Tratamiento con opiáceos para el dolor de la artritis reumatoide
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Cross‐over trial. Method of randomization of treatment order not described Blinding: subjects and investigator blinded; matching tablets used WIthdrawals: 6 participants Sample size calculation: not reported | |
| Participants | 51 participants with "definite or classical" rheumatoid arthritis and "moderate to severe arthritic pain" (not further defined) Disease duration: not reported Age and gender not reported DMARD therapy: not reported | |
| Interventions | Two interventions of duration one week each in cross‐over design; no washout period Intervention 1: Aspirin tablet 500mg tds for 2 days, then 1g tds for 5 days Intervention 2: Aspirin plus dextropropoxyphene tablet: aspirin 500mg plus dextropropoxyphene 50mg tds for 2 days, then aspirin 1g plus dextropropoxyphene 100mg tds for 5 days | |
| Outcomes | Assessed at end of week 1 and week 2: Patient assessment of treatment efficacy ("better", "no change", "worse") | |
| Notes | Random allocation of treatment order is implied but not made explicit. No method for randomization is described. Quote: "The hospital pharmacist alone knew what drug each patient had received." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described. Not clear that randomization occurred at all. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Quote: "Matching tablets...were used", "The trial...was double‐blind". Comment: No further detail given; maintenance of blind doubtful. |
| Incomplete outcome data (attrition bias) | High risk | 3/51 dropped out due to AE and were excluded from data analysis. A further 3/51 were lost to follow‐up and also excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided regarding adverse events. |
| Other bias | High risk | No washout period. Potential for carry‐over effects. |
| Methods | Randomized controlled trial Duration: 7 days Randomization method: not described Blinding: both participants and investigators (presumed to be the outcome assessors) were blinded WIthdrawals: one participant Sample size calculation: 40 patients "estimated" to have been sufficient; no calculation reported | |
| Participants | 40 participants, 36 females and 4 males Mean age 56.9 years Diagnosis: rheumatoid arthritis (ARA criteria) on stable doses of background therapy for at least one month (mean disease duration 96.3 months) DMARD therapy: stable therapy permitted to continue (proportion not reported) Inclusion criteria: persistent residual pain; pain at least 'moderate' on 5‐point verbal rating scale in 24 hours prior to randomization Exclusion criteria: contraindication to codeine or paracetamol; history of opioid abuse; use of an anti‐inflammatory drug other than usual therapy in 48 hours prior to randomization | |
| Interventions | Group 1: codeine 30mg plus paracetamol 500mg three times daily (7am, 1pm, 8pm) Group 2: placebo | |
| Outcomes | Primary efficacy measures: 1. Patient assessment of overall efficacy (5‐point verbal rating scale) at one week 2. Physician assessment of overall efficacy (5‐point verbal rating scale) at one week Secondary efficacy measures: 1. Daily pain score (100mm visual analogue scale) 2. Daily disability score (4‐point scale) 3. Number of pain‐related nocturnal awakenings, recorded daily 4. Pain relief (unchanged, worse, <50% relief, >50% relief) at one week | |
| Notes | One participant was mistakenly enrolled despite reporting only "slight" pain in the 24 hours before randomization ‐ this subject was excluded from the efficacy analysis but was included in the safety analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This...randomized...study versus placebo was carried out in two parallel groups..." Comment: Method of sequence generation not described in text. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "...double‐blind study versus placebo..." Comment: Blinding method not described. |
| Incomplete outcome data (attrition bias) | High risk | One subject randomized to placebo group excluded from efficacy analysis due to insufficient pain at baseline, but included in safety analysis. Efficacy data for dropouts imputed by LOCF. No information given on timing of dropouts or dropouts from active treatment group. |
| Selective reporting (reporting bias) | High risk | Daily pain VAS scores not reported in text. |
| Other bias | Low risk | |
| Methods | Randomized controlled trial Duration: 6 weeks (2 weeks dose titration phase, 4 weeks treatment phase) Randomization method: not described Blinding: participants and investigators were blinded WIthdrawals: one subject, excluded from all analyses Sample size calculation: not reported | |
| Participants | 19 participants, 13 females and 6 males Mean age 58 years (intervention group), 56 years (placebo group); age range 52‐71 years (intervention group), 38‐79 years (placebo group) Diagnosis: Rheumatoid arthritis (ACR criteria) Disease duration: not reported DMARD therapy: stable therapy permitted to continue (proportion not reported) Inclusion criteria: Pain on 10cm VAS >5cm; pain for at least one month prior to entry; pain for at least 6 hours per day Exclusion criteria: not reported | |
| Interventions | Group 1: Tilidine/naloxone 50mg/4mg or 100mg/8mg or 150mg/12mg Group 2: Placebo | |
| Outcomes | All outcomes assessed at 6 weeks: 1. Pain (10cm visual analogue scale) 2. Number of swollen joints 3. Number of tender joints 4. Pain‐related sleep disturbance 5. Quality of life (McGill pain questionnaire) 6. Global efficacy (better, no change, worse) | |
| Notes | Pain VAS scores at 6 weeks were reported as median and range only. An estimate of mean and SD was not made from these data, in accordance with the recommendations in the Cochrane Handbook (Ch 7.7.3.6) (Higgins 2009c), and these data were not used for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | One participant who withdrew immediately after randomization but before any treatment was received did not provide any data and was not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | High risk | Intervention and control groups had significantly different disease duration. |
| Methods | Randomized controlled trial Duration: 7 days Randomization method: not described Blinding: described as "double‐blind"; no further details given WIthdrawals: 2 participants Sample size calculation: 60 participants "estimated...on the basis of previous available studies". No calculation reported | |
| Participants | 60 participants, 50 females, 10 males Mean age (SD): 54.8 (16.1) years (paracetamol‐codeine group); 59.4 (9.9) years (diclofenac group) Diagnosis: rheumatoid arthritis (ACR criteria) Mean disease duration (SD): 10.6 (9.6) years (paracetamol‐codeine group); 7 (4.8) years (diclofenac group) DMARD therapy: stable therapy permitted to continue (53 of 60 participants) Inclusion criteria: stable background antirheumatic therapy for at least 2 months; pain in 24 hours before randomization at least 'moderate' on a 5‐point verbal rating scale Exclusion criteria: relative contraindication to paracetamol or codeine; previous opioid abuse | |
| Interventions | Group 1: Paracetamol 500mg + codeine 30mg tablet three times daily, plus diclofenac 50mg at 7pm, plus placebo (matching diclofenac) at 8am Group 2: Diclofenac 50mg tablet at 8am and 7pm, plus placebo (matching paracetamol+codeine) three times daily | |
| Outcomes | 1. Patient global efficacy rating (5‐point verbal rating scale) at 7 days 2. Physician global efficacy rating (5‐point verbal rating scale) at 7 days 3. Pain (100mm VAS) at 7 days 4. Daily pain (100mm VAS), measured daily 5. Disability score (4‐point verbal rating scale), measured daily 6. Number of nocturnal awakenings, measured daily 7. Duration of morning stiffness, measured daily 8. Intention to resume treatment, at 7 days | |
| Notes | No details given for power calculation. Results reported as for an equivalence study, although not apparently powered as such. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This...randomized...study was carried out in two parallel groups" Comment: Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy method |
| Incomplete outcome data (attrition bias) | High risk | Quote: "In the event of withdrawal, the last recorded score was extrapolated to D(ay) 7." Comment: End of study assessments (primary outcome) not available for 2 withdrawals from paracetamol/codeine group due to adverse effects. |
| Selective reporting (reporting bias) | High risk | Quote: "…the principal criterion of efficacy was the assessment by the patient and physician of the overall efficacy of the 7‐day treatment using a verbal 5‐point scale". Comment 1: One set of efficacy data presented; unclear whether patient or physician or combined scores. Comment 2: Other outcome measures not clearly reported in results: Patient‐reported 5‐point verbal pain scale at end of trial, duration of morning stiffness. |
| Other bias | Low risk | |
| Methods | Sequential cross‐over study of single doses of five different analgesic drugs Sequence of administration randomized by hospital pharmacist Blinding: participants, study personnel and investigators were blinded. The code for the sequence of drug administration was kept by the hospital pharmacist Withdrawals: zero Sample size calculation: not reported | |
| Participants | 30 participants Age and gender not reported Diagnosis: rheumatoid arthritis (ARA criteria) Disease duration: not reported DMARD therapy: stable therapy permitted to continue (proportion not reported) Inclusion criteria: persistent pain in at least 4 joints; no recent improvement in disease control Exclusion criteria: regular glucocorticoid use; regular narcotic analgesic use; severe mechanical pain due to degenerative disease; DMARDs commenced within 6 months of entry to study | |
| Interventions | Each participant received each of 6 capsules (5 active agents, below, plus placebo) sequentially as required for pain, no sooner than 6 hours after the most recent study drug: 1. Aspirin 650mg 2. Paracetamol 650mg 3. Codeine sulphate 65mg 4. Propoxyphene hydrochloride 65mg 5. Pentazocine hydrochloride 50mg | |
| Outcomes | 1. Analgesic effectiveness by rank, measured at 6 hours after 6th capsule administered 2. Pain relief (as proportion of complete abolition of pain) 3. Time to maximum analgesia 4. Time to recurrence of pain | |
| Notes | Data from 30 participants who completed the study were presented. Unclear whether there were participants who did not complete the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sets of [identical] capsules of each of these 6 agents were prepared in advance by a hospital pharmacist, varying the sequence of the agents from set to set." Comment: Probably adequate, although method of sequence generation not specified. |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐based sequence generation |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "All doses of all test agents were standard hospital preparations and were concealed in large opaque gelatin capsules". Quote: "Records of the sequence of the drugs in each set were kept by the pharmacist and were not available to the patients' physicians until completion of the study". |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Thirty subjects took and evaluated their responses to all 6 test agents, and only these 30 responses were considered in analyzing the data". Comment: Unclear whether there were other subjects who commenced treatment but did not complete the protocol. |
| Selective reporting (reporting bias) | Unclear risk | Three outcome measures listed in methods and all reported. |
| Other bias | Unclear risk | Insufficient information |
| Methods | Sequential cross‐over study of single doses of three active interventions plus placebo Randomization method: sequence of administration randomized by latin square design over sets of four patients Blinding: participants and investigators blinded to treatment sequence Withdrawals: zero Sample size calculation: not reported | |
| Participants | 40 participants Age range 20‐70 years Gender not reported Diagnosis: rheumatoid arthritis (diagnostic criteria not reported) Disease duration: not reported DMARD therapy: not reported Inclusion criteria: requirement for regular analgesic medications | |
| Interventions | Each participant received each of four treatments sequentially on four consecutive mornings: 1. Codeine phosphate 8mg + acetylsalicylic acid 250mg + phenacetin 250mg, two tablets, plus two placebo tablets 2. Isopropylantipyrine 150mg + phenacetin 250mg + caffeine 50mg, 2 tablets, plus two placebo tablets 3. Isopropylantipyrine 150mg + phenacetin 250mg + caffeine 50mg, 4 tablets 4. Placebo, 4 tablets | |
| Outcomes | Pain relief (5‐point verbal rating scale) at 30, 60, 90, 120 minutes, 4.5 hours and 6 hours after administration of study drug | |
| Notes | All participants were administered placebo before entry into the study, those who reported "definite" pain relief were excluded prior to randomization. The number of participants who were excluded was not reported. Outcome measures not clearly reported. Maximum pain relief score (of six scores after each dose of study drug) appears to have been used for statistical analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Order of administration randomized by latin square design in sets of four patients. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "…the trial was conducted under double‐blind conditions". Quote: "All treatments appeared to be identical and were presented to patients in the form of four white tablets". Comment: unclear whether study personnel were adequately blinded to treatment allocation. Comment: Single outcome assessor (lead author on study); unclear whether blinding was maintained. |
| Incomplete outcome data (attrition bias) | Unclear risk | Data collected for 40 completers; unclear whether other subjects commenced the study. |
| Selective reporting (reporting bias) | High risk | Reported outcomes not clearly specified in methods. |
| Other bias | High risk | An initial dose of placebo was given to all participants and those who 'responded' to placebo were excluded from randomization into the trial. Judgement of 'placebo responder' was made subjectively by the investigators. |
| Methods | Three consecutive trials of similar design Trial 1: Comparison of single doses of 4 interventions (3 analgesic tablets and placebo) in a sequential pairwise cross‐over design Trial 2: Comparison of single doses of 4 interventions (3 soluble analgesics and placebo) in a sequential pairwise cross‐over design Trial 3: Comparison of single doses of 3 interventions (2 analgesic tablets and placebo) in a sequential pairwise cross‐over design Randomization method: order of treatment allocation in each pairwise comparison was randomized, but the method of randomization is not described Blinding: participants and investigators blinded to treatment sequence Withdrawals: 78 participants were randomized across the three trials but data from 67 participants were analyzed Sample size calculation: not reported | |
| Participants | 30 participants in trial 1, 24 participants in trial 2, 24 participants in trial 3 Age and gender not reported Diagnosis: rheumatoid arthritis (ARA criteria) Disease duration: not reported DMARD therapy: not reported Inclusion criteria: pain of sufficient severity to require "on‐demand" analgesic medications at least once a day | |
| Interventions | For each participant, single doses of different interventions were compared in pairs, where each intervention was given once, on consecutive days. Each participant within a trial received all possible treatment pairs (6 comparisons in trials 1 and 2, 3 comparisons in trial 3), with one day of 'usual therapy' between each paired comparison. The interventions were: Trial 1: 1. paracetamol 325mg + dextropropoxyphene 32.5mg, 2 tablets, plus 2 placebo tablets 2. paracetamol 500mg, 2 tablets, plus 2 placebo tablets 3. pentazocine 25mg, 2 tablets, plus 2 placebo tablets 4. placebo, 4 tablets Trial 2: 1. aspirin 500mg + codeine 8mg, 2 soluble tablets, plus 2 soluble placebo tablets 2. paracetamol 325mg + dextropropoxyphene 32.5mg, 2 soluble tablets, plus 2 soluble placebo tablets 3. aspirin 300mg, 2 soluble tablets, plus 2 soluble placebo tablets 4. placebo, 4 soluble tablets Trial 3: 1. 'Ciba 44,328' (an experimental compound related to propoxyphene) 50mg, 2 tablets, plus 2 placebo tablets 2. paracetamol, 2 tablets, plus 2 placebo tablets 3. placebo, 4 tablets | |
| Outcomes | 1. pain relief (4‐point verbal rating scale), measured hourly for six hours after administration of study drug 2. patient preference, after each pairwise comparison | |
| Notes | It is not clear whether any subjects participated in more than one trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Treatment order was randomized and balanced so far as possible". Comment: Not clear that treatment allocation was random. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The double‐placebo method was used to ensure that the trial was double‐blind…" Comment: Method of blinding of study personnel and outcome assessors is not clear. |
| Incomplete outcome data (attrition bias) | High risk | 78 patients were randomized, but only 67 were included in the analysis. No information provided on the remaining subjects. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | High risk | Three separate trials reported. It is unclear whether participants could be recruited into more than one trial. Not all participants appeared to complete each trial, but reasons not given. No mention of adverse events. |
| Methods | Randomized controlled trial Duration: 7 days Randomization method: participants were randomized to active treatment or placebo in 3:1 ratio. Randomization method not described Blinding: participants, investigators and clinical personnel were blinded to treatment assignment Withdrawals: 10 participants were excluded from the 'intention to treat' efficacy analysis after randomization; 5 participants who had been randomized were not included in the tolerability analysis Sample size calculation: 126 in the active treatment group and 42 in the placebo group were estimated to be required to detect a difference in mean daily pain relief of 0.4, at alpha=0.05, power=0.8 | |
| Participants | 277 participants randomized, 267 (227 females and 40 males) included in the analysis Active treatment group: 171 females and 30 males, mean age 51.55 (range 24‐76) Placebo group: 56 females and 10 males, mean age 52.02 (range 26‐74) Diagnosis: rheumatoid arthritis (ACR criteria), diagnosed at least 6 months prior to enrolment in trial Disease duration: not reported (diagnosis at least 6 months prior required for study entry) DMARD therapy: stable therapy permitted to continue (94.0% in active treatment group, 92.4% in placebo group) Inclusion criteria: Pain due to rheumatoid arthritis of severity at least 40mm on 100mm VAS for at least 2 days prior to randomization; stable background DMARD and NSAID therapy for at least 30 days Exclusion criteria: concomitant use of SSRI antidepressants, short‐acting analgesics, topical analgesics or anaesthetics within 5 half‐lives prior to study entry; intra‐articular glucocorticoids within 2 months prior to study entry; commencement of new DMARD therapy within 3 months or oral glucocorticoids within 4 weeks prior to study entry; other active articular disease; previous failure of, or intolerance to, tramadol; major psychiatric morbidity; history of substance abuse | |
| Interventions | Group 1: tramadol 37.5mg + paracetamol 325mg, one tablet three times daily Group 2: placebo, one tablet three times daily | |
| Outcomes | Primary: 1. Mean pain relief score (6‐point scale), measured daily Secondary: 1. Mean pain score (100mm VAS), measured daily 2. Pain (100mm VAS) at 7 days 3. Pain relief (6‐point scale) at 7 days 4. Patient global assessment of efficacy (5‐point Likert scale), measured at 7 days 5. Investigator global assessment of efficacy (5‐point Likert scale), measured at 7 days 6. Function (Health Assessment Questionnaire), measured at 7 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized in a 3:1 ratio..." Comment: Method of randomization unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Quote: "All subjects, investigators and clinical personnel were blinded to treatment assignment until the trial was completed…". Comment: Matching placebo used. Blinding probably adequate. |
| Incomplete outcome data (attrition bias) | High risk | 277 individuals randomized. Text states that 267 subjects for whom at least one efficacy measurement was available were analysed (ITT) but only 248 included in the primary efficacy analysis. Missing data imputed by LOCF, although BOCF may have been more appropriate. |
| Selective reporting (reporting bias) | High risk | One of the secondary outcome measures specified in the methods section ("pain intensity at the second visit") was not reported. |
| Other bias | Low risk | |
| Methods | Placebo‐controlled cross‐over trial Duration: 10 weeks (5 weeks per phase) Randomization method: order of intervention randomly allocated; the method of randomization was not described Blinding: placebo was used but no further details of blinding procedure were provided Withdrawals: 16 participants failed to complete the study, 11 of whom withdrew during the first phase Sample size calculation: not reported | |
| Participants | 20 participants (19 males and one female) Age not reported Diagnosis: rheumatoid arthritis (diagnostic criteria not reported) Disease duration: not reported DMARD therapy: not reported Inclusion criteria: pain poorly controlled by non‐narcotic analgesics Exclusion criteria: pregnancy or lactation; history of drug abuse; severe hepatic, renal, cardiac or respiratory comorbidity; prior sensitivity to trial medications | |
| Interventions | Two intervention phases of duration 5 weeks each in cross‐over design; no washout period: Intervention 1: Controlled‐release morphine sulphate 10mg 12‐hourly, titrated by the investigator in weeks 2 and 3 to achieve adequate analgesia (maximum dose 60mg 12‐hourly), and gradually reduced in dose in the fifth week until cessation at the completion of 5 weeks Intervention 2: placebo | |
| Outcomes | All outcomes were assessed at the completion of weeks 2, 4 and 5 in each intervention phase: 1. Pain (100mm VAS) 2. Pain verbal rating scale (4‐point) 3. Function (Health Assessment Questionnaire) 4. Joint inflammation (Ritchie index) 5. Grip strength (sum of both hands, in mm Hg) | |
| Notes | Due to the unexpectedly high number of withdrawals, the study was analysed as a parallel‐group controlled trial at the completion of phase one, rather than as a cross‐over trial. Data were analysed at the completion of weeks 2 and 4 of phase one, and only completers were included in the efficacy analysis. The protocol allowed for titration of the morphine sulphate dose to a maximum of 120mg per day. The doses received by participants were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...patients were randomly allocated..." Comment: Method of randomization unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | High rate of adverse effects and drop‐outs suggests blinding may not have been adequately maintained. |
| Incomplete outcome data (attrition bias) | High risk | High drop‐out rate. Initial cross‐over design but data only analysed for first period. Completers only were analysed for efficacy outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | High risk | Planned as cross‐over trial but only the results of phase I were analysed due to unexpected number of withdrawals. |
| Methods | Placebo‐controlled cross‐over trial Duration: 2 weeks (one week per phase) Randomization method: order of intervention randomly allocated; the method of randomization was not described Blinding: placebo was used but no further details of blinding procedure were provided Withdrawals: 5 participants Sample size calculation: not reported | |
| Participants | 40 participants (29 females and 11 males) Mean age 51.95 years (range 26‐73) Diagnosis: rheumatoid arthritis (1958 ARA criteria) Mean disease duration 8.1 years (SD 8.2 years) DMARD therapy: not permitted Inclusion criteria: inadequate pain relief despite stable doses of NSAIDs for at least one month Exclusion criteria: current use of glucocorticoids, chrysotherapy or anti‐malarial drugs | |
| Interventions | Two intervention phases of duration one week each in cross‐over design; no washout period: Intervention 1: pentazocine 25mg, one tablet four‐hourly by day plus 2 tablets on retiring Intervention 2: placebo | |
| Outcomes | All outcomes were assessed at the completion of each one‐week intervention phase: 1. Pain (5‐point verbal rating scale) 2. Stiffness (5‐point verbal rating scale) 3. Joint tenderness (Ritchie index) 4. Grip strength At the completion of the second intervention phase, participants also recorded a preference for one of the interventions | |
| Notes | 5 participants withdrew prior to completion of the study and were excluded from analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to initial pentazocine or placebo treatment periods". Comment: method of randomization unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The trial was of the double‐blind cross‐over type" Quote: "identical placebo tablets" Comment: Blinding of participants probably adequate; blinding of study personnel and outcome assessors unclear. |
| Incomplete outcome data (attrition bias) | High risk | Five out of forty participants failed to complete the study and were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | High risk | No washout period. Potential for carry‐over effects. |
| Methods | Placebo‐controlled cross‐over trial Duration: Four one‐week intervention phases, commenced at two‐week intervals Randomization method: order of interventions was randomly allocated; the method of randomization was not described Blinding: placebo was used but no further details of blinding procedure were provided Withdrawals: one participant Sample size calculation: not reported | |
| Participants | 16 participants (14 females and 2 males) Age range 21‐67 years Diagnosis: rheumatoid arthritis (ARA criteria) Mean disease duration 6.2 years (SD 8.0 years) DMARD therapy: stable therapy permitted to continue (proportion not reported) Inclusion criteria: daily anti‐inflammatory analgesic use Exclusion criteria: gastrointestinal, hepatic, renal or psychiatric disease; previous intolerance to study medications | |
| Interventions | Four intervention phases of duration one week each in cross‐over design; one week washout period between each intervention phase: Intervention 1: paracetamol 500mg twice daily on days 1‐3, indomethacin 25mg three times daily on days 4‐5, indomethacin 50mg on the morning of day 6 Intervention 2: paracetamol 500mg twice daily on days 1‐3, dextropropoxyphene 65mg three times daily on days 4‐5, dextropropoxyphene 130mg on the morning of day 6 Intervention 3: paracetamol 500mg twice daily on days 1‐3, dextropropoxyphene 65mg mane and 2pm, plus amitriptyline 25mg nocte on days 4‐5, dextropropoxyphene 65mg plus amitriptyline 25mg on the morning of day 6 Intervention 4: paracetamol 500mg twice daily on days 1‐3, placebo three times daily on days 4‐5, placebo on the morning of day 6 | |
| Outcomes | All outcomes were assessed at 2 hours and 4 hours after the administration of the study drug on day 6 of each intervention phase: Primary: psychomotor tests Secondary: 1. pain (100mm VAS) 2. other subjective assessments, including drowsiness, mood, anxiety, contentment | |
| Notes | One participant withdrew from the study and was not included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The trial comprised four randomized double‐blind crossover treatment periods started at two‐week intervals". Comment: randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: study drugs were given in "identical gelatin capsules". Comment: Blinding of participants probably adequate; blinding of study personnel and outcome assessors unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1/16 subjects dropped out "due to reasons unrelated to the trial" and was not included in data analysis. No further information on missing data provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided, although it appears that only one pain outcome measure was planned, and was reported in full. |
| Other bias | Unclear risk | Insufficient information |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No comparator group | |
| Same study as Boureau 1991, published in a different language | |
| No pain outcome measure | |
| Mixed population of OA and RA; data not able to be separated for analysis | |
| Not published as a full‐text article | |
| Not published as a full‐text article | |
| Mixed population of OA and RA; data not able to be separated for analysis | |
| Mixed population of OA and RA; data not able to be separated for analysis | |
| Comparison of steroid versus no steroid; all participants received the same background opioid therapy | |
| No comparator group | |
| No pain outcome measure | |
| No participants with rheumatoid arthritis | |
| Mixed population of OA and RA; data not able to be separated for analysis | |
| Mixed population; data not able to be separated for analysis | |
| No comparator group | |
| No comparator group | |
| Trial of tramadol in chronic joint pain but participants with rheumatoid arthritis were specifically excluded | |
| Mixed population; data not able to be separated for analysis | |
| Mixed population of OA and RA; data not able to be separated for analysis | |
| Mixed population; data not able to be separated for analysis | |
| Trial of intra‐articular morphine in temporomandibular joint disorders; participants with inflammatory arthritis were excluded. | |
| No comparator group |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Opioid versus placebo, Outcome 1 Pain VAS. | ||||
| 1.1 Morphine, measured at 2 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Morphine, measured at 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tilidine/naloxone, measured at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean daily pain intensity Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Opioid versus placebo, Outcome 2 Mean daily pain intensity. | ||||
| 2.1 Tramadol/paracetamol | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean daily pain relief (maximum score = 4, negative score = pain worse) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Opioid versus placebo, Outcome 3 Mean daily pain relief (maximum score = 4, negative score = pain worse). | ||||
| 3.1 Tramadol/paracetamol | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pain relief of 50% or better Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Opioid versus placebo, Outcome 4 Pain relief of 50% or better. | ||||
| 4.1 Codeine/paracetamol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Withdrawal due to inadequate analgesia Show forest plot | 4 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.34, 2.01] |
| Analysis 1.5  Comparison 1 Opioid versus placebo, Outcome 5 Withdrawal due to inadequate analgesia. | ||||
| 5.1 Codeine/paracetamol | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.73] |
| 5.2 Tilidine/naloxone | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.13, 4.13] |
| 5.3 Tramadol/paracetamol | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 5.18] |
| 5.4 Morphine | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.40, 4.49] |
| 6 Patient reported global impression of clinical change (PGIC) 'good' or 'very good' Show forest plot | 3 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.03, 2.03] |
| Analysis 1.6  Comparison 1 Opioid versus placebo, Outcome 6 Patient reported global impression of clinical change (PGIC) 'good' or 'very good'. | ||||
| 6.1 Tilidine/naloxone | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.45, 2.63] |
| 6.2 Tramadol/paracetamol | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.97, 1.82] |
| 6.3 Codeine/paracetamol | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [1.14, 4.77] |
| 7 Desire to resume treatment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Opioid versus placebo, Outcome 7 Desire to resume treatment. | ||||
| 7.1 Codeine/paracetamol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Functional status (HAQ) Show forest plot | 2 | 243 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| Analysis 1.8  Comparison 1 Opioid versus placebo, Outcome 8 Functional status (HAQ). | ||||
| 8.1 Tramadol/paracetamol | 1 | 223 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.32, 0.18] |
| 8.2 Morphine | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.94, 0.34] |
| 9 Participants reporting adverse events Show forest plot | 4 | Odds Ratio (Random, 95% CI) | 3.90 [2.31, 6.56] | |
| Analysis 1.9  Comparison 1 Opioid versus placebo, Outcome 9 Participants reporting adverse events. | ||||
| 9.1 Codeine/paracetamol | 1 | Odds Ratio (Random, 95% CI) | 2.33 [0.64, 8.55] | |
| 9.2 Tilidine/naloxone | 1 | Odds Ratio (Random, 95% CI) | 2.00 [0.31, 12.85] | |
| 9.3 Tramadol/paracetamol | 1 | Odds Ratio (Random, 95% CI) | 4.70 [2.48, 8.91] | |
| 9.4 Pentazocine | 1 | Odds Ratio (Random, 95% CI) | 4.33 [0.79, 23.70] | |
| 10 Serious adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Opioid versus placebo, Outcome 10 Serious adverse events. | ||||
| 10.1 Tramadol/paracetamol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Dextropropoxyphene plus aspirin versus aspirin alone | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Withdrawal due to adverse events Show forest plot | 3 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.52, 13.75] |
| Analysis 1.11  Comparison 1 Opioid versus placebo, Outcome 11 Withdrawal due to adverse events. | ||||
| 11.1 Codeine/paracetamol | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.57] |
| 11.2 Tilidine/naloxone | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 5.25 [0.31, 89.35] |
| 11.3 Tramadol/paracetamol | 1 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 6.37 [1.58, 25.69] |
| 12 Withdrawal due to adverse event or inefficacy Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 Opioid versus placebo, Outcome 12 Withdrawal due to adverse event or inefficacy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in 100mm pain VAS Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Opioid versus NSAID, Outcome 1 Change in 100mm pain VAS. | ||||
| 2 Global efficacy Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Opioid versus NSAID, Outcome 2 Global efficacy. | ||||
| 3 Desire to resume treatment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Opioid versus NSAID, Outcome 3 Desire to resume treatment. | ||||
| 4 Withdrawal due to inadequate analgesia Show forest plot | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.4  Comparison 2 Opioid versus NSAID, Outcome 4 Withdrawal due to inadequate analgesia. | ||||
| 5 Participants reporting adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Opioid versus NSAID, Outcome 5 Participants reporting adverse events. | ||||
| 6 Withdrawal due to adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Opioid versus NSAID, Outcome 6 Withdrawal due to adverse events. | ||||
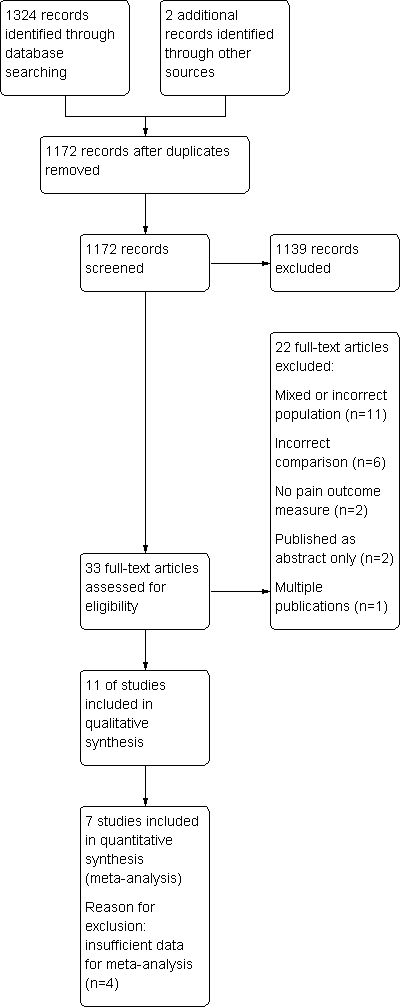
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
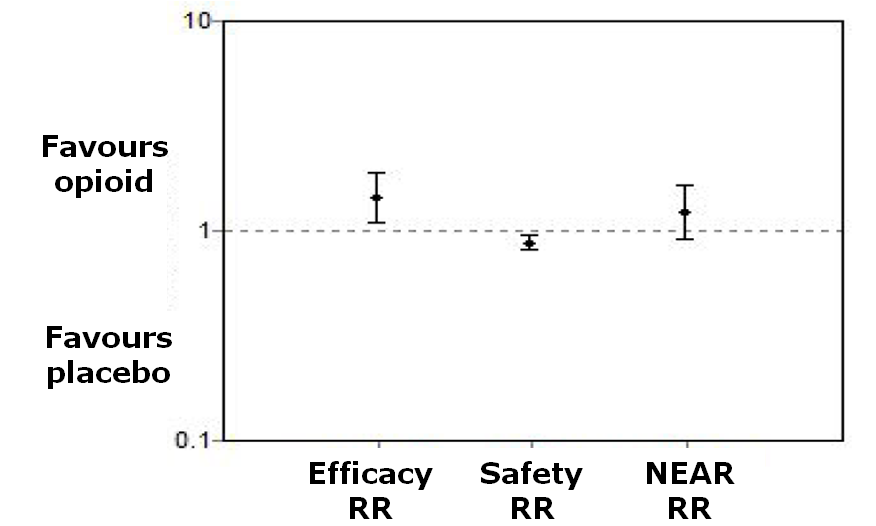
Net efficacy adjusted for risk

Comparison 1 Opioid versus placebo, Outcome 1 Pain VAS.

Comparison 1 Opioid versus placebo, Outcome 2 Mean daily pain intensity.
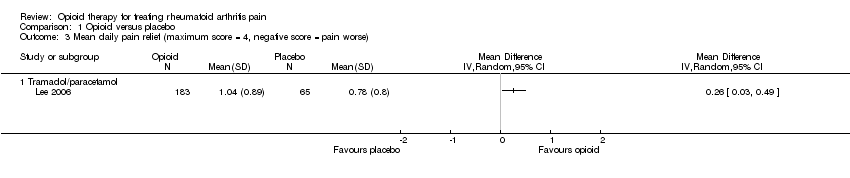
Comparison 1 Opioid versus placebo, Outcome 3 Mean daily pain relief (maximum score = 4, negative score = pain worse).

Comparison 1 Opioid versus placebo, Outcome 4 Pain relief of 50% or better.
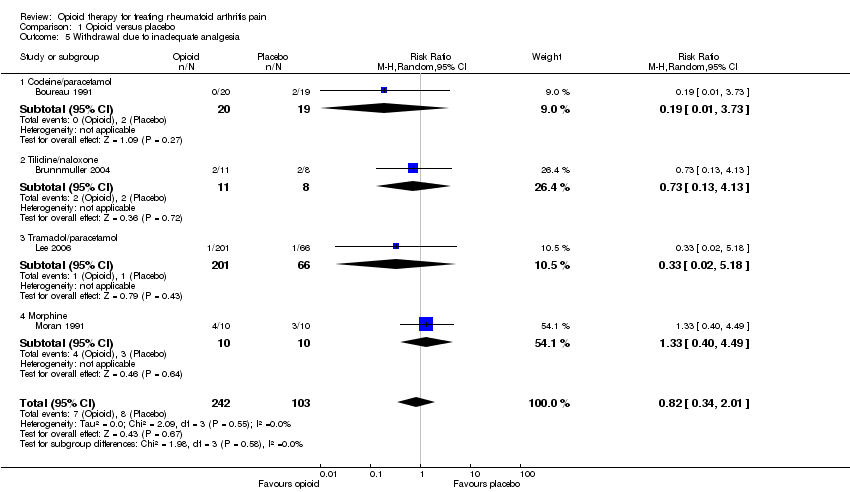
Comparison 1 Opioid versus placebo, Outcome 5 Withdrawal due to inadequate analgesia.

Comparison 1 Opioid versus placebo, Outcome 6 Patient reported global impression of clinical change (PGIC) 'good' or 'very good'.
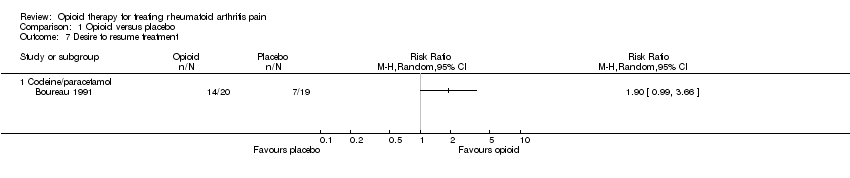
Comparison 1 Opioid versus placebo, Outcome 7 Desire to resume treatment.

Comparison 1 Opioid versus placebo, Outcome 8 Functional status (HAQ).

Comparison 1 Opioid versus placebo, Outcome 9 Participants reporting adverse events.
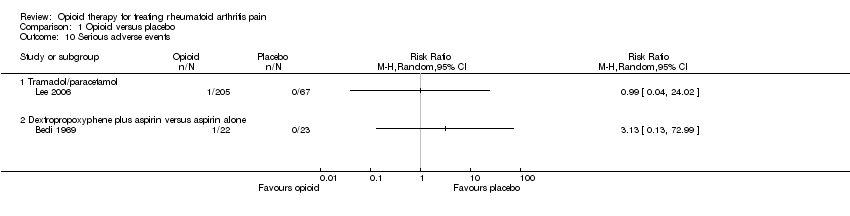
Comparison 1 Opioid versus placebo, Outcome 10 Serious adverse events.

Comparison 1 Opioid versus placebo, Outcome 11 Withdrawal due to adverse events.

Comparison 1 Opioid versus placebo, Outcome 12 Withdrawal due to adverse event or inefficacy.
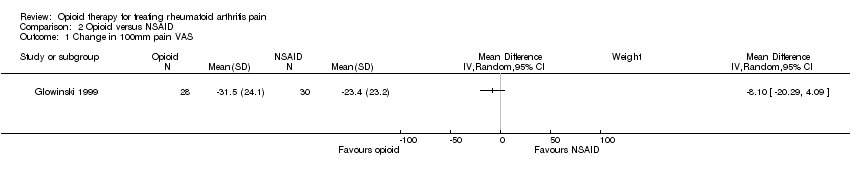
Comparison 2 Opioid versus NSAID, Outcome 1 Change in 100mm pain VAS.

Comparison 2 Opioid versus NSAID, Outcome 2 Global efficacy.

Comparison 2 Opioid versus NSAID, Outcome 3 Desire to resume treatment.

Comparison 2 Opioid versus NSAID, Outcome 4 Withdrawal due to inadequate analgesia.

Comparison 2 Opioid versus NSAID, Outcome 5 Participants reporting adverse events.

Comparison 2 Opioid versus NSAID, Outcome 6 Withdrawal due to adverse events.
| Opioids for rheumatoid arthritis pain | ||||||
| Patient or population: patients with rheumatoid arthritis pain | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Opioids | |||||
| Patient reported global impression of clinical change (PGIC) 'good' or 'very good' | 398 per 1000 | 573 per 1000 | RR 1.44 | 324 | ⊕⊕⊝⊝ | Absolute risk difference = 18% (1% to 41%). Relative percent change = 44% (3% to 103%). NNT = 6 (3 to 84)4 |
| Withdrawal due to inadequate analgesia | 78 per 1000 | 64 per 1000 | RR 0.82 | 345 | ⊕⊕⊝⊝ | Not statistically significant4 |
| Functional status (HAQ) | The mean Functional status (HAQ) in the intervention groups was | 243 | ⊕⊕⊝⊝ | HAQ only reported in two studies (of duration one week and two weeks). | ||
| Withdrawal due to adverse events | 53 per 1000 | 142 per 1000 | RR 2.67 | 331 | ⊕⊕⊝⊝ | Not statistically significant4 |
| Participants reporting adverse events | 209 per 1000 | 508 per 1000 | OR 3.90 | 371 | ⊕⊕⊝⊝ | Absolute risk difference = 30% (17% to 42%). Relative percent change = 143% (81% to 203%). NNTH = 4 (3 to 6)4 |
| Serious adverse events | See comment | See comment | Not estimable | 317 | ⊕⊕⊝⊝ | Two studies reported serious adverse events. In each study, one participant in the opioid group experienced an SAE. There were no deaths. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There was heterogeneity in the choice of opioid and the use of an adjunctive non‐opioid analgesic. 10 of the 11 included studies used a weak opioid. Oral morphine was the only strong opioid used. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Morphine, measured at 2 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Morphine, measured at 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tilidine/naloxone, measured at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean daily pain intensity Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Tramadol/paracetamol | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean daily pain relief (maximum score = 4, negative score = pain worse) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Tramadol/paracetamol | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pain relief of 50% or better Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Codeine/paracetamol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Withdrawal due to inadequate analgesia Show forest plot | 4 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.34, 2.01] |
| 5.1 Codeine/paracetamol | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.73] |
| 5.2 Tilidine/naloxone | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.13, 4.13] |
| 5.3 Tramadol/paracetamol | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 5.18] |
| 5.4 Morphine | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.40, 4.49] |
| 6 Patient reported global impression of clinical change (PGIC) 'good' or 'very good' Show forest plot | 3 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.03, 2.03] |
| 6.1 Tilidine/naloxone | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.45, 2.63] |
| 6.2 Tramadol/paracetamol | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.97, 1.82] |
| 6.3 Codeine/paracetamol | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [1.14, 4.77] |
| 7 Desire to resume treatment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Codeine/paracetamol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Functional status (HAQ) Show forest plot | 2 | 243 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 8.1 Tramadol/paracetamol | 1 | 223 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.32, 0.18] |
| 8.2 Morphine | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.94, 0.34] |
| 9 Participants reporting adverse events Show forest plot | 4 | Odds Ratio (Random, 95% CI) | 3.90 [2.31, 6.56] | |
| 9.1 Codeine/paracetamol | 1 | Odds Ratio (Random, 95% CI) | 2.33 [0.64, 8.55] | |
| 9.2 Tilidine/naloxone | 1 | Odds Ratio (Random, 95% CI) | 2.00 [0.31, 12.85] | |
| 9.3 Tramadol/paracetamol | 1 | Odds Ratio (Random, 95% CI) | 4.70 [2.48, 8.91] | |
| 9.4 Pentazocine | 1 | Odds Ratio (Random, 95% CI) | 4.33 [0.79, 23.70] | |
| 10 Serious adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Tramadol/paracetamol | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Dextropropoxyphene plus aspirin versus aspirin alone | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Withdrawal due to adverse events Show forest plot | 3 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.52, 13.75] |
| 11.1 Codeine/paracetamol | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.57] |
| 11.2 Tilidine/naloxone | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 5.25 [0.31, 89.35] |
| 11.3 Tramadol/paracetamol | 1 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 6.37 [1.58, 25.69] |
| 12 Withdrawal due to adverse event or inefficacy Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in 100mm pain VAS Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Global efficacy Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Desire to resume treatment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Withdrawal due to inadequate analgesia Show forest plot | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Participants reporting adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Withdrawal due to adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |

