Agentes dopaminérgicos para la encefalopatía hepática
Resumen
Antecedentes
Los pacientes con encefalopatía hepática pueden presentarse a la consulta con síntomas extrapiramidales y cambios en los ganglios basales. Estos cambios son similares a los observados en pacientes con enfermedad de Parkinson. Por lo tanto, se han evaluado los agentes dopaminérgicos (como bromocriptina y levodopa, utilizados en pacientes con enfermedad de Parkinson) como un tratamiento potencial para los pacientes con encefalopatía hepática.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales de los agentes dopaminérgicos versus placebo o ninguna intervención para los pacientes con encefalopatía hepática.
Métodos de búsqueda
Los ensayos se identificaron mediante el registro de ensayos controlados del Grupo Cochrane Hepatobiliar (Cochrane Hepato‐Biliary Group) (enero 2014), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL) (número 12 de 12, 2013), MEDLINE (1946 hasta enero 2014), EMBASE (1974 hasta enero 2014), y en Science Citation Index‐Expanded (1900 hasta enero 2014). También se realizaron búsquedas manuales en listas de referencias, actas de congresos y registros de ensayos en línea.
Criterios de selección
Se incluyeron ensayos aleatorios, independientemente del estado de la publicación o del idioma. Los análisis primarios incluyeron datos de los ensayos aleatorios que utilizaron un diseño de grupos paralelos o el primer período de los ensayos cruzados. Los datos pareados de los ensayos cruzados se incluyeron en los análisis de sensibilidad.
Obtención y análisis de los datos
Tres autores de la revisión extrajeron los datos de forma independiente. Los metanálisis de efectos aleatorios se realizaron como resultado de una heterogeneidad clínica esperada. Se realizaron metanálisis de efectos fijos, análisis de metarregresión, análisis de subgrupos y análisis de sensibilidad para evaluar las fuentes de heterogeneidad y de sesgo (errores sistemáticos). Se utilizó el análisis secuencial de los ensayos para controlar el riesgo de intervención del azar (errores aleatorios).
Resultados principales
Se incluyeron cinco ensayos que asignaron al azar a 144 participantes con encefalopatía hepática evidente publicados durante 1979 a 1982. Tres ensayos evaluaron levodopa y dos ensayos evaluaron bromocriptina. La dosis media diaria fue de 4 g para la levodopa y de 15 g para la bromocriptina. La duración mediana del tratamiento fue de 14 días (rango de siete a 56 días). Ninguno de los ensayos hizo un seguimiento de los participantes después del final del tratamiento. Sólo un ensayo informó un control adecuado del sesgo; se consideró que los cuatro ensayos restantes tenían un alto riesgo de sesgo. Los metanálisis del modelo de efectos aleatorios indicaron que los agentes dopaminérgicos no tuvieron ningún efecto beneficioso ni perjudicial sobre la encefalopatía hepática en los análisis primarios (15/80 [19%] versus 14/80 [18%]; odds ratio [OR] 2,99; intervalo de confianza [IC] del 95%: 0,09 a 100,55; dos ensayos) o al incluir los datos pareados de los ensayos cruzados (OR 1,04; IC del 95%: 0,75 a 1,43). Se identificaron pruebas claras de heterogeneidad entre los ensayos en el análisis primario (I2 = 65%) y al incluir los datos pareados de los ensayos cruzados (I2 = 40%).
Los agentes dopaminérgicos no tuvieron ningún efecto beneficioso ni perjudicial sobre la mortalidad (42/144 [29%] versus 38/144 [26%]; OR 1,11; IC del 95%: 0,35 a 3,54; cinco ensayos). Los análisis secuenciales de los ensayos demostraron que se carece de información para refutar o recomendar las intervenciones para todos los resultados. Los agonistas dopaminérgicos no parecieron aumentar el riesgo de eventos adversos.
Conclusiones de los autores
Esta revisión no halló pruebas para recomendar o refutar la administración de agentes dopaminérgicos para la encefalopatía hepática. Parece necesaria la realización de más ensayos clínicos aleatorios controlados con placebo sin riesgos de errores sistemáticos ni riesgos de errores aleatorios para obtener pruebas firmes sobre los agentes dopaminérgicos para los pacientes con encefalopatía hepática.
PICO
Resumen en términos sencillos
Agentes dopaminérgicos para la encefalopatía hepática
La encefalopatía hepática es una complicación grave de las enfermedades hepáticas graves. La enfermedad a menudo es fluctuante y presenta un espectro amplio de síntomas que varían desde signos menores que no son fácilmente discernibles hasta el coma profundo. Los síntomas a menudo se presentan en conexión con el estrés relacionado con la infección, la deshidratación, el estreñimiento o la hemorragia gastrointestinal. No se conocen los mecanismos exactos que subyacen al desarrollo de la enfermedad. Los estudios experimentales indican que los cambios mentales observados en la encefalopatía hepática reflejan alteraciones en los neurotransmisores del cerebro.
La dopamina desempeña una función principal en la neurotransmisión. Varias enfermedades del sistema nervioso, incluida la enfermedad de Parkinson, son causadas por una disfunción en el sistema de dopamina. Algunos pacientes con encefalopatía hepática presentan síntomas similares a los observados en los pacientes con enfermedad de Parkinson (cerebración lenta; rigidez en los movimientos; temblor). Para los pacientes con enfermedad de Parkinson, los fármacos conocidos como agentes dopaminérgicos (fármacos que imitan el efecto del neurotransmisor dopamina) alivian claramente los síntomas. También se han evaluado estos fármacos para los pacientes con encefalopatía hepática.
Se realizó la presente revisión sistemática para determinar los efectos beneficiosos y perjudiciales de los agentes dopaminérgicos para los pacientes con encefalopatía hepática. Los análisis incluyeron cinco pequeños ensayos publicados en 1982 o anteriormente. Todos los ensayos excepto uno tuvieron riesgos altos de sesgo (es decir, riesgos de errores sistemáticos o riesgos de sobrestimación de los efectos beneficiosos o riesgos de subestimación de los efectos perjudiciales). Sólo se incluyeron 144 pacientes en los cinco ensayos, y en consecuencia, se observa la presencia de riesgos de errores aleatorios (es decir, intervención del azar). Los análisis no mostraron ninguna diferencia significativa con respecto a los síntomas de la encefalopatía hepática o la mortalidad en los pacientes tratados con agentes dopaminérgicos en comparación con los pacientes que recibieron un placebo inactivo o ninguna intervención. El número de pacientes con eventos adversos parecía comparable en los dos grupos de intervención. En base a las pruebas disponibles, se establece la conclusión de que no pueden encontrarse datos que permitan recomendar o refutar la administración de agentes dopaminérgicos para la encefalopatía hepática. Parece necesaria la realización de más ensayos clínicos aleatorios controlados con placebo sin riesgos de errores sistemáticos ni riesgos de errores aleatorios para obtener pruebas firmes sobre los agentes dopaminérgicos para los pacientes con encefalopatía hepática.
Authors' conclusions
Summary of findings
| Dopamine agonists versus placebo or no intervention for hepatic encephalopathy | ||||||
| Patient or population: patients with hepatic encephalopathy. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (placebo or no intervention) | Dopamine agonists | |||||
| Mortality | Study population | OR 1.11 | 144 | ⊕⊕⊝⊝ | ||
| 535 per 1000 | 561 per 1000 | |||||
| Moderate | ||||||
| 395 per 1000 | 420 per 1000 | |||||
| Hepatic encephalopathy | Study population | OR 2.99 | 80 | ⊕⊕⊝⊝ | ||
| 350 per 1000 | 617 per 1000 | |||||
| Moderate | ||||||
| 184 per 1000 | 403 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1The randomisation methods were classed as adequate in two trials, and three trials were double‐blind. | ||||||
Background
Description of the condition
Hepatic encephalopathy is a complex neuropsychiatric syndrome seen in severe liver failure (Gitlin 1996; Ferenci 2002). Symptoms range from minor neuropsychiatric changes to deep coma (Conn 1979). Hepatic encephalopathy may be clinically overt or may consist of mild neurocognitive impairments, which have been identified in a substantial percentage of patients with liver disease (Randolph 2009). The course of the disease may be episodic, with recurrent symptoms, or chronic, with more stable symptoms (Bajaj 2011). The exact underlying pathophysiology is not known. Experimental studies suggest that symptoms develop as the result of accumulation of toxic agents that have not been metabolised by the liver (Gitlin 1996). Other potential mechanisms include the generation of false neurotransmitters and an abnormal interaction between astrocytes and other cellular elements with cerebral oedema and alterations in glioneural communication (Haussinger 2000; Cordoba 2001).
Description of the intervention
Many patients with hepatic encephalopathy present with extrapyramidal symptoms and have changes in the basal ganglia, as detected by magnetic resonance imaging and proton spectroscopy (Spahr 2000). These symptoms are comparable with those seen in Parkinson's disease and suggest an impairment of dopamine neurotransmission (Blei 1999; Jover 2003). Patients with Parkinson's disease are less likely to experience dyskinesia and dystonia when treated with levodopa (Stowe 2008). Uncontrolled trials suggest that levodopa or bromocriptine could be beneficial in the treatment of patients with hepatic encephalopathy (Parkes 1970; Jorge 1973). The effects of dopamine agents have also been assessed in randomised clinical trials (Uribe 1979; Michel 1980; Morgan 1980), and previous guidelines suggested that the intervention may be considered in patients with chronic hepatic encephalopathy (Blei 1999; Lizardi‐Cervera 2003).
Why it is important to do this review
We have previously published a systematic review on dopamine agents for hepatic encephalopathy (Als‐Nielsen 2004a). The results of this review were inconclusive. We have been unable to identify any further meta‐analyses or systematic reviews on the topic. To determine the strengths and weaknesses of the current evidence, we have updated our previous review (Als‐Nielsen 2004a).
Objectives
To evaluate the beneficial and harmful effects of dopamine agents versus placebo or no intervention for patients with hepatic encephalopathy.
Methods
Criteria for considering studies for this review
Types of studies
This review included all randomised trials, regardless of publication status, language, or blinding. Unpublished trials were included if the methodology and the data were available in written form. We planned to include observational studies reporting harms, but we identified no observational studies reporting relevant data.
Types of participants
Patients with hepatic encephalopathy were included, irrespective of the aetiology of the underlying liver disease. The diagnostic criteria could include psychometric tests, clinical scoring systems (such as the West‐Haven criteria), electroencephalography (Guerit 2009), or biochemical findings (including ammonia levels). Based on the diagnostic criteria used in the included trials, participants were classified as having overt or minimal hepatic encephalopathy, and the latter was classified further as recurrent or chronic.
Types of interventions
The intervention comparisons assessed were dopamine agents (e.g., levodopa, bromocriptine) versus placebo or no intervention. Studies were included irrespective of the dose or duration of therapy.
Types of outcome measures
Primary outcomes
-
Mortality (all‐cause).
-
All cause non‐fatal serious adverse events.
-
Morbidity. This outcome measure was assessed on the basis of the number of participants who showed no improvement in manifestations of hepatic encephalopathy as defined by the authors of included trials.
Secondary outcomes
-
All‐cause non‐serious adverse events (number and type) (ICH‐GCP 1997).
-
Qualitiy of life.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and Science Citation Index‐Expanded (Royle 2003). Search strategies with time spans of the searches are given in Appendix 1.
Searching other resources
Reference lists in relevant articles and conference proceedings were scanned for additional trials not identified in the electronic searches. We wrote to authors of identified trials and pharmaceutical companies to enquire about additional trials. Ongoing and completed trials were also identified through searches in the World Health Organization Trial Search Portal (www.who.int/trialsearch/).
Data collection and analysis
Selection of studies
All review authors participated in the selection of trials. AEJ listed the potentially eligible trials. Subsequently, trials that fulfilled all inclusion criteria were identified. Excluded trials were listed along with the reasons for exclusion.
Data extraction and management
Three review authors (AEJ, BA‐N, and LLG) extracted data independently. All disagreements were resolved through discussion before analyses.
We extracted data on the design of the trial (country of origin, parallel or cross‐over design, and bias control), participant characteristics (aetiology of underlying liver diseases and type of hepatic encephalopathy, mean age, proportion of men), and the intervention regimen assessed (type, dose, and duration of therapy).
Assessment of risk of bias in included studies
We assessed the risk of bias in the trials independently in accordance with the instructions provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2013). Because of the risk of overestimation of intervention effects in randomised trials with high risk of bias (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savovic 2012, Savovic 2012a), we assessed the influence of risk of bias on trial results using the following domains.
Allocation sequence generation
-
Low risk of bias: Sequence generation was achieved by using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
-
Uncertain risk of bias: The method of sequence generation was not specified.
-
High risk of bias: The sequence generation method was not random.
Allocation concealment
-
Low risk of bias: The participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g., if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
-
Uncertain risk of bias: The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
-
High risk of bias: The allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants, personnel, and outcome assessors
-
Low risk of bias: Blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
-
Uncertain risk of bias: Information was insufficient to permit assessment of whether blinding was likely to induce bias on the results.
-
High risk of bias: No blinding or incomplete blinding was performed, and assessment of outcomes was likely to be influenced by lack of blinding.
Incomplete outcome data
-
Low risk of bias: Missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, had been employed to handle missing data.
-
Uncertain risk of bias: Information was insufficient to permit assessment of whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
-
High risk of bias: The results were likely to be biased as the result of missing data.
Selective outcome reporting
-
Low risk of bias: All outcomes were predefined and reported, or all clinically relevant and reasonably expected outcomes were reported. The trial was registered on the www.clinicaltrials.gov web site or on a similar register, or the protocol was published.
-
Uncertain risk of bias: It was unclear whether all predefined and clinically relevant and reasonably expected outcomes were reported.
-
High risk of bias: One or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
Other bias
-
Low risk of bias: The trial appears to be free of other components that could put it at risk of bias.
-
Uncertain risk of bias: The trial may or may not be free of other components that could put it at risk of bias.
-
High risk of bias: Other factors in the trial could put it at risk of bias (e.g., for‐profit involvement, authors conducting trials on the same topic).
Trials with unclear or high risk of bias methodology in one or more of the above domains were considered trials with high risk of bias. The remaining were considered trials with low risk of bias.
Measures of treatment effect
All outcome measures were dichotomised and were expressed using odds ratios (ORs) with 95% confidence intervals (CIs).
Unit of analysis issues
The primary analyses included data from trials using a parallel‐group design and from the first treatment period of cross‐over trials. Additional analyses were performed that included paired data from the cross‐over trials (Becker 1993; Elbourne 2002).
Dealing with missing data
Data on all participants randomly assigned were sought to allow intention‐to‐treat analyses that included participants irrespective of compliance or follow‐up. For participants with missing data, carry‐forward of the last observed response was used. We originally planned to analyse the influence of missing data using imputation (Higgins 2008). We planned to impute missing values as failures, successes, same as control group, same as experimental group, and same as own group (Higgins 2008). We did not perform these analyses because no losses to follow‐up were described.
Assessment of heterogeneity
Intertrial heterogeneity was assessed on the basis of I2 values.
Assessment of reporting biases
We planned to evaluate the risk of reporting bias by comparing trial protocols and published reports. Furthermore, reporting biases were assessed on the basis of the extent to which clinically relevant outcome measures (hepatic encephalopathy, mortality, and adverse events) were reported.
Data synthesis
Analyses were performed in Review Manager 5 (RevMan 2012) and in STATA 12 (STATA 12). Primary meta‐analyses were performed by using random‐effects models because of anticipated variability between trials regarding participants and interventions.
Subgroup analysis and investigation of heterogeneity
Originally, we planned to perform several subgroup analyses to assess sources of intertrial heterogeneity (bias control, participant characteristics, and intervention regimens). However, because of the limited number of trials in the meta‐analyses of the primary outcomes, we were able to perform these subgroup analyses only for the outcome measure of mortality. Likewise, regression analyses (Egger's test) that were planned to estimate the risk of publication bias and other biases (small‐study effects) were performed only for the outcome measure of mortality.
Trial sequential analysis
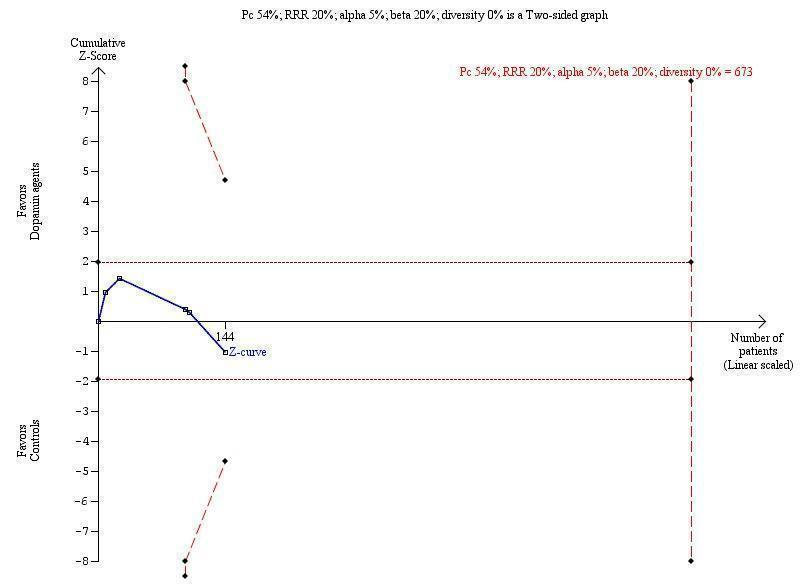
We performed trial sequential analysis (CTU 2011; Thorlund 2011) to control risks of random errors due to sparse data and repetitive testing of cumulative data (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). To minimise the risk of random error, we calculated the required information size, defined as the required sample size necessary to detect or reject intervention effects after adjusting for diversity (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009;Wetterslev 2009; Thorlund 2010). The information size was calculated on the basis of a risk ratio (RR) reduction of 20% or the results of included trials with a low risk of bias (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009;Wetterslev 2009; Thorlund 2010). We presented the results of the analysis in a graph. with individual trials added on the basis of their year of publication. If more than one trial was published in a year, trials were added alphabetically according to the first author's family name. The results of the trials were presented as a cumulative Z‐curve. The trial sequential monitoring boundaries were constructed and the diversity‐adjusted required information size calculated with a type 1 error of 5% and a type 2 error of 20%. The results were displayed as a graph with the cumulative meta‐analysis results entered. The trial sequential analysis shows firm evidence of intervention effects (or no intervention effects) if the cumulative Z‐curve crosses the monitoring boundaries; it also shows that additional trials may be needed if the boundaries are not crossed.
Sensitivity analysis
The robustness of the results was assessed by repeating the meta‐analyses using a fixed‐effect model. No additional sensitivity analyses were performed because of the limited number of trials identified.
Results
Description of studies
Results of the search
In total, 294 references were identified through the literature searches (Appendix 1). After duplicates and clearly irrelevant references (references to papers that did not describe trials of dopaminergic agents for participants with hepatic encephalopathy) were excluded, 17 references were retrieved for further assessment (Figure 1). Of these, eight references referred to five randomised trials that were eligible for inclusion (Uribe 1979; Vij 1979; Michel 1980; Morgan 1980; Koshy 1982). Through correspondence with the authors of two trials (Uribe 1979; Morgan 1980), additional information was obtained on trial results and methods. For the remaining trials, data were gathered from published reports.
Included studies
All of the included trials were described in at least one full‐paper article published from 1979 to 1982. Three trials used a parallel‐group design (Vij 1979; Michel 1980; Koshy 1982), and two trials used a cross‐over design (Uribe 1979; Morgan 1980).
In total, 144 participants with overt hepatic encephalopathy were included. Three trials (66 participants in the treatment group versus 65 participants in the control group) assessed acute episodes of hepatic encephalopathy (Vij 1979; Michel 1980;Koshy 1982). Two trials (seven participants in the treatment group versus six participants in the control group) assessed chronic hepatic encephalopathy (Uribe 1979; Morgan 1980). Two trials included participants with acute fulminant liver failure due to viral hepatitis (Vij 1979; Koshy 1982). Three trials included participants with cirrhosis (Uribe 1979; Michel 1980; Morgan 1980). The proportion of participants with alcoholic liver disease ranged from 0 to 80%. The proportion of participants with viral hepatitis ranged from 0 to 100%, and mean age ranged from 32 years to 57 years.
Three trials assessed levodopa (Vij 1979; Michel 1980;Koshy 1982), and two trials assessed bromocriptine (Uribe 1979; Morgan 1980). The mean daily dose was 4 grams for levodopa and 15 grams for bromocriptine. The median duration of treatment was 14 days (range seven to 56 days). None of the trials followed participants after the end of treatment. None of the included trials assessed health economics.
Excluded studies
Nine references to eight trials were excluded because they turned out not to be randomised or referred to cross‐over trials that compared dopamine agents versus interventions for hepatic encephalopathy considered potentially active (Characteristics of included studies).
Risk of bias in included studies
All trials had a high risk of bias in the assessment of one or more than one of the bias risk domains.
Allocation
Randomisation methods (allocation sequence generation and allocation concealment) were classed as adequate in two trials (Uribe 1979; Morgan 1980) and unclear in the remaining trials (Figure 2).

Figure 2. Risk of bias graph: review authors' judgements about all risk of bias items presented as percentages across all included studies.
Blinding
Three trials were blinded using a placebo (Uribe 1979; Michel 1980; Morgan 1980). No blinding was described in the remaining trials.
Incomplete outcome data
Two trials accounted for all participants with missing outcome data (Uribe 1979; Morgan 1980). In the remaining three trials, no dropouts or withdrawals were described, giving the impression that no losses to follow‐up occurred, although this was not specifically stated.
Selective reporting
We were able to extract data on hepatic encephalopathy from only three trials (Uribe 1979; Michel 1980; Morgan 1980).
Other potential sources of bias
No sample size calculations were reported. None of the included trials received industry funding.
Effects of interventions
See: Summary of findings for the main comparison Dopamine agonists for hepatic encephalopathy
Mortality
Random‐effects meta‐analyses found no difference in mortality between participants randomly assigned to dopamine agents versus controls (OR 1.11, 95% CI 0.34 to 3.54; Analysis 1.1). Little intertrial heterogeneity was noted (I2 = 28%). The result was confirmed in a fixed‐effect meta‐analysis (OR 1.24, 95% CI 0.59 to 2.59). No evidence of small‐study effects was identified in regression analysis (Egger's test P value 0.35). In subgroup analyses, no clear differences were seen between trials on participants with acute episodes compared with chronic hepatic encephalopathy (Analysis 1.2) or participants with fulminant liver failure or cirrhosis (Analysis 1.3), trials on levodopa or bromocriptine (Analysis 1.4), trials with a low or unclear risk of bias (Analysis 1.5), or trials using a parallel or cross‐over design (Analysis 1.6).
The trial sequential analysis graph showed that the cumulative Z‐curve does not cross the monitoring boundary (Figure 3). The analysis showed a diversity‐adjusted required information size of 673 participants (the number of participants needed to reach firm evidence of an intervention effect of 20% risk ratio reduction). The number of participants included corresponds to only 21% of the diversity‐adjusted required information size. Accordingly, we lack evidence to recommend or refute dopamine agents for hepatic encephalopathy.

Trial sequential analysis of dopamine agents versus placebo or no intervention in participants with hepatic encephalopathy.
The outcome measure is mortality. The analysis was performed with an event rate of 54% (Pc) in the control group, a risk ratio (RR) reduction of 20%, alpha 5%, beta 20%, and diversity 0%. The cumulative Z‐curve does not cross the naive 5% statistical boundaries (dotted horizontal lines) or the trial sequential boundaries for benefits or harms (inward sloping etched lines). The results show that the diversity‐adjusted required information size was 673 participants, corresponding to 21% of the total sample size in the included trials. The programme did not even draw futility boundaries. Accordingly, the meta‐analysis does not recommend or refute an intervention effect; data are simply too few.
Hepatic encephalopathy
The primary random‐effects meta‐analyses showed no significant effects of dopamine agents on hepatic encephalopathy compared with placebo or no intervention when data from parallel‐group trials were analysed (OR 0.33, 95% CI 0.01 to 11.25; Analysis 1.7) or when paired data from the cross‐over trial reporting this outcome measure were included (OR 0.68, 95% CI 0.17 to 2.67; Analysis 1.8). The results were confirmed by fixed‐effect meta‐analyses including data from parallel‐group trials (OR 1.08, 95% CI 0.45 to 2.62), but also when paired data from the two cross‐over trials reporting this outcome measure were included (OR 1.04, 95% CI 0.75 to 1.43).
Adverse events
We were able to retrieve data on adverse events only from the two cross‐over trials (Uribe 1979; Morgan 1980). In total, seven of 13 participants experienced non‐serious adverse events during treatment with dopamine agents. No adverse events were reported during control periods. No clear difference was observed between intervention and control groups (Analysis 1.9). No serious adverse events were registered. Adverse events included hypomania (n = 1), hallucinations and headache (n = 1), constipation (n = 3), and nausea and vomiting (n = 2).
Quality of life
None of the included trials reported data on quality of life.
Discussion
Summary of main results
Patients with cirrhosis may present with extrapyramidal symptoms similar to those seen in Parkinson's disease (Jover 2003). Further similarities between participants with hepatic encephalopathy and participants with Parkinson's disease include alterations in the basal ganglia (Spahr 2000). In theory, dopamine agents that are effective in Parkinson's disease could alleviate manifestations of hepatic encephalopathy. However, the present systematic review found no evidence to recommend or refute the use of dopamine agents for patients with hepatic encephalopathy. The available evidence includes only a limited number of small trials published before 1983. No clear effects were identified for any of the outcome measures assessed. Additional analyses found no specific subgroups that indicated potential effects when the results of included trials were separated on the basis of the type of hepatic encephalopathy at inclusion, the type of underlying liver disease, or the intervention assessed. The dose and duration of the interventions assessed were similar across trials. Data from participants with Parkinson's disease (Miyasaki 2002) show that the dose of both levodopa and bromocriptine and the duration of the intervention regimens assessed in included trials should be sufficiently high to detect a clinical response. The combined evidence is not promising. However, the statistical power is low, and evidence is insufficient to support or refute beneficial or harmful effects of the interventions assessed.
Overall completeness and applicability of evidence
To ensure completeness of the evidence, we performed extensive literature searches. Our regression analyses showed no clear evidence of publication bias or other small‐study effects. Still, the regression analysis was not sensitive because of the limited number of trials.
The main problem with the included trials is the fact that a number of potentially effective interventions for patients with decompensated liver disease have been identified after the trials were completed. These interventions include treatments for hepatic encephalopathy (Bass 2010), bleeding oesophageal varices (Abraldes 2007), and spontaneous bacterial peritonitis (Wiest 2012). Likewise, the diagnostic assessment and nomenclature for hepatic encephalopathy have been updated (Bajaj 2011). Accordingly, extrapolation of results from the present review to current clinical practice is of limited value.
Quality of the evidence
Adequate internal validity depends on the control of bias and random errors. Because three trials had unclear randomisation (Michel 1980; Morgan 1980; Koshy 1982) and consequently an unclear control of selection bias, the internal validity of their results and of the results of our meta‐analyses can be questioned. The use of a cross‐over design as applied in two of the included trials (Uribe 1979; Morgan 1980) is also debatable. Even chronic hepatic encephalopathy may have a fluctuating course (Basile 1991); therefore, manifestations of hepatic encephalopathy may change during the course of the trial, irrespective of the interventions assessed. The underlying condition and the ability to respond to treatment may not remain stable from the first to the second treatment period. We therefore used only data from the first study period of the cross‐over trials in our primary analyses. Unfortunately, these data were available for only one trial (Morgan 1980). The sensitivity analysis on paired data did not change our overall result.
Potential biases in the review process
Identification and selection of trials are essential to the assessment of bias in the review process. To limit bias in the selection process, we included trials irrespective of language or publication status. We also chose to include trials regardless of the dose or duration of the interventions assessed. This led to a relatively heterogeneous group of trials. We did, however, choose to exclude trials with an active comparison group. This choice was made on the basis of lack of evidence supporting several of the interventions assessed for patients with hepatic encephalopathy. The strategy resulted in the exclusion of two small, low‐quality, cross‐over trials on chronic hepatic encephalopathy (Messner 1982; Uribe 1983). The control groups in these trials received lactulose or neomycin, which could affect the course of hepatic encephalopathy. The total number of participants randomly assigned in these two trials was only 15, and this limits the value of these results.
Agreements and disagreements with other studies or reviews
At present, dopamine agents are not recommended for patients with hepatic encephalopathy. Previous guidelines state that bromocriptine may be considered for patients with chronic hepatic encephalopathy that is unresponsive to other interventions (Blei 1999). In agreement with more recent recommendations (Phongsamran 2010), the present review contradicts these recommendations, suggesting that no evidence is available to support the use of dopamine agents for chronic hepatic encephalopathy.

Figure 2. Risk of bias graph: review authors' judgements about all risk of bias items presented as percentages across all included studies.
Trial sequential analysis of dopamine agents versus placebo or no intervention in participants with hepatic encephalopathy.
The outcome measure is mortality. The analysis was performed with an event rate of 54% (Pc) in the control group, a risk ratio (RR) reduction of 20%, alpha 5%, beta 20%, and diversity 0%. The cumulative Z‐curve does not cross the naive 5% statistical boundaries (dotted horizontal lines) or the trial sequential boundaries for benefits or harms (inward sloping etched lines). The results show that the diversity‐adjusted required information size was 673 participants, corresponding to 21% of the total sample size in the included trials. The programme did not even draw futility boundaries. Accordingly, the meta‐analysis does not recommend or refute an intervention effect; data are simply too few.

Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 1 Mortality.

Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 2 Mortality stratified by type of hepatic encephalopathy.
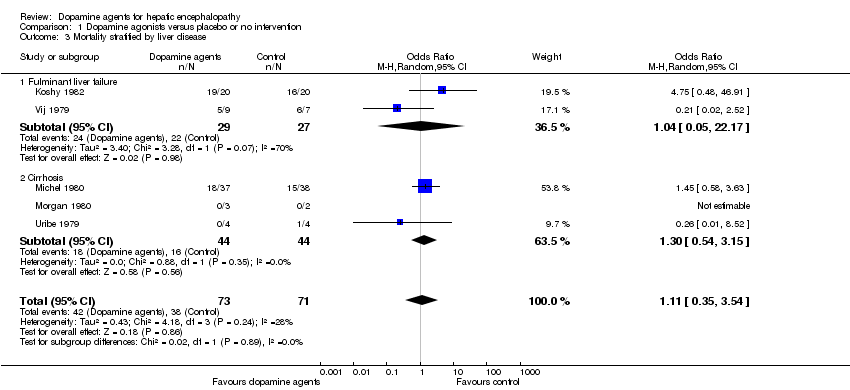
Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 3 Mortality stratified by liver disease.

Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 4 Mortality stratified by intervention.

Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 5 Mortality stratified by bias risk.
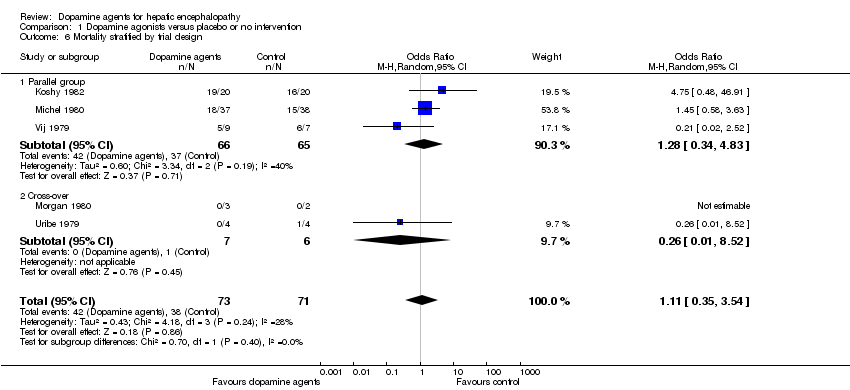
Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 6 Mortality stratified by trial design.

Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 7 Hepatic encephalopathy.

Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 8 Hepatic encephalopathy paired data.

Comparison 1 Dopamine agonists versus placebo or no intervention, Outcome 9 Adverse events paired data.
| Dopamine agonists versus placebo or no intervention for hepatic encephalopathy | ||||||
| Patient or population: patients with hepatic encephalopathy. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (placebo or no intervention) | Dopamine agonists | |||||
| Mortality | Study population | OR 1.11 | 144 | ⊕⊕⊝⊝ | ||
| 535 per 1000 | 561 per 1000 | |||||
| Moderate | ||||||
| 395 per 1000 | 420 per 1000 | |||||
| Hepatic encephalopathy | Study population | OR 2.99 | 80 | ⊕⊕⊝⊝ | ||
| 350 per 1000 | 617 per 1000 | |||||
| Moderate | ||||||
| 184 per 1000 | 403 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1The randomisation methods were classed as adequate in two trials, and three trials were double‐blind. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 5 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.35, 3.54] |
| 2 Mortality stratified by type of hepatic encephalopathy Show forest plot | 5 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.35, 3.54] |
| 2.1 Acute episode of hepatic encephalopathy | 3 | 131 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.34, 4.83] |
| 2.2 Chronic hepatic encephalopathy | 2 | 13 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 8.52] |
| 3 Mortality stratified by liver disease Show forest plot | 5 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.35, 3.54] |
| 3.1 Fulminant liver failure | 2 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.05, 22.17] |
| 3.2 Cirrhosis | 3 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.54, 3.15] |
| 4 Mortality stratified by intervention Show forest plot | 5 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.35, 3.54] |
| 4.1 Levodopa | 3 | 131 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.34, 4.83] |
| 4.2 Bromocriptine | 2 | 13 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 8.52] |
| 5 Mortality stratified by bias risk Show forest plot | 5 | 144 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.59, 2.59] |
| 5.1 Low bias risk | 2 | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.01, 8.52] |
| 5.2 Unclear bias risk | 3 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.63, 2.91] |
| 6 Mortality stratified by trial design Show forest plot | 5 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.35, 3.54] |
| 6.1 Parallel group | 3 | 131 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.34, 4.83] |
| 6.2 Cross‐over | 2 | 13 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 8.52] |
| 7 Hepatic encephalopathy Show forest plot | 2 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 2.99 [0.09, 100.55] |
| 8 Hepatic encephalopathy paired data Show forest plot | 3 | Odds Ratio (Random, 95% CI) | 0.68 [0.17, 2.67] | |
| 9 Adverse events paired data Show forest plot | 2 | OR (Random, 95% CI) | 2.12 [‐0.99, 5.24] | |



