Le traitement de l'hypertension dans la maladie artérielle périphérique
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | DAPHNE study: single‐blind placebo medication for first six weeks, followed by double‐blind randomisation | |
| Participants | Location: outpatient clinics at two hospitals in the Netherlands | |
| Interventions | Doxazosin 1 mg, 2 mg, 4 mg, 8 mg, 16 mg once daily (od) or HCTZ 12.5 mg, 25 mg, 50 mg, 100 mg. The dose of either doxazosin or HCTZ was increased at two‐weekly intervals until the target blood pressure was achieved Duration: three years | |
| Outcomes | Arterial intima‐media thickness (IMT) of 20 longitudinal arterial wall segments of the carotid and femoral arteries | |
| Notes | For the outcome arterial (IMT) the data presented are the change from baseline to three‐years follow‐up, for the average of 20 mean far and near walls of carotid and femoral arteries combined for the two treatment groups. However, data are also presented for carotid and femoral arteries separately, and combined arteries for each of the following: average of maximum far and near walls, average of far walls, and average of near walls. There was a significant change in IMT for each of the groups of arteries examined, for both the HCTZ and doxazosin groups. Data for the change in arterial IMT from baseline to three‐years follow‐up are only available for 27 patients receiving HCTZ and 29 patients receiving doxazosin. For the outcome peripheral vascular surgery, the type of surgery was not specified. Five patients in each treatment group (HCTZ n = 31; doxazosin n = 33) received peripheral vascular surgery for progressive PAD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description given, only that patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | No description given, only that patients were randomised |
| Blinding (performance bias and detection bias) | Unclear risk | Trial described as double‐blind but no description given |
| Blinding (performance bias and detection bias) | Unclear risk | Trial described as double‐blind but no description given |
| Blinding (performance bias and detection bias) | Unclear risk | No information given regarding the blinding of the outcome assessor |
| Incomplete outcome data (attrition bias) | High risk | Similar number of dropouts in both groups but follow‐up data only available for 69.2% and 70.7% in the HCTZ and doxazosin groups, respectively |
| Selective reporting (reporting bias) | Unclear risk | The authors reported all the data for the measures they stated in the methods section of the paper |
| Other bias | Unclear risk | Measurement bias: measurements of the IMT from the carotid and femoral arteries were "quality controlled by repeated measurement procedure", but intra‐ and inter‐observer variability was not reported in this paper. |
| Methods | Multi‐centre, prospective, randomised, double‐blind, active‐controlled trial | |
| Participants | Location: multi‐centre, all sites in Germany, participants recruited April 2006 to December 2008 Although 177 participants were randomised only results report intention‐to‐treat (ITT) analysis on n = 163. 163 participants, mean (SD) age 66.3 (9.2), all Caucasian and 125 (76.7%) men | |
| Interventions | Nebivolol 5 mg od or HCTZ 25 mg od Duration: 6 months | |
| Outcomes | Primary outcome: % change in IC distance (distance walked in metres until onset of pain) between baseline and week 28 Secondary outcomes: ACD (pain‐free metres plus distance walked with pain) between baseline and week 28; quality of life; adverse events and serious adverse events (which included cardiovascular outcomes). Although not listed as secondary endpoints the paper also reports on ABI and all‐cause mortality which are of interest to this Cochrane review | |
| Notes | Although 177 participants were randomised, results only report ITT analysis on n = 163. No detail given on the 14 patients randomised who are not included in the ITT analyses. Both treatment groups were well‐balanced in terms of baseline differences. The only significant baseline difference was in concomitant use of amlodipine (25.3% versus 10.3% in the nebivolol vs HCTZ group, respectively). PAD defined as history of typical IC for ≥ 6 months; actual proven PAD by objective means such as haemodynamics and non‐invasive imaging or angiography; history of previous lower extremity vascular intervention; ABI of the worse leg < 0.90. All data reported in this Cochrane review are for ITT analyses (as is the convention for RCTs). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised 1:1 using a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Randomised 1:1 using a computer‐generated randomisation list |
| Blinding (performance bias and detection bias) | Low risk | Trial described as double‐blind and the nebivolol and HCTZ tablets were manufactured to be identical in size and appearance |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind trial, tablets identical in appearance and allocation done by computer‐generated randomisation list |
| Blinding (performance bias and detection bias) | Unclear risk | No detail given on the blinding of the outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | 177 participants were randomised yet ITT analyses were only conducted on n = 163. Per protocol analyses were conducted on n = 127 (n = 65 on nebivolol and n = 62 on HCTZ); therefore 36 participants discontinued study medication (n = 19 on nebivolol and n = 17 on HCTZ) |
| Selective reporting (reporting bias) | Unclear risk | Data were reported on all the outcomes mentioned in the Methods of the study. Additional outcomes are reported in the results section. Data were not available for 14 randomised participants with no explanation given for this missing data |
| Other bias | Unclear risk | Of the 266 participants screened only 177 (66.5%) were randomised |
| Methods | Mutli‐centre, randomised, double‐blind, placebo‐controlled, two‐by‐two factorial trial | |
| Participants | Location: 267 centres in 19 countries in North and South America and Europe HOPE participants were aged ≥ 55 years with existing cardiovascular disease or diabetes mellitus and an additional coronary risk factor (smoking, hypertension, hypercholesterolaemia/low HDL/microalbuminuria) but no heart failure or left ventricular dysfunction Number of patients: 1725 PAD patients Number of PAD patients randomised to ramipril or placebo not stated in HOPE 2004 paper. Unpublished data provided by the authors Ramipril: 836 (48.5%) Placebo: 889 (51.5%) | |
| Interventions | Single‐blind run‐in period for 7‐10 days with ramipril 2.5 mg daily. If tolerated, randomised to ramipril 10 mg od (starting dose of 2.5 mg for 7 days, 5 mg for three weeks, then 10 mg) or matching placebo Duration: 4.5 years | |
| Outcomes | Primary outcomes: cardiovascular death, non‐fatal myocardial infarction, or non‐fatal stroke | |
| Notes | Heart Outcomes Prevention Evaluation (HOPE) We wish to thank Professor Östergren and colleagues for providing additional data on the PAD patients included in the HOPE trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a central telephone service |
| Allocation concealment (selection bias) | Low risk | Randomised using a central telephone service |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind trial. 'Randomised using a central telephone service and patients given either ramipril or matching placebo' |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind trial. 'Randomised using a central telephone service and patients given either ramipril or matching placebo' |
| Blinding (performance bias and detection bias) | Low risk | Endpoint classification committee of two masked clinicians reviewed clinical records of all cardiovascular events reported by recruiting centres to determine whether they met endpoint criteria |
| Incomplete outcome data (attrition bias) | Unclear risk | Permanent discontinuation of medication was similar in the ramipril (28.9%) and placebo group (27.3%) |
| Selective reporting (reporting bias) | Low risk | No apparent problems |
| Other bias | Low risk | No apparent problems |
| Methods | Post hoc analysis of the INternational VErapamil‐SR/Trandolapril STudy (INVEST). Multicentre, international, randomised, open‐label, blinded endpoint study | |
| Participants | Location: 862 centres in 14 countries; patients enrolled September 1997 to February 2003 In the subgroup analysis of patients with concomitant PAD at baseline the following were randomised to receive: Calcium antagonist‐based strategy: 1345 participants, mean (SD not reported) age 68.6 years; 48.2% men; 49.3% white Beta‐adrenoreceptor blocker‐based strategy: 1354 participants, mean (SD not reported) age 68.8 years; 47.6% men; 46.2% white. 2.5% participants in total were lost to follow‐up (no further detail available on these participants) | |
| Interventions | Calcium antagonist‐based strategy (verapamil SR ± trandolapril) or a beta‐adrenoreceptor blocker‐based strategy (atenolol ± HCTZ). The first step was either verapamil SR 240 mg daily or atenolol 50 mg daily. Doses could be adjusted downwards if necessary by physician. Other anti‐hypertensive drugs were added in stepped fashion to achieve blood pressure control Step 2: verapamil SR/trandolapril 240/2 (4) mg daily or atenolol 50 mg daily plus HCTZ 25 mg daily Step 3: verapamil SR/trandolapril 180/2 (4) mg twice daily or atenolol 50 mg twice daily plus HCTZ 25 mg twice daily Step 4: verapamil SR/trandolapril 180/2 (4) mg twice daily plus HCTZ 25 mg daily or atenolol 50 mg twice daily plus HCTZ 25 mg twice daily plus trandolapril 2 (4) mg daily Step 5: any other non‐study anti‐hypertensive drug (except beta‐adrenoreceptor blockers for calcium antagonist‐based strategy patients and calcium antagonists for beta‐adrenoreceptor blocker strategy patients) could be added in if needed Duration of treatment: 24 months | |
| Outcomes | Primary outcome: composite endpoint of the first occurrence of all‐cause death, non‐fatal MI or stroke by ITT analysis Other outcomes: all‐cause mortality, cardiovascular mortality, total MI (fatal plus non‐fatal), and total stroke (fatal plus non‐fatal) Two additional composite outcomes: an event in the primary outcome or poor/fair QoL at the final visit or an event in the primary outcome or first occurrence of a vascular procedure (carotid endarterectomy/stent, amputation, percutaneous peripheral vascular intervention, or aortic aneurysm resection/repair/stent) during follow‐up | |
| Notes | Definition of PAD for patients included in this post hoc analysis was a documented history of PAD on the baseline INVEST form. No detailed information regarding the diagnosis of PAD was available. There are no significant baseline differences between the treatment groups for those patients with concomitant PAD but patients with PAD were significantly older and had more co‐morbidities at baseline than those without PAD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using an electronic system available via the Internet |
| Allocation concealment (selection bias) | Unclear risk | Randomised using an electronic system available via the Internet and investigator informed electronically of treatment allocation. However treatment was open label |
| Blinding (performance bias and detection bias) | High risk | Open‐label trial |
| Blinding (performance bias and detection bias) | High risk | Open‐label trial. Physicians could down titrate initial dose of verapamil or HCTZ monotherapy or up‐titrate in a stepped fashion as detailed in the Intervention section. The final step allowed the physician to add in any non‐study anti‐hypertensive (except beta‐adrenoreceptor blockers for calcium antagonist‐based strategy patients and calcium antagonists for beta‐adrenoreceptor blocker strategy patients) at their discretion to control blood pressure |
| Blinding (performance bias and detection bias) | Low risk | Outcomes adjudicated by a blinded events committee |
| Incomplete outcome data (attrition bias) | Unclear risk | 2.5% participants with concomitant PAD were lost to follow‐up (no further detail available on these participants) |
| Selective reporting (reporting bias) | High risk | Data analysed on the ITT population. In the INVEST protocol and main results papers the individual components of the composite endpoints are given by treatment strategy but only two composite endpoints are reported in the subgroup analysis in PAD patients |
| Other bias | High risk | Post hoc analysis and therefore the patients were not randomised to treatment on the basis of their PAD diagnosis. Definition of PAD for patients included in this post hoc analysis was a documented history of PAD on the baseline INVEST form. No detailed information regarding the diagnosis of PAD was available |
| Methods | Nebivolol or Metoprolol in Arterial Occlusive Disease (NORMA) Trial (ISRCTN06278310), prospective, randomised, double‐blind, single‐centre trial | |
| Participants | Location: single‐centre in Germany 128 participants randomised to receive nebivolol (n = 65) or metoprolol (n = 63) but endpoint analysis (and demographics) only available on n = 109 (85.2%) Nebivolol group: 52 (13 dropouts); mean (SD) age 66.7 (8.3) years; 45 (86.5%) men Metoprolol group: 57 (6 dropouts); mean (SD) age 65.9 (7.9) years; 41 (71.9%) men | |
| Interventions | Nebivolol 5 mg od or metoprolol 95 mg od. Duration: 48 weeks of treatment | |
| Outcomes | Outcomes not specified as primary and secondary. However, the study was powered to detect a difference in flow‐mediated dilatation (FMD) between the groups. Outcomes were the change in ABI, IC distance (metres until onset of pain), ACD (distance beyond which exercise could not be continued because of claudication pain), QoL, and endothelial‐dependent FMD between baseline and 48 weeks of treatment Although not listed as secondary endpoints the paper also reports on all‐cause mortality and revascularisation (number requiring a revascularisation procedure) which are of interest to this Cochrane review. | |
| Notes | Only post‐menopausal women were recruited (to exclude the effect of female hormones on endothelial function). No significant differences in any baseline characteristics between the two treatment groups; borderline significant difference (P = 0.06) in the number of men in each group (with more men in the nebivolol group). In the data analysis section the study authors state that analyses were performed for two populations: safety population (those who received ≥ 1 dose of double‐blind medication) and an endpoint population (all patients for whom the endpoint variables were available). However, only the endpoint analyses are reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how the treatment allocation was generated other than that the patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | No description of how the treatment allocation was generated other than that the patients were randomised |
| Blinding (performance bias and detection bias) | Unclear risk | Trial described as double‐blind but no description given |
| Blinding (performance bias and detection bias) | Unclear risk | Trial described as double‐blind but no description given |
| Blinding (performance bias and detection bias) | Unclear risk | Clinical monitoring, data management, and statistical analysis were performed by a company but unclear it the study authors were involved in the outcome assessments |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors estimated a 20% dropout rate after screening. Endpoint data were not available on 19 randomised patients. 13 participants randomised to nebivolol dropped out. Six participants randomised to metoprolol dropped out |
| Selective reporting (reporting bias) | High risk | The study authors reported on all of the outcomes stated in the methods but although 128 participants were randomised, endpoint analyses were only available on n = 109. In the data analysis section the study authors state that analyses were performed for two populations: safety population (those who received ≥ 1 dose of double‐blind medication) and an endpoint population (all patients for whom the endpoint variables were available). However, only the endpoint analyses are reported. Authors state that the study had 80% power to detect a 2.0% change in FMD between the treatment groups, based on their planned sample size of n = 51 in each group |
| Other bias | Unclear risk | Only post‐menopausal women were recruited (to exclude the effect of female hormones on endothelial function). No significant differences in any baseline characteristics between the two treatment groups; borderline significant difference (P = 0.06) in the number of men in each group (with more men in the nebivolol group) |
| Methods | Two‐centre, randomised, double‐blind, placebo‐controlled trial Dropout rate: 3.2% in treatment group; 1.7% in placebo group | |
| Participants | Location: two centres supervising five general practices, Germany Inclusion criteria: patients with newly diagnosed or pre‐treated essential hypertension, defined as sitting DBP of 95 ‐104 mmHg and one of nine concomitant diseases or therapies: hyperlipidaemia, type II diabetes mellitus, ischaemic heart disease, cardiac arrhythmias, PAD, nephropathy with proteinuria, chronic obstructive pulmonary disease (COPD), or degenerative joint disease treated with non‐steroidal anti‐inflammatory drugs (NSAIDS). Subgroup with PAD patients, defined as a history of IC ≥ 6 months, a pain‐free walking distance of 80 to 200 m (Fontaine Stage IIb), and angiographic or ultrasound evidence of iliac or femoral occlusion Number of patients: 490 (only 54 with PAD) Males: 263; females: 227 Age: 40‐75 years Perindopril group: 253 (26 with PAD) | |
| Interventions | Perindopril 4 mg od or placebo Duration: six weeks | |
| Outcomes | PAD patients: Doppler ankle pressures, pain‐free and MWD, SBP, DBP and heart rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how the treatment allocation was generated other than that the patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | No description of how treatment allocation was concealed other than that the patients were randomised |
| Blinding (performance bias and detection bias) | Unclear risk | No description of how the participant was blinded to treatment allocation other than that the trial was double‐blind |
| Blinding (performance bias and detection bias) | Unclear risk | No description of how the physician was blinded to treatment allocation other than that the trial was double‐blind |
| Blinding (performance bias and detection bias) | Unclear risk | Blood pressure readings were measured using an automated device which printed out the values and dated them. These printouts were included with the follow‐up records. All data analyses were conducted centrally in Munich, Germany using the DataEase system. There was no other description of blinding to the other outcome measures including Doppler index, and claudication and MWD |
| Incomplete outcome data (attrition bias) | Unclear risk | There was overall dropout rate of 3.2% and 1.7% in the treatment and placebo groups, respectively, but the actual number of PAD patients with follow‐up data was not clear |
| Selective reporting (reporting bias) | High risk | The study authors do not report all the data collected for all the various subgroups, just the most disease‐specific or relevant outcomes |
| Other bias | Low risk | All data analyses were conducted centrally in Munich using the DataEase system |
| Methods | Randomised, placebo‐controlled trial. Method of randomisation not mentioned. Dropouts: one from the treatment group; two from the placebo group | |
| Participants | Location: Germany Inclusion criteria: patients with Fontaine Stage IIb (walking distance < 200 m on treadmill at 4 km/h at a 10° gradient) PAD and concomitant chronic stable angina pectoris and mild hypertension plus one other risk factor from undergoing percutaneous transluminal coronary angioplasty (PTCA). PAD was diagnosed based on arterial angiography and colour‐coded duplex ultrasound, with occlusions (diameter ≤ 5 cm) or subtotal stenoses in the distal superficial femoral artery present for > 6 months Number of patients: 98 Verapamil group: 49 (1 dropout = 48) | |
| Interventions | Verapamil 240 mg twice daily, or placebo Duration: six months | |
| Outcomes | Assessments before, immediately after, and at six weeks and six months from baseline of: the degree of stenosis measured by angiography; superficial femoral artery IMT measured by colour duplex ultrasound; SBP was measured in the arm and posterior tibial artery by means of continuous wave Doppler ultrasound in the PTCA‐treated leg ‐ the ankle/brachial SBP ratio was then calculated; distance to claudication; SBP, DBP and ventricular septal thickness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description other than being random |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment other than in a randomised, double‐blind manner |
| Blinding (performance bias and detection bias) | Unclear risk | No description of blinding of the participant except for the study being double‐blind |
| Blinding (performance bias and detection bias) | Unclear risk | No description of blinding of the physician except for the study being double‐blind |
| Blinding (performance bias and detection bias) | Unclear risk | The colour‐coded duplex scans were reviewed by two independent experts unaware of which group the participant was allocated to |
| Incomplete outcome data (attrition bias) | Low risk | One verapamil patient and two placebo patients were placed on other medication during the trial and their data were not included in the analyses |
| Selective reporting (reporting bias) | High risk | Distance to claudication after treatment not reported |
| Other bias | Unclear risk | Measurement of the layer thickness: in separate experiments with three observers and four repeated measures of layer thickness for 36 patients, intra‐observer variability was 3.1% and inter‐observer variability was 5.9% |
| Methods | Pilot study, single‐centre, prospective, single‐blind | |
| Participants | Location: single centre at an academic hospital in Germany, recruited April 2004 to February 2006 | |
| Interventions | Telmisartan 40 mg or 80 mg od or placebo. Initial dose of telmisartan was 40 mg od. After 4 weeks of treatment, study medication uptitrated to 80 mg daily if SBP was ≥ 150 mmHg at this visit Duration: 12 months | |
| Outcomes | Primary endpoint: AWD Secondary endpoints: FMD, carotid IMT, ABI, and disease‐related QoL | |
| Notes | n = 40 randomised to treatment but only 36 completed the study and were analysed (attrition bias). The four who dropped were out due to premature discontinuation of the study medication, due to non‐adherence. No detail is given on which drug they were randomised to. IMT was assessed by more than one sonographer but they were trained using a standardised protocol (not specified in paper) and used a common and extensively validated imaging protocol. A minimum of three measurements at different sites on each common carotid artery were obtained and IMT values were averaged. Power calculation suggests that n = 18 in each arm had 80% power to detect a 1.5% difference in MWD. Study supported by Bayer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description given, only that patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | No description given, only that patients were randomised |
| Blinding (performance bias and detection bias) | Unclear risk | Single‐blind but no description given on blinding |
| Blinding (performance bias and detection bias) | Unclear risk | Physician was unaware of treatment allocation but no description of blinding given |
| Blinding (performance bias and detection bias) | Unclear risk | No detail is given on the blinding of the outcome assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | n = 40 randomised to treatment but only 36 completed the study and were analysed. The four dropped out due to premature discontinuation of the study medication due to non‐adherence. No detail is given on which drug they were randomised to |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods are included in the results. Data not available for the four (10%) participants who dropped out |
| Other bias | Unclear risk | Baseline differences between the two groups despite randomisation (not reported) but ANCOVA was performed which found no confounding effects of differences in baseline values on treatment. Very small sample size |
ABI: ankle brachial index
ACD: absolute claudication distance
CAD: coronary artery disease
COPD: chronic obstructive pulmonary disease
DBP: diastolic blood pressure
FMD: flow‐mediated dilatation
HCTZ: hydrochlorothiazide
HDL: high‐density lipoprotein
IC: intermittent claudication
IMT: intima‐media thickness
ITT: intention‐to‐treat
MI: myocardial infarction
NSAIDS: non‐steroidal anti‐inflammatory drugs
od: once daily
PAD: peripheral arterial disease
PTCA: percutaneous transluminal coronary angioplasty
PVD: peripheral vascular disease
SBP: systolic blood pressure
SD: standard deviation
QoL: quality of life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The data for PAD patients were not available. All patients had concomitant diabetes mellitus | |
| Patients were not hypertensive at baseline | |
| The data for PAD patients were not available | |
| The treatment period for each arm in this trial was two weeks only and the minimum for inclusion is one month | |
| This study fits the inclusion criteria except that the data were presented as a line graph in the results. The author has been successfully contacted but the relevant data are no longer available | |
| Poorly designed study with only seven patients and no clear outcome measure. Attempts to contact the authors have been unsuccessful | |
| The active drug, ifenprodil, is not an anti‐hypertensive drug although it blocks alpha1‐adrenoreceptors | |
| This study is of treatment titration and not of outcome | |
| Walking distance data were presented as a line graph and attempts to contact the authors have been unsuccessful | |
| The active drug in this double‐blind, placebo‐controlled trial was an antiplatelet drug, not anti‐hypertensive medication | |
| This study was not a randomised controlled trial | |
| This study is a randomised controlled trial but the results are confounded by the potential addition of a nitrate to lower blood pressure further | |
| The active drug in this study was not an anti‐hypertensive medication | |
| Only published as an abstract but the abstract was not available | |
| The treatment period for this trial was two weeks only and the minimum for inclusion is one month | |
| This study was not a randomised controlled trial | |
| The active drug in this study was not an anti‐hypertensive medication | |
| The treatment in this trial comprised a single dose only, and the minimum for inclusion is one month | |
| The treatment period for this trial was only 25 days and the minimum for inclusion is one month | |
| This trial did not compare two anti‐hypertensive therapies or one therapy against placebo | |
| Hypertensive patients were actively excluded from this study | |
| This paper was concerned with abdominal aortic aneurysms and not peripheral occlusive disease | |
| Although this trial measured calf blood flow under treatment with beta‐blockers, the patient cohort did not have PAD | |
| The treatment period for this trial was three weeks only and the minimum for inclusion is one month | |
| The treatment period for this trial was 10 days only and the minimum for inclusion is one month | |
| This is a retrospective case control study and not a randomised controlled study | |
| This is a comment on the results of the ONTARGET trial not an original data paper | |
| The drug in this study, beraprost, is not an accepted anti‐hypertensive medication | |
| The data for PAD patients were not available | |
| The treatment in this trial comprised only one dose and the minimum for inclusion is one month | |
| This paper describes a study design only and contains no results | |
| The treatment period was 10 days only in this study and the minimum for inclusion is one month | |
| The active drug in this study was not an anti‐hypertensive medication | |
| This was not a randomised, controlled trial of anti‐hypertensive therapy in peripheral vascular disease | |
| Only one patient in this study was symptomatic of peripheral vascular disease. Attempts to contact the authors have been unsuccessful | |
| Cross‐over trial of captopril against ticlopidine and placebo, never tested against either drug though it was included in a sequence of treatments but at the same time in both treatment groups | |
| The data for PAD patients were not available | |
| PAD patients not included in this study | |
| Patients with peripheral vascular disease were not studied | |
| Wrong disease group. This study included patients with atherosclerotic disease undergoing non‐cardiac surgery | |
| The treatment period was two weeks only and not the minimum of one month | |
| Cross‐over trial, with the data from both or all phases presented lumped together, and not analysed separately | |
| Cross‐over trial with the data from both or all phases presented lumped together, and not separately | |
| Hypertensive patients accounted for 40% of the cohort. The data were not presented for the hypertensive patients alone and thus the study could not be included. Attempts to contact the authors have been unsuccessful | |
| Patients were not hypertensive at the outset of this study | |
| The outcome measures in this study did not include any specific for PVD, i.e. walking distance | |
| The treatment period for this trial was two weeks only and the minimum for inclusion is one month | |
| This study is of adequate design but the standard deviation was missing from the published data and the authors have failed to respond to requests for further data | |
| Patients were not hypertensive at the outset of this study | |
| The active drug in this study was not an anti‐hypertensive medication | |
| This paper included patients with PAD but does not use measurements of PAD as outcome measures | |
| This paper does not contain patients with PVD as a main or subgroup | |
| This is a good study of mortality with anti‐hypertensive therapy but the therapies were mixed and the paper did not specify which patient was on which dose etc. Also, carotid stenosis and PAD were presented together in the results. Attempts to contact the authors have failed | |
| Cross‐over trial, with the data from both or all phases presented lumped together, and not separately | |
| This study did not include PAD patients | |
| The data for PAD patients were not available | |
| This study was not a randomised controlled trial | |
| This study did not include PAD patients as a study or subgroup | |
| This study was not a randomised controlled trial |
PAD: peripheral arterial disease
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | Location: three hospitals in Australia (Melbourne, Townsville and Brisbane), participants recruited between 10 May 2008 and 23 August 2011 Ramipril group: 106 (7 drop‐outs). Mean (SD) age 65.5 (5.3) years; 87 (82.1%) men |
| Interventions | Ramipril 10 mg od or matching placebo Duration: 24 weeks |
| Outcomes | Primary outcomes: pain‐free walking time (time to onset of claudication pain) and maximum walking time Secondary outcomes: ABI; stenosis severity (duplex ultrasound of lower limb arteries); patient‐reported symptoms and functional status (assessed by the Walking Impairment Questionnaire); and health‐related QoL |
| Notes | Randomisation process reported as 'tamper‐proof', with participants randomised into blocks of 10 to receive ramipril or matching placebo. All investigators, analysts and participants were blinded to drug assignment and baseline data when they performed follow‐up measurements. No cross‐over. Not all participants were hypertensive at baseline. |
ABI: ankle brachial index
IC: intermittent claudication
od: once daily
QoL: quality of life
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 ACE inhibitors versus placebo, Outcome 1 Cardiovascular events. | ||||
| 2 Claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 ACE inhibitors versus placebo, Outcome 2 Claudication distance. | ||||
| 3 Maximum walking distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 ACE inhibitors versus placebo, Outcome 3 Maximum walking distance. | ||||
| 4 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 ACE inhibitors versus placebo, Outcome 4 Ankle brachial pressure index. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Degree of diameter stenosis Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 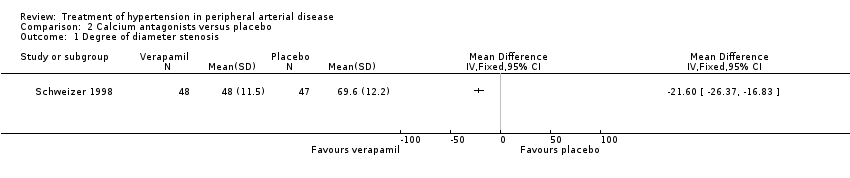 Comparison 2 Calcium antagonists versus placebo, Outcome 1 Degree of diameter stenosis. | ||||
| 2 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2 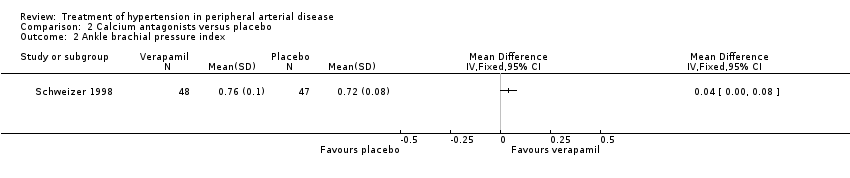 Comparison 2 Calcium antagonists versus placebo, Outcome 2 Ankle brachial pressure index. | ||||
| 3 Arterial intima‐media thickness Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Calcium antagonists versus placebo, Outcome 3 Arterial intima‐media thickness. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Arterial intima‐media thickness (IMT) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 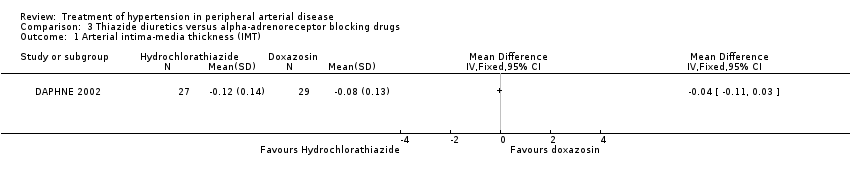 Comparison 3 Thiazide diuretics versus alpha‐adrenoreceptor blocking drugs, Outcome 1 Arterial intima‐media thickness (IMT). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Maximum walking distance at 12 months (m) Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 4.1
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 1 Maximum walking distance at 12 months (m). | ||||||||||||||||
| 2 Intima‐media thickness at 12 months (cm) Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 4.2
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 2 Intima‐media thickness at 12 months (cm). | ||||||||||||||||
| 3 Ankle‐brachial pressure index Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 4.3
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 3 Ankle‐brachial pressure index. | ||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in intermittent claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 1 Change in intermittent claudication distance. | ||||
| 2 Absolute claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2 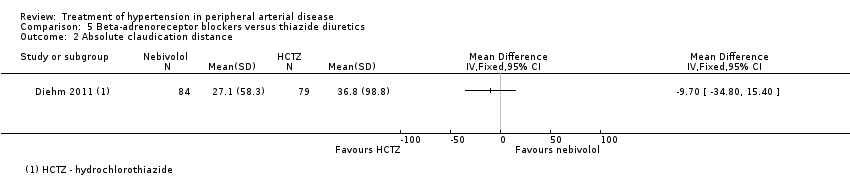 Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 2 Absolute claudication distance. | ||||
| 3 Change in ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 3 Change in ankle brachial pressure index. | ||||
| 4 All‐cause mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 4 All‐cause mortality. | ||||
| 5 Non‐fatal cardiovascular events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.5 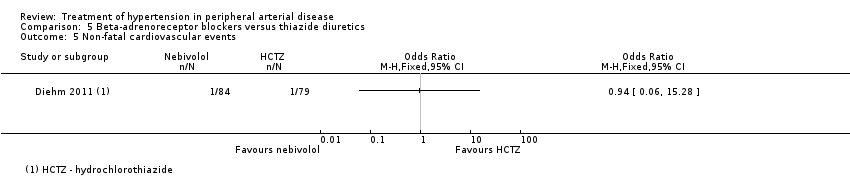 Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 5 Non‐fatal cardiovascular events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 1 All‐cause mortality. | ||||
| 2 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 2 Ankle brachial pressure index. | ||||
| 3 Intermittent claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 3 Intermittent claudication distance. | ||||
| 4 Absolute claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 4 Absolute claudication distance. | ||||
| 5 Need for revascularisation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.5 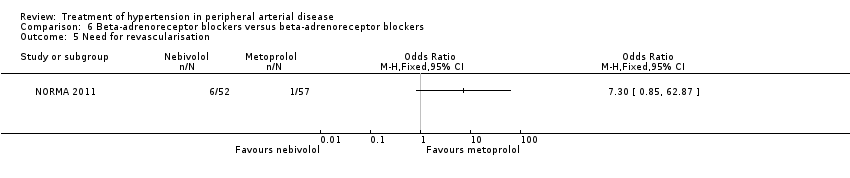 Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 5 Need for revascularisation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Composite endpoint of death, non‐fatal MI, or non‐fatal stroke Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Calcium antagonist‐based strategy versus beta‐adrenoreceptor blocker‐based strategy, Outcome 1 Composite endpoint of death, non‐fatal MI, or non‐fatal stroke. | ||||
| 2 Composite endpoint of death, non‐fatal MI or stroke, and revascularisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Calcium antagonist‐based strategy versus beta‐adrenoreceptor blocker‐based strategy, Outcome 2 Composite endpoint of death, non‐fatal MI or stroke, and revascularisation. | ||||
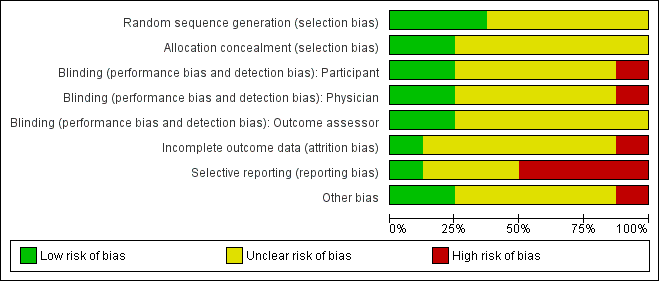
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
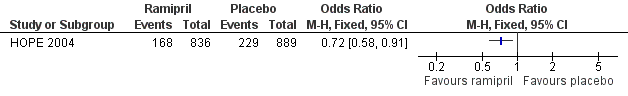
Forest plot of comparison: 1 ACE inhibitors versus placebo, outcome: 1.1 Cardiovascular events.
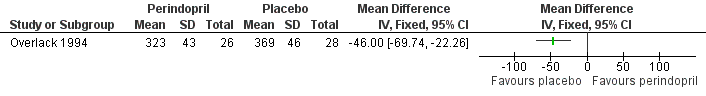
Forest plot of comparison: 1 ACE inhibitors versus placebo, outcome: 1.3 Maximum walking distance.

Forest plot of comparison: 2 Calcium antagonists versus placebo, outcome: 2.1 Degree of diameter stenosis.
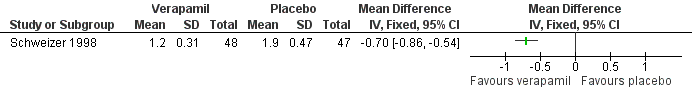
Forest plot of comparison: 2 Calcium antagonists versus placebo, outcome: 2.3 Arterial intima‐media thickness.
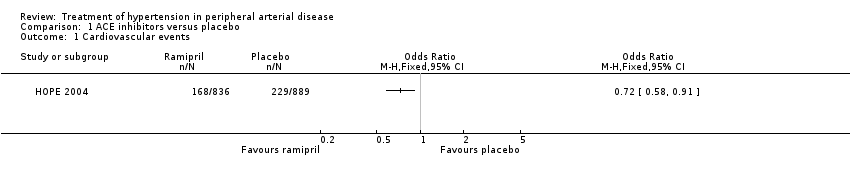
Comparison 1 ACE inhibitors versus placebo, Outcome 1 Cardiovascular events.

Comparison 1 ACE inhibitors versus placebo, Outcome 2 Claudication distance.

Comparison 1 ACE inhibitors versus placebo, Outcome 3 Maximum walking distance.

Comparison 1 ACE inhibitors versus placebo, Outcome 4 Ankle brachial pressure index.
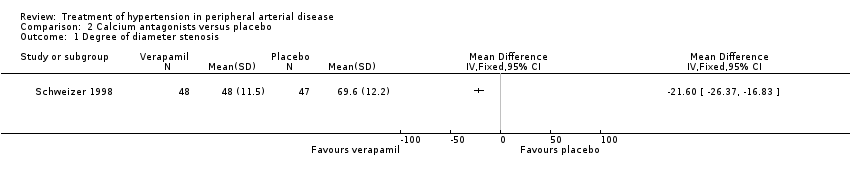
Comparison 2 Calcium antagonists versus placebo, Outcome 1 Degree of diameter stenosis.
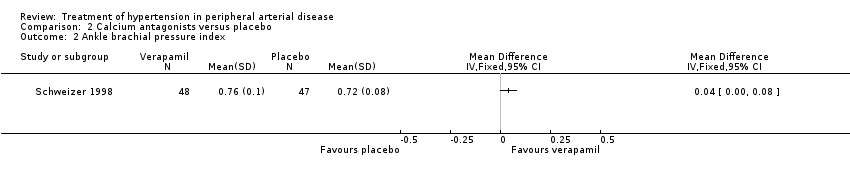
Comparison 2 Calcium antagonists versus placebo, Outcome 2 Ankle brachial pressure index.
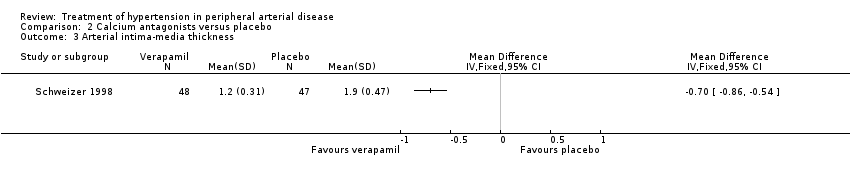
Comparison 2 Calcium antagonists versus placebo, Outcome 3 Arterial intima‐media thickness.

Comparison 3 Thiazide diuretics versus alpha‐adrenoreceptor blocking drugs, Outcome 1 Arterial intima‐media thickness (IMT).
| Study | Telmisartan (n = 18) | Placebo (n = 18) |
| Zankl 2010 | Median (95% CI) | Median (95% CI) |
| Zankl 2010 | 191 (157 ‐ 226) | 132 (103 ‐ 192) |
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 1 Maximum walking distance at 12 months (m).
| Study | Telmisartan (n = 18) | Placebo (n = 18) |
| Zankl 2010 | Median (95% CI) | Median (95% CI) |
| Zankl 2010 | 0.08 (0.07 ‐ 0.09) | 0.09 (0.08 ‐ 1.00) |
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 2 Intima‐media thickness at 12 months (cm).
| Study | Telmisartan (n = 18) | Placebo (n = 18) |
| Zankl 2010 | Median (95% CI) | Median (95% CI) |
| Zankl 2010 | 0.60 (0.60 ‐ 0.77) | 0.52 (0.48 ‐ 0.67) |
Comparison 4 Angiotensin‐II receptor antagonist versus placebo, Outcome 3 Ankle‐brachial pressure index.

Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 1 Change in intermittent claudication distance.

Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 2 Absolute claudication distance.

Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 3 Change in ankle brachial pressure index.

Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 4 All‐cause mortality.
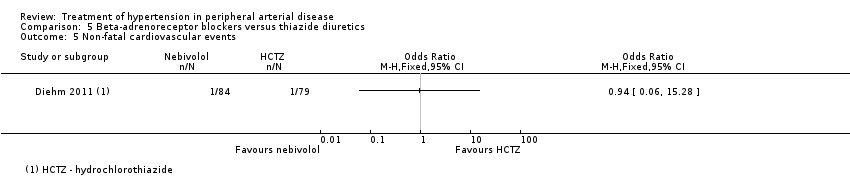
Comparison 5 Beta‐adrenoreceptor blockers versus thiazide diuretics, Outcome 5 Non‐fatal cardiovascular events.

Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 1 All‐cause mortality.
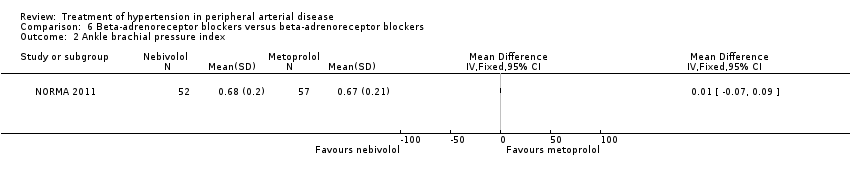
Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 2 Ankle brachial pressure index.
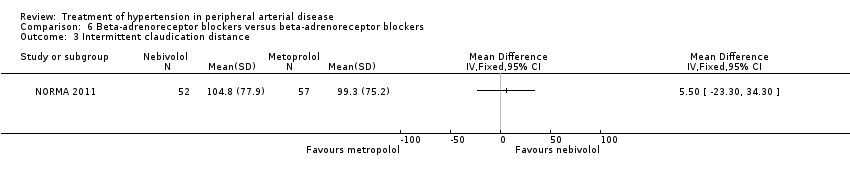
Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 3 Intermittent claudication distance.

Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 4 Absolute claudication distance.
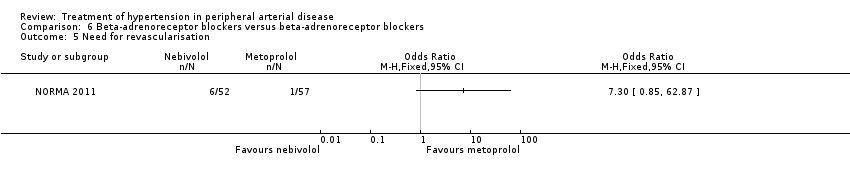
Comparison 6 Beta‐adrenoreceptor blockers versus beta‐adrenoreceptor blockers, Outcome 5 Need for revascularisation.

Comparison 7 Calcium antagonist‐based strategy versus beta‐adrenoreceptor blocker‐based strategy, Outcome 1 Composite endpoint of death, non‐fatal MI, or non‐fatal stroke.

Comparison 7 Calcium antagonist‐based strategy versus beta‐adrenoreceptor blocker‐based strategy, Outcome 2 Composite endpoint of death, non‐fatal MI or stroke, and revascularisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Maximum walking distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Degree of diameter stenosis Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Arterial intima‐media thickness Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Arterial intima‐media thickness (IMT) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maximum walking distance at 12 months (m) Show forest plot | Other data | No numeric data | ||
| 2 Intima‐media thickness at 12 months (cm) Show forest plot | Other data | No numeric data | ||
| 3 Ankle‐brachial pressure index Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in intermittent claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Absolute claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 All‐cause mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Non‐fatal cardiovascular events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Ankle brachial pressure index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Intermittent claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Absolute claudication distance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Need for revascularisation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Composite endpoint of death, non‐fatal MI, or non‐fatal stroke Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Composite endpoint of death, non‐fatal MI or stroke, and revascularisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

