Colocación de espirales endovasculares versus clips neuroquirúrgicos para pacientes con hemorragia subaracnoidea por un aneurisma
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 2) (searched 26 March 2017)
#1MeSH descriptor: [Subarachnoid Hemorrhage] this term only
#2MeSH descriptor: [Intracranial Hemorrhages] this term only
#3MeSH descriptor: [Cerebral Hemorrhage] this term only
#4MeSH descriptor: [Intracranial Aneurysm] this term only
#5MeSH descriptor: [Rupture, Spontaneous] this term only
#6#4 and #5
#7MeSH descriptor: [Aneurysm, Ruptured] this term only
#8MeSH descriptor: [Brain] explode all trees
#9MeSH descriptor: [Meninges] explode all trees
#10#8 or #9
#11#7 and #10
#12(subarachnoid or arachnoid) near/6 (haemorrhage* or hemorrhage* or bleed* or blood*)
#13MeSH descriptor: [Vasospasm, Intracranial] this term only
#14(cerebral or intracranial or cerebrovascular) near/6 (vasospasm or spasm)
#15(brain or cereb* or intracranial) near/3 aneurysm* near/3 ruptur*
#16SAH
#17#1 or #2 or #3 or #6 or #11 or #12 or #13 or #14 or #15 or #16
#18MeSH descriptor: [Embolization, Therapeutic] this term only
#19MeSH descriptor: [Endovascular Procedures] this term only
#20MeSH descriptor: [Prostheses and Implants] this term only
#21MeSH descriptor: [Blood Vessel Prosthesis] this term only
#22MeSH descriptor: [Vascular Surgical Procedures] this term only
#23MeSH descriptor: [Blood Vessel Prosthesis Implantation] this term only
#24coil* or hydrocoil* or Guglielmi*
#25#18 or #19 or #20 or #21 or #22 or #23 or #24
#26MeSH descriptor: [Neurosurgical Procedures] this term only
#27MeSH descriptor: [Craniotomy] this term only
#28MeSH descriptor: [Neurosurgery] this term only
#29MeSH descriptor: [Aneurysm] this term only and with qualifier(s): [Surgery ‐ SU]
#30MeSH descriptor: [Aneurysm, Ruptured] this term only and with qualifier(s): [Surgery ‐ SU]
#31MeSH descriptor: [Intracranial Aneurysm] this term only and with qualifier(s): [Surgery ‐ SU]
#32MeSH descriptor: [Subarachnoid Hemorrhage] this term only and with qualifier(s): [Surgery ‐ SU]
#33clip*
#34#26 or #27 or #28 or #29 or #30 or #31 or #32 or #33
#35#17 and #25 and #34
Appendix 2. MEDLINE Ovid (1966 to 26 March 2018)
1. Subarachnoid Hemorrhage/
2. intracranial hemorrhages/ or cerebral hemorrhage/ or vasospasm, intracranial/
3. Intracranial Aneurysm/
4. Rupture, Spontaneous/
5. 3 and 4
6. Aneurysm, Ruptured/
7. exp brain/
8. 6 and 7
9. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw.
10. ((brain or cereb$ or intracranial) adj3 aneurysm$ adj3 ruptur$).tw.
11. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw.
12. sah.tw.
13. 1 or 2 or 5 or 8 or 9 or 10 or 11 or 12
14. Embolization, Therapeutic/ or endovascular procedures/
15. "prostheses and implants"/ or blood vessel prosthesis/
16. vascular surgical procedures/ or blood vessel prosthesis implantation/
17. (coil$ or hydrocoil$ or Guglielmi$).tw.
18. or/14‐17
19. 13 and 18
20. neurosurgical procedures/ or craniotomy/
21. Neurosurgery/
22. aneurysm/su or aneurysm, ruptured/su or intracranial aneurysm/su
23. Subarachnoid Hemorrhage/su [Surgery]
24. clip$.tw.
25. or/20‐24
26. 19 and 25
27. Randomized Controlled Trials as Topic/
28. random allocation/
29. Controlled Clinical Trials as Topic/
30. control groups/
31. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
32. double‐blind method/
33. single‐blind method/
34. Research Design/
35. randomized controlled trial.pt.
36. controlled clinical trial.pt.
37. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
38. random$.tw.
39. (controlled adj5 (trial$ or stud$)).tw.
40. (clinical$ adj5 trial$).tw.
41. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
42. (surgical adj5 (group$ or subject$ or patient$)).tw.
43. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
44. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
45. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
46. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw.
47. controls.tw.
48. trial.ti.
49. or/27‐48
50. 26 and 49
Appendix 3. Embase Ovid (1980 to 26 March 2018)
1. subarachnoid hemorrhage/
2. brain hemorrhage/ or brain vasospasm/ or intracranial aneurysm/ or brain artery aneurysm/
3. brain artery aneurysm rupture/
4. aneurysm rupture/ and exp brain/
5. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw.
6. ((brain or cereb$ or intracranial) adj3 aneurysm$ adj3 ruptur$).tw.
7. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw.
8. sah.tw.
9. or/1‐8
10. Artificial Embolism/ or coil embolization/
11. blood vessel prosthesis/
12. endovascular surgery/
13. endovascular coiling/
14. (coil$ or hydrocoil$ or Guglielmi$).tw.
15. or/10‐14
16. aneurysm surgery/ or aneurysm clip/
17. subarachnoid hemorrhage/su
18. brain hemorrhage/su or brain vasospasm/su or intracranial aneurysm/su or brain artery aneurysm/su or aneurysm rupture/su
19. neurosurgery/ or craniotomy/
20. clip/ or clip$.tw.
21. or/16‐20
22. 9 and 15 and 21
23. Randomized Controlled Trial/
24. Randomization/
25. Controlled Study/
26. control group/
27. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
28. Double Blind Procedure/
29. Single Blind Procedure/ or triple blind procedure/
30. "types of study"/
31. (random$ or RCT$).tw. or trial.ti.
32. (controlled adj5 (trial$ or stud$)).tw.
33. (clinical$ adj5 trial$).tw.
34. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
35. (surgical adj5 (group$ or subject$ or patient$)).tw.
36. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
37. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
38. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
39. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw.
40. controls.tw.
41. or/23‐40
42. 22 and 41
Appendix 4. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform search strategy
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform search strategy:
ClinicalTrials.gov: subarachnoid AND (coiling OR clipping)
WHO International Clinical Trials Registry Platform: subarachnoid AND (coiling OR clipping)
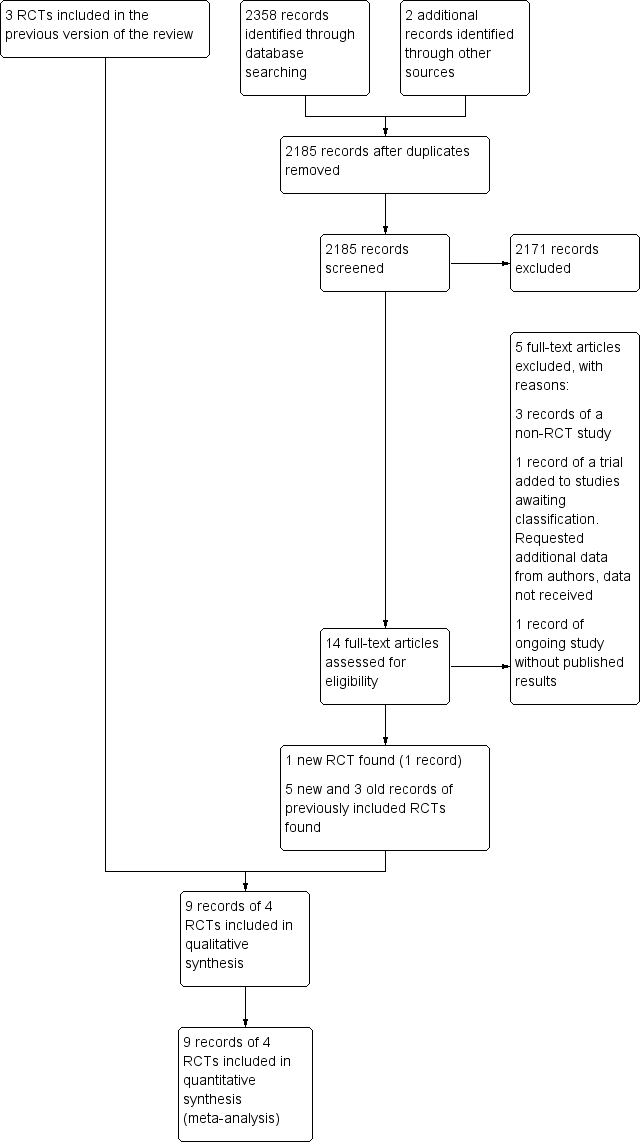
Study flow diagram. RCT: randomised controlled trial.
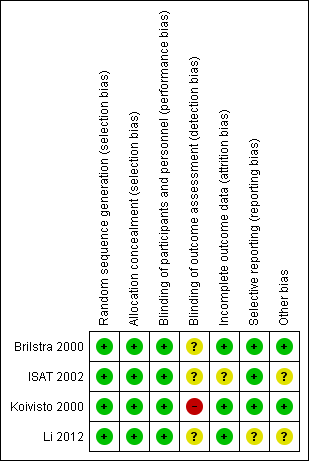
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
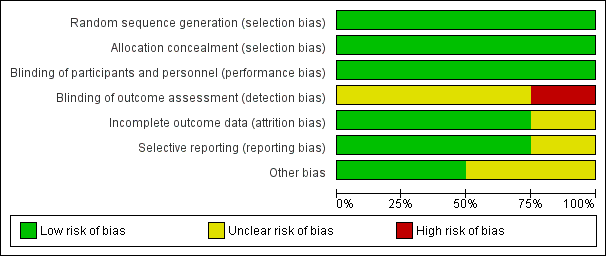
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
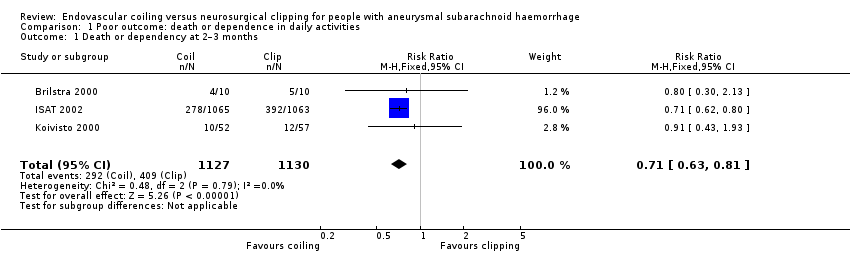
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 1 Death or dependency at 2–3 months.
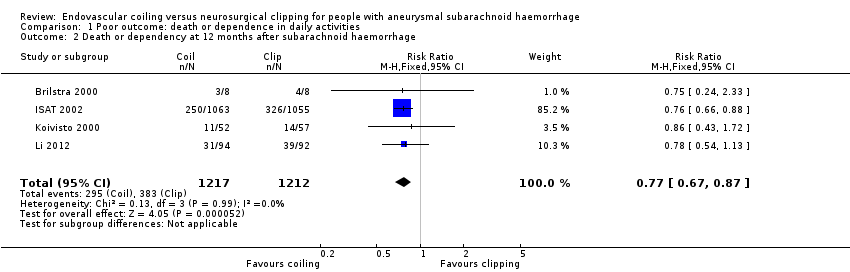
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 2 Death or dependency at 12 months after subarachnoid haemorrhage.
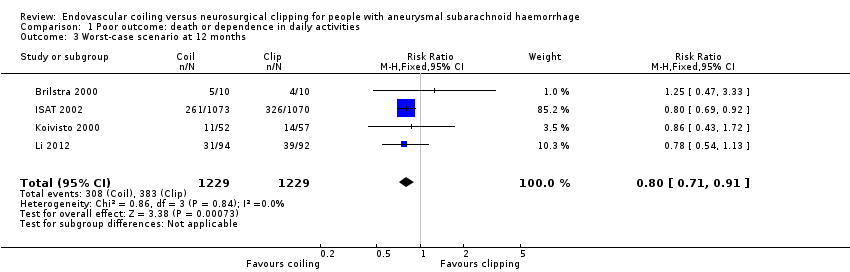
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 3 Worst‐case scenario at 12 months.

Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 4 Death or dependency at 5 years.
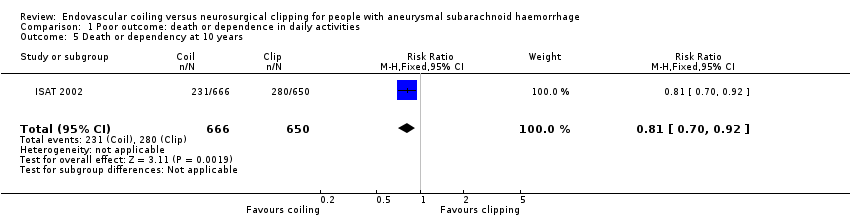
Comparison 1 Poor outcome: death or dependence in daily activities, Outcome 5 Death or dependency at 10 years.

Comparison 2 Death from any cause, Outcome 1 Death from any cause 2–3 months.

Comparison 2 Death from any cause, Outcome 2 Death from any cause between randomisation and 1 year after SAH.

Comparison 2 Death from any cause, Outcome 3 Death from any cause up to 5 years.

Comparison 2 Death from any cause, Outcome 4 Death from any cause up to 10 years.
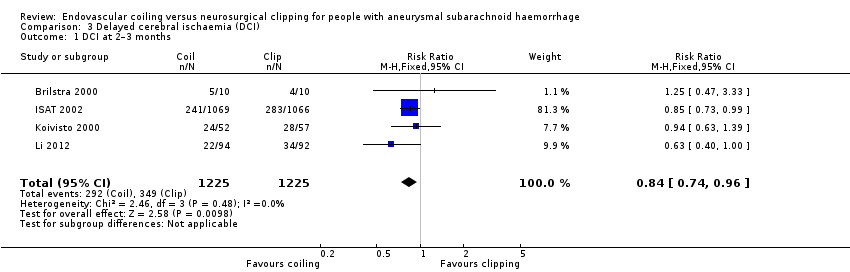
Comparison 3 Delayed cerebral ischaemia (DCI), Outcome 1 DCI at 2–3 months.

Comparison 4 Rebleeding, Outcome 1 Rebleed before treatment.
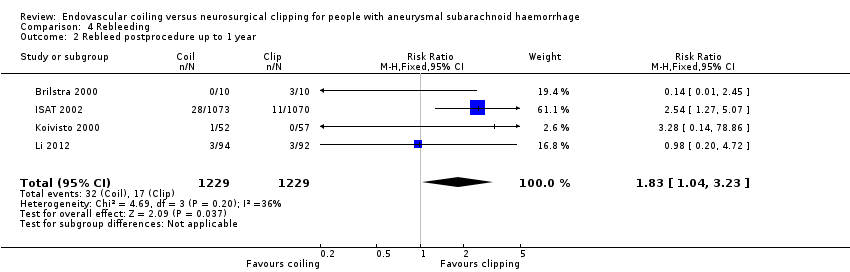
Comparison 4 Rebleeding, Outcome 2 Rebleed postprocedure up to 1 year.
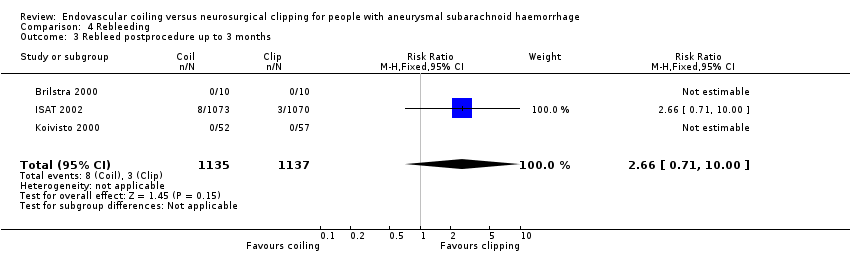
Comparison 4 Rebleeding, Outcome 3 Rebleed postprocedure up to 3 months.

Comparison 4 Rebleeding, Outcome 4 Rebleed postprocedure up to 5 years.
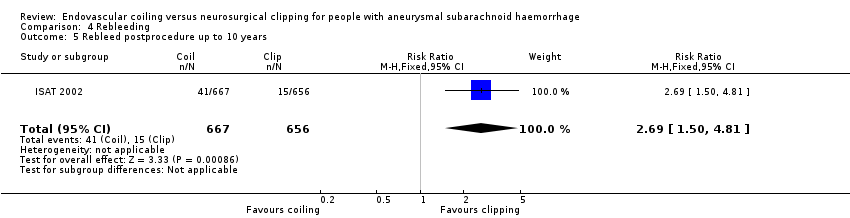
Comparison 4 Rebleeding, Outcome 5 Rebleed postprocedure up to 10 years.

Comparison 5 Complications from intervention, Outcome 1 Complications from intervention.
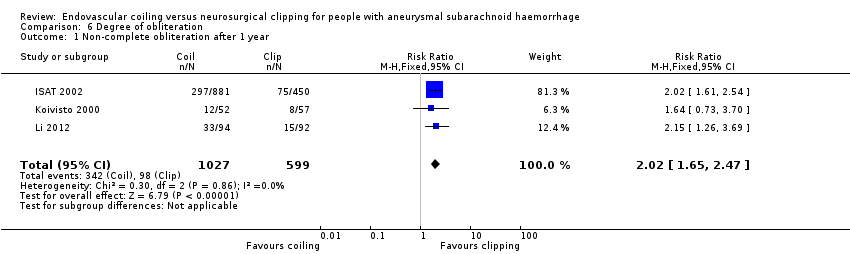
Comparison 6 Degree of obliteration, Outcome 1 Non‐complete obliteration after 1 year.

Comparison 6 Degree of obliteration, Outcome 2 < 90% occlusion after 1 year.

Comparison 7 Subgroup analysis: aneurysm location, Outcome 1 Poor outcome at 12 months: posterior and anterior circulation.
| Endovascular coiling compared with neurosurgical clipping for subarachnoid haemorrhage | ||||||
| Patient or population: people with subarachnoid haemorrhage from a ruptured intracranial aneurysm Settings: tertiary care Intervention: endovascular coiling of aneurysm Comparison: neurosurgical clipping of aneurysm | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Neurosurgical clipping | Endovascular coiling | |||||
| Poor outcome: death or dependence in daily activities (12 months) | Study population | RR 0.77 (0.67 to 0.87) | 2429 | ⊕⊕⊕⊝ | — | |
| 366 per 1000 | 281 per 1000 | |||||
| Poor outcome (death or dependence) (10 years) | Study population | RR 0.81 (0.70 to 0.92) | 1316 | ⊕⊕⊝⊝a,b | Based on subgroup of participants in 1 large RCT only | |
| 430 per 1000 | 348 per 1000 | |||||
| Death from any cause (12 months) | Study population | RR 0.80 (0.63 to 1.02) | 2429 | ⊕⊕⊕⊝ | — | |
| 154 per 1000 | 123 per 1000 | |||||
| Delayed cerebral ischaemia (2–3 months) | Study population | RR 0.84 (0.74 to 0.96) | 2450 (4 RCTs) | ⊕⊕⊕⊝ | — | |
| 384 per 1000 | 322 per 1000 | |||||
| Rebleeding postprocedure up to 1 year | Study population | RR 1.83 (1.04 to 3.23) | 2458 (4 RCTs) | ⊕⊕⊕⊕ | — | |
| 21 per 1000 | 38 per 1000 (21 to 67) | |||||
| Rebleeding postprocedure up to 10 years | Study population | RR 2.69 (1.50 to 4.81) | 1323 (1 RCT) | ⊕⊕⊝⊝a,b | Based on 1 large RCT only | |
| 22 per 1000 | 61 per 1000 | |||||
| Complications from the intervention | Study population | RR 1.05 (0.44 to 2.53) | 129 (2 RCTs) | ⊕⊕⊝⊝c | Based on 2 small RCTs only | |
| 235 per 1000 | 246 per 1000 (103 to 594) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived from the studies included in the meta‐analysis. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to indirectness of evidence: participants in poor condition on admission under‐represented in the largest RCT. bDowngraded one level due to risk of bias: long‐term outcome data available for only a subgroup of participants. cDowngraded two levels due to risk of bias: underpowered due to data availability from only two small trials, unclear definition of complication from intervention. | ||||||
| Number of participants per treatment | Extent of occlusion | ||
| 100% | 90% to 100% | < 90% | |
| Endovascular coiling: 881 | 584 (66%) | 228 (26%) | 69 (8%) |
| Neurosurgical clipping: 450 | 370 (82%) | 55 (12%) | 25 (6%) |
| Endovascular coiling: 52 | 40 (77%) | 10 (19%) | 2 (4%) |
| Neurosurgical clipping: 57 | 49 (86%) | 7 (12%) | 1 (2%) |
| Total | |||
| Endovascular coiling: 933 | 624 (67%) | 238 (26%) | 71 (8%) |
| Neurosurgical clipping: 507 | 419 (83%) | 62 (12%) | 26 (5%) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency at 2–3 months Show forest plot | 3 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.63, 0.81] |
| 2 Death or dependency at 12 months after subarachnoid haemorrhage Show forest plot | 4 | 2429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.87] |
| 3 Worst‐case scenario at 12 months Show forest plot | 4 | 2458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.91] |
| 4 Death or dependency at 5 years Show forest plot | 1 | 1724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.01] |
| 5 Death or dependency at 10 years Show forest plot | 1 | 1316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death from any cause 2–3 months Show forest plot | 3 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.18] |
| 2 Death from any cause between randomisation and 1 year after SAH Show forest plot | 4 | 2429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.02] |
| 3 Death from any cause up to 5 years Show forest plot | 1 | 2087 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.98] |
| 4 Death from any cause up to 10 years Show forest plot | 1 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DCI at 2–3 months Show forest plot | 4 | 2450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rebleed before treatment Show forest plot | 3 | 2272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.37, 1.12] |
| 2 Rebleed postprocedure up to 1 year Show forest plot | 4 | 2458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.04, 3.23] |
| 3 Rebleed postprocedure up to 3 months Show forest plot | 3 | 2272 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.71, 10.00] |
| 4 Rebleed postprocedure up to 5 years Show forest plot | 1 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.51, 5.02] |
| 5 Rebleed postprocedure up to 10 years Show forest plot | 1 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.50, 4.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complications from intervention Show forest plot | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.44, 2.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐complete obliteration after 1 year Show forest plot | 3 | 1626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.65, 2.47] |
| 2 < 90% occlusion after 1 year Show forest plot | 2 | 1440 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.93, 2.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Poor outcome at 12 months: posterior and anterior circulation Show forest plot | 2 | 2226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.66, 0.88] |
| 1.1 Poor outcome at 12 months: posterior circulation | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.92] |
| 1.2 Poor outcome at 12 months: anterior circulation | 2 | 2157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.68, 0.90] |

