Benzodiazepines for psychosis‐induced aggression or agitation
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (DSM‐III R). | |
| Interventions | 1. Alprazolam + haloperidol: dose alprazolam 1mg/oral, haloperidol 5 mg/oral. N=14. 2. Haloperidol + placebo: dose 5 mg/bid. N=14. Repeat dose given within first 24 hours if psychotic subscale was>11. Total dose administered on day 1 repeated days 2 and 3. Each patient received a minimum of 2 doses. Mean number of doses: haloperidol 2.1, combination 3.2. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ITT: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised ‐ computer generated random numbers. | |
| Participants | Diagnosis: psychosis and uncontrolled behaviour (agitated, aggressive, destructive, assault or restless behaviour). | |
| Interventions | 1. Lorazepam + haloperidol: dose lorazepam 2 mg/IM, haloperidol 5 mg/IM/max. 6 doses. N=32. 2. Lorazepam: dose 2 mg/IM/max. 6 doses. N=31. 3. Haloperidol: dose 5 mg/IM/max. 6 doses. N=35. Administered during first 12 hrs of study. First three injections at least 1 hr apart and remainder 2 hrs apart. Total not to exceed 6 doses. Need for subsequent doses made by blinded evaluator. Most patients had < 3 doses (lorazepam: 74%; haloperidol: 71%; combination: 91%). | |
| Outcomes | Global impression: Additional medication. Unable to use ‐ | |
| Notes | ITT: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: acutely agitated behaviour. | |
| Interventions | 1. Lorazepam + haloperidol: dose lorazepam 2 mg/IM, haloperidol 5 mg/IM. N=9. 2. Lorazepam: dose 2 mg/IM. N=11. Repeated once at 60 min if patients were still severely agitated. | |
| Outcomes | Leaving the study early. | |
| Notes | ITT: not applicable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: bipolar affective illness, manic phase schizoaffective disorder, schizophreniform disorder, brief reactive disorder or atypical psychosis, (DSM‐III). | |
| Interventions | 1. Clonazepam: dose 1‐2 mg/IM. N=8. 2. Haloperidol: dose 5‐10 mg/IM. N=8. Given at 0, 0.5 and 1 hour; mean dose clonazepam (5.4 mg), haloperidol (19.4 mg). Dosage adjusted by blinded psychiatrist; procyclidine given to haloperidol group and procyclidine placebo given to clonazepam group. Both groups still received medications as usual. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ITT: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised, table of random numbers. | |
| Participants | Diagnosis: schizophrenia (N=19), schizoaffective disorder (N=7), bipolar (N=2) (DSM IV, axis 1 SCID). | |
| Interventions | 1. Flunitrazepam: dose 1 mg/IM/single dose. N=15. 2. Haloperidol: dose 5 mg/IM/single dose. N=13. Patients were monitored for 120 minutes. Subjects on routine psychotropic treatment. | |
| Outcomes | Leaving the study early. | |
| Notes | ITT: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (N=13), bipolar (N=13), schizoaffective (N=4), psychotic disorder not otherwise specified (N=7). | |
| Interventions | 1. Lorazepam: dose 2 mg/oral or IM/every 30 minutes for 4h. N=17. 2. Haloperidol: dose 5 mg/oral or IM/every 30 minutes for 4h. N=20. Medication was administered every 30 min for 4h until the patient was sedated or no longer posed a danger to themselves or staff. | |
| Outcomes | Global impression: Sedation, CGI. | |
| Notes | ITT: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised*. | |
| Participants | Diagnosis: paranoid schizophrenia (N=9), schizoaffective schizophrenia (N=9), schizophrenia subtypes (N=5), paranoid states (N=2), manic (N=5), no diagnosis (N=10). | |
| Interventions | 1. Diazepam: dose mean 5‐15 mg/IM/tds. N=20. 2. Haloperidol: dose 10‐15 mg/IM/tds. N=20. All free from neuroleptics for 48 hours before the study began. | |
| Outcomes | Global impression: Sedation, CGI. Unable to use ‐ | |
| Notes | ITT: not applicable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: bipolar disorder (DSM‐IV). | |
| Interventions | 1. Lorazepam: dose 2‐5 mg/IM. N=51. 2. Olanzapine: dose 10‐25 mg/IM. N=99. 3. Placebo. N=51. Randomised 2:1:1; patients received 1‐3 doses over 3‐20 hours after the first injection based on clinical judgement of investigator. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ITT: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: not stated. | |
| Participants | Diagnosis: schizophrenia, bipolar, schizoaffective, psychotic depression. | |
| Interventions | 1. Lorazepam: dose 2 mg/IM/mean no. of injections 1.13. N=30. 2. Haloperidol: dose 5 mg/IM/mean no. of injections 1.10. N=30. Not clear when additional doses were given. | |
| Outcomes | Global impression: Sedation. Unable to use ‐ | |
| Notes | ITT: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder or bipolar disorder. | |
| Interventions | 1. Lorazepam: dose 2 mg/IM. N=30. 2. Haloperidol: dose 5 mg/IM. N=28. Appears to have been given as single dose. | |
| Outcomes | Adverse events: EPS. Unable to use ‐ | |
| Notes | ITT: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: psychoactive substance abuse (N=24), schizophrenia (N=16) or bipolar disorder (N=14) (DSM‐III R) no diagnosis (N=6). | |
| Interventions | 1. Clothiapine + *haloperidol: dose clothiapine 40 mg/IM, haloperidol 10 mg/IM. N=30. 2. Lorazepam + *haloperidol: dose lorazepam 4 mg/IM, haloperidol 10 mg/IM. N=30. Dose repeated 6 hourly "if warranted". | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | *haloperidol was given to both groups at a fixed dose and assumptions have been made that the effect of this is 'background noise', and therefore this study has been included under the comparison of 'antipsychotics versus benzodiazepines'. ITT: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Diagnostic tools:
DSM ‐ Diagnostic Statistical Manual.
SCID ‐ Structured clinical interview.
Rating Scales:
Behaviour ‐
ABS ‐ Agitated Behavior Scale.
ACES ‐ Agitation‐Calmness Evaluation Scale.
OAS ‐ Overt Agression Scale.
TMBS ‐ Target Manic Behaviour Scale.
Global state ‐
CGI ‐ Clinical Global Impression.
MCGRS ‐ Modified Clinical Global Rating Scale.
Mental state ‐
BPRS ‐ Brief Psychiatric Rating Scale.
IMPS ‐ Inpatient Multidimensional Psychiatric Scale.
MBPRS ‐ Modified Brief Psychiatric Rating Scale.
NOSIE ‐ Nurses' Observation Scale for Inpatient Evaluation.
PANSS ‐ Positive and Negative syndrome Scale.
YMRS ‐ Young Mania Rating.
Side‐Effects ‐
EPS ‐ Extrapyramidal Side Effects.
SAS ‐ Simpson Angus Score.
VAS ‐ Visual Analogue Scale.
ITT ‐ Intention to Treat Analysis.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: unclear. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: 1. Need for additional medication ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.40] |
| Analysis 1.1  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 1 Global impression: 1. Need for additional medication ‐ medium term. | ||||
| 2 Global impression: 2. Sedation ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.61] |
| Analysis 1.2  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 2 Global impression: 2. Sedation ‐ medium term. | ||||
| 3 Global impression: 3. Average change ‐ medium term (CGI‐S, high = poor) Show forest plot | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.46, 0.60] |
| Analysis 1.3  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 3 Global impression: 3. Average change ‐ medium term (CGI‐S, high = poor). | ||||
| 4 Global impression: 4. Leaving the study early ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.38] |
| Analysis 1.4  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 4 Global impression: 4. Leaving the study early ‐ medium term. | ||||
| 5 Mental state: 1. Remaining excited ‐ medium term (PANSS‐excited component) Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.40, 0.97] |
| Analysis 1.5  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 5 Mental state: 1. Remaining excited ‐ medium term (PANSS‐excited component). | ||||
| 6 Mental state: 2. Average change ‐ medium term (PANSS, high = poor) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐2.57 [‐6.23, 1.09] |
| Analysis 1.6  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 6 Mental state: 2. Average change ‐ medium term (PANSS, high = poor). | ||||
| 7 Mental state: 3. Average change ‐ medium term (PANSS‐excited component, high = poor) Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐3.83, 0.01] |
| Analysis 1.7  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 7 Mental state: 3. Average change ‐ medium term (PANSS‐excited component, high = poor). | ||||
| 8 Adverse events: 1. Extrapyramidal effects Show forest plot | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 2.96] |
| Analysis 1.8  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 8 Adverse events: 1. Extrapyramidal effects. | ||||
| 9 Adverse events: 2. Requiring anticholinergic medication ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.10] |
| Analysis 1.9  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 9 Adverse events: 2. Requiring anticholinergic medication ‐ medium term. | ||||
| 10 Adverse events: 3. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 10 Adverse events: 3. General. | ||||
| 10.1 dizziness | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.89, 54.87] |
| 10.2 nausea | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.50, 162.97] |
| 10.3 vomiting | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 1 Global impression: 1. Need for additional medication ‐ medium term Show forest plot | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.51, 3.22] | ||||||||||||||||||||
| Analysis 2.1  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 1 Global impression: 1. Need for additional medication ‐ medium term. | ||||||||||||||||||||||||
| 2 Global impression: 2. Sedation ‐ medium term Show forest plot | 6 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.21] | ||||||||||||||||||||
| Analysis 2.2  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 2 Global impression: 2. Sedation ‐ medium term. | ||||||||||||||||||||||||
| 3 Global impression: 3. Average score (CGI‐S, high = poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 2.3  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 3 Global impression: 3. Average score (CGI‐S, high = poor). | ||||||||||||||||||||||||
| 3.1 Short term | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.58, 1.98] | ||||||||||||||||||||
| 3.2 Medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐1.36, 0.12] | ||||||||||||||||||||
| 4 Global impression: 4. Average change ‐ medium term (CGI‐S, high = poor) Show forest plot | 2 | 189 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.07, 0.47] | ||||||||||||||||||||
| Analysis 2.4  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 4 Global impression: 4. Average change ‐ medium term (CGI‐S, high = poor). | ||||||||||||||||||||||||
| 5 Global impression: 5. Average score ‐ medium term (CGI‐S, skewed) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 2.5
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 5 Global impression: 5. Average score ‐ medium term (CGI‐S, skewed). | ||||||||||||||||||||||||
| 6 Global impression: 6. Leaving the study early ‐medium term Show forest plot | 4 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.11, 27.35] | ||||||||||||||||||||
| Analysis 2.6  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 6 Global impression: 6. Leaving the study early ‐medium term. | ||||||||||||||||||||||||
| 7 Behaviour: 1. Not improved ‐ medium term (OAS) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.31, 22.05] | ||||||||||||||||||||
| Analysis 2.7  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 7 Behaviour: 1. Not improved ‐ medium term (OAS). | ||||||||||||||||||||||||
| 8 Behaviour: 2. Average aggression score ‐ medium term (OAS, skewed) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 2.8
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 8 Behaviour: 2. Average aggression score ‐ medium term (OAS, skewed). | ||||||||||||||||||||||||
| 9 Mental state: 1. Not improved ‐ medium term (IMPS) Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.34, 6.70] | ||||||||||||||||||||
| Analysis 2.9  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 9 Mental state: 1. Not improved ‐ medium term (IMPS). | ||||||||||||||||||||||||
| 10 Mental state: 2. Average score (BPRS, high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 2.10  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 10 Mental state: 2. Average score (BPRS, high = poor). | ||||||||||||||||||||||||
| 10.1 Short term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐3.26 [‐10.65, 4.13] | ||||||||||||||||||||
| 10.2 Medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐4.07 [‐10.76, 2.62] | ||||||||||||||||||||
| 11 Mental state: 3. Average change ‐ medium term (BPRS, high = poor) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐7.60 [‐13.87, ‐1.33] | ||||||||||||||||||||
| Analysis 2.11  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 11 Mental state: 3. Average change ‐ medium term (BPRS, high = poor). | ||||||||||||||||||||||||
| 12 Mental state: 4. Average change ‐ medium term (BPRS‐psychosis subscale, high score = poor) Show forest plot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐4.65, 4.05] | ||||||||||||||||||||
| Analysis 2.12  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 12 Mental state: 4. Average change ‐ medium term (BPRS‐psychosis subscale, high score = poor). | ||||||||||||||||||||||||
| 13 Mental state: 5. Remaining excited ‐ medium term (PANSS‐excited component) Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.06, 3.18] | ||||||||||||||||||||
| Analysis 2.13  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 13 Mental state: 5. Remaining excited ‐ medium term (PANSS‐excited component). | ||||||||||||||||||||||||
| 14 Mental state: 6. Average change ‐ medium term (PANSS, high = poor) Show forest plot | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 5.64 [2.20, 9.08] | ||||||||||||||||||||
| Analysis 2.14  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 14 Mental state: 6. Average change ‐ medium term (PANSS, high = poor). | ||||||||||||||||||||||||
| 15 Mental state: 7. Average change ‐ medium term (PANSS‐Excited component, high = poor) Show forest plot | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | 2.85 [1.14, 4.56] | ||||||||||||||||||||
| Analysis 2.15  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 15 Mental state: 7. Average change ‐ medium term (PANSS‐Excited component, high = poor). | ||||||||||||||||||||||||
| 16 Adverse events: 1. Extrapyramidal effects Show forest plot | 7 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.43] | ||||||||||||||||||||
| Analysis 2.16  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 16 Adverse events: 1. Extrapyramidal effects. | ||||||||||||||||||||||||
| 17 Adverse events: 2. Requiring anticholinergic medication ‐ medium term Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 1.89] | ||||||||||||||||||||
| Analysis 2.17  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 17 Adverse events: 2. Requiring anticholinergic medication ‐ medium term. | ||||||||||||||||||||||||
| 18 Adverse events: 3. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 2.18  Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 18 Adverse events: 3. General. | ||||||||||||||||||||||||
| 18.1 ataxia | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.22, 23.71] | ||||||||||||||||||||
| 18.2 dizziness | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.63, 3.07] | ||||||||||||||||||||
| 18.3 dry mouth | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.49, 7.24] | ||||||||||||||||||||
| 18.4 nausea | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.76 [0.89, 67.67] | ||||||||||||||||||||
| 18.5 speech disorder | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.11, 2.87] | ||||||||||||||||||||
| 18.6 vomiting | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.46 [0.71, 255.70] | ||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 1 Global impression: 1. Need for additional medication ‐ medium term Show forest plot | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] | ||||||||||||||||||||
| Analysis 3.1  Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 1 Global impression: 1. Need for additional medication ‐ medium term. | ||||||||||||||||||||||||
| 2 Global impression: 2. Not improved ‐ short term (CGI) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.66, 3.25] | ||||||||||||||||||||
| Analysis 3.2  Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 2 Global impression: 2. Not improved ‐ short term (CGI). | ||||||||||||||||||||||||
| 3 Global impression: 3. Leaving the study early ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||
| Analysis 3.3  Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 3 Global impression: 3. Leaving the study early ‐ medium term. | ||||||||||||||||||||||||
| 4 Behaviour: 1. Not improved ‐ short term (OAS) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.74] | ||||||||||||||||||||
| Analysis 3.4  Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 4 Behaviour: 1. Not improved ‐ short term (OAS). | ||||||||||||||||||||||||
| 5 Mental state: 1. Average change ‐ medium term (BPRS‐psychosis subscale, high = poor, skewed) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 3.5
Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 5 Mental state: 1. Average change ‐ medium term (BPRS‐psychosis subscale, high = poor, skewed). | ||||||||||||||||||||||||
| 6 Adverse events: 1. Extrapyramidal effects Show forest plot | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.18, 20.30] | ||||||||||||||||||||
| Analysis 3.6  Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 6 Adverse events: 1. Extrapyramidal effects. | ||||||||||||||||||||||||
| 7 Adverse events: 2. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 3.7  Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 7 Adverse events: 2. General. | ||||||||||||||||||||||||
| 7.1 ataxia | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.26, 8.11] | ||||||||||||||||||||
| 7.2 dizziness | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.12, 3.61] | ||||||||||||||||||||
| 7.3 dry mouth | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.23] | ||||||||||||||||||||
| 7.4 speech disorder | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.26, 8.11] | ||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 1 Global impression: 1. Need for additional medication ‐ medium term Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.15] | ||||||||||||||||||||
| Analysis 4.1  Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 1 Global impression: 1. Need for additional medication ‐ medium term. | ||||||||||||||||||||||||
| 2 Global impression: 2. Leaving the study early ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||
| Analysis 4.2  Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 2 Global impression: 2. Leaving the study early ‐ medium term. | ||||||||||||||||||||||||
| 3 Mental state: 1. Average score ‐ medium term (BPRS, high = poor) Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐7.26, 7.28] | ||||||||||||||||||||
| Analysis 4.3  Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 3 Mental state: 1. Average score ‐ medium term (BPRS, high = poor). | ||||||||||||||||||||||||
| 4 Mental state: 2. Average score ‐ medium term (BPRS‐psychosis subscale, high = poor) Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐1.93 [‐5.73, 1.87] | ||||||||||||||||||||
| Analysis 4.4  Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 4 Mental state: 2. Average score ‐ medium term (BPRS‐psychosis subscale, high = poor). | ||||||||||||||||||||||||
| 5 Mental state: 3. Average change ‐ medium term (BPRS‐psychosis subscale, high = poor, skewed) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 4.5
Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 5 Mental state: 3. Average change ‐ medium term (BPRS‐psychosis subscale, high = poor, skewed). | ||||||||||||||||||||||||
| 6 Adverse events: 1. Extrapyramidal effects Show forest plot | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.94] | ||||||||||||||||||||
| Analysis 4.6  Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 6 Adverse events: 1. Extrapyramidal effects. | ||||||||||||||||||||||||
| 7 Adverse events: 2. Requiring anticholinergic medication ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.25, 1.24] | ||||||||||||||||||||
| Analysis 4.7  Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 7 Adverse events: 2. Requiring anticholinergic medication ‐ medium term. | ||||||||||||||||||||||||
| 8 Adverse events: 3. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 4.8  Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 8 Adverse events: 3. General. | ||||||||||||||||||||||||
| 8.1 ataxia | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.28 [0.36, 29.97] | ||||||||||||||||||||
| 8.2 dizziness | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.13, 4.09] | ||||||||||||||||||||
| 8.3 dry mouth | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.24, 5.04] | ||||||||||||||||||||
| 8.4 speech disorder | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.20, 3.39] | ||||||||||||||||||||
| 9 Hospital and service outcome: Unfit for early discharge Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.54, 1.50] | ||||||||||||||||||||
| Analysis 4.9  Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 9 Hospital and service outcome: Unfit for early discharge. | ||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: 1. Sedation ‐ medium term Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 SENSITIVITY ANALYSIS: 1. HIGH vs LOW ATTRITION, Outcome 1 Global impression: 1. Sedation ‐ medium term. | ||||
| 1.1 high | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 1.2 low | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.73] |
| 2 Global impression: 2. Leaving the study early ‐ medium term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 SENSITIVITY ANALYSIS: 1. HIGH vs LOW ATTRITION, Outcome 2 Global impression: 2. Leaving the study early ‐ medium term. | ||||
| 2.1 high | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 2.2 low | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.82 [0.62, 54.58] |
| 3 Adverse events: Extrapyramidal effects Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 SENSITIVITY ANALYSIS: 1. HIGH vs LOW ATTRITION, Outcome 3 Adverse events: Extrapyramidal effects. | ||||
| 3.1 high | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.62] |
| 3.2 low | 2 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: Sedation ‐ medium term Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 SENSITIVITY ANALYSIS: 2. RANDOMISED vs UNKNOWN, Outcome 1 Global impression: Sedation ‐ medium term. | ||||
| 1.1 randomised | 4 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.34, 1.49] |
| 1.2 unknown | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.45, 1.44] |
| 2 Adverse events: 1. Extrapyramidal effects Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 SENSITIVITY ANALYSIS: 2. RANDOMISED vs UNKNOWN, Outcome 2 Adverse events: 1. Extrapyramidal effects. | ||||
| 2.1 randomised | 6 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.63] |
| 2.2 unknown | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.65] |

Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 1 Global impression: 1. Need for additional medication ‐ medium term.
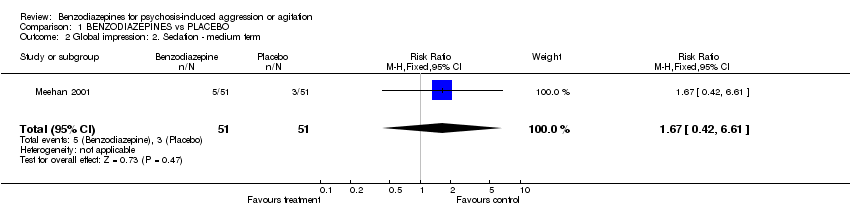
Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 2 Global impression: 2. Sedation ‐ medium term.

Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 3 Global impression: 3. Average change ‐ medium term (CGI‐S, high = poor).

Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 4 Global impression: 4. Leaving the study early ‐ medium term.

Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 5 Mental state: 1. Remaining excited ‐ medium term (PANSS‐excited component).
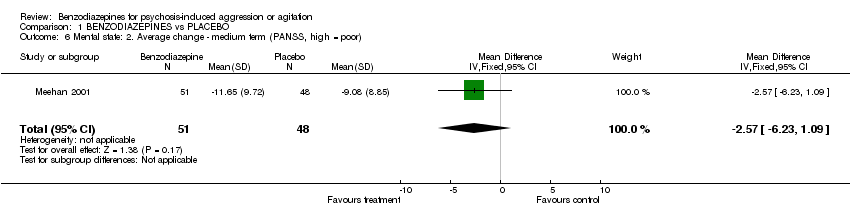
Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 6 Mental state: 2. Average change ‐ medium term (PANSS, high = poor).
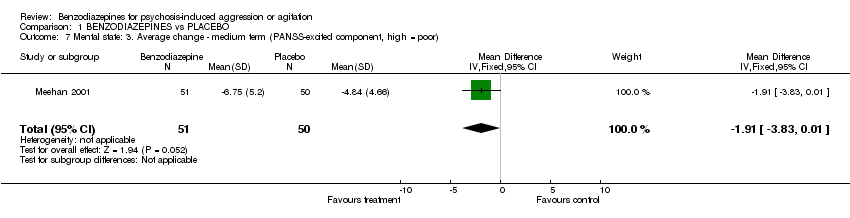
Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 7 Mental state: 3. Average change ‐ medium term (PANSS‐excited component, high = poor).
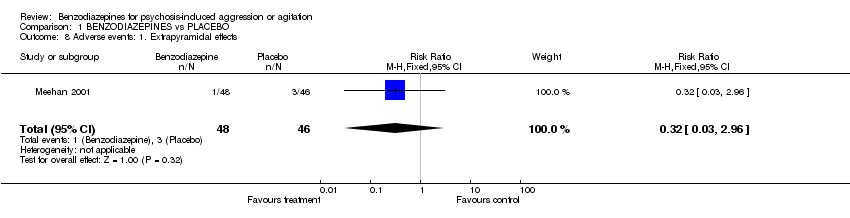
Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 8 Adverse events: 1. Extrapyramidal effects.

Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 9 Adverse events: 2. Requiring anticholinergic medication ‐ medium term.
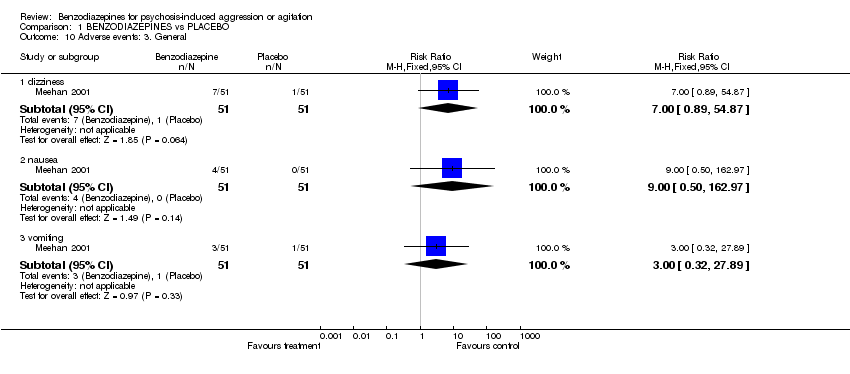
Comparison 1 BENZODIAZEPINES vs PLACEBO, Outcome 10 Adverse events: 3. General.

Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 1 Global impression: 1. Need for additional medication ‐ medium term.

Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 2 Global impression: 2. Sedation ‐ medium term.
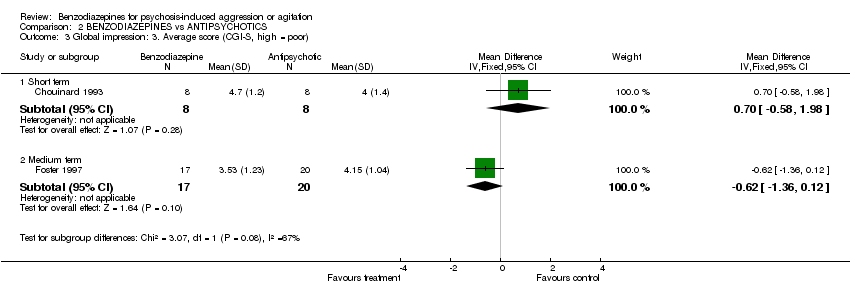
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 3 Global impression: 3. Average score (CGI‐S, high = poor).

Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 4 Global impression: 4. Average change ‐ medium term (CGI‐S, high = poor).
| Study | Intervention | mean | SD | N |
| Chouinard 1993 | Clonazepam | 2.60 | 1.70 | 8 |
| Chouinard 1993 | haloperidol | 2.80 | 0.60 | 8 |
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 5 Global impression: 5. Average score ‐ medium term (CGI‐S, skewed).
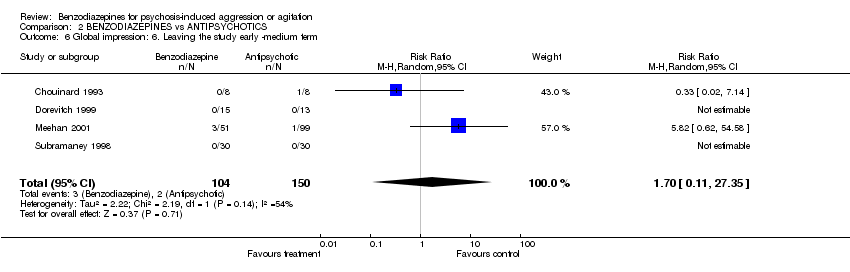
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 6 Global impression: 6. Leaving the study early ‐medium term.

Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 7 Behaviour: 1. Not improved ‐ medium term (OAS).
| Study | Intervention | mean | SD | N |
| Subramaney 1998 | Lorazepam | 1.83 | 3.14 | 30 |
| Subramaney 1998 | Clothiapine | 1.33 | 2.78 | 30 |
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 8 Behaviour: 2. Average aggression score ‐ medium term (OAS, skewed).

Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 9 Mental state: 1. Not improved ‐ medium term (IMPS).
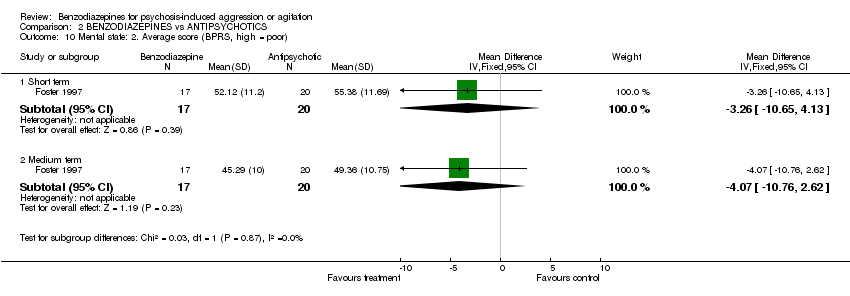
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 10 Mental state: 2. Average score (BPRS, high = poor).
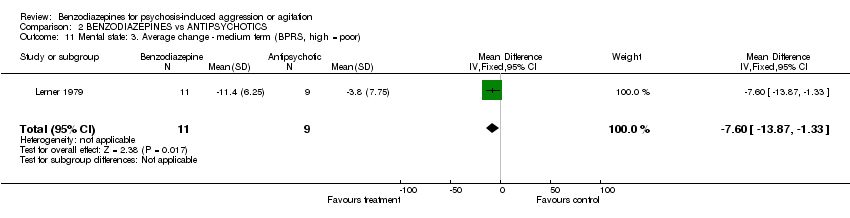
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 11 Mental state: 3. Average change ‐ medium term (BPRS, high = poor).
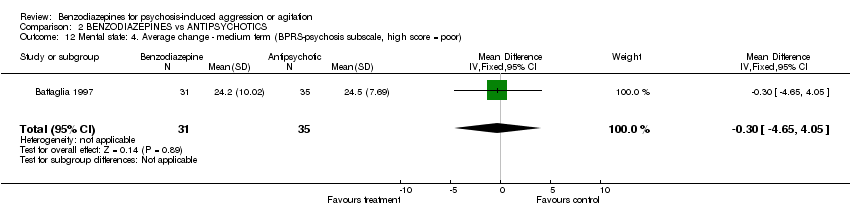
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 12 Mental state: 4. Average change ‐ medium term (BPRS‐psychosis subscale, high score = poor).
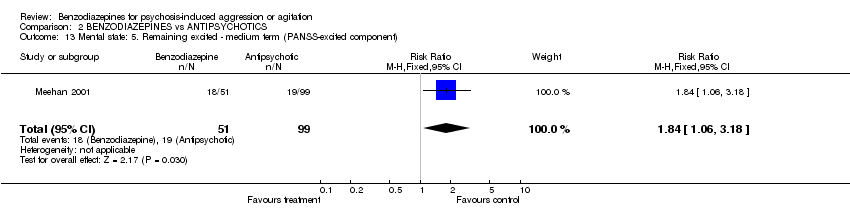
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 13 Mental state: 5. Remaining excited ‐ medium term (PANSS‐excited component).
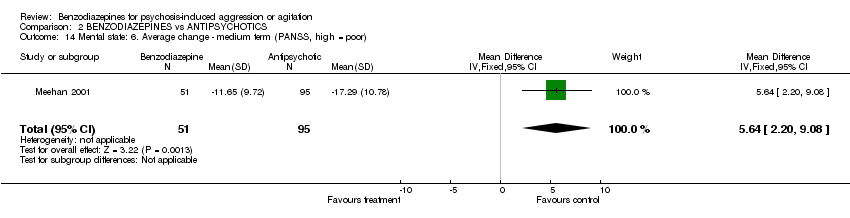
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 14 Mental state: 6. Average change ‐ medium term (PANSS, high = poor).

Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 15 Mental state: 7. Average change ‐ medium term (PANSS‐Excited component, high = poor).
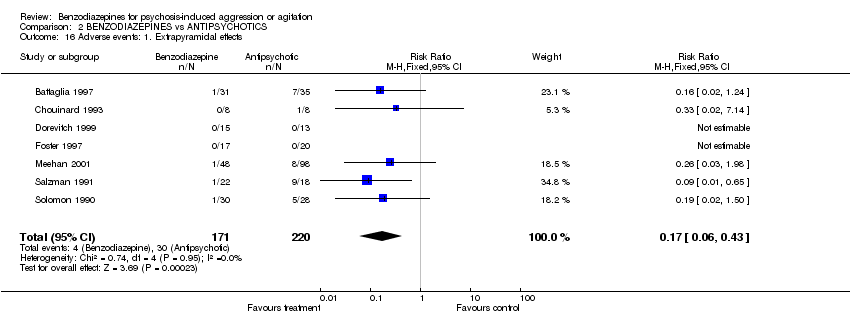
Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 16 Adverse events: 1. Extrapyramidal effects.

Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 17 Adverse events: 2. Requiring anticholinergic medication ‐ medium term.

Comparison 2 BENZODIAZEPINES vs ANTIPSYCHOTICS, Outcome 18 Adverse events: 3. General.
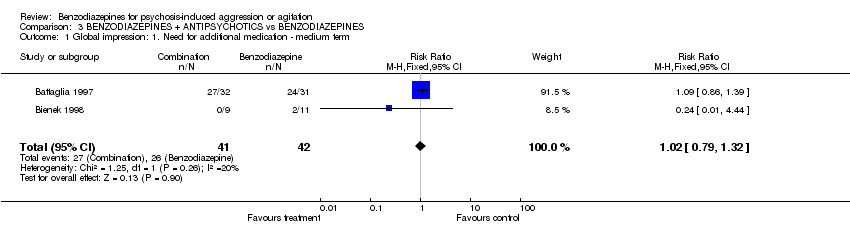
Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 1 Global impression: 1. Need for additional medication ‐ medium term.

Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 2 Global impression: 2. Not improved ‐ short term (CGI).

Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 3 Global impression: 3. Leaving the study early ‐ medium term.
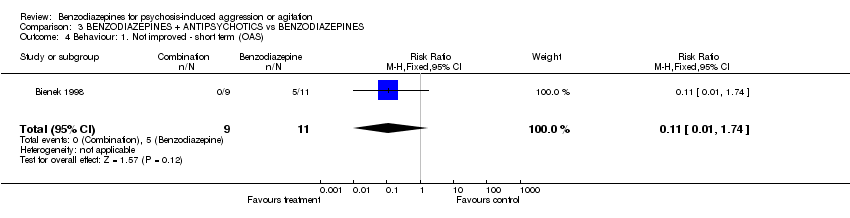
Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 4 Behaviour: 1. Not improved ‐ short term (OAS).
| Study | Intervention | mean | SD | N |
| Battaglia 1997 | Lorazepam + haloperidol | 17.50 | 10.18 | 32 |
| Battaglia 1997 | Lorazepam | 24.20 | 10.02 | 31 |
Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 5 Mental state: 1. Average change ‐ medium term (BPRS‐psychosis subscale, high = poor, skewed).

Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 6 Adverse events: 1. Extrapyramidal effects.
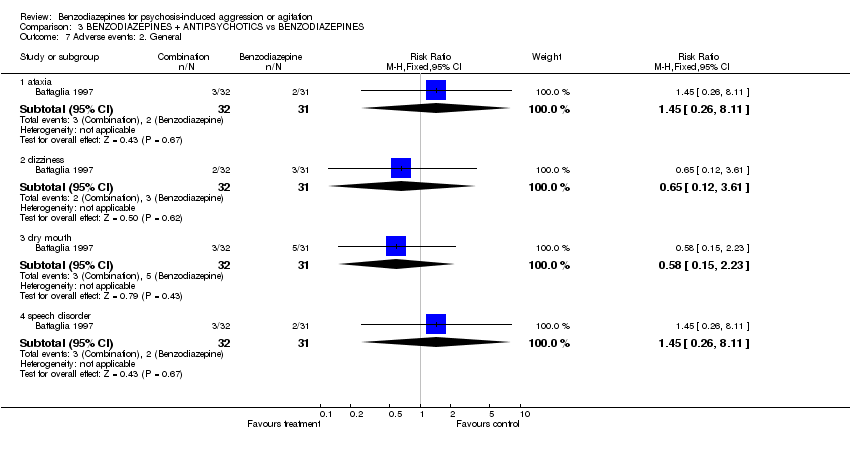
Comparison 3 BENZODIAZEPINES + ANTIPSYCHOTICS vs BENZODIAZEPINES, Outcome 7 Adverse events: 2. General.
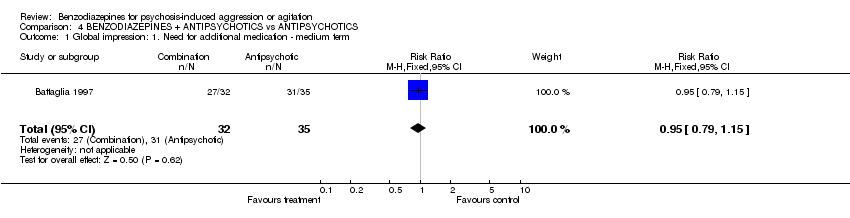
Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 1 Global impression: 1. Need for additional medication ‐ medium term.

Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 2 Global impression: 2. Leaving the study early ‐ medium term.
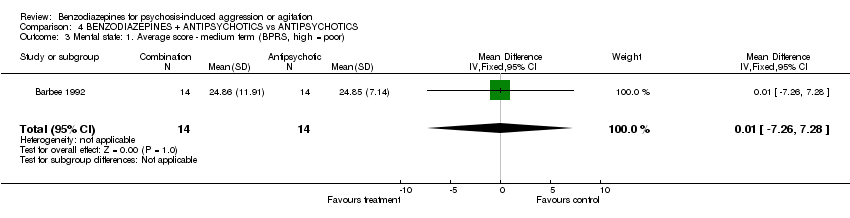
Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 3 Mental state: 1. Average score ‐ medium term (BPRS, high = poor).

Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 4 Mental state: 2. Average score ‐ medium term (BPRS‐psychosis subscale, high = poor).
| Study | Intervention | mean | SD | N |
| Battaglia 1997 | Lorazepam + haloperidol | 17.50 | 10.18 | 32 |
| Battaglia 1997 | Haloperidol | 24.50 | 7.69 | 35 |
Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 5 Mental state: 3. Average change ‐ medium term (BPRS‐psychosis subscale, high = poor, skewed).
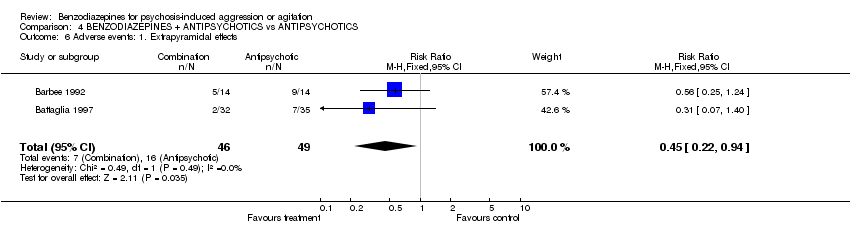
Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 6 Adverse events: 1. Extrapyramidal effects.

Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 7 Adverse events: 2. Requiring anticholinergic medication ‐ medium term.
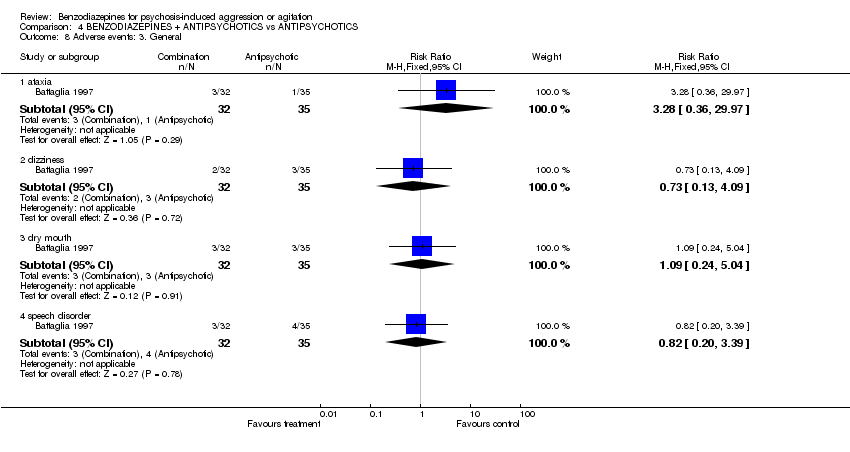
Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 8 Adverse events: 3. General.

Comparison 4 BENZODIAZEPINES + ANTIPSYCHOTICS vs ANTIPSYCHOTICS, Outcome 9 Hospital and service outcome: Unfit for early discharge.
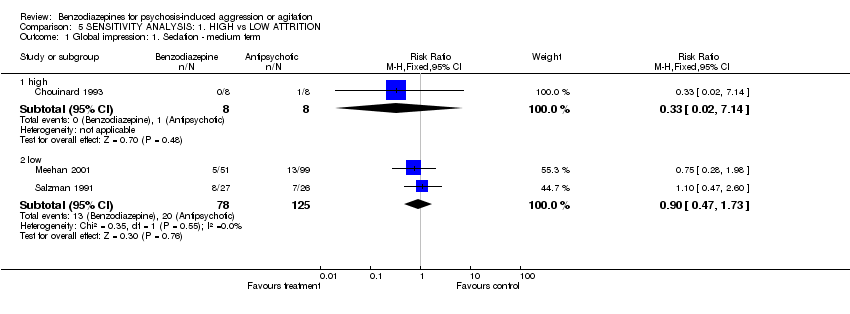
Comparison 5 SENSITIVITY ANALYSIS: 1. HIGH vs LOW ATTRITION, Outcome 1 Global impression: 1. Sedation ‐ medium term.
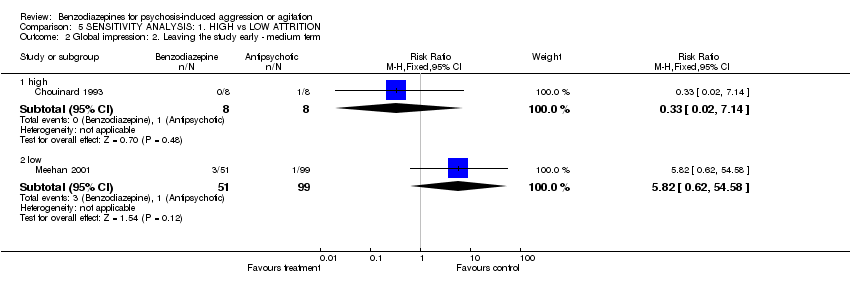
Comparison 5 SENSITIVITY ANALYSIS: 1. HIGH vs LOW ATTRITION, Outcome 2 Global impression: 2. Leaving the study early ‐ medium term.

Comparison 5 SENSITIVITY ANALYSIS: 1. HIGH vs LOW ATTRITION, Outcome 3 Adverse events: Extrapyramidal effects.
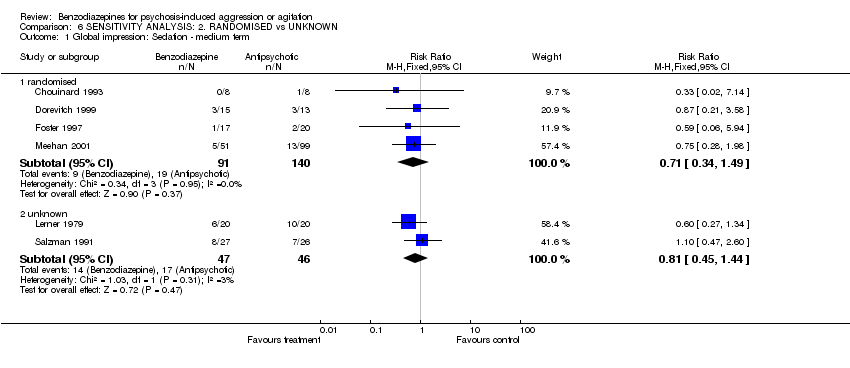
Comparison 6 SENSITIVITY ANALYSIS: 2. RANDOMISED vs UNKNOWN, Outcome 1 Global impression: Sedation ‐ medium term.

Comparison 6 SENSITIVITY ANALYSIS: 2. RANDOMISED vs UNKNOWN, Outcome 2 Adverse events: 1. Extrapyramidal effects.
| Drug of choice | Mean dose |
| diazepam* | 27 (10‐80) |
| haloperidol | 22 (10‐60) |
| chlorpromazine | 162 (50‐400) |
| droperidol | 14 (10‐20) |
| paraldehyde | U/K |
| amytal | U/K |
| lorazepam | U/K |
| nitrazepam** | U/K |
| *most frequent | |
| ** least frequent |
| Drug of choice | Mean dose | Frequency of use |
| haloperidol + promethazine | 5 (2.5‐10) + 50 (25‐100) | 61% |
| haloperidol + promethazine + diazepam | 5 (2.5‐10) + 50 (25‐100) +10 | 15% |
| diazepam | 10 | 9% |
| haloperidol + promethazine + chlorpromazine | 5 + 50 + 25 | 7% |
| chlorpromazine + diazepam + promethazine | 25 + 10 + 50 | 1% |
| chlorpromazine + promethazine | 25 + 50 | 1% |
| chlorpromazine | 25 | 1% |
| diazepam + promethazine | 10 + 50 | 1% |
| haloperidol + diazepam | 5 + 10 | 1% |
| promethazine | 50 | 1% |
| Attrition | Study | % loss | Duration | Notes |
| High | Barbee 1992 | 31 | 72 hours | |
| Chouinard 1993 | 12 | 2 hours | ||
| Salzman 1991 | 33 | 48 hours | ‐ for EPS outcome; 12% loss for 'sedation' | |
| Low | Bienek 1998 | 0 | 7 days | |
| Meehan 2001 | 4 | 24 hours | ||
| Solomon 1990 | 0 | 7 days | ||
| Subramaney 1998 | 0 | 7 days |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: 1. Need for additional medication ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.40] |
| 2 Global impression: 2. Sedation ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.61] |
| 3 Global impression: 3. Average change ‐ medium term (CGI‐S, high = poor) Show forest plot | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.46, 0.60] |
| 4 Global impression: 4. Leaving the study early ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.38] |
| 5 Mental state: 1. Remaining excited ‐ medium term (PANSS‐excited component) Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.40, 0.97] |
| 6 Mental state: 2. Average change ‐ medium term (PANSS, high = poor) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐2.57 [‐6.23, 1.09] |
| 7 Mental state: 3. Average change ‐ medium term (PANSS‐excited component, high = poor) Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐3.83, 0.01] |
| 8 Adverse events: 1. Extrapyramidal effects Show forest plot | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 2.96] |
| 9 Adverse events: 2. Requiring anticholinergic medication ‐ medium term Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.10] |
| 10 Adverse events: 3. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dizziness | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.89, 54.87] |
| 10.2 nausea | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.50, 162.97] |
| 10.3 vomiting | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: 1. Need for additional medication ‐ medium term Show forest plot | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.51, 3.22] |
| 2 Global impression: 2. Sedation ‐ medium term Show forest plot | 6 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.21] |
| 3 Global impression: 3. Average score (CGI‐S, high = poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short term | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.58, 1.98] |
| 3.2 Medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐1.36, 0.12] |
| 4 Global impression: 4. Average change ‐ medium term (CGI‐S, high = poor) Show forest plot | 2 | 189 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.07, 0.47] |
| 5 Global impression: 5. Average score ‐ medium term (CGI‐S, skewed) Show forest plot | Other data | No numeric data | ||
| 6 Global impression: 6. Leaving the study early ‐medium term Show forest plot | 4 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.11, 27.35] |
| 7 Behaviour: 1. Not improved ‐ medium term (OAS) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.31, 22.05] |
| 8 Behaviour: 2. Average aggression score ‐ medium term (OAS, skewed) Show forest plot | Other data | No numeric data | ||
| 9 Mental state: 1. Not improved ‐ medium term (IMPS) Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.34, 6.70] |
| 10 Mental state: 2. Average score (BPRS, high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Short term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐3.26 [‐10.65, 4.13] |
| 10.2 Medium term | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐4.07 [‐10.76, 2.62] |
| 11 Mental state: 3. Average change ‐ medium term (BPRS, high = poor) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐7.60 [‐13.87, ‐1.33] |
| 12 Mental state: 4. Average change ‐ medium term (BPRS‐psychosis subscale, high score = poor) Show forest plot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐4.65, 4.05] |
| 13 Mental state: 5. Remaining excited ‐ medium term (PANSS‐excited component) Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.06, 3.18] |
| 14 Mental state: 6. Average change ‐ medium term (PANSS, high = poor) Show forest plot | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 5.64 [2.20, 9.08] |
| 15 Mental state: 7. Average change ‐ medium term (PANSS‐Excited component, high = poor) Show forest plot | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | 2.85 [1.14, 4.56] |
| 16 Adverse events: 1. Extrapyramidal effects Show forest plot | 7 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.43] |
| 17 Adverse events: 2. Requiring anticholinergic medication ‐ medium term Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 1.89] |
| 18 Adverse events: 3. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 ataxia | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.22, 23.71] |
| 18.2 dizziness | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.63, 3.07] |
| 18.3 dry mouth | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.49, 7.24] |
| 18.4 nausea | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.76 [0.89, 67.67] |
| 18.5 speech disorder | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.11, 2.87] |
| 18.6 vomiting | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.46 [0.71, 255.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: 1. Need for additional medication ‐ medium term Show forest plot | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] |
| 2 Global impression: 2. Not improved ‐ short term (CGI) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.66, 3.25] |
| 3 Global impression: 3. Leaving the study early ‐ medium term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Behaviour: 1. Not improved ‐ short term (OAS) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.74] |
| 5 Mental state: 1. Average change ‐ medium term (BPRS‐psychosis subscale, high = poor, skewed) Show forest plot | Other data | No numeric data | ||
| 6 Adverse events: 1. Extrapyramidal effects Show forest plot | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.18, 20.30] |
| 7 Adverse events: 2. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 ataxia | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.26, 8.11] |
| 7.2 dizziness | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.12, 3.61] |
| 7.3 dry mouth | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.23] |
| 7.4 speech disorder | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.26, 8.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: 1. Need for additional medication ‐ medium term Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.15] |
| 2 Global impression: 2. Leaving the study early ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mental state: 1. Average score ‐ medium term (BPRS, high = poor) Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐7.26, 7.28] |
| 4 Mental state: 2. Average score ‐ medium term (BPRS‐psychosis subscale, high = poor) Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐1.93 [‐5.73, 1.87] |
| 5 Mental state: 3. Average change ‐ medium term (BPRS‐psychosis subscale, high = poor, skewed) Show forest plot | Other data | No numeric data | ||
| 6 Adverse events: 1. Extrapyramidal effects Show forest plot | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.94] |
| 7 Adverse events: 2. Requiring anticholinergic medication ‐ medium term Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.25, 1.24] |
| 8 Adverse events: 3. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 ataxia | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.28 [0.36, 29.97] |
| 8.2 dizziness | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.13, 4.09] |
| 8.3 dry mouth | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.24, 5.04] |
| 8.4 speech disorder | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.20, 3.39] |
| 9 Hospital and service outcome: Unfit for early discharge Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.54, 1.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: 1. Sedation ‐ medium term Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 high | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 1.2 low | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.73] |
| 2 Global impression: 2. Leaving the study early ‐ medium term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 high | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.14] |
| 2.2 low | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.82 [0.62, 54.58] |
| 3 Adverse events: Extrapyramidal effects Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 high | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.62] |
| 3.2 low | 2 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global impression: Sedation ‐ medium term Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 randomised | 4 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.34, 1.49] |
| 1.2 unknown | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.45, 1.44] |
| 2 Adverse events: 1. Extrapyramidal effects Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 randomised | 6 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.63] |
| 2.2 unknown | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.65] |

