Etosuccimida, valproato de sodio o lamotrigina para las crisis de ausencia en niños y adolescentes
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003032.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Epilepsia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Data were extracted by Francesco Brigo and Stanley C. Igwe. Analyses were undertaken by Francesco Brigo. Text of the final review was written by Francesco Brigo and Stanley C. Igwe.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
None known.
Acknowledgements
We wish to acknowledge the hard and valuable work that went in to the original version of the review by Ewa Posner, Khalid Mohamed and Tony Marson.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Jan 21 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Francesco Brigo, Stanley C Igwe, Simona Lattanzi | |
| 2019 Feb 08 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Francesco Brigo, Stanley C Igwe, Simona Lattanzi | |
| 2017 Feb 14 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Francesco Brigo, Stanley C Igwe | |
| 2005 Oct 19 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Ewa B Posner, Khalid K Mohamed, Anthony G Marson | |
| 2003 Jul 21 | Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents | Review | Ewa B Posner, Khalid K Mohamed, A G Marson, Tony G Marson | |
Differences between protocol and review
Compared to the protocol originally describing the methods for the review, when updating the review we performed a more comprehensive assessment of bias, focusing on the following methodological issues and risk of bias: random sequence generation (selection bias); allocation concealment (selection bias); blinding (performance bias and detection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); and selective reporting (reporting bias).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Humans;
PICO
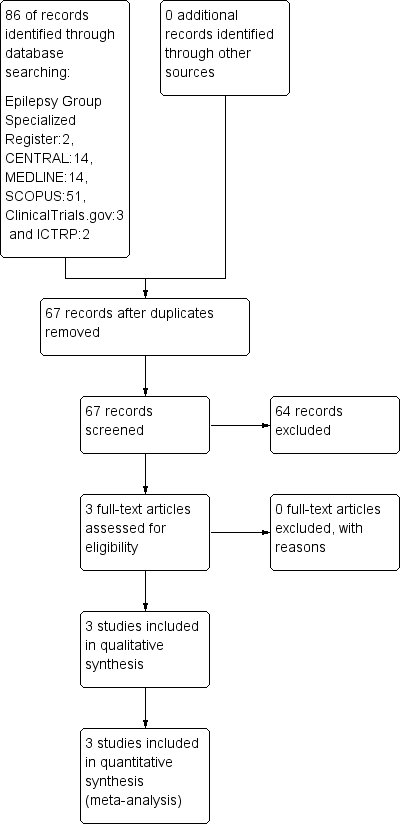
Study flow diagram (results refer only to the updated version of the review).
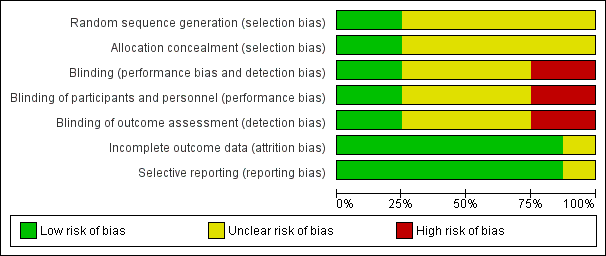
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
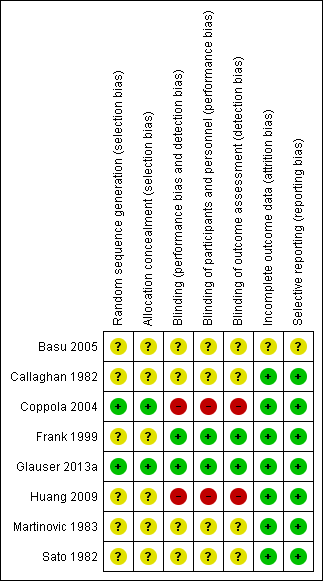
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
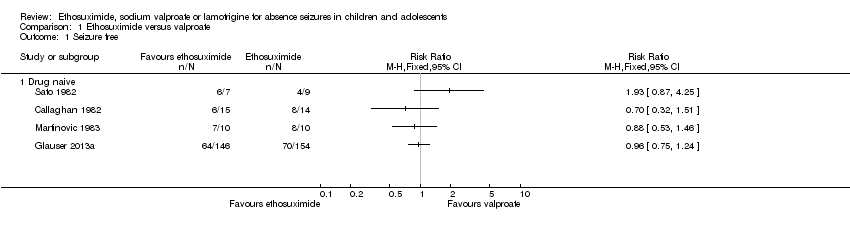
Comparison 1 Ethosuximide versus valproate, Outcome 1 Seizure free.
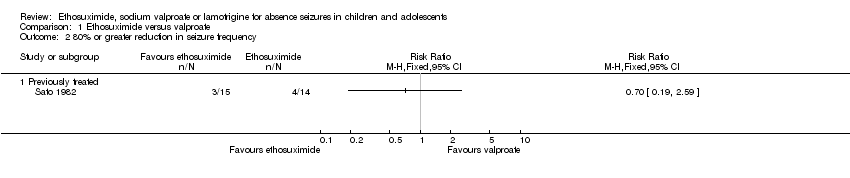
Comparison 1 Ethosuximide versus valproate, Outcome 2 80% or greater reduction in seizure frequency.

Comparison 1 Ethosuximide versus valproate, Outcome 3 50% or greater reduction in seizure frequency.

Comparison 2 Lamotrigine versus valproate, Outcome 1 Seizure free.
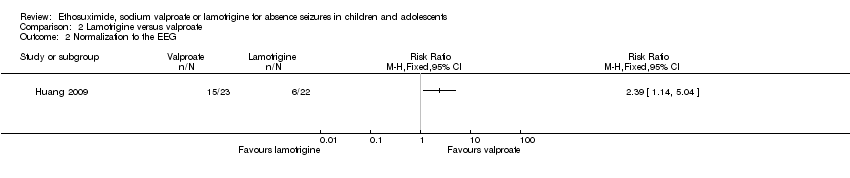
Comparison 2 Lamotrigine versus valproate, Outcome 2 Normalization fo the EEG.
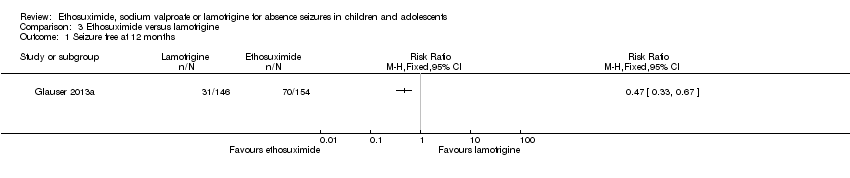
Comparison 3 Ethosuximide versus lamotrigine, Outcome 1 Seizure free at 12 months.
| Event | Callaghan 1982 | Sato 1982 | Martinovic 1983 | Coppola 2004 | Huang 2009 | Glauser 2013 |
| Acute pancreatitis | 1 | |||||
| Obesity/Weight gain | 1 | 1 | 14 | |||
| Drowsiness | 4 | |||||
| Nausea | 5 | 3 | 12* | |||
| Vomiting | 1 | 2 | 12* | |||
| Decreased platelet numbers | 2 | 4 | ||||
| Increased appetite | 15 | |||||
| Poor appetite | 1 | 8 | ||||
| Diarrhoea | 1 | 7 | ||||
| Dizziness | 1 | 2 | ||||
| Hyperactivity | 23 | |||||
| Attention problems | 24 | |||||
| Hostility | 22 | |||||
| Concentration decreased | 18 | |||||
| Personality change | 17 | |||||
| Sleep problem | 17 | |||||
| Depression | 11 | |||||
| Slow process speed | 11 | |||||
| Memory problem | 10 | |||||
| Apathy | 9 | |||||
| Fatigue | 27 | |||||
| Headache | 1 | 18 | ||||
| Leukopenia | 2 | |||||
| Elevated liver function tests | 1 | 7 | ||||
| Elevated LDH | 1 | |||||
| Rash | 2 | |||||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking valproate: 14 (Callaghan 1982), 22 (Sato 1982), 10 (Martinovic 1983), 19 (Coppola 2004), 23 (Huang 2009), 146 (Glauser 2013a). | ||||||
| Event | Callaghan 1982 | Sato 1982 | Martinovic 1983 | Glauser 2013 |
| Drowsiness | 1 | 5 | ||
| Tiredness | 2 | |||
| Nausea | 3 | 2 | 29* | |
| Vomiting | 3 | 29* | ||
| Increased appetite | 6 | |||
| Poor appetite | 1 | 10 | ||
| Diarrhoea | 9 | |||
| Dizziness | 1 | 10 | ||
| Headache | 2 | 23 | ||
| Leukopenia | 3 | |||
| Hiccups | 1 | |||
| Moodiness | 1 | |||
| Hyperactivity | 13 | |||
| Attention problems | 8 | |||
| Hostility | 4 | |||
| Concentration decreased | 6 | |||
| Personality change | 6 | |||
| Sleep problem | 11 | |||
| Depression | 4 | |||
| Slow process speed | 3 | |||
| Memory problem | 0 | |||
| Apathy | 4 | |||
| Fatigue | 26 | |||
| Rash | 6 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking ethosuximide: 14 (Callaghan 1982), 23 (Sato 1982), 10 (Martinovic 1983), 154 (Glauser 2013a). | ||||
| Event | Frank 1999 | Coppola 2004 | Huang 2009 | Glauser 2013 |
| Abdominal pain | 5 | |||
| Headache | 2 | 2 | 14 | |
| Nausea | 3 | 2* | ||
| Vomiting | 2* | |||
| Poor appetite | 2 | 9 | ||
| Increased appetite | 1 | 10 | ||
| Diarrhoea | 2 | |||
| Dizziness | 3 | 5 | 5 | |
| Hyperkinesia | 2 | |||
| Hyperactivity | 12 | |||
| Attention problems | 11 | |||
| Hostility | 11 | |||
| Concentration decreased | 9 | |||
| Personality change | 10 | |||
| Sleep problem | 5 | |||
| Depression | 11 | |||
| Slow process speed | 7 | |||
| Memory problem | 8 | |||
| Apathy | 3 | |||
| Fatigue | 1 | 18 | ||
| Rash | 10 | 1 | 2 | 6 |
| Nervousness | 1 | |||
| Diplopia | 1 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking lamotrigine: 15 (Frank 1999), 19 (Coppola 2004), 24 (Huang 2009), 146 (Glauser 2013a). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Drug naive | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 80% or greater reduction in seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Previously treated | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 50% or greater reduction in seizure frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Seizure free at 1 month | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [2.77, 25.59] | |
| 1.2 Seizure free at 3 months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.16, 2.31] | |
| 1.3 Seizure freedom at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.88, 2.28] | |
| 1.4 Seizure free at 12 months | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.32, 2.11] | |
| 2 Normalization fo the EEG Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

