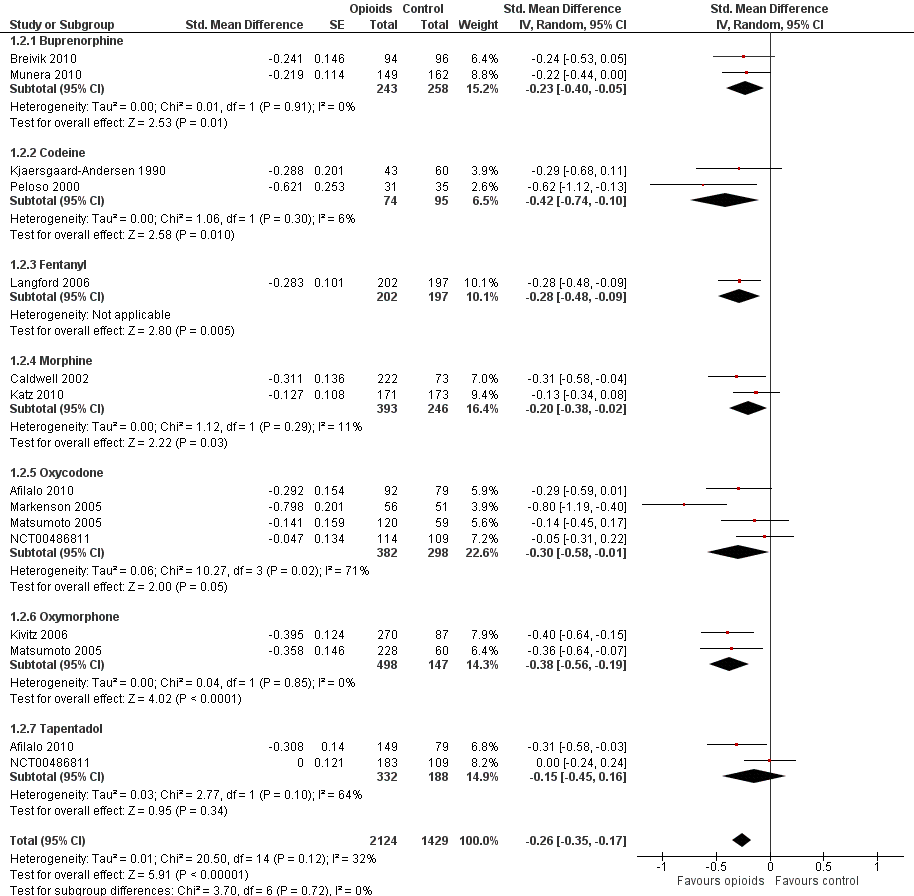
Forest plot of 12 trials comparing the effects of any type of opioids and control (placebo or no intervention) on function. Values on x axis denote standardised mean differences. The plot is stratified according to type of opioids. Matsumoto 2005 contributed with two comparisons and the standard error was inflated and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis. Data relating to the 3, 3, and 2 active intervention arms in Caldwell 2002, Kivitz 2006, and Matsumoto 2005, respectively, were pooled.

Funnel plot for effects on functioning of the knee or hip.
Numbers on x axis refer to standardised mean differences (SMDs), on y axis to standard errors of SMDs

Standardised mean differences of function (y axis) are plotted against total daily dose of morphine equivalents (x axis). The size of the circles is proportional to the random‐effects weights that were used in the meta‐regression. The dotted line indicates predicted treatment effects (regression line) from univariable meta‐regression by using daily morphine equivalence doses the explanatory variable, and dashed lines represent the 95% confidence intervals.

Forest plot of 10 trials comparing participants experiencing any adverse event between any opioid and control (placebo or no intervention). Values on x axis denote risks ratios. The plot is stratified according to type of opioid. Matsumoto 2005, Hartrick 2009, Afilalo 2010, Etropolski 2011, and NCT00486811 contributed with two comparisons and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis.

Risk ratios of participants experiencing any adverse event between opioids and control groups (y axis) are plotted against total daily dose of morphine equivalents (x axis). The size of the circles is proportional to the random‐effects weights that were used in the meta‐regression. The dotted line indicates predicted treatment effects (regression line) from univariable meta‐regression by using daily morphine equivalence doses the explanatory variable, and dashed lines represent the 95% confidence intervals.
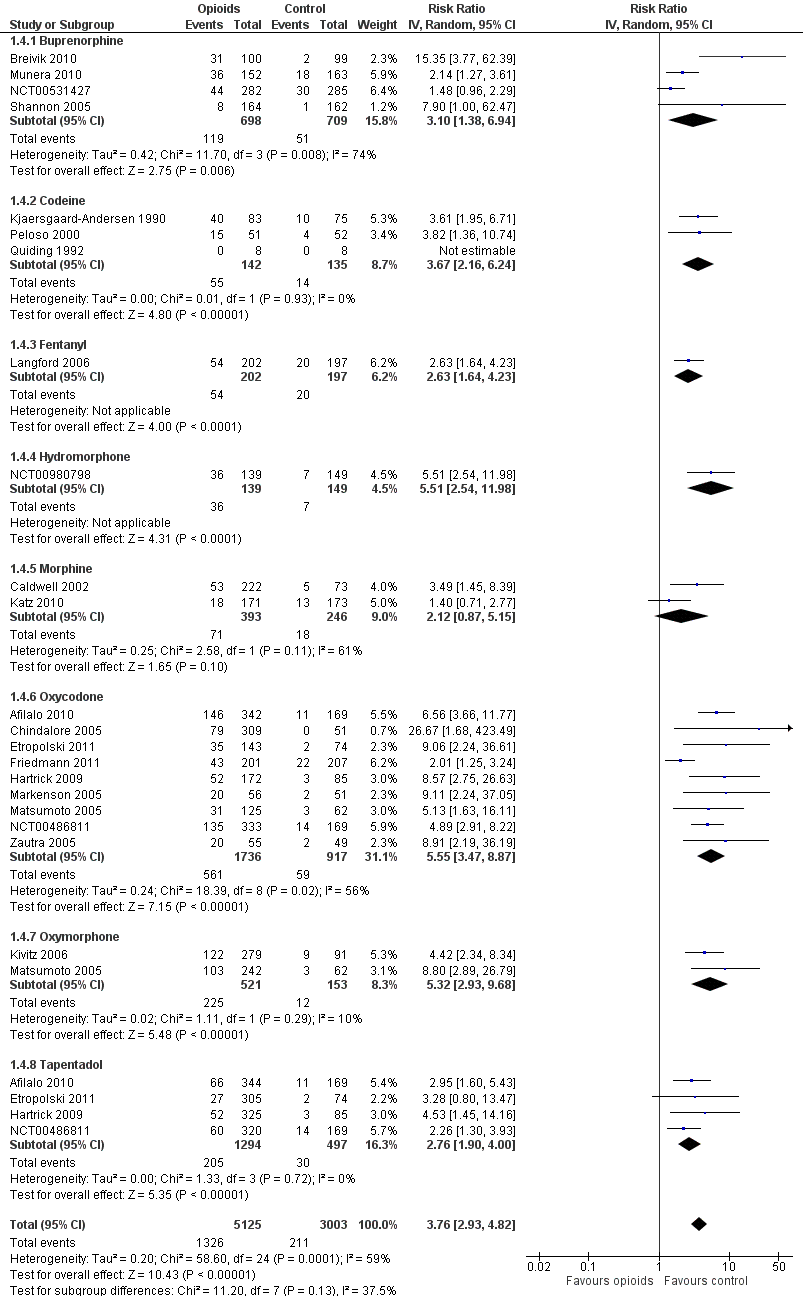
Forest plot of 21 trials comparing participants withdrawn or dropped out because of adverse events between any opioid and control (placebo or no intervention). Values on x axis denote risks ratios. The plot is stratified according to type of opioid. Matsumoto 2005, Hartrick 2009, Afilalo 2010, Etropolski 2011, and NCT00486811 contributed with two comparisons and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis. The risk ratio in one trial could not be estimated because no withdrawals or drop‐outs because of adverse events occurred in either group.

Risk ratios of participants withdrawn or dropped out because of adverse events between opioids and control groups (y axis) are plotted against total daily dose of morphine equivalents (x axis). The size of the circles is proportional to the random‐effects weights that were used in the meta‐regression. The dotted line indicates predicted treatment effects (regression line) from univariable meta‐regression by using daily morphine equivalence doses the explanatory variable, and dashed lines represent the 95% confidence intervals.
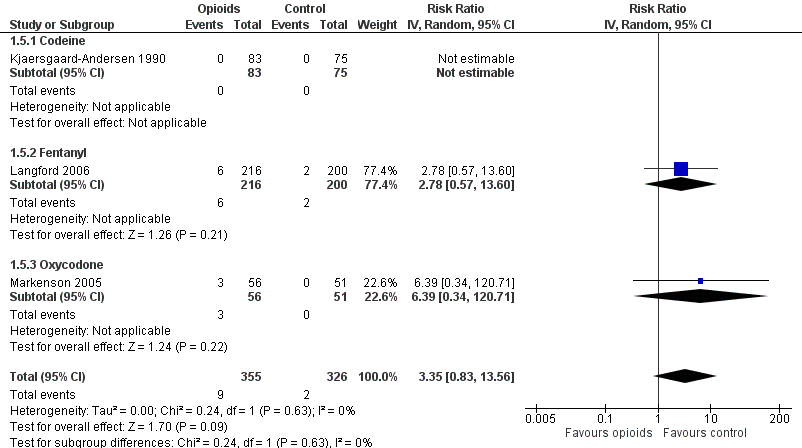
Forest plot of three trials comparing participants experiencing any serious adverse event between any opioid and control (placebo or no intervention). Values on x axis denote risks ratios. The plot is stratified according to type of opioid. The risk ratio in one trial could not be estimated because no serious adverse event occurred in either group.

Forest plot of 4 comparisons in three trials comparing participants experiencing withdrawal symptoms between any opioid and control (placebo or no intervention). Values on x axis denote odds ratios. The plot is stratified according to type of opioid. Afilalo 2010 contributed with two comparisons and the number of participants in the placebo group was halved to avoid duplicate counting of participants when including both comparisons in the overall meta‐analysis.
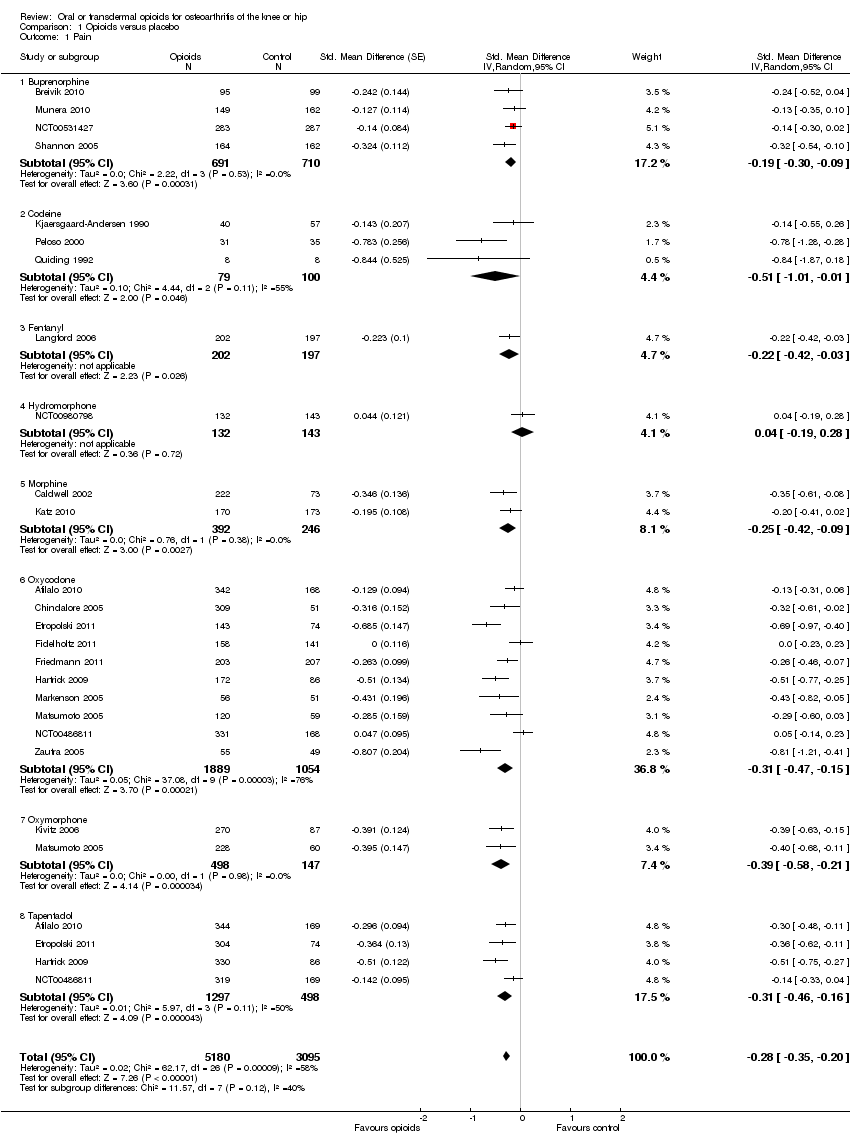
Comparison 1 Opioids versus placebo, Outcome 1 Pain.

Comparison 1 Opioids versus placebo, Outcome 2 Function.
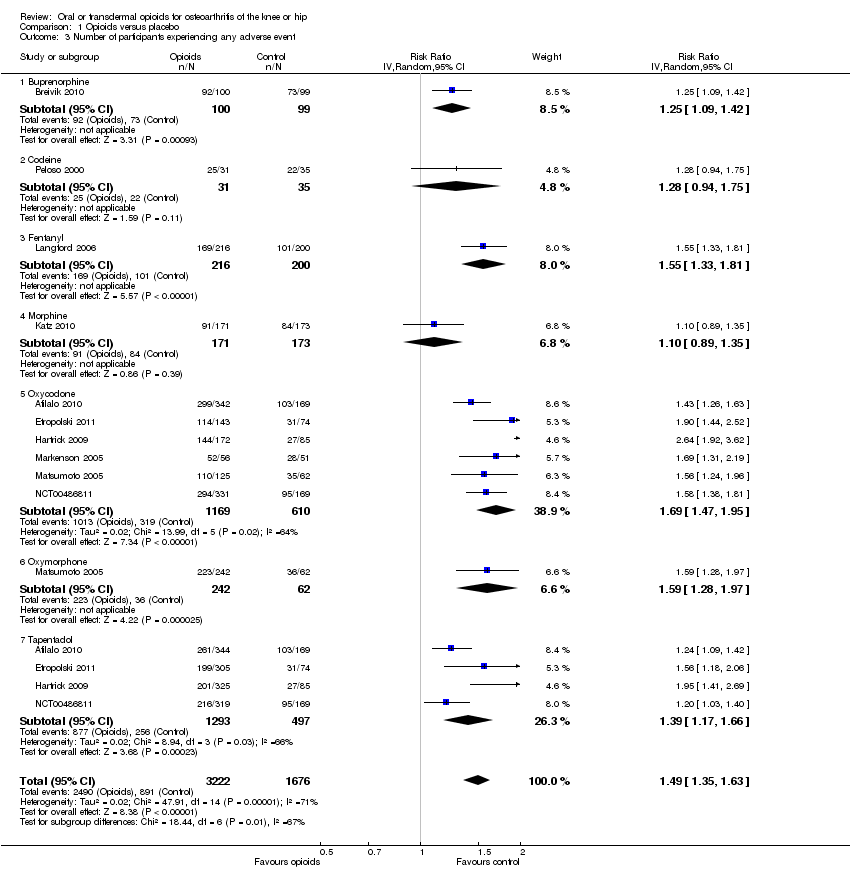
Comparison 1 Opioids versus placebo, Outcome 3 Number of participants experiencing any adverse event.
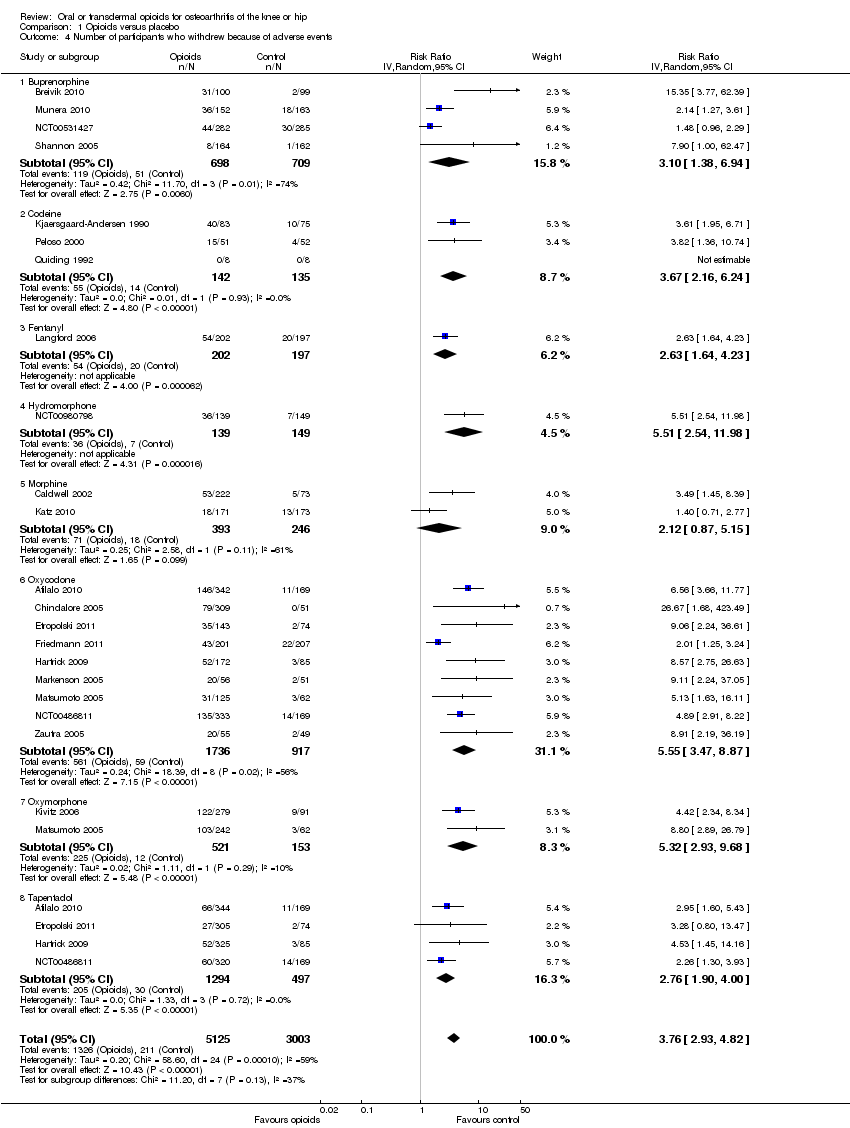
Comparison 1 Opioids versus placebo, Outcome 4 Number of participants who withdrew because of adverse events.

Comparison 1 Opioids versus placebo, Outcome 5 Number of participants experiencing any serious adverse event.

Comparison 1 Opioids versus placebo, Outcome 6 Withdrawal symptoms.
| Oral or transdermal opioids compared with placebo for osteoarthritis of the knee or hip | ||||||
| Patient or population: participants with osteoarthritis of the knee or hip Settings: various orthopaedic or rheumatology clinics Intervention: oral or transdermal opioids Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Opioids | |||||
| Pain intensity Various pain scales. (median follow‐up: 4 weeks) | ‐1.8 cm change 29% improvement | ‐2.5 cm change 41% improvement | SMD ‐0.28 (‐0.35 to ‐0.20) | 8275 | ++++ | NNTB 10 (95% CI 8 to 14)4 |
| Function Various validated function scales. (median follow‐up: 5 weeks) | ‐1.2 units 21% improvement | ‐1.8 units on WOMAC 32% improvement | SMD ‐0.26 (‐0.35 to ‐0.17) | 3553 | ++++ | NNTB 12 (95% CI 10 to 18)7 |
| Number of participants experiencing any adverse event (median follow‐up: 8 weeks) | 150 per 1000 participant‐years8 | 224 per 1000 participant‐years | RR 1.49 (1.35 to 1.63) | 4898 | +++O | NNTH 14 (95% CI 11 to 19) |
| Number of participants who withdrew because of adverse events (median follow‐up: 6 weeks) | 17 per 1000 participant‐years8 | 64 per 1000 participant‐years | RR 3.76 (2.93 to 4.82) | 7712 | ++++ | NNTH 21 (95% CI 15 to 30) |
| Number of participants experiencing any serious adverse event (median follow‐up: 8 weeks) | 4 per 1000 participant‐years8 | 13 per 1000 participant‐years | RR 3.35 (0.83 to 13.56) | 681 | ++OO | Little evidence of harmful effect (NNTH not statistically significant) |
| Withdrawal symptoms (median follow‐up: 16 weeks) | 9 per 1000 participant‐years11 | 24 per 100 | OR 2.67 (2.02 to 3.77) | 1151 | +++O | NNTH 65 (95% CI 42 to 110) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence 1 Median reduction as observed across placebo groups in large osteoarthritis trials (see methods section, Nüesch 2009). | ||||||
| Variable | Number of | N of participants | N of participants | Pain intensity | Heterogeneity | P value* |
| All trials | 22 | 5180 | 3095 | ‐0.28 (‐0.35 to ‐0.20) | 58% | |
| Analgesic potency | 0.32 | |||||
| Weak | 3 | 79 | 100 | ‐0.51 (‐1.01 to ‐0.01) | 55% | |
| Strong | 19 | 5101 | 2995 | ‐0.26 (‐0.35 to ‐0.18) | 64% | |
| Route of administration | 0.36 | |||||
| Oral | 17 | 4287 | 2188 | ‐0.30 (‐0.41 to ‐0.20) | 70% | |
| Transdermal | 5 | 893 | 907 | ‐0.20 (‐0.29 to ‐0.11) | 0% | |
| Allocation concealment | 0.31 | |||||
| Adequate | 8 | 1981 | 1141 | ‐0.32 (‐0.44 to ‐0.21) | 48% | |
| Inadequate or unclear | 14 | 3199 | 1954 | ‐0.24 (‐0.35 to ‐0.13) | 67% | |
| Blinding of participants | 0.23 | |||||
| Adequate | 15 | 3050 | 1616 | ‐0.32 (‐0.42 to ‐0.22) | 53% | |
| Inadequate or unclear | 7 | 2130 | 1479 | ‐0.21 (‐0.34 to ‐0.08) | 73% | |
| Intention‐to‐treat analysis | 0.43 | |||||
| Yes | 1 | 283 | 287 | ‐0.14 (‐0.30 to 0.02) | N/A | |
| No or unclear | 21 | 4897 | 2808 | ‐0.29 (‐0.37 to ‐0.20) | 63% | |
| Type of control intervention | 0.97 | |||||
| Placebo | 20 | 5132 | 3030 | ‐0.28 (‐0.36 to ‐0.19) | 65% | |
| No intervention | 2 | 48 | 65 | ‐0.33 (‐0.93 to 0.28) | 35% | |
| Number of participants randomised | 0.08 | |||||
| > 200 | 16 | 4895 | 2796 | ‐0.24 (‐0.33 to ‐0.16) | 64% | |
| ≤ 200 | 6 | 285 | 299 | ‐0.47 (‐0.71 to ‐0.23) | 48% | |
| Duration of treatment | 0.001 | |||||
| > 1 month | 10 | 2635 | 1972 | ‐0.15 (‐0.22 to ‐0.08) | 25% | |
| ≤ 1 month | 12 | 2545 | 1123 | ‐0.40 (‐0.50 to ‐0.30) | 37% | |
| Use of analgesic co‐interventions | 0.59 | |||||
| Similar between groups | 6 | 1189 | 891 | ‐0.31 (‐0.46 to ‐0.16) | 60% | |
| Unclear | 16 | 3991 | 2204 | ‐0.26 (‐0.36 to ‐0.16) | 65% | |
| Type of osteoarthritis | 0.77 | |||||
| Hip only | 2 | 48 | 65 | ‐0.33 (‐0.93 to 0.28) | 35% | |
| Knee only | 4 | 1674 | 1010 | ‐0.22 (‐0.41 to ‐0.04) | 78% | |
| Knee and hip | 16 | 3458 | 2020 | ‐0.29 (‐0.38 to ‐0.20) | 56% | |
| *P value for interaction. N/A: not available. | ||||||
| Variable | Number of | N of participants | N of participants | Function | Heterogeneity | P value* |
| All trials | 12 | 2124 | 1429 | ‐0.26 (‐0.35 to ‐0.17) | 32% | |
| Analgesic potency | 0.42 | |||||
| Weak | 2 | 74 | 95 | ‐0.42 (‐0.74 to ‐0.10) | 6% | |
| Strong | 10 | 2050 | 1334 | ‐0.26 (‐0.36 to ‐0.16) | 48% | |
| Route of administration | 0.76 | |||||
| Oral | 9 | 1679 | 974 | ‐0.30 (‐0.43 to ‐0.16) | 58% | |
| Transdermal | 3 | 445 | 455 | ‐0.25 (‐0.38 to ‐0.12) | 0% | |
| Allocation concealment | 0.43 | |||||
| Adequate | 6 | 1034 | 762 | ‐0.32 (‐0.45 to ‐0.18) | 47% | |
| Inadequate or unclear | 6 | 1090 | 667 | ‐0.23 (‐0.37 to ‐0.09) | 39% | |
| Blinding of participants | 0.008 | |||||
| Adequate | 10 | 1656 | 1038 | ‐0.32 (‐0.40 to ‐0.24) | 0% | |
| Inadequate or unclear | 2 | 468 | 391 | ‐0.07 (‐0.20 to 0.07) | 0% | |
| Intention‐to‐treat analysis | 0.34 | |||||
| Yes | 1 | 171 | 173 | ‐0.13 (‐0.34 to 0.08) | N/A | |
| No or unclear | 11 | 1953 | 1256 | ‐0.29 (‐0.40 to ‐0.19) | 44% | |
| Type of control intervention | 0.96 | |||||
| Placebo | 11 | 2081 | 1369 | ‐0.28 (‐0.38 to ‐0.18) | 49% | |
| No intervention | 1 | 43 | 60 | ‐0.29 (‐0.68 to 0.11) | N/A | |
| Number of participants randomised | 0.11 | |||||
| > 200 | 8 | 1900 | 1187 | ‐0.23 (‐0.32 to ‐0.14) | 26% | |
| ≤ 200 | 4 | 224 | 242 | ‐0.46 (‐0.73 to ‐0.19) | 51% | |
| Duration of treatment | 0.41 | |||||
| > 1 month | 6 | 1061 | 893 | ‐0.25 (‐0.41 to ‐0.09) | 66% | |
| ≤ 1 month | 6 | 1063 | 536 | ‐0.31 (‐0.42 to ‐0.20) | 0% | |
| Use of analgesic co‐interventions | 0.38 | |||||
| Similar between groups | 4 | 460 | 456 | ‐0.40 (‐0.67 to ‐0.13) | 71% | |
| Unclear | 8 | 1664 | 973 | ‐0.24 (‐0.33 to ‐0.15) | 16% | |
| Type of osteoarthritis | 0.45 | |||||
| Hip only | 1 | 43 | 60 | ‐0.29 (‐0.68 to 0.11) | N/A | |
| Knee only | 2 | 538 | 376 | ‐0.16 (‐0.43 to 0.11) | 76% | |
| Knee and hip | 9 | 1543 | 993 | ‐0.31 (‐0.41 to ‐0.20) | 31% | |
| *P value for interaction. N/A: not available. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 22 | 8275 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.35, ‐0.20] |
| 1.1 Buprenorphine | 4 | 1401 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.30, ‐0.09] |
| 1.2 Codeine | 3 | 179 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐1.01, ‐0.01] |
| 1.3 Fentanyl | 1 | 399 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.42, ‐0.03] |
| 1.4 Hydromorphone | 1 | 275 | Std. Mean Difference (Random, 95% CI) | 0.04 [‐0.19, 0.28] |
| 1.5 Morphine | 2 | 638 | Std. Mean Difference (Random, 95% CI) | ‐0.25 [‐0.42, ‐0.09] |
| 1.6 Oxycodone | 10 | 2943 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.47, ‐0.15] |
| 1.7 Oxymorphone | 2 | 645 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.58, ‐0.21] |
| 1.8 Tapentadol | 4 | 1795 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.46, ‐0.16] |
| 2 Function Show forest plot | 12 | 3553 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.35, ‐0.17] |
| 2.1 Buprenorphine | 2 | 501 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.40, ‐0.05] |
| 2.2 Codeine | 2 | 169 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐0.74, ‐0.10] |
| 2.3 Fentanyl | 1 | 399 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.48, ‐0.09] |
| 2.4 Morphine | 2 | 639 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 2.5 Oxycodone | 4 | 680 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.58, ‐0.01] |
| 2.6 Oxymorphone | 2 | 645 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.56, ‐0.19] |
| 2.7 Tapentadol | 2 | 520 | Std. Mean Difference (Random, 95% CI) | ‐0.15 [‐0.45, 0.16] |
| 3 Number of participants experiencing any adverse event Show forest plot | 10 | 4898 | Risk Ratio (IV, Random, 95% CI) | 1.49 [1.35, 1.63] |
| 3.1 Buprenorphine | 1 | 199 | Risk Ratio (IV, Random, 95% CI) | 1.25 [1.09, 1.42] |
| 3.2 Codeine | 1 | 66 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.94, 1.75] |
| 3.3 Fentanyl | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.33, 1.81] |
| 3.4 Morphine | 1 | 344 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.89, 1.35] |
| 3.5 Oxycodone | 6 | 1779 | Risk Ratio (IV, Random, 95% CI) | 1.69 [1.47, 1.95] |
| 3.6 Oxymorphone | 1 | 304 | Risk Ratio (IV, Random, 95% CI) | 1.59 [1.28, 1.97] |
| 3.7 Tapentadol | 4 | 1790 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.17, 1.66] |
| 4 Number of participants who withdrew because of adverse events Show forest plot | 21 | 8128 | Risk Ratio (IV, Random, 95% CI) | 3.76 [2.93, 4.82] |
| 4.1 Buprenorphine | 4 | 1407 | Risk Ratio (IV, Random, 95% CI) | 3.10 [1.38, 6.94] |
| 4.2 Codeine | 3 | 277 | Risk Ratio (IV, Random, 95% CI) | 3.67 [2.16, 6.24] |
| 4.3 Fentanyl | 1 | 399 | Risk Ratio (IV, Random, 95% CI) | 2.63 [1.64, 4.23] |
| 4.4 Hydromorphone | 1 | 288 | Risk Ratio (IV, Random, 95% CI) | 5.51 [2.54, 11.98] |
| 4.5 Morphine | 2 | 639 | Risk Ratio (IV, Random, 95% CI) | 2.12 [0.87, 5.15] |
| 4.6 Oxycodone | 9 | 2653 | Risk Ratio (IV, Random, 95% CI) | 5.55 [3.47, 8.87] |
| 4.7 Oxymorphone | 2 | 674 | Risk Ratio (IV, Random, 95% CI) | 5.32 [2.93, 9.68] |
| 4.8 Tapentadol | 4 | 1791 | Risk Ratio (IV, Random, 95% CI) | 2.76 [1.90, 4.00] |
| 5 Number of participants experiencing any serious adverse event Show forest plot | 3 | 681 | Risk Ratio (IV, Random, 95% CI) | 3.35 [0.83, 13.56] |
| 5.1 Codeine | 1 | 158 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Fentanyl | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 2.78 [0.57, 13.60] |
| 5.3 Oxycodone | 1 | 107 | Risk Ratio (IV, Random, 95% CI) | 6.39 [0.34, 120.71] |
| 6 Withdrawal symptoms Show forest plot | 3 | Odds Ratio (Random, 95% CI) | 2.76 [2.02, 3.77] | |
| 6.1 Oxycodone | 1 | Odds Ratio (Random, 95% CI) | 2.18 [0.61, 7.81] | |
| 6.2 Morphine | 1 | Odds Ratio (Random, 95% CI) | 3.05 [0.12, 75.52] | |
| 6.3 Tapentadol | 1 | Odds Ratio (Random, 95% CI) | 0.99 [0.25, 3.97] | |
| 6.4 Fentanyl | 1 | Odds Ratio (Random, 95% CI) | 2.97 [2.13, 4.14] | |

