Dopaminergic agonists for hepatic encephalopathy
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel group trial. | |
| Participants | 40 patients with fulminant hepatic failure were randomised. | |
| Interventions | Experimental: levodopa 4 gram/day + standard HE regime. | |
| Outcomes | Mortality. | |
| Notes | Number of dropouts: uncertain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Parallel group trial. | |
| Participants | 75 patients with cirrhosis and acute hepatic encephalopathy were randomised. | |
| Interventions | Experimental 1: levodopa (2 gram on day 1, 4 gram/day the next 6 days). | |
| Outcomes | Clinical improvement. | |
| Notes | Number of dropouts: uncertain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Crossover trial. | |
| Participants | Five patients with cirrhosis and chronic hepatic encephalopathy were randomised. | |
| Interventions | Experimental: bromocriptine 15 mg/day. | |
| Outcomes | Clinical improvement. | |
| Notes | Number of dropouts: 0. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Crossover trial. | |
| Participants | Eight patients with cirrhosis and chronic hepatic encephalopathy were randomised. | |
| Interventions | Experimental: bromocriptine 15 mg/day. | |
| Outcomes | Clinical improvement. | |
| Notes | Number of dropouts: 1 patient died in the first treatment period while receiving placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Parallel group trial. | |
| Participants | 16 patients with fulminant hepatic failure were randomised. | |
| Interventions | Experimental: levodopa 3‐4 gram/day + supportive therapy. | |
| Outcomes | Mortality. | |
| Notes | Number of dropouts: uncertain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Randomised trial assessing the effect of bromocriptine for alcohol withdrawal symptoms. Excluded because patients did not have hepatic encephalopathy at entry and this was not assessed as an outcome. | |
| Controlled crossover study including five patients with chronic hepatic encephalopathy comparing bromocriptine + lactulose, levodopabenserazide + lactulose, and lactulose during five treatment periods. Excluded because the study does not appear to be randomised. We have contacted the authors, but have not obtained a response yet. We urge anyone with knowledge about the design of this study to contact us with information on the design. | |
| Observational study on four patients with fulminant hepatic failure given L‐dopa. Excluded due to lack of randomisation. | |
| Observational study on three patients with hepatic encephalopathy given levodopa. Excluded due to lack of randomisation. | |
| Controlled crossover study including three patients with chronic hepatic encephalopathy comparing levodopa with placebo. Excluded because the study does not appear to be randomised. We have contacted the authors, but have not obtained a response yet. We urge anyone with knowledge about the design of this study to contact us with information on the design. | |
| Randomised crossover trial including 11 patients with chronic hepatic encephalopathy comparing bromocriptine with lactulose. Excluded because the control group received lactulose. | |
| Controlled crossover study including seven patients with chronic hepatic encephalopathy comparing bromocriptine with placebo. Excluded because the study does not appear to be randomised. We have contacted the authors, but have not obtained a response yet. We urge anyone with knowledge about the design of this study to contact us with information on the design. | |
| Controlled crossover study including ten patients with chronic hepatic encephalopathy comparing amantadine + lactulose, levodopabenserazide + lactulose, and lactulose during six treatment periods. Excluded because the study does not appear to be randomised. We have contacted the authors, but have not obtained a response yet. We urge anyone with knowledge about the design of this study to contact us with information on the design. | |
| Controlled crossover study including six patients with chronic hepatic encephalopathy comparing bromocriptine with placebo during four treatment periods. Excluded due to lack of randomisation. | |
| Randomised trial assessing the effect of metoclopramide, a dopamine‐antagonist, in four patients with cirrhosis and hepatic encephalopathy. Excluded because the experimental group did not receive a dopamine agonist. | |
| Randomised crossover trial including four patients with chronic hepatic encephalopathy comparing bromocriptine with neomycin. Excluded because the control group received antibiotics. | |
| Narrative review. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients without improvement ‐ including data from 1. treatment period in crossover trials Show forest plot | 2 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 11.25] |
| Analysis 1.1  Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 1 Number of patients without improvement ‐ including data from 1. treatment period in crossover trials. | ||||
| 1.1 Levodopa for acute hepatic encephalopathy ‐ parallel trial | 1 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.47, 3.15] |
| 1.2 Bromocriptine for chronic hepatic encephalopathy ‐ first treatment period result | 1 | 5 | Odds Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 1.99] |
| 2 Number of patients without improvement ‐ including paired data from crossover trials Show forest plot | 3 | OR (Random, 95% CI) | 0.68 [0.17, 2.67] | |
| Analysis 1.2  Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 2 Number of patients without improvement ‐ including paired data from crossover trials. | ||||
| 2.1 Levodopa for acute hepatic encephalopathy ‐ parallel trial | 1 | OR (Random, 95% CI) | 1.07 [0.77, 1.49] | |
| 2.2 Bromocriptine for chronic hepatic encephalopathy ‐ result from paired data | 2 | OR (Random, 95% CI) | 0.15 [0.00, 6.44] | |
| 3 Mortality Show forest plot | 4 | 139 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.35, 3.54] |
| Analysis 1.3  Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 3 Mortality. | ||||
| 3.1 Levodopa for acute hepatic encephalopathy | 1 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.58, 3.63] |
| 3.2 Bromocriptine for chronic hepatic encephalopathy | 1 | 8 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 8.52] |
| 3.3 Levodopa for fulminant hepatic failure | 2 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.05, 22.17] |
| 4 Adverse events Show forest plot | 2 | OR (Random, 95% CI) | 8.33 [0.37, 187.74] | |
| Analysis 1.4  Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 4 Adverse events. | ||||
| 5 Sensitivity analysis ‐ methodological quality, number of patients without improvement Show forest plot | 3 | OR (Random, 95% CI) | 0.68 [0.17, 2.67] | |
| Analysis 1.5  Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 5 Sensitivity analysis ‐ methodological quality, number of patients without improvement. | ||||
| 5.1 High quality | 1 | OR (Random, 95% CI) | 0.01 [0.00, 2.11] | |
| 5.2 Low quality | 2 | OR (Random, 95% CI) | 1.05 [0.76, 1.46] | |

Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 1 Number of patients without improvement ‐ including data from 1. treatment period in crossover trials.

Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 2 Number of patients without improvement ‐ including paired data from crossover trials.

Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 3 Mortality.
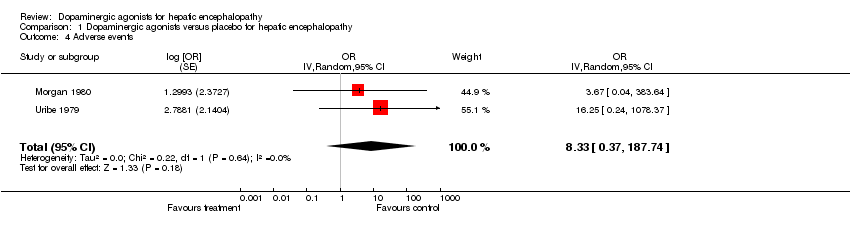
Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 4 Adverse events.

Comparison 1 Dopaminergic agonists versus placebo for hepatic encephalopathy, Outcome 5 Sensitivity analysis ‐ methodological quality, number of patients without improvement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients without improvement ‐ including data from 1. treatment period in crossover trials Show forest plot | 2 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 11.25] |
| 1.1 Levodopa for acute hepatic encephalopathy ‐ parallel trial | 1 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.47, 3.15] |
| 1.2 Bromocriptine for chronic hepatic encephalopathy ‐ first treatment period result | 1 | 5 | Odds Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 1.99] |
| 2 Number of patients without improvement ‐ including paired data from crossover trials Show forest plot | 3 | OR (Random, 95% CI) | 0.68 [0.17, 2.67] | |
| 2.1 Levodopa for acute hepatic encephalopathy ‐ parallel trial | 1 | OR (Random, 95% CI) | 1.07 [0.77, 1.49] | |
| 2.2 Bromocriptine for chronic hepatic encephalopathy ‐ result from paired data | 2 | OR (Random, 95% CI) | 0.15 [0.00, 6.44] | |
| 3 Mortality Show forest plot | 4 | 139 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.35, 3.54] |
| 3.1 Levodopa for acute hepatic encephalopathy | 1 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.58, 3.63] |
| 3.2 Bromocriptine for chronic hepatic encephalopathy | 1 | 8 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 8.52] |
| 3.3 Levodopa for fulminant hepatic failure | 2 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.05, 22.17] |
| 4 Adverse events Show forest plot | 2 | OR (Random, 95% CI) | 8.33 [0.37, 187.74] | |
| 5 Sensitivity analysis ‐ methodological quality, number of patients without improvement Show forest plot | 3 | OR (Random, 95% CI) | 0.68 [0.17, 2.67] | |
| 5.1 High quality | 1 | OR (Random, 95% CI) | 0.01 [0.00, 2.11] | |
| 5.2 Low quality | 2 | OR (Random, 95% CI) | 1.05 [0.76, 1.46] | |


