Le traitement de l'hypertension dans la maladie artérielle périphérique
Résumé scientifique
Contexte
La maladie artérielle périphérique (MAP) provoque une morbidité et une mortalité considérables. L'hypertension est un facteur de risque de la MAP. Le traitement de l'hypertension doit être compatible avec les symptômes de la MAP. La controverse sur les effets des bloquants bêta‐adreno récepteurs dans l'hypertension chez les patients avec une MAP a conduit à des nombreux médecins à arrêter la prescription de bêta‐bloquants. ‐adreno récepteurs et on sait peu sur les effets des autres classes d'anti‐hypertenseurs en présence d'une MAP. Ceci est la deuxième mise à jour d'une revue Cochrane publiée pour la première fois en 2003.
Objectifs
Déterminer les effets de médicaments anti hyper tenseurs chez des patients présentant une pression artérielle élevée et une MAP symptomatique en termes de taux d'événements cardio‐vasculaires et les décès, les symptômes de claudication et l'ischémie aiguë des membres inférieurs et la progression de l'athérosclérose avec MAP, telle que mesurée par l'indice de pression systolique (IPS) et le besoin de la revascularisation (une chirurgie reconstructrice ou l'angioplastie) ou une amputation.
Stratégie de recherche documentaire
Pour cette mise à jour, le coordonnateur du registre des essais du groupe Cochrane sur les maladies vasculaires périphériques a effectué des recherches dans le registre spécialisé du groupe Cochrane sur les maladies vasculaires périphériques (dernière recherche mars 2013) et CENTRAL (2013, numéro 2).
Critères de sélection
Les essais contrôlés randomisés (ECR) d'au moins un traitement anti‐hypertenseur par rapport à un placebo ou entre deux anti‐hypertenseurs avec des interventions d'une durée d'au moins un mois. Les essais devaient inclure des patients avec une MAP symptomatique.
Recueil et analyse des données
Les données ont été extraites par un auteur (DAL) et vérifiées par le second (GYHL). Des études potentiellement éligibles ont été exclus lorsque la présentation des résultats empêchait une extraction appropriée des données et lorsque les enquêtes auprès des auteurs n'ont pas fourni de données brutes.
Résultats principaux
Huit ECR ont été inclus avec un total de 3610 des patients avec une MAP. Quatre études comparaient une classe reconnue de traitement anti‐hyper tenseur à un placebo et quatre études comparaient deux traitements anti hyper tenseurs. Les études n'ont pas été combinées en raison de la variation des comparaisons et les résultats présentés. La qualité globale des preuves disponibles n'était pas claire, principalement en raison d'un manque de détail dans les rapports d'étude sur les procédures de randomisation et de mise en aveugle et des données de résultats incomplets. Deux études comparaient l'enzyme de conversion de l'angiotensine (ECA) par rapport à un placebo. Dans une étude, il y avait une réduction significative du nombre d'événements cardio‐vasculaires chez les patients recevant du ramipril (rapport des cotes (RC) 0,72, intervalle de confiance (IC) à 95%, de 0,58 à 0,91 ; n = 1725). Dans le deuxième essai utilisant le perindopril (n = 52), il y avait une augmentation marginale de la distance de claudication mais aucun changement d'IPS et une réduction de la distance de marche maximale. Un essai comparant le vérapamil, un antagoniste du calcium par rapport à un placebo chez les patients subissant une angioplastie (n = 96) a suggéré que le vérapamil, réduisait la re sténose (pourcentage de diamètre de la sténose (±SD) 48,0 % ± 11,5 versus 69,6 % ±12,2 ; P < ± 0,01), bien que cela ne se reflétait pas dans le maintien d'un IPTB élevé (0.76 ± 0.10 versus 0,72 ± 0,08 pour le vérapamil par rapport à un placebo). Une autre étude (n = 80) n'a démontré aucune différence significative dans l'épaisseur intima‐média artérielle (EIM) chez des hommes recevant le diurétique thiazidique hydrochlorothiazide (HCTZ) par rapport à ceux recevant le bloquant alpha‐adrénorécepteur, doxazosine (‐0,12 ± 0,14 mm et ‐0,08 ± 0,13 mm, respectivement ; P = 0,66). Une étude (n = 36) comparant le telmisartan à un placebo a trouvé une amélioration significative de la distance de marche maximale à 12 mois avec le telmisartan (médiane (intervalle interquartile (IQR)) de 191 m (de 157 à 226) versus 103 m (76 à 164) ; P < 0,001) mais aucune différence en termes de IPS (médiane (IQR) de 0,60 (0,60 à 0,77) versus 0,52 (0,48 à 0,67)) ou artérielle IMT (médiane (IQR) de 0,08 cm (de 0,07 à 0,09) versus 0,09 cm (de 0,08 à 0,10)). Deux études comparaient les bêta‐bloquant‐adrenoreceptor nebivolol avec soit le diurétique thiazidique HCTZ ou avec le métroprolol. Les deux études n'ont trouvé aucune différence significative dans la distance de claudication intermittente ou absolue, CIA, ou dans la mortalité, toutes causes confondures, entre les anti‐hypertenseurs. Une analyse en sous‐groupe de la MAP patients (n = 2699) dans une étude qui comparait une stratégie d'antagonistes calciques (le vérapamil (SR) à libération lente ± trandolapril) à un bêta‐bloquant‐adrenoreceptor de base (aténolol ± hydrochlorothiazide) n'a trouvé aucune différence significative dans les critères de jugement composite de décès, infarctus du myocarde mortel ou non mortel, un accident vasculaire cérébral (AVC) avec ou sans la revascularisation (RC 0,90, IC à 95 %, de 0,76 à 1,07 et RC 0,96, IC à 95 %, de 0,82 à 1,13, respectivement).
Conclusions des auteurs
Les évidences sur l'utilisation de différents médicaments anti hyper tenseurs chez les personnes atteintes d'une MAP sont pauvres de sorte que nous ignorons si des bénéfices significatifs ou une augmentation de risques. Cependant, le manque de données examinant spécifiquement les résultats chez les patients avec une MAP ne devrait pas nuire aux preuves irréfutables sur le bénéfice du traitement de l'hypertension et la diminution de la pression artérielle.
PICO
Résumé simplifié
Le traitement de l'hypertension artérielle chez les personnes souffrant d'une maladie artérielle périphérique
La tension artérielle élevée systématiquement peut entraîner des complications telles qu'une crise cardiaque (infarctus du myocarde) ou un AVC. La maladie artérielle périphérique (MAP), une pathologie qui affecte les vaisseaux sanguins (artères transportant le sang vers les jambes, les bras et l'aire stomacale) et la pression artérielle élevée (hypertension) sont associées à l'athérosclérose. Ceci est un durcissement des artères qui est causé par les dépôts de lipides, de cholestérol et d'autres substances à l'intérieur des vaisseaux sanguins. La MAP est diagnostiquée lorsque l'apport sanguin vers les jambes est restreint, ce qui entraîne des douleurs et des crampes qui limitent la marche (claudication intermittente). Elle est mesurée par la distance de marche (sur un tapis roulant) avant l'apparition de la douleur (distance de claudication) ou l'index brachial jambier(IBJ) qui est le rapport de la pression artérielle dans les bras et les jambes. La pression artérielle plus faible dans les jambes par rapport au bras (IBJ à moins de 1,0) indique des artères obstruées dans les jambes (ou une MAP). Une MAP peut évoluer vers une douleur en repos et une ischémie aiguë des membres inférieurs (perte soudaine de la vascularisation d'un membre provoquée par une obstruction ; un caillot de sang ou d'un dépôt de stéatose) qui nécessite une revascularisation (restaurer le flux sanguin en élargissant le vaisseau sanguin) ou une amputation. Le traitement de l'hypertension pour réduire les événements cardiovasculaires (crise cardiaque ou un AVC) et les décès doivent tenir soigneusement compte de la MAP. Les anti‐hyper tenseurs peuvent aggraver les symptômes de la MAP en réduisant le débit sanguin et l'apport en oxygène des membres et peut avoir des effets à long terme sur la progression de la maladie. Les preuves issues des essais contrôlés randomisés (ECR) portant sur les risques et bénéfices de différents anti‐hyper tenseurs sur les mesures de la MAP sont insuffisantes.
Nous avons identifié huit ECR avec un total de 3610 personnes avec une MAP symptomatique où les participants étaient randomisés pour recevoir un traitement anti‐hyper tenseur pendant au moins un mois ou à un placebo ou à l'absence de traitement. Quatre études comparaient un traitement anti‐hyper tenseur à un placebo et quatre études comparaient deux traitements anti hypertenseurs. Les études n'ont pas été combinés en raison de la variation des comparaisons et les critères de résultats présentés. Un essai avec 1725 participants a montré que le ramipril, un antagoniste sélectif de l'enzyme de conversion de l'angiotensine (ECA), était efficace pour réduire le nombre d'événements cardiovasculaires de 28 % par rapport à un placebo. Dans une autre étude (n = 52), le perindopril, un inhibiteur de l'ECA, a montré une petite augmentation de la distance de claudication mais aucun changement d'IPS et une réduction de la distance de marche maximale (DMM). Chez les patients bénéficiant d'une angioplastie artérielle périphérique (une procédure consistant à ouvrir les vaisseaux sanguins rétrécis ou obstrués) les résultats d'un essai avec 96 participants ont suggéré que l'inhibiteur des canaux calciques vérapamil, réduisait la resténose (nouvelle obstruction de l'artère) au bout de six mois. Dans une petite étude (n = 80) l'épaisseur de la paroi artérielle périphérique était similaire si les hommes recevaient le diurétique thiazidique l’hydrochlorothiazide (HCTZ) ou le bloquant alpha‐adrénorécepteur doxazosine. Dans une autre étude de petite taille (n = 36), la DMM était améliorée à 12 mois dans le groupe le telmisartan, un antagoniste des récepteurs de l'angiotensine‐II par rapport au groupe placebo, mais il n'y avait aucune différence significative en termes d'IPS ou de l'épaisseur de la paroi artérielle. Une autre étude (n = 163) n'a trouvé aucune différence significative dans la distance de claudication intermittente ou absolue, CIA, la mortalité toutes causes ou les événements cardiovasculaires non mortels, après 24 semaines dans le groupe traité avec le bêta‐bloquant adrénorécepteur nebivolol et le groupe HCTZ. Une étude comparant deux bêta‐bloquants,le nebivolol et le métroprolol, n'a trouvé aucune différence claire dans la distance de claudication intermittente ou absolue, l'ABI, la mortalité toutes causes confondues ou la revascularisation après 36 semaines de traitement. Une analyse en sous‐groupe de patients avec MAP (n = 2699) dans la dernière étude n'a révélé aucune différence significative dans les critères de jugement combiné de décès, infarctus du myocarde mortel ou non mortel, un accident vasculaire cérébral (AVC) avec ou sans revascularisation entre une stratégie avec l'antagoniste du calcium vérapamil (libération lente LL) avec ou sans trandolapril par rapport à l'utilisation d'un bêta‐bloquant‐adrenoreceptor (aténolol avec ou sans HCTZ).
Les évidences sur l'utilisation de différents médicaments antihypertenseurs chez les personnes atteintes d'une MAP sont pauvres de sorte qu'on sait peu sur des avantages ou des risques de le favoriser. Néanmoins, le manque de données examinant spécifiquement les résultats chez les patients hypertendus avec une MAP ne devrait pas nuire aux preuves irréfutables sur le bénéfice du traitement de l'hypertension et la diminution de la pression artérielle.
Authors' conclusions
Background
Description of the condition
Peripheral arterial disease (PAD) is a condition that affects the blood vessels (arteries) carrying the blood to the legs, arms, and stomach area. PAD occurs when these arteries start to narrow because of a build‐up of fatty deposits inside the blood vessels. PAD mainly affects the arteries in the legs. It can cause discomfort or pain in the lower legs when walking because the narrowing in the blood vessels means that less oxygen‐rich blood reaches the legs. This discomfort or pain is called intermittent claudication. Not everyone has these symptoms.
PAD is the third leading cause of morbidity from atherosclerotic disease (disease of the arteries caused by the build‐up of fatty deposits on the inner lining of the blood vessels) after coronary heart disease and stroke (Fowkes 2013). PAD is the cause of a large number of hospital admissions each year. In the United States of America, 9.6% of cardiovascular events are due to PAD, resulting in 63,000 annual hospital admissions (Kannel 1996). In addition, PAD is associated with significant morbidity and mortality (Criqui 1992; Clement 2007; Fowkes 2013). Patients with PAD, with and without intermittent claudication, have almost three times the risk of dying or having a major cardiovascular event (stroke or heart attack) compared to people without PAD (Pande 2011; Fowkes 2013). Although the risk of non‐fatal cardiovascular events (morbidity) among patients with intermittent claudication is reported to be approximately five to 10 in every 100 patients (Dormandy 2000) the risk of death is much higher. For example, the Framingham study showed that two out of every five patients with intermittent claudication died within 10 years of being diagnosed with PAD (Murabito 2005); this high adverse outcome is also reported in several other studies (Reunanen 1982; Bowlin 1997; Dormandy 2000; Feringa 2006) and the risk is similar whether the patient is symptomatic or not (Criqui 1992).
Hypertension is the name given to high blood pressure that is consistently at such a level that it can cause disease or lead to complications (for example myocardial infarction (MI) (known as a heart attack) or stroke). Hypertension is a common and important risk factor for all vascular disorders including PAD (Kannel 1974; Fowkes 1992; Clement 2007). At presentation, between 2% and 5% of hypertensive patients have intermittent claudication, and this prevalence increases with age. Similarly, 35% to 55% of patients with PAD at presentation also have hypertension (Johnston 1988; Novo 1992; Binaghi 1994; Violi 1996; Cheng 1999; Hirsch 2001; Clement 2007; Singer 2008). Patients who suffer from either hypertension or PAD have a high risk of MI and stroke and when hypertension and PAD are both present the risk is greatly increased (Makin 2001; Clement 2007; Singer 2008).
Hypertension contributes to the pathogenesis (progression of disease) of atherosclerosis (the basic pathological process underlying PAD) (Simon 1986; Bauwens 1989; McGill 1998). Indeed, both hypertension and PAD are associated with abnormal levels of lipid and coagulation factors in the blood (Makin 2002).
Description of the intervention
Given the co‐existence of PAD and hypertension, the overlap in risk factors between the two conditions, the increased risk of non‐fatal cardiovascular events in PAD, and the greater risk of death (Criqui 1992; Murabito 2005; Clement 2007; Fowkes 2013) treatment guidelines highlight the importance of reducing the patient's overall cardiovascular risk as well as targeting the individual risk factors (Clement 2007; ESH 2013). Treatment of hypertension in patients with symptoms of PAD is an obvious therapeutic target but this needs careful consideration because of the possibility that the anti‐hypertensive medication will cause secondary effects and alter the process(es) causing PAD. While beta‐adrenoreceptor blockers (which block the action of noradrenaline and adrenaline on the heart and thus help to reduce blood pressure) are recommended for use in MI to increase survival (Hjalmarson 1997), there has been some concern that they may worsen the symptoms of intermittent claudication for people with PAD although several studies have shown that there is no evidence to support this (Bogaert 1983; Hiatt 1985; Radack 1991). In addition to beta‐adrenoreceptor blockers, there are other classes of anti‐hypertensives that are commonly used to reduce blood pressure including diuretics, calcium channel blockers (CCBs), angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and alpha‐adrenoreceptor blockers.
How the intervention might work
The mechanism thought to cause the worsening of PAD symptoms with beta‐adrenoreceptor blockers is peripheral vasoconstriction (narrowing of peripheral blood vessels) leading to further ischaemia in an already ischaemic limb (reducing blood flow to limbs that already have a poor oxygen supply). There is only scant evidence for this with studies showing either no change in walking distance when one beta‐adrenoreceptor blocker is used (Solomon 1991) or a deterioration (Schweizer 1996), particularly when two beta‐adrenoreceptor blockers are combined (Solomon 1991). Some beta‐adrenoreceptor blockers (for example nebivolol or carvedilol) have 'intrinsic sympathomimetic activity' (ISA) that mimics the effects of the sympathetic nervous system causing the blood vessels away from the heart (for example in the feet) to open up (vasodilation) thus allowing greater blood flow. This might be useful for patients with impaired blood flow to the lower extremities (the legs).
If too much sodium is retained by the body, this can lead to fluid overload and increases the blood pressure. Diuretics work by increasing the flow of urine thereby removing sodium and chloride (and in turn water) from the body and can help to lower blood pressure. Control of blood pressure can help reduce the risk of cardiovascular events which are common in PAD patients.
Dihydropyridine calcium channel blockers (for example, amlodipine, felodipine, nifedipine) cause vasodilation thereby helping to lower blood pressure. CCBs such as verapamil and diltiazem also vasodilate blood vessels but they can reduce cardiac output by lowering the heart rate and impair cardiac systolic function. As a result, the 'cardioselective' CCBs should not be used in patients with concomitant heart failure.
ACE inhibitors inhibit the conversion of the inactive form of the hormone angiotensin to the active form. The active form raises blood pressure, thus blocking its formation with ACE inhibitors helps to reduce blood pressure. There is some evidence that ACE inhibitors increase peripheral perfusion (blood flow) and claudication distance (walking distance before onset of pain in people who have this symptom, known as claudicants) in normotensive PAD patients (Breckenridge 1992; Ahimastos 2006) although concerns about exacerbating renal artery stenosis (blockage) arise (Makin 2001). These agents are also increasingly recognised for their cardioprotective effects (HOPE 1996).
Angiotensin receptor blockers (ARBs) have very similar properties to ACE inhibitors. ARBs selectively block the receptor for angiotensin‐II to inhibit vasoconstriction (narrowing of the blood vessels), secretion of aldosterone, and sodium and water retention, thereby helping to lower blood pressure.
Cardioselective beta‐adrenoreceptor blockers, CCBs, ACE inhibitors and ARBs may potentially help to reduce leg pain by increasing the blood flow to the legs thereby increasing pain‐free walking distance. In addition, these drugs have been shown to reduce blood pressure and the risk of cardiovascular events in hypertensive populations and in those with coronary heart disease (ABCD 2003; HOPE 2004; INVEST 2006; VALUE 2006) some of which have included patients with concomitant PAD.
Long‐acting alpha‐adrenoreceptor blockers such as doxazosin also have vasodilating properties (increasing the blood flow) and are recommended as fourth line drugs, in addition to other anti‐hypertensive drugs, in people with resistant hypertension (ESH 2013).
Why it is important to do this review
Current guidelines for treating hypertension recommend an individualised approach using anti‐hypertensive agents (ESH 2013). There is scant evidence from randomised controlled trials of the benefit or harm of one class of anti‐hypertensive medication over another in PAD patients and subsequently there are no specific national guidelines on the choice of blood pressure medication in patients with PAD. However, a consensus document for the management of PAD (TASC‐II 2007) highlights that controlling a patient's total cardiovascular risk is paramount in such patients. Identification of individual cardiovascular risks, of which hypertension is prevalent, is the first step in the recommendations with subsequent optimal management of each risk factor. Addressing overall cardiovascular risk necessitates polypharmacy and a regimen that includes anti‐platelet agents, statins and an ACE inhibitor (unless contraindicated) plus lifestyle modification (smoking cessation, reduction of salt intake, weight control and exercise) (Clement 2007; TASC‐II 2007). Co‐morbidities need to be considered when making decisions about treatment (Clement 2007; Singer 2008) as these may indicate that one anti‐hypertensive is preferable to another in a particular patient, for example use of a beta‐adrenoreceptor blocker or ACE inhibitor following an acute coronary syndrome or avoidance of the CCB verapamil or diltiazem in those with concomitant heart failure. Anti‐hypertensive drugs have been used for many years in the treatment of hypertension although little is known of their effect in PAD. Therefore, before prescribing anti‐hypertensive medication for people suffering with PAD, the short‐term effects on symptoms and the longer‐term effects on disease progression and cardiovascular outcomes should be considered.
Objectives
To conduct a systematic review to determine the effects of anti‐hypertensive drugs in patients with both raised blood pressure and symptomatic peripheral arterial disease (PAD) in terms of:
-
rate of cardiovascular events and death;
-
symptoms of claudication and critical leg ischaemia;
-
progression of atherosclerotic PAD as measured by ankle brachial index changes and the need for revascularisation (reconstructive surgery or angioplasty) or amputation.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing at least one recognised class of anti‐hypertensive treatment against placebo or two anti‐hypertensive medications against each other and including patients with symptomatic PAD as either the study population or a subgroup. The intervention must have been for at least one month in each group. Studies were excluded when the trial design was adequate but the published data were inadequate for the purposes of the review, or if all attempts to contact the authors to obtain further data were unsuccessful. Cross‐over trials were excluded as they would not be suitable for measures of disease severity and progression in view of the erroneous assumption that any effects are fully reversible after each treatment.
Types of participants
Patients with PAD and hypertension. For the purposes of this review PAD was restricted to the effects of PAD thus including those patients with intermittent claudication (IC) and critical limb ischaemia (CLI) (as defined by ischaemic rest pain) and excluding patients with aortic aneurysm, carotid disease, thrombophlebitis, and vasospasm. Hypertension was defined as elevated blood pressure (based on the current definition of hypertension at the time of recruitment) or was assumed in patients receiving anti‐hypertensive medication.
Types of interventions
To be eligible for inclusion, trials had to include an intervention of at least one recognised class of anti‐hypertensive treatment against placebo or two anti‐hypertensive medications against each other and include patients with symptomatic PAD. The intervention in each group had to last for at least one month.
Types of outcome measures
Primary outcomes
-
All‐cause mortality
-
Cardiovascular deaths (stroke, myocardial infarction (MI), pulmonary embolism, peripheral arterial embolism)
-
Non‐fatal cardiovascular events (stroke, MI, pulmonary embolism, peripheral arterial embolism)
Secondary outcomes
-
Measurements of disease severity, including claudication distance on a treadmill and ankle‐brachial index (ABI)
-
Measurements of disease progression (e.g. ABI and arterial intima‐media thickness (IMT))
-
Need for revascularisation and other vascular interventions (e.g. bypass surgery, angioplasty, amputation)
Search methods for identification of studies
There were no language restrictions and relevant papers that were in languages other than English were translated.
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Cochrane Peripheral Vascular Diseases Group Specialised Register (last searched March 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 2), part of The Cochrane Library (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL and AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
For the original review, the authors searched the National Health Service Database of Abstracts of Reviews of Effectiveness (DARE) (last searched August 2002) to identify potentially eligible studies and review articles.
Searching other resources
Reference lists of relevant articles were screened.
Data collection and analysis
Selection of studies
Both review authors (DAL, GYHL) independently selected trials for inclusion in the review. Disagreements were resolved by discussion.
Data extraction and management
DAL extracted the data, which were checked by GYHL. The data extracted included information relating to the complexities of the topic area, such as patient characteristics and concomitant treatments, as well as data relating to study eligibility, quality and outcomes. Disagreements between review authors on data extraction were resolved by discussion.
Assessment of risk of bias in included studies
For the updated review, risk of bias was assessed independently by the review authors using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the domains listed below. There were three possible judgements: 'low' risk of bias, 'high' risk of bias, and if insufficient detail was reported the judgement on risk of bias was judged to be 'unclear'. We assessed the new studies included in the updated review and we reassessed the studies already included in the previous versions of the review using these criteria.
Sequence generation
Low risk of bias, if the allocation sequence was generated using techniques such as a random number table; a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice.
High risk of bias, if the allocation sequence was generated using techniques such as odd or even date of birth; date (or day) of admission; hospital or clinic record number.
Unclear risk of bias, if there was insufficient information about the sequence generation process to permit judgement.
Allocation concealment
Low risk of bias, if the allocation concealment used methods such as central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered opaque, sealed envelopes.
High risk of bias, if the participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on using an open random allocation schedule (for example a list of random numbers); assignment envelopes used without appropriate safeguards (for example if envelopes were unsealed or non‐opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number.
Unclear risk of bias, if there was insufficient information to permit judgement of low or high risk of bias. This was usually the case if the method of concealment was not described or not described in sufficient detail to allow a definite judgement (for example if the use of assignment envelopes was described but it remained unclear whether envelopes were sequentially numbered, opaque and sealed).
Where the method of allocation was unclear, we planned to contact study authors for them to provide further details.
Blinding
Low risk of bias, if there was no blinding but the review authors judged that the outcome and the outcome measurement were not likely to be influenced by lack of blinding; blinding of participants and key study personnel was ensured and it was unlikely that the blinding could have been broken; either participants or some key study personnel were not blinded but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias, if there was no blinding or incomplete blinding and the outcome or outcome measurement was likely to be influenced by lack of blinding; blinding of key study participants and personnel was attempted but it was likely that the blinding could have been broken; either participants or some key study personnel were not blinded and the non‐blinding of others was likely to introduce bias.
Unclear risk of bias, if there was insufficient information to permit judgement of low or high risk of bias or the study did not address this outcome (for example where the blinding was described only as double‐blind without any other details).
Incomplete data assessment (loss of participants, for example with withdrawals, dropouts, protocol deviations)
Low risk of bias, if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome; missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size; missing data were imputed using appropriate methods.
High risk of bias, if the reasons for missing outcome data were likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was enough to introduce clinically relevant bias in the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to introduce clinically relevant bias in observed effect size; 'as‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Unclear risk of bias, if there was insufficient reporting of attrition or exclusions to permit judgement of low or high risk of bias (for example numbers randomised were not stated, no reasons for missing data were provided); or the study did not address this.
Selective outcome reporting
Low risk of bias, if the study protocol was available and all of the study’s pre‐specified (primary and secondary) outcomes that were of interest in the review were reported in the pre‐specified way; the study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified.
High risk of bias, if not all of the study’s pre‐specified primary outcomes were reported; one or more primary outcomes were reported using measurements, analysis methods or subsets of the data (for example subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis; the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Unclear risk of bias, if there was insufficient information to permit judgement of low or high risk of bias.
Other sources of bias
Low risk of bias, if the study appeared to be free of other sources of bias.
High risk of bias, if there was at least one important risk of bias (for example the study had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal stopping rule); had extreme baseline imbalance; had been claimed to be fraudulent; had some other problem).
Unclear risk of bias, if there was either insufficient information to assess whether an important risk of bias existed or if there was insufficient rationale or evidence that an identified problem would introduce bias.
Measures of treatment effect
Statistical analyses were undertaken as follows. For continuous variables (for example the change in IC distance between baseline and follow‐up) where the data were presented as mean ± standard deviation (SD) the weighted mean difference with 95% confidence interval (CI) was used. Where continuous variables were presented as median (95% CI) the original data from the study were reported as 'other data'. As a summary measure of effectiveness (for example reduction in all‐cause mortality) the odds ratio (OR) with 95% CI was calculated for dichotomous variables.
Unit of analysis issues
In each of the included studies, participants were individually randomised to one of two intervention groups and the analyses were conducted with each patient as the individual unit of analysis, that is with a single measurement for each outcome collected for each patient and analysed. There were no cross‐over trials, cluster‐randomised trials or any other non‐standard designs.
Dealing with missing data
For unpublished studies, or where data were incomplete in published papers, attempts were made to contact authors or researchers to obtain further details. For the original review, no reply was received from six authors who were approached (Bostrom 1986; Novo 1986; Rouffy 1989; Solomon 1991; Sutton‐Tyrell 1995; Casiglia 1997). A reply was received from one (Bogaert 1983) saying that the original data were no longer available. For the 2009 review update, five large RCTs of anti‐hypertensive treatment in hypertensive patients where there were significant numbers of patients with PAD were also identified (LIFE 2002; INVEST 2003; ASCOT 2005; VALUE 2006; ONTARGET 2008). The steering committees for each of these trials were contacted to obtain the raw data for those hypertensive patients with PAD. However, these committees were waiting for publication of the subgroup data before making the data available to us. For this updated review (2013), a subgroup analysis of the INVEST 2003 trial participants with hypertension and PAD was identified (Bavry 2010a) and the data were included. Data on the subgroup of hypertensive patients with PAD from the remaining four large RCTs were still unavailable for this updated review (LIFE 2002; ASCOT 2005; VALUE 2006; ONTARGET 2008). Should the data become available, these trials will be included in the next update of this review.
Assessment of heterogeneity
We were not able to perform any meta‐analysis as included studies compared different types of anti‐hypertensive drugs, to each other or to placebo, and outcomes measured were not common among all studies. However, if meta‐analysis had been possible, we would have applied tests of heterogeneity between trials, as appropriate, using the I2 statistic. Where high levels of heterogeneity among the trials (I2 > 50%) were identified, we would have explored it using subgroup analysis and by performing sensitivity analysis. We would have used a random‐effects model meta‐analysis for an overall summary, if this had been considered appropriate.
Assessment of reporting biases
There were not enough studies in this review to test for reporting bias, thus the review is discussed as a narrative review.
Data synthesis
It was not possible to perform any meta‐analysis as included studies compared different types of anti‐hypertensive drugs, to each other or to placebo, and outcomes were not common among all studies. Results of the individual studies were combined into a narrative review.
Subgroup analysis and investigation of heterogeneity
There were insufficient studies to carry out subgroup analyses. Future revisions of the review may examine different medications within one class of drug (for example atenolol, metoprolol, nebivolol) where the comparator is identical, the duration of the treatment, or treatment effects in men versus women. This will be dependent upon the availability of such data in the included study reports.
Sensitivity analysis
There were insufficient studies to carry out sensitivity analyses. However, future revisions of the review may employ sensitivity analyses to examine factors that may lead to differences between the results of individual trials including poor quality versus good quality trials.
Results
Description of studies
Results of the search
For this 2013 update, following screening of titles and abstracts full texts of 77 references to 57 trials were obtained and considered. Four new studies were included in this update and seven new studies were excluded.
Included studies
See the table Characteristics of included studies.
In total, eight trials were finally included in this review (Overlack 1994; Schweizer 1998; DAPHNE 2002; INVEST 2003; HOPE 2004; Zankl 2010; Diehm 2011; NORMA 2011). A subgroup analysis of the PAD patients enrolled in the INVEST 2003 trial was published in 2010 (Bavry 2010a), therefore this study was moved from the excluded studies to the included studies for this update.
Interventions
Four studies compared anti‐hypertensive therapy against placebo (Overlack 1994; Schweizer 1998; HOPE 2004; Zankl 2010), with two of these studies comparing angiotensin converting enzyme (ACE) inhibitors with placebo (Overlack 1994; HOPE 2004), another (Schweizer 1998) comparing a calcium channel blocker (CCB) with placebo, and one (Zankl 2010) comparing an angiotensin‐II receptor antagonist with placebo. Three studies compared two anti‐hypertensive agents against each other (DAPHNE 2002; Diehm 2011; NORMA 2011); one study compared a thiazide diuretic with an alpha‐adrenoreceptor blocking agent (DAPHNE 2002), one a beta‐adrenoreceptor blocking agent with a thiazide diuretic (Diehm 2011) and another compared two beta‐adrenoreceptor blocking agents against each other (NORMA 2011). Another study, a subgroup analysis of the INVEST 2003 trial (Bavry 2010a), compared a calcium antagonist‐based strategy with a beta‐adrenoreceptor blocking strategy.
Description of PAD
The description of how PAD was diagnosed differed between the included studies. In the INVEST (INternational VErapamil‐SR/Trandolapril) study (INVEST 2003), PAD was defined as a documented history of peripheral vascular disease on the baseline form. Schweizer et al (Schweizer 1998) used the Fontaine Stage IIb classification (Fontaine 1954), that is, a pain‐free walking distance of less than 200 m without ischaemic rest pain or trophic changes (changes in skin and muscle related to chronic ischaemia, although how this was quantified was not explained); in addition to arterial angiography and colour‐coded duplex ultrasound occlusion (diameter ≤ 5 cm) or subtotal stenoses in the distal superficial femoral artery that were present for > six months. Overlack et al (Overlack 1994) included patients with a history of IC for ≥ six months and Fontaine Stage IIb disease who had a proven occlusion on angiography. Zankl et al (Zankl 2010) included patients with documented PAD and at least Stage Fontaine IIa. The NORMA (Nebivolol or Metoprolol in Arterial Occlusive Disease) trial (NORMA 2011) defined PAD as stable IC (Fontaine Stage II) for ≥ six months and an ABI < 0.9 (measured as the ratio of systolic blood pressure in the ankle and arm). The HOPE trial (HOPE 2004) defined PAD as either a history of IC with an ABI < 0.9 (measured by manual palpation of the foot pulse and not ultrasound doppler) or previous vascular intervention or limb amputation for PAD. Diehm et al (Diehm 2011) included patients with one or more of the following: a history of typical IC for at ≥ six months; actual proven PAD by objective means such as haemodynamics and non‐invasive imaging or angiography; history of previous vascular intervention; or ABI of the worse leg < 0.9. In the DAPHNE (Doxazosin Atherosclerosis Progression in Hypertension in the NEtherlands) study (DAPHNE 2002) PAD was defined as IC or peripheral vascular surgery because of atherosclerosis.
Definition of hypertension
The studies used a variety of different criteria to define hypertension. Schweizer (Schweizer 1998) and Overlack (Overlack 1994) included patients with mild essential hypertension, which was quantified in one study (Overlack 1994) as a sitting diastolic blood pressure of 95 to 104 mmHg on three different occasions. The DAPHNE study defined hypertension on the basis of diastolic blood pressure alone, 95 to 115 mmHg in two out of three supine readings (DAPHNE 2002). The HOPE study defined hypertension as blood pressure > 160/90 mmHg or on the basis that people were on anti‐hypertensive treatment (HOPE 2004). Zankl et al (Zankl 2010) included patients with arterial hypertension which was not defined but those with uncontrolled hypertension (systolic blood pressure ≥ 170 mmHg and diastolic blood pressure ≥ 95 mmHg or both) were excluded. The NORMA study (NORMA 2011) included patients with stage I arterial hypertension (systolic blood pressure (SBP) 140 to 159 mmHg and diastolic blood pressure (DBP) 90 to 99 mmHg) or a previous diagnosis of stage I arterial hypertension who were under current treatment. At the time of inclusion, SBP had to be > 100 mmHg and < 160 mmHg, with DBP < 100 mmHg (NORMA 2011). Diehm et al (Diehm 2011) included patients with a SBP between 140 and 179 mmHg and a DBP 90 to 109 mmHg with or without treatment with anti‐hypertensive drugs at screening. After a four‐week run‐in period, the second eligibility criterion for hypertension was a SBP > 130 mmHg and DBP pressure > 85 mmHg (NORMA 2011). The INVEST study (INVEST 2003) included patients with essential hypertension defined as SBP > 140 mmHg and DBP > 90 mmHg (if diabetes or renal impairment was present hypertension was defined as SBP > 130 mmHg and DBP > 85 mmHg) requiring treatment.
Excluded studies
See the table Characteristics of excluded studies.
Fifty‐nine studies were excluded for the following reasons.
-
Ten studies were excluded because the treatment period was shorter than the month specified in the review protocol (Reichert 1975; Smith 1982; Lepantalo 1984; Hiatt 1985; Lepantalo 1985a; Klieber 1986; Bernardi 1988; Natali 1989; Kalus 1995; Liu 1997).
-
Seven studies were eligible for inclusion but the data presentation in the papers was inadequate and attempts to obtain the specific data failed. For six of these trials the authors were contacted but did not respond (Bostrom 1986; Novo 1986; Rouffy 1989; Solomon 1991; Sutton‐Tyrell 1995; Casiglia 1997). For one study (Bogaert 1983) the author was successfully contacted but the data were not available as the study had been conducted several years ago.
-
Nine studies did not include patients with PAD (Lepantalo 1983; Panzner 1992; Weibull 1992; Stumpe 1993; Leeman 1995; Stumpe 1995; OPERA 2002; POISE 2008; Takeda 2010).
-
Six studies were not RCTs (Ingram 1982; Winterfeld 1984; Lepantalo 1985b; Novo 1985; Coto 1991; Van de Ven 1994).
-
Eight studies did not use anti‐hypertensive medication (Larsen 1969; Domschky 1977; Jageneau 1977; Nelson 1978; Staessen 1978; Coto 1989; Branchereau 1995; Lievre 1996).
-
Two studies were excluded because none of the outcome measures matched those predefined for this review (Siniscalchi 1993; Brown 1998).
-
Four studies were excluded because the patients were not hypertensive at the outset of the trial, or hypertensive patients were excluded (Spence 1993; Laurent 1994; Schweizer 1996; Ahimastos 2006).
-
Two trials (Svendsen 1986; Roberts 1987) were cross‐over trials with a washout period at the start of the trial; the post‐intervention data from both or all phases were presented lumped together and not separately. Another trial (Roberts 1992) was a cross‐over trial with no washout phase between treatments; the data from both phases were presented lumped together.
-
Another trial compared captopril against ticlopidine but did not compare captopril against placebo (Novo 1996). In addition, this trial (Novo 1996) only used ABI to confirm diagnosis although the exact value used was not mentioned.
-
One study involved open‐label administration of vasodilating agents to control blood pressure, if necessary, thus making the results of dubious value (Diehm 1993).
-
One publication (Mann 1998) was excluded because it was a protocol for an RCT with no data. Another study was excluded as it was a comment on the results of the ONTARGET trial and did not present any data (Liakishev 2008). One publication was excluded because it was only published as an abstract although the full abstract was not available (Gastmann 1987).
-
The data for PAD patients were not available from five RCTs (LIFE 2002; ABCD 2003; ASCOT 2005; VALUE 2006; ONTARGET 2008).
Risk of bias in included studies
See the table Characteristics of included studies.
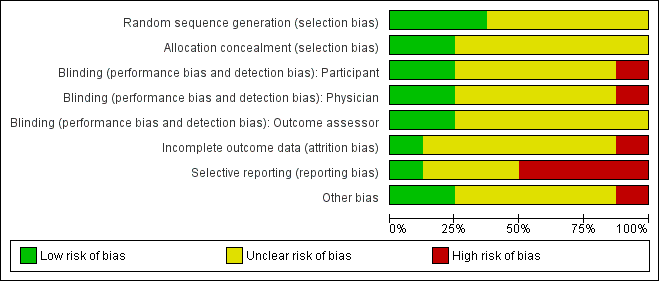
For the updated review, risk of bias was assessed independently by two review authors using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The results are displayed in Figure 1 and Figure 2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Two (HOPE 2004; Diehm 2011) of the included studies provided information about adequate random sequence generation and allocation concealment. Patients were randomised using a central telephone service in the HOPE study (HOPE 2004), and Diehm used a computer‐generated randomisation list (Diehm 2011). There was a low risk of bias in allocation concealment in both these studies (HOPE 2004; Diehm 2011) as treatment was double‐blind. In the subgroup analysis of the INVEST study (INVEST 2003) there was a low risk of bias in random sequence generation because patients were randomised using an electronic system available via the Internet. However, allocation concealment was unclear because, although the investigator was informed electronically of treatment allocation, the treatment was open label. In five of the included studies (Overlack 1994; Schweizer 1998; DAPHNE 2002; Zankl 2010; NORMA 2011) patients were randomised to the treatment arms but it was unclear if there was any selection bias because no further description of the random sequence generation or allocation concealment process was provided.
Blinding
The HOPE study (HOPE 2004) demonstrated a low risk of performance bias and detection bias because the trial was double‐blind and the outcome of cardiovascular events was classified by a committee of two clinicians blinded to treatment allocation who reviewed clinical records of all cardiovascular events reported by recruiting centres to determine whether they met the endpoint criteria.
In one study (Diehm 2011) the risk of performance bias and detection bias for patients and physicians was low because both were blinded to treatment allocation and the nebivolol and hydrochlorothiazide (HCTZ) tablets were manufactured to be identical in size and appearance. However, no detail was given on the blinding of the outcome assessors (Diehm 2011).
In four studies (Overlack 1994; Schweizer 1998; DAPHNE 2002; NORMA 2011) both the patients and the physicians were blinded to treatment but no further details were given on patient or physician blinding resulting in an unclear risk of bias. In addition, no details were given on the blinding of outcome assessments in three of these studies (Overlack 1994; DAPHNE 2002; NORMA 2011). In the study by Schweizer (Schweizer 1998) two of the three outcomes (assessed by colour‐coded duplex scan) were reviewed by two independent experts who were unaware of which group the participant was allocated to. In another study (Zankl 2010) the risk of performance bias and detection bias was unclear as the trial design was single‐blind and, although the physician was not aware of treatment allocation, no information was available on the blinding process of the patients, physicians or outcome assessors.
The INVEST study (INVEST 2003) had a high risk of performance bias and detection bias in terms of the patients and physicians as INVEST was an open‐label trial. Physicians could down‐titrate the initial dose of verapamil or HCTZ monotherapy or up‐titrate medication in a stepped fashion and the final step of treatment titration allowed the physician to add in any non‐study anti‐hypertensive (except beta‐adrenoreceptor blockers for calcium antagonist‐based strategy patients and calcium antagonists for beta‐adrenoreceptor blocker strategy patients) at their discretion to control blood pressure. However, outcomes were adjudicated by a blinded events committee resulting in a low risk of bias in outcome assessment (INVEST 2003).
Incomplete outcome data
The attrition rate varied from 2.5% (INVEST 2003) to 31% (DAPHNE 2002). One study had a low risk of attrition bias as only three patients (verapamil n = 1, placebo n = 2) who were randomised to treatment were not included in the outcome analyses (Schweizer 1998). Another study (DAPHNE 2002) had a high risk of attrition bias as 25% of patients dropped out overall but the attrition rate was higher with HCTZ (30.8%) than doxazosin (19.5%).
In the other seven studies (Overlack 1994; DAPHNE 2002; INVEST 2003; HOPE 2004; Zankl 2010; Diehm 2011; NORMA 2011) the risk of attrition bias was unclear. In the subgroup analysis of INVEST (INVEST 2003) 2.5% of patients with concomitant PAD were lost to follow‐up and no further detail was available on these participants. In the study by Diehm, 14 (9.2%) patients who were randomised were not included in the intention‐to‐treat (ITT) analysis and no detail was given on these patients (Diehm 2011). In the NORMA trial (NORMA 2011) 19 (14.8%) patients dropped out, approximately twice as many in the nebivolol group (n = 13) compared to the metoprolol group (n = 6). In the HOPE study (HOPE 2004) the permanent discontinuation rate was around 30% but it was similar between the treatment arms (ramipril 28.9% versus placebo 27.3%) and the data were analysed on an ITT basis. In the Overlack study (Overlack 1994) the overall dropout rate was 3.2% and 1.7% in the treatment and placebo groups, respectively, but the actual number of PAD patients with follow‐up data was not clear. In the Zankl study (Zankl 2010) the attrition rate was 10% (n = 4) due to premature discontinuation of the study medication because of non‐adherence but no detail was given on which drug they were randomised to.
Selective reporting
The HOPE study (HOPE 2004) had a low risk of reporting bias as data were presented on all the pre‐specified outcome measures listed in the methods by the trialists and analyses were ITT.
Four studies (Overlack 1994; Schweizer 1998; INVEST 2003; NORMA 2011) had a high risk of reporting bias. Overlack did not report all the data collected for the various subgroups, just the most disease‐specific or relevant outcomes (Overlack 1994). Two studies (Schweizer 1998; INVEST 2003) did not report on all outcomes. Schweizer did not report distance to claudication after treatment (Schweizer 1998) and the subgroup analysis of the PAD patients from the INVEST study (INVEST 2003) only reported the two composite endpoints whereas the INVEST protocol and main results papers stated that the individual components of the composite endpoints were also outcomes. In the NORMA study (NORMA 2011) the data analysis section stated that analyses were performed for two populations: the safety population (those who received ≥ one dose of double‐blind medication) and an endpoint population (all patients for whom the endpoint variables were available). However, only the endpoint analyses on 109 (85.2%) patients were reported.
Despite providing data on all endpoints, three studies (DAPHNE 2002; Zankl 2010; Diehm 2011) had an unclear risk of reporting bias as outcome data were not available on those who dropped out.
Other potential sources of bias
Inclusion bias
Four studies (INVEST 2003; Zankl 2010; Diehm 2011; NORMA 2011) had a potential risk of bias relating to patient inclusion. In the subgroup analysis of PAD patients enrolled in INVEST (INVEST 2003), patients were not randomised to treatment based on the presence of PAD, however there were no significant baseline differences between PAD patients randomised to the two treatment arms. In addition, the definition of PAD employed in INVEST was a documented history of PAD at baseline and no detailed information regarding the diagnosis of PAD was available (INVEST 2003). Zankl (Zankl 2010) stated that there were baseline differences between the two groups despite randomisation (not reported) but analysis of covariance (ANCOVA) was performed, which found no confounding effects of differences in baseline values on treatment. Diehm (Diehm 2011) only randomised 66.5% of patients screened. In the NORMA study (NORMA 2011) only post‐menopausal women were eligible to be included and there was a borderline significant difference in the number of men in the two treatment groups, with a trend towards more men in the nebivolol group.
Measurement bias
One study (DAPHNE 2002) had an unclear risk of bias relating to measurement. In the DAPHNE study (DAPHNE 2002), measurements of the IMT from the carotid and femoral arteries were "quality controlled by repeated measurement procedure", but intra‐ and inter‐observer variability was not reported.
Effects of interventions
We did not conduct a meta‐analysis because the eight studies compared different types of anti‐hypertensive drugs to each other or to placebo and outcomes were not common among all studies. Therefore, we have presented the data and discussed results separately for the eight included studies (Overlack 1994; Schweizer 1998; DAPHNE 2002; INVEST 2003; HOPE 2004; Zankl 2010; Diehm 2011; NORMA 2011). In future updates of the review, as new studies emerge and previously unavailable data from five identified studies (LIFE 2002; ABCD 2003; ASCOT 2005; VALUE 2006; ONTARGET 2008) become available, it may be possible to add further comparisons and, where appropriate, combine findings in a meta‐analysis.
Comparison 1 ‐ ACE inhibitors versus placebo
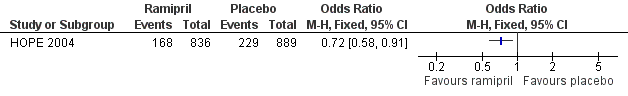
Two trials compared the effects of the ACE inhibitors and placebo (Overlack 1994; HOPE 2004). In the HOPE study (HOPE 2004), when the 836 patients receiving ramipril were compared to the 889 patients receiving placebo there was a significant reduction in the number of cardiovascular events (combined endpoint of cardiovascular death, non‐fatal MI and non‐fatal stroke) with ramipril (OR 0.72, 95% CI 0.58 to 0.91) (HOPE 2004) (Figure 3). In the other study (Overlack 1994), when the 26 patients treated with perindopril were compared to the 28 patients on placebo there was a marginal increase in claudication distance (MD 8.0 m, 95% CI 0.49 to 15.51) and a reduction in maximal walking distance (MD ‐46.00 m, 95 % CI ‐69.74 to ‐22.26) but no change in ABI (MD 0, 95% CI ‐0.03 to 0.03) (Figure 4).

Forest plot of comparison: 1 ACE inhibitors versus placebo, outcome: 1.1 Cardiovascular events.
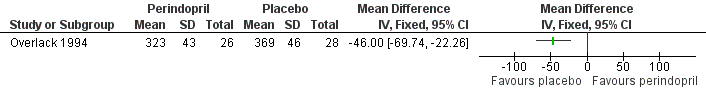
Forest plot of comparison: 1 ACE inhibitors versus placebo, outcome: 1.3 Maximum walking distance.
Data on IMT and revascularisation were not reported in these studies.
Comparison 2 ‐ calcium antagonists versus placebo
The third trial compared the calcium antagonist verapamil against placebo in 96 patients who had undergone peripheral arterial angioplasty (Schweizer 1998). SBP was measured in the arm and posterior tibial artery by means of continuous wave Doppler ultrasound in the PTCA‐treated leg. The ankle brachial SBP ratio was then calculated. In terms of ABI there was no statistically significant difference between the active drug and placebo immediately after angioplasty or at six weeks. However, six months after the angioplasty there was a marginal benefit on ABI in favour of the calcium antagonist, although the 95% CI fell exactly on zero (MD 0.04, 95% CI 0.00 to 0.08). The degree of diameter stenosis and arterial IMT six months after angioplasty showed a significant reduction with the use of verapamil (MD ‐21.6%, 95% CI ‐26.4 to ‐16.8; and MD ‐0.70 mm, 95% CI ‐0.86 to ‐0.54, respectively) (Figure 5; Figure 6, respectively) compared to placebo. Data on other outcomes were not reported in this study.

Forest plot of comparison: 2 Calcium antagonists versus placebo, outcome: 2.1 Degree of diameter stenosis.
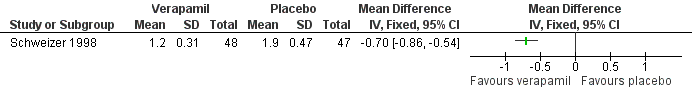
Forest plot of comparison: 2 Calcium antagonists versus placebo, outcome: 2.3 Arterial intima‐media thickness.
Comparison 3 ‐ thiazide diuretics versus alpha‐adrenoceptor blocking drugs
In the fourth study there was no significant difference in arterial IMT when the 27 men receiving the thiazide diuretic hydrochlorothiazide (HCTZ) were compared with the 29 men receiving the alpha‐adrenoreceptor blocker doxazosin (MD ‐0.04 mm, 95% CI ‐0.11 to 0.03) (DAPHNE 2002). Data for other outcomes were not reported in this study.
Comparison 4 ‐ angiotensin‐II receptor antagonist versus placebo
The fifth study comparing the angiotensin‐II receptor antagonist telmisartan against placebo in 36 patients (Zankl 2010) found a significant improvement in MWD after 12 months in the 18 patients treated with telmisartan compared to those receiving placebo (median 191 m, 95% CI 157 to 226 and 132 m, 95% CI 103 to 192, respectively; P < 0.001). There were no significant differences in ABI (median 0.60, 95% CI 0.60 to 0.77 and 0.60, 95% CI 0.56 to 0.77, respectively) or IMT (median 0.08 cm, 95% CI 0.07 to 0.09 and 0.09 cm, 95% CI 0.08 to 0.09, respectively) after 12 months between those receiving telmisartan or placebo. Data on other outcomes were not reported in this study.
Comparison 5 ‐ beta‐adrenoreceptor blockers versus thiazide diuretics
In the sixth study there were no significant differences between the 84 patients receiving the beta‐adrenoreceptor blocker nebivolol and the 79 patients receiving the thiazide diuretic HCTZ after 24 weeks of treatment in change in IC distance (MD ‐1.3 m, 95% CI ‐19.04 to 16.44), change in absolute claudication distance (ACD) (MD ‐9.70 m, 95% CI ‐34.80 to 15.40), change in ABI (MD ‐0.02, 95% CI ‐0.06 to 0.03), all‐cause mortality (OR 2.86, 95% CI 0.11 to 71.15) or non‐fatal cardiovascular events (OR 0.94, 95% CI 0.06 to 15.28) (Diehm 2011). Data on other outcomes were not reported in this study.
Comparison 6 ‐ beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers
In the NORMA study (NORMA 2011) comparing two beta‐adrenoreceptor blockers there were no significant differences after 36 weeks of treatment between the 52 patients receiving nebivolol and the 57 patients receiving metoprolol in IC distance (MD 5.50 m, 95% CI ‐23.30 to 34.30), ACD (MD ‐18.70 m, 95% CI ‐77.31 to 39.91), ABI (MD 0.01, 95% CI ‐0.07 to 0.09), all‐cause mortality (OR 11.78, 95% CI 0.62 to 224.14) or revascularisation (OR 7.30, 95% CI 0.85 to 62.87). Data on other outcomes were not reported in this study.
Comparison 7 ‐ calcium antagonist‐based strategy versus beta‐adrenoreceptor blocker‐based strategy
A subgroup analysis (INVEST 2003) among 2699 PAD patients in the INVEST study revealed no significant differences in the composite endpoints of death, non‐fatal MI or non‐fatal stroke (OR 0.90, 95% CI 0.76 to 1.07) or death, non‐fatal MI or non‐fatal stroke and revascularisation (OR 0.96, 95% CI 0.82 to 1.13) between the 1345 patients receiving a calcium antagonist‐based strategy (verapamil SR ± trandolapril) compared to the 1354 patients receiving a beta‐adrenoreceptor blocker strategy (atenolol ± HCTZ). Data on other outcomes were not reported in this study.
Discussion
Summary of main results
This review found eight RCTs (Overlack 1994; Schweizer 1998; DAPHNE 2002; INVEST 2003; HOPE 2004; Zankl 2010; Diehm 2011; NORMA 2011) comparing at least one recognised class of anti‐hypertensive treatment against placebo (Overlack 1994; Schweizer 1998; HOPE 2004; Zankl 2010) or two anti‐hypertensive medications against each other (DAPHNE 2002; INVEST 2003; Diehm 2011; NORMA 2011) in patients with PAD as either the study population (Overlack 1994; Schweizer 1998; DAPHNE 2002; Zankl 2010; Diehm 2011; NORMA 2011) or a subgroup (INVEST 2003; HOPE 2004). Compared to placebo, ramipril significantly reduced the risk of cardiovascular events (combined endpoint of cardiovascular death, non‐fatal MI or non‐fatal stroke) (OR 0.72, 95% 0.58 to 0.91) (HOPE 2004) but there was no significant difference between anti‐hypertensive treatment strategies on cardiovascular events (death, non‐fatal MI or non‐fatal stroke) in the subgroup analysis of PAD patients in the INVEST study (INVEST 2003). Verapamil significantly improved arterial IMT compared to placebo following arterial angioplasty (Schweizer 1998) but two other studies found no differences in arterial IMT with telmisartan compared to placebo (Zankl 2010) or HCTZ and doxazosin (DAPHNE 2002). Anti‐hypertensives do not appear to improve pain‐free walking distance (Overlack 1994; Diehm 2011; NORMA 2011) or ABI (Overlack 1994; Schweizer 1998; Zankl 2010; Diehm 2011; NORMA 2011).
Overall completeness and applicability of evidence
Although there can be little question about the validity of outcome measures such as walking distance and cardiovascular events, some of the measures used in published studies (for example calf blood flow and ABI) might be regarded as surrogate endpoints. While both these measures are useful for measuring blood flow objectively in the affected limb, thus obtaining a diagnosis of the disease, subjective reporting of the progression of the disease by patients might well be the more important consideration in clinical practice.
Two (INVEST 2003; HOPE 2004) of the eight included studies (Overlack 1994; Schweizer 1998; DAPHNE 2002; INVEST 2003; HOPE 2004; Zankl 2010; Diehm 2011; NORMA 2011) looked at hard clinical endpoints, such as cardiovascular events (INVEST 2003; HOPE 2004). Two other studies also reported data for all‐cause mortality (Diehm 2011; NORMA 2011) or revascularisation (NORMA 2011), although these endpoints were not listed as outcomes in the study methods. In the HOPE study, ramipril significantly reduced the risk of cardiovascular events (combined endpoint of cardiovascular death, non‐fatal MI and non‐fatal stroke) by 28% when compared to placebo (OR 0.72, 95% CI 0.58 to 0.91) (Figure 3). In the PAD subgroup analysis of the INVEST study (INVEST 2003) there was no significant difference between a calcium antagonist‐based strategy (verapamil ± trandolapril) and a beta‐adrenoreceptor blocker‐based strategy (atenolol ± HCTZ) in the composite endpoints of death, non‐fatal MI or non‐fatal stroke (OR 0.90, 95% CI 0.76 to 1.07) or death, non‐fatal MI or non‐fatal stroke plus revascularisation (OR 0.96, 95% CI 0.82 to 1.13). One study (Diehm 2011) also reported no significant differences in all‐cause mortality or non‐fatal cardiovascular events between nebivolol and HCTZ (OR 2.86, 95% CI 0.11 to 71.15 and OR 0.94, 95% CI 0.06 to 15.28, respectively). In the NORMA study (NORMA 2011) there were no significant differences between nebivolol and metoprolol in all‐cause mortality (OR 10.67, 95% CI 0.56 to 203.18) and revascularisation (OR 7.30, 95% CI 0.85 to 62.87).
Three of the studies examining the effect of anti‐hypertensives on arterial IMT reported conflicting results (Schweizer 1998; DAPHNE 2002; Zankl 2010). One (Schweizer 1998) demonstrated a significant improvement in arterial IMT with verapamil compared to placebo six weeks and six months following peripheral arterial angioplasty (Figure 6), while the other two (DAPHNE 2002; Zankl 2010) found no difference in arterial IMT between HCTZ and doxazosin (DAPHNE 2002) or telmisartan and placebo (Zankl 2010). Verapamil was also associated with a reduction in the degree of restenosis compared to placebo (Schweizer 1998) (Figure 5). Five studies (Overlack 1994; Schweizer 1998; Zankl 2010; Diehm 2011; NORMA 2011) examined the effects of the anti‐hypertensives on ABI. Three (Overlack 1994; Schweizer 1998; Zankl 2010) compared the anti‐hypertensives perindopril (Overlack 1994), verapamil (Schweizer 1998) and telmisartan (Zankl 2010) to placebo and all three found no significant difference in ABI between the active drug and placebo (Overlack 1994; Schweizer 1998; Zankl 2010) (Figure 4). Two studies (Diehm 2011; NORMA 2011) compared nebivolol to either HCTZ (Diehm 2011) or metoprolol (NORMA 2011) and both found no significant difference in ABI between the two anti‐hypertensives. The reduction in the degree of narrowing within the affected artery that was seen with verapamil (Schweizer 1998) should be considered in the light of a very marginal improvement in ABI six months after angioplasty, and no data on symptoms. It is possible that although there was restenosis on placebo little deterioration in blood flow occurred due to other mechanisms such as the formation of new blood vessels, called collaterals, to take the blood to the feet.
Three studies (Overlack 1994; Diehm 2011; NORMA 2011) investigating the effects of the anti‐hypertensives ramipril (Overlack 1994) and nebivolol (Diehm 2011; NORMA 2011) compared to placebo (Overlack 1994), HCTZ (Diehm 2011) or metoprolol (NORMA 2011) reported no significant improvement in IC distance with treatment. These three studies (Overlack 1994; Diehm 2011; NORMA 2011) plus one other (Zankl 2010) also examined the effect of anti‐hypertensives on MWD, IC distance plus metres walked in pain. Two studies (Diehm 2011; NORMA 2011) comparing two anti‐hypertensives treatments reported no significant differences in MWD, while the other two studies (Overlack 1994; Zankl 2010) comparing active drug to placebo reported conflicting results. Overlack reported a significant improvement in MWD with placebo compared to ACE inhibitor (MD ‐46.00 m, 95% CI ‐69.74 to ‐22.26) (Overlack 1994) (Figure 4), while the other (Zankl 2010) found a significant improvement in the MWD with telmisartan compared to placebo (median 191 m, 95% CI 157 to 226 and 132 m, 95% CI 103 to 192, respectively; P < 0.001).
Therefore, it appears that ACE inhibitors are effective in reducing the number of cardiovascular events (HOPE 2004), with no significant differences in cardiovascular events between anti‐hypertensive regimens (INVEST 2003). In one study (Schweizer 1998) with blinded outcome assessment in hypertensive patients with PAD, verapamil appears to significantly improve arterial IMT and reduce the degree of restenosis six months after peripheral arterial angioplasty (Schweizer 1998). Anti‐hypertensives do not appear to improve ABI or IC distance. There is conflicting evidence on the benefit of anti‐hypertensives in improving MWD, with one study (Zankl 2010) reporting an improvement in MWD with telmisartan compared to placebo while another (Overlack 1994) demonstrated an improvement in MWD with placebo compared to ramipril. However, caution is warranted when interpreting the results given that four (Overlack 1994; Schweizer 1998; DAPHNE 2002; Zankl 2010) of the eight included studies had relatively small numbers of patients, ranging from 36 (Zankl 2010) to 98 (Schweizer 1998), and two (INVEST 2003; HOPE 2004) were subgroup analyses; many were underpowered for some or all of the outcomes assessed.
Quality of the evidence
Overall the quality of the available evidence was unclear, primarily as a result of a lack of detail in the study reports on the randomisation and blinding procedures and incomplete outcome data (Figure 1; Figure 2). The one exception was the HOPE study (HOPE 2004), which demonstrated a low risk of bias except for a high attrition rate of 30%, although it used an ITT analysis. The DAPHNE study (DAPHNE 2002) also had a high risk of attrition bias with one quarter of all patients discontinuing study medication but a higher attrition rate with HCTZ than doxazosin.
The PAD subgroup analysis of the INVEST study (INVEST 2003) was at high risk of performance and detection bias (Figure 2) as the trial was an open‐label trial and medication could be up‐titrated at the discretion of the physician, however the cardiovascular outcomes were independently adjudicated by a panel blinded to treatment allocation (INVEST 2003). In addition, the definition of PAD employed in INVEST was a documented history of PAD at baseline and no detailed information regarding the diagnosis of PAD was available (INVEST 2003). Further, there was a high risk of reporting bias in the subgroup analysis of INVEST (INVEST 2003) as only the two composite endpoints were reported and not the individual components of the composite endpoints which were also listed as outcomes. Three other studies (Overlack 1994; Schweizer 1998; NORMA 2011) also had a high risk of selective reporting (Figure 1; Figure 2). Overlack did not report the data collected for all the various subgroups, just the most disease‐specific or relevant outcomes (Overlack 1994), and Schweizer did not report distance to claudication after treatment (Schweizer 1998). In the NORMA study (NORMA 2011) only the endpoint analyses were reported; the safety population data (those who received ≥ one dose of double‐blind medication) described in the data analysis section was not included.
Potential biases in the review process
Our search strategy included a comprehensive search of several electronic databases. In addition, we wrote to the authors of included and excluded studies requesting further data. Further, the titles and abstracts of all the studies identified by the search strategy were reviewed independently by two review authors and disagreements were resolved by consensus. Data extraction of the included studies was also undertaken independently by two review authors. Therefore, we believe that the potential for bias in the review process was minimal and that it is unlikely that we have missed important studies.
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Forest plot of comparison: 1 ACE inhibitors versus placebo, outcome: 1.1 Cardiovascular events.
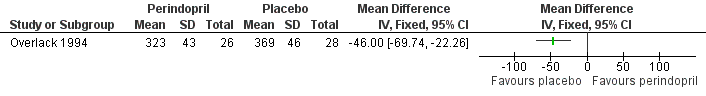
Forest plot of comparison: 1 ACE inhibitors versus placebo, outcome: 1.3 Maximum walking distance.

Forest plot of comparison: 2 Calcium antagonists versus placebo, outcome: 2.1 Degree of diameter stenosis.
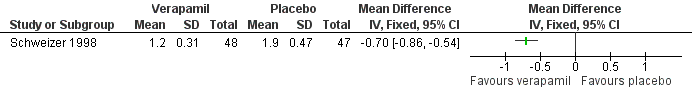
Forest plot of comparison: 2 Calcium antagonists versus placebo, outcome: 2.3 Arterial intima‐media thickness.
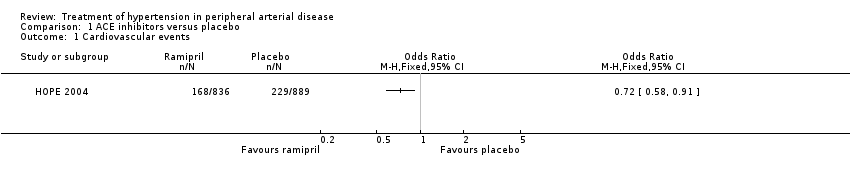
Comparison 1 ACE inhibitors versus placebo, Outcome 1 Cardiovascular events.

Comparison 1 ACE inhibitors versus placebo, Outcome 2 Claudication distance.

Comparison 1 ACE inhibitors versus placebo, Outcome 3 Maximum walking distance.

Comparison 1 ACE inhibitors versus placebo, Outcome 4 Ankle brachial pressure index.
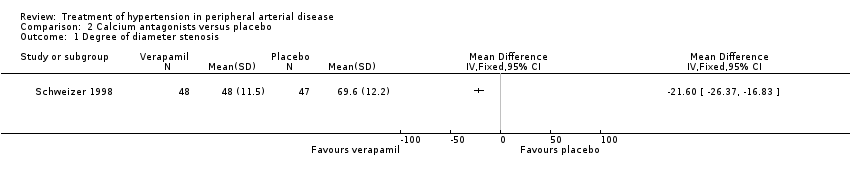
Comparison 2 Calcium antagonists versus placebo, Outcome 1 Degree of diameter stenosis.
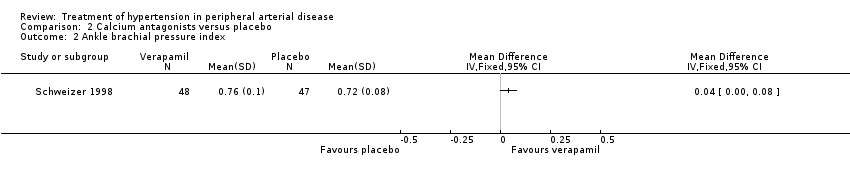
Comparison 2 Calcium antagonists versus placebo, Outcome 2 Ankle brachial pressure index.
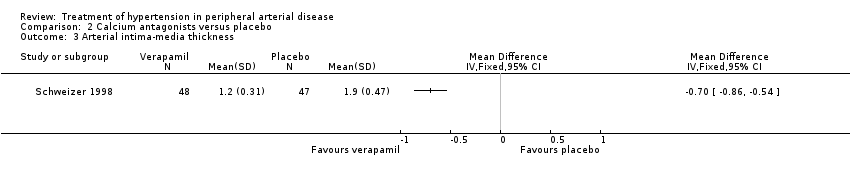
Comparison 2 Calcium antagonists versus placebo, Outcome 3 Arterial intima‐media thickness.

Comparison 3 Thiazide diuretics versus alpha‐adrenoreceptor blocking drugs, Outcome 1 Arterial intima‐media thickness (IMT).
| Study | Telmisartan (n = 18) | Placebo (n = 18) |
| Zankl 2010 | Median (95% CI) | Median (95% CI) |
| Zankl 2010 | 191 (157 ‐ 226) | 132 (103 ‐ 192) |
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 1 Maximum walking distance at 12 months (m).
| Study | Telmisartan (n = 18) | Placebo (n = 18) |
| Zankl 2010 | Median (95% CI) | Median (95% CI) |
| Zankl 2010 | 0.08 (0.07 ‐ 0.09) | 0.09 (0.08 ‐ 1.00) |
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 2 Intima‐media thickness at 12 months (cm).
| Study | Telmisartan (n = 18) | Placebo (n = 18) |
| Zankl 2010 | Median (95% CI) | Median (95% CI) |
| Zankl 2010 | 0.60 (0.60 ‐ 0.77) | 0.52 (0.48 ‐ 0.67) |
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 3 Ankle‐brachial pressure index.

Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 1 Change in intermittent claudication distance.

Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 2 Absolute claudication distance.

Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 3 Change in ankle brachial pressure index.

Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 4 All‐cause mortality.
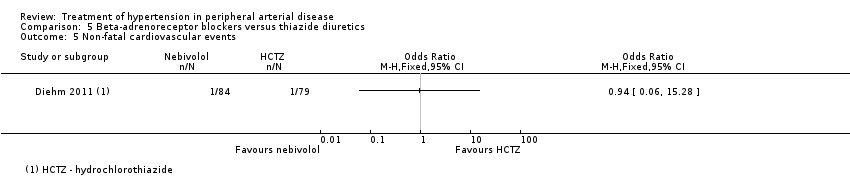
Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 5 Non‐fatal cardiovascular events.

Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 1 All‐cause mortality.
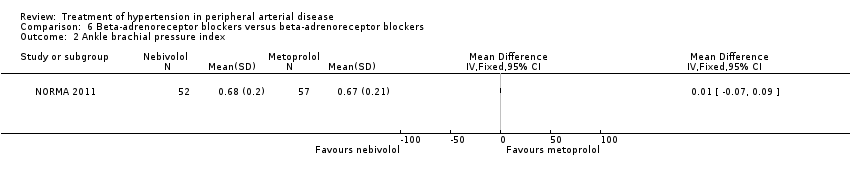
Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 2 Ankle brachial pressure index.
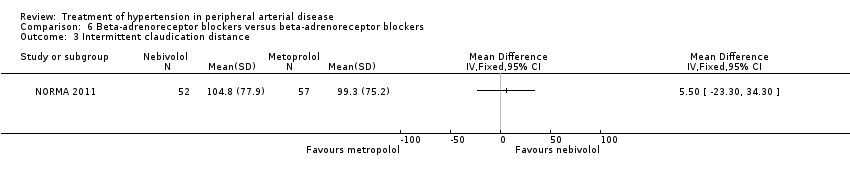
Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 3 Intermittent claudication distance.

Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 4 Absolute claudication distance.
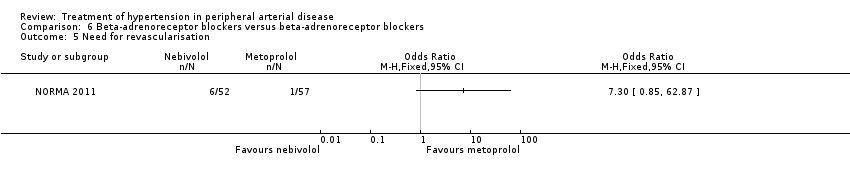
Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 5 Need for revascularisation.

Comparison 7 Calcium antagonist‐based strategy versus beta‐adrenoreceptor blocker‐based strategy, Outcome 1 Composite endpoint of death, non‐fatal MI, or non‐fatal stroke.

Comparison 7 Calcium antagonist‐based strategy versus beta‐adrenoreceptor blocker‐based strategy, Outcome 2 Composite endpoint of death, non‐fatal MI or stroke, and revascularisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Maximum walking distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Degree of diameter stenosis Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Arterial intima‐media thickness Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Arterial intima‐media thickness (IMT) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maximum walking distance at 12 months (m) Show forest plot | Other data | No numeric data | ||
| 2 Intima‐media thickness at 12 months (cm) Show forest plot | Other data | No numeric data | ||
| 3 Ankle‐brachial pressure index Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in intermittent claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Absolute claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 All‐cause mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Non‐fatal cardiovascular events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Intermittent claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Absolute claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Need for revascularisation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Composite endpoint of death, non‐fatal MI, or non‐fatal stroke Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Composite endpoint of death, non‐fatal MI or stroke, and revascularisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

