Je li bolje hranjenje formulom ili doniranim majčinim mlijekom za nedonoščad i djecu rođenu s niskom porođajnom težinom
Abstract
Background
When sufficient maternal breast milk is not available, alternative sources of enteral nutrition for preterm or low birth weight infants are donor breast milk or artificial formula. Donor breast milk may retain some of the non‐nutritive benefits of maternal breast milk for preterm or low birth weight infants. However, feeding with artificial formula may ensure more consistent delivery of optimal levels of nutrients. Uncertainty exists about the balance of risks and benefits of feeding formula versus donor breast milk for preterm or low birth weight infants.
Objectives
To determine the effect of feeding with formula compared with donor breast milk on growth and development in preterm or low birth weight infants.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 3), MEDLINE (1966 to March 2014), EMBASE (1980 to March 2014), CINAHL (1982 to March 2014), conference proceedings and previous reviews.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing feeding with formula versus donor breast milk in preterm or low birth weight infants.
Data collection and analysis
We extracted data using the standard methods of the Cochrane Neonatal Group, with separate evaluation of trial quality and data extraction by two review authors.
Main results
Nine trials, in which 1070 infants participated, fulfilled the inclusion criteria. Four trials compared standard term formula versus donor breast milk and five compared nutrient‐enriched preterm formula versus donor breast milk. Only the two most recent trials used nutrient‐fortified donor breast milk. The trials contain various methodological quality weaknesses, specifically uncertainty about adequate allocation concealment methods in three trials and lack of blinding in most of the trials.
Formula‐fed infants had higher in hospital rates of increase in weight [mean difference (MD): 2.58 (95% confidence interval (CI) 1.98 to 3.71) g/kg/day], length [MD 1.93 (95% CI 1.23 to 2.62) mm/week] and head circumference [MD 1.59 (95% CI 0.95 to 2.24) mm/week]. We did not find evidence of an effect on post‐discharge growth rates or neurodevelopmental outcomes. Formula feeding increased the risk of necrotising enterocolitis: typical risk ratio 2.77 (95% CI 1.40 to 5.46); risk difference 0.04 (95% CI 0.02 to 0.07).
Authors' conclusions
In preterm and low birth weight infants, feeding with formula compared with donor breast milk results in a higher rate of short‐term growth but also a higher risk of developing necrotising enterocolitis. Limited data on the comparison of feeding with formula versus nutrient‐fortified donor breast milk are available. This limits the applicability of the findings of this review as nutrient fortification of breast milk is now a common practice in neonatal care. Future trials may compare growth, development and adverse outcomes in infants who receive formula milk versus nutrient‐fortified donor breast milk given as a supplement to maternal expressed breast milk or as a sole diet.
PICO
Laički sažetak
Je li bolje hranjenje formulom ili doniranim majčinim mlijekom za nedonoščad i djecu rođenu s niskom porođajnom težinom
Kad majčino mlijeko nije dostupno za hranjenje nedonoščeta ili djeteta niske porođajne težine, preostaje hranjenje formulom ili izdojenim mlijekom druge majke koja donira svoje mlijeko (donirano majčino mlijeko). Ovaj Cochrane sustavni pregled uključio je 9 randomiziranih kontroliranih istraživanja čiji rezultati pokazuju da hranjenje formulom povećava kratkoročnu stopu rasta, ali je povezano s većim rizikom od razvoja teškog poremećaja crijeva koji se naziva "nekrotizirajući enterokolitis". Nema dokaza o dugoročnom učinku na rast i razvoj djeteta. Potrebna su daljnja istraživanja koja će usporediti ta dva načina hranjenja. Trebalo bi usporediti formulu koja je prilagođena za hranjenje nedonoščadi s doniranim majčinim mlijekom koje sadrži dodane hranjive tvari.
Authors' conclusions
Background
Maternal breast milk is the recommended form of enteral nutrition for preterm or low birth weight (LBW) infants. Breast milk contains non‐nutrient factors including immunoglobulins that may promote intestinal adaptation and maturation, improve enteral feed tolerance, and protect against infective and inflammatory disorders (Agostoni 2010).
When sufficient maternal breast milk is not available, the two common alternatives available for feeding preterm or LBW infants are artificial formula and donor breast milk (donated by other lactating women). These may be given either as the sole form of enteral feeding or as a supplement to maternal breast milk.
Description of the condition
Providing appropriate nutrition for preterm or LBW infants is a critical component of neonatal care. Early enteral nutrition, particularly the use of donor breast milk or formula, may have a substantial impact on clinically important outcomes such as necrotising enterocolitis, invasive infection and short‐term growth. These infectious and inflammatory complications may increase the risk of mortality and other morbidities and adversely affect long‐term growth and neurodevelopmental outcomes.
Description of the intervention
A variety of artificial formulas (usually adapted from cow's milk) are available. These vary in energy, protein and mineral content but can, broadly, be considered as:
-
standard 'term' formula, designed for term infants based on the composition of mature breast milk: the typical energy content is between about 67 to 70 kcal/100 ml;
-
nutrient‐enriched 'preterm' formula, designed to provide nutrient intakes to match intrauterine accretion rates (Tsang 1993): these are energy‐enriched (typically up to about 80 kcal/100 ml) and variably protein‐ and mineral‐enriched (Fewtrell 1999).
The comparison arm for the intervention is donor breast milk. Expressed breast milk from donor mothers, usually mothers who have delivered at term, generally has a lower content of energy and protein than term formula milk (Gross 1980; Gross 1981). The nutrient content of donor breast milk may be further compromised by pasteurisation (Wight 2001). Donor human milk also varies with regard to fat, energy and protein content depending upon the stage of lactation at which it is collected. Milk expressed from the donor's lactating breast usually has a higher energy and protein content than that collected from the contralateral breast ('drip' breast milk) (Lucas 1978).
How the intervention might work
There is concern that the nutritional requirements of preterm or LBW infants, who are born with relatively impoverished nutrient reserves and are subject to additional metabolic stresses compared with term infants, may not be fully met by enteral feeding with donor breast milk (Hay 1994; Schanler 1995). These deficiencies may have adverse consequences for growth and development. However, a major putative benefit of donor breast milk is that the delivery of immuno‐protective and growth factors to the immature gut mucosa may prevent serious adverse outcomes, including necrotising enterocolitis and invasive infection (Beeby 1992; Lucas 1990).
Why it is important to do this review
Given the potential for the type of enteral nutrition to affect important outcomes for preterm or LBW infants, and since uncertainty exists about the balance between the putative benefits and harms, an attempt to detect, appraise and synthesise evidence from randomised controlled trials is merited.
Objectives
To determine the effect of feeding with formula compared with donor breast milk on growth and development in preterm or LBW infants.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials utilising either random or quasi‐random participant allocation.
Types of participants
Preterm (less than 37 weeks' gestation) or low birth weight (less than 2.5 kg) infants.
Types of interventions
Enteral feeding with formula versus donor breast milk. The allocated milk feed may have been a supplement to maternal breast milk or have formed the entire enteral intake (sole diet).
Trials in which parenteral nutritional support was available during the period of advancement of enteral feeds were acceptable provided that the groups received similar treatment other than the type of milk feed.
Types of outcome measures
Primary outcomes
Growth
-
Time to regain birth weight and subsequent rates of weight gain, linear growth, head growth or skinfold thickness growth up to six months post‐term.
-
Long‐term growth: weight, height or head circumference (and/or proportion of infants who remain below the 10th percentile for the index population's distribution) assessed at intervals from six months post‐term.
Neurodevelopment
-
Death or severe neurodevelopmental disability defined as any one or combination of the following: non‐ambulant cerebral palsy, developmental delay (developmental quotient less than 70), auditory and visual impairment. We analysed each component individually as well as part of the composite outcome.
-
Neurodevelopmental scores in survivors aged greater than, or equal to, 12 months of age measured using validated assessment tools.
-
Cognitive and educational outcomes in survivors aged more than five years old.
Secondary outcomes
-
All‐cause mortality during the neonatal period and prior to hospital discharge.
-
Necrotising enterocolitis confirmed at surgery or autopsy or diagnosed by at least two of the following clinical features:
-
abdominal radiograph showing pneumatosis intestinalis or gas in the portal venous system or free air in the abdomen;
-
abdominal distension with abdominal radiograph with gaseous distension or frothy appearance of bowel lumen (or both);
-
blood in stool;
-
lethargy, hypotonia or apnoea (or combination of these).
-
-
Days after birth to establish full enteral feeding (independently of parenteral nutrition).
-
Feeding intolerance defined as a requirement to cease enteral feeds and commence parenteral nutrition.
-
Incidence of invasive infection as determined by culture of bacteria or fungus from blood, cerebrospinal fluid, urine or from a normally sterile body space.
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Group (http://neonatal.cochrane.org/).
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 3), MEDLINE (1966 to March 2014), EMBASE (1980 to March 2014) and CINAHL (1982 to March 2014) using a combination of the following text words and MeSH terms: [Infant, Newborn OR Infant, Premature OR Infant, Low Birth Weight OR Infant, Very Low Birth Weight/ OR infan* OR neonat* OR preterm OR prem*] AND "Infant‐Nutrition"/ all subheadings OR Infant Formula OR milk OR formula]. The search outputs were limited with the filters for clinical trials recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not apply any language restrictions.
We searched ClinicalTrials.gov for completed or ongoing trials.
Searching other resources
We examined the references in all studies identified as potentially relevant.
We searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993 to 2013), the European Society for Pediatric Research (1995 to 2013), the UK Royal College of Paediatrics and Child Health (2000 to 2014) and the Perinatal Society of Australia and New Zealand (2000 to 2013). Trials reported only as abstracts were eligible if sufficient information was available from the report, or from contact with the authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Group.
Selection of studies
Two review authors screened the title and abstract of all studies identified by the above search strategy. We assessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed any disagreements until consensus was achieved.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Two review authors extracted the data separately. We discussed any disagreements until consensus was achieved. We contacted the investigators for further information if data from the trial reports were insufficient.
Assessment of risk of bias in included studies
We used the criteria and standard methods of the Cochrane Neonatal Group to assess the methodological quality of any included trials. We requested additional information from the trial authors to clarify methodology and results as necessary. We evaluated and reported the following issues in the 'Risk of bias' tables:
1. Sequence generation: We categorised the method used to generate the allocation sequence as:
-
low risk: any random process e.g. random number table; computer random number generator;
-
high risk: any non random process e.g. odd or even date of birth; patient case‐record number;
-
unclear.
2. Allocation concealment: We categorised the method used to conceal the allocation sequence as:
-
low risk: e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes;
-
high risk: open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth;
-
unclear.
3. Blinding: We assessed blinding of participants, clinicians and caregivers, and outcome assessors separately for different outcomes and categorised the methods as:
-
low risk;
-
high risk;
-
unclear.
4. Incomplete outcome data: We described the completeness of data including attrition and exclusions from the analysis for each outcome and any reasons for attrition or exclusion where reported. We assessed whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised completeness as:
-
low risk: less than 20% missing data;
-
high risk: 20% or more missing data;
-
unclear.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CI). When it was deemed appropriate to combine two or more study arms, we obtained the treatment effects from the combined data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We determined the number needed to treat to benefit (NNTB) or harm (NNTH) for a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials and the neonatal unit for cluster‐randomised trials.
Dealing with missing data
We requested missing data from trial investigators where possible.
Assessment of heterogeneity
If more than one trial was included in a meta‐analysis, we examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and describe the variability in effect estimates that may be due to heterogeneity rather than sampling error. If substantial (I² > 50%) heterogeneity was detected, we explored the possible causes (for example, differences in study quality, participants, intervention regimens or outcome assessments) in sensitivity and subgroup analyses.
Assessment of reporting biases
If more than five trials were included in a meta‐analysis, we inspected a funnel plot for asymmetry.
Data synthesis
We used fixed‐effect models for meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses of trials to compare:
-
formula versus donor breast milk given as (i) a sole diet or (ii) a supplement to maternal expressed breast milk;
-
formula versus donor breast milk that is (i) unfortified or (ii) nutrient‐fortified (defined as supplementation with more than one of the following components: protein, fat, carbohydrate or minerals).
Results
Description of studies
Results of the search
We identified 16 reports of studies for full‐text screening. We included nine trials and excluded seven studies.
We found three on‐going trials (Characteristics of ongoing studies).
Included studies
Nine trials fulfilled the review eligibility criteria (Raiha 1976; Davies 1977; Schultz 1980; Gross 1983; Tyson 1983; Lucas 1984a; Lucas 1984b; Schanler 2005; Cristofalo 2013). Most of these trials were undertaken during the late 1970s and early 1980s by investigators attached to neonatal units in Europe and North America. Two trials have been undertaken since the year 2000 (Schanler 2005; Cristofalo 2013). For further details see Characteristics of included studies.
Participants
In total, 1070 infants participated in the included trials. Most participants were clinically stable preterm infants of gestational age less than 32 weeks or birth weight less than 1800 g. Most of the trials specifically excluded infants who were small for gestational age at birth and infants with congenital anomalies, or gastrointestinal or neurological problems.
Interventions
The trials varied according to type of formula, whether donor breast milk feeds were fortified and whether the intervention was a sole diet or a supplement to mother's own milk:
-
Four trials compared feeding with term formula milk versus donor breast milk (Davies 1977; Gross 1983; Raiha 1976; Schultz 1980). In all of these trials term formula or donor breast milk was the sole diet.
-
Five trials compared feeding with preterm formula milk versus donor breast milk (Lucas 1984a; Lucas 1984b; Schanler 2005; Tyson 1983; Cristofalo 2013). In three of these trials preterm formula milk or donor breast milk was the sole diet (Lucas 1984a; Tyson 1983; Cristofalo 2013). In the other two trials preterm formula milk or donor breast milk was given as a supplement to maternal breast milk (Lucas 1984b; Schanler 2005).
Five trials used donor breast milk collected from mothers who had delivered an infant at term (Davies 1977; Lucas 1984a; Lucas 1984b; Raiha 1976; Schultz 1980). Two of these trials used 'drip' breast milk (Lucas 1984a; Lucas 1984b). One trial used preterm donor breast milk (Schanler 2005), one trial used both term and preterm milk (Gross 1983) and two trials did not specify the type of donor breast milk (Tyson 1983; Cristofalo 2013). In all trials except Tyson 1983, the donor breast milk was pasteurised.
Only the two more recent trials used nutrient‐fortified donor breast milk (Schanler 2005;Cristofalo 2013).
In general, feeds were allocated for several weeks, or until participating infants reached a specified weight (generally over 2 kg).
Outcomes
The most commonly reported outcomes were growth parameters during the study period or until hospital discharge. Most reports also gave information on adverse outcomes, including feeding intolerance and the incidence of necrotising enterocolitis. Only two trials reported long‐term growth and neurodevelopmental outcomes for surviving infants (Lucas 1984a; Lucas 1984b).
Excluded studies
We excluded seven studies (Cooper 1984; Jarvenpaa 1983; Narayanan 1982; O'Connor 2003; Putet 1984; Sullivan 2010; Svenningsen 1982). The reasons for exclusion are described in the table Characteristics of excluded studies.
Risk of bias in included studies
Quality assessments are detailed in the table Characteristics of included studies ans summarised in Figure 1.
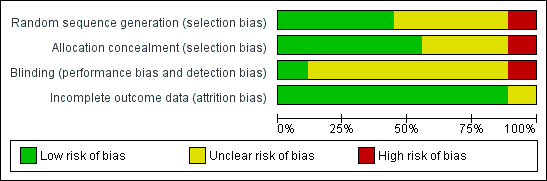
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three trials reported adequate allocation concealment methods (sealed, numbered envelopes; central randomisation in blocks) (Lucas 1984a; Lucas 1984b; Tyson 1983). Five trials did not report details of allocation concealment. One trial randomly allocated participants to one of the four formula arms, but allocated every fifth infant to the donor breast milk arm (Raiha 1976).
Blinding
Two trials blinded the staff or caregivers to the feeding arms (Schanler 2005; Cristofalo 2013). Three trials did not blind the staff (Lucas 1984a; Lucas 1984b; Tyson 1983). Four trials did not report whether staff were blinded.
Most of the trials did not specify whether the outcome assessors were blind to the feeding arms. In two trials staff were blind to the post‐discharge outcomes (Lucas 1984a; Lucas 1984b).
Incomplete outcome data
Six trials reported 100% follow‐up for the short‐term outcomes. In the other three trials infants who developed complications (5% to 10% of the total enrolled) were withdrawn from the study and therefore the short‐term growth data for these infants were not presented (Gross 1983; Raiha 1976; Tyson 1983). In the two trials which assessed long‐term outcomes more than 80% of participants were assessed (Lucas 1984a; Lucas 1984b).
Selective reporting
Some of the outcomes in this review were reported as adverse outcomes in some of the studies rather than as a predefined outcome, but there is no evidence of selective reporting.
Effects of interventions
Growth
See Analysis 1.1 to Analysis 1.15.
Time to regain birth weight
Meta‐analysis of data from Gross 1983 and Raiha 1976 found that the formula‐fed group regained birth weight more quickly: mean difference (MD) ‐4.0 days (95% confidence interval (CI) ‐5.8 to ‐2.2) (Analysis 1.1; Figure 2).

Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.1 Time to regain birth weight (days from birth).
Schultz 1980 did not detect a statistically significant difference, but standard deviations were not reported and the data could not be included in the meta‐analysis.
Lucas 1984a reported the median time to regain birth weight as statistically significantly lower in the formula‐fed infants (10 versus 16 days). Lucas 1984b did not find a statistically significant difference (13 versus 15 days). Standard deviations were not reported and these data were not included in a meta‐analysis.
Weight gain
Formula‐fed infants had a statistically significant higher rate of weight gain but with substantial heterogeneity in the size of this effect [MD 2.58 (95% CI 1.98 to 3.17) g/kg/day, I² = 91%, 8 trials, 702 participants (Analysis 1.2; Figure 3)]. This effect existed in separate comparisons of feeding with term formula versus donor breast milk [MD 1.74 (95% CI 0.96 to 2.53) g/kg/day] and preterm formula versus donor breast milk [MD 3.71 (95% CI 2.79 to 4.63) g/kg/day].
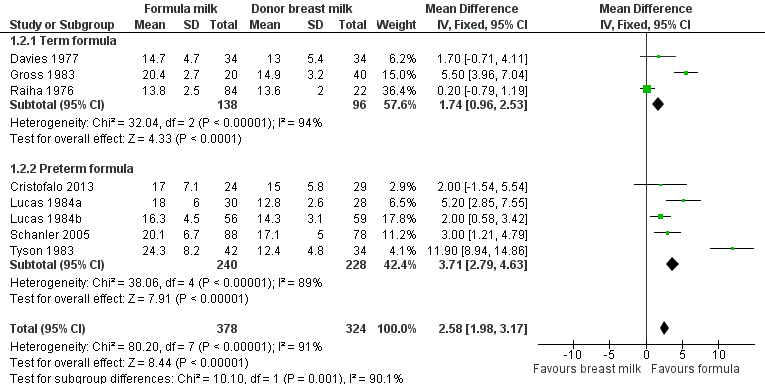
Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.2 Short‐term weight change (g/kg/day).
Linear growth
Formula‐fed infants had a statistically significant higher rate of increase in crown‐heel length but with substantial heterogeneity in the size of this effect [MD 1.36 (95% CI 0.87 to 1.85) mm/week, I² = 70%, 7 trials, 492 participants (Analysis 1.3; Figure 4)]. This effect existed in separate comparisons of feeding with term formula versus donor breast milk [MD 0.80 (95% CI 0.10 to 1.50) mm/week] and preterm formula versus donor breast milk [MD 1.93 (95% CI 1.23 to 2.62) mm/week].
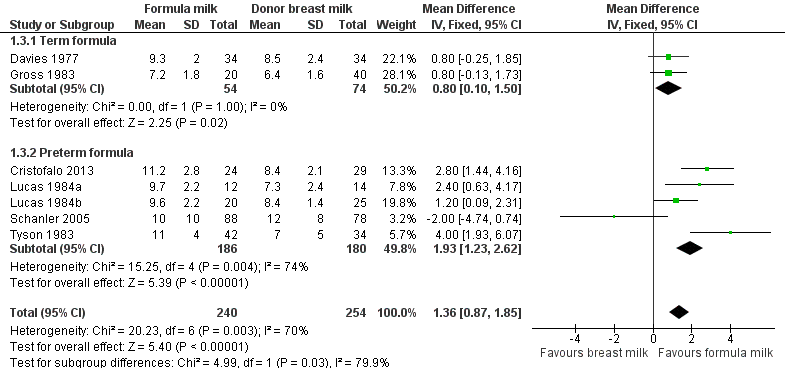
Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.3 Short‐term change in crown‐heel length (mm/week).
Head growth
Formula‐fed infants had a statistically significant higher rate of increase in occipito‐frontal head circumference but with substantial heterogeneity in the size of this effect [MD 1.21 (95% CI 0.75 to 1.67) mm/week, I² = 69%, 7 trials, 568 participants (Analysis 1.6; Figure 5)]. This effect existed in separate comparisons of feeding with term formula versus donor breast milk [MD 0.81 (95% CI 0.15 to 1.47) mm/week] and preterm formula versus donor breast milk [MD 1.59 (95% CI 0.95 to 2.24) mm/week ].
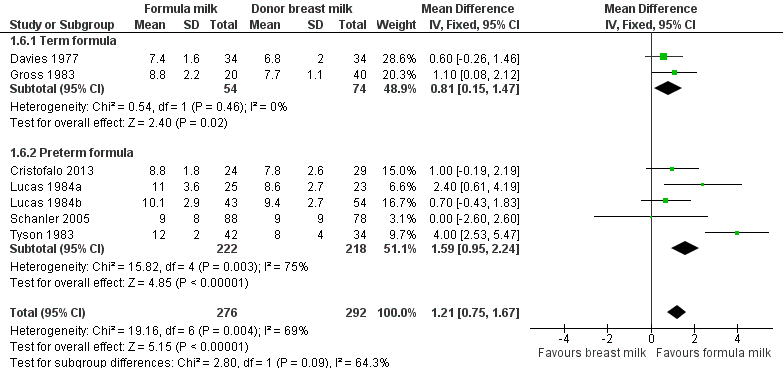
Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.6 Short‐term change in head circumference (mm/week).
Long‐term growth
Post‐hospital discharge growth was reported by Lucas 1984a and Lucas 1984b. Neither individual study, nor meta‐analyses of data from both studies, found any statistically significant differences in the weight, length or head circumference at nine months, 18 months or 7.5 to eight years post‐term.
Neurodevelopment
See Analysis 1.16 to Analysis 1.18.
Neurodevelopmental outcomes were reported by two trials. Neither Lucas 1984a nor Lucas 1984b, nor a meta‐analysis of data from both, found statistically significant differences in Bayley Psychomotor and Mental Development Indices at 18 months corrected age:
-
Bayley Mental Development Index: MD 1.24 (95% CI ‐2.6 to 5.1).
-
Bayley Psychomotor Development Index: MD ‐0.3 (95% CI ‐3.8 to 3.9).
Gross 1983 stated that there was "no difference" in Bayley Mental or Psychomotor Developmental Indices at 15 months post‐term (study published as abstract only).
Severe neurodevelopmental disability (Amiel‐Tison 1986 classification) was assessed in two trials. Neither Lucas 1984a nor Lucas 1984b, nor a meta‐analysis of data from both trials, demonstrated a statistically significant difference in the incidence of neurological impairment at 18 months post‐term: typical RR 1.2 (95% CI 0.6 to 2.3); RD ‐0.02 (95% CI ‐0.04 to 0.17).
Lucas 1984a and Lucas 1984b assessed cognitive outcomes (verbal and performance intelligence quotient) in about 20% of participants at ages eight and 16 years. However, numerical data were not reported for the individual trials but rather were combined with data from another trial undertaken by the same investigators that compared feeding preterm infants with nutrient‐enriched versus standard formula (Isaacs 2009).
Secondary outcomes
All‐cause mortality
See Analysis 1.19.
Data were available from four trials. Two trials reported mortality until nine months post‐term (Lucas 1984a; Lucas 1984b). Two trials reported mortality until hospital discharge (Schanler 2005; Cristofalo 2013). None of the trials found a statistically significant difference in mortality between the feeding groups. Since it is likely that most infant mortality in this population occurred before hospital discharge, we combined the data from the trials in a meta‐analysis. This analysis did not demonstrate a statistically significant difference: typical RR 1.33 (95% CI 0.79 to 2.25); RD 0.02 (95% CI ‐0.02 to 0.06). There was no evidence of statistical heterogeneity (I² = 0%).
Necrotising enterocolitis
See Analysis 1.20.
Meta‐analysis of data from six trials (including both term and preterm formula) found a statistically significant higher incidence of necrotising enterocolitis in the formula‐fed group: typical RR 2.77 (95% CI 1.40 to 5.46); RD 0.04 (95% CI 0.02 to 0.07); number needed to treat to benefit (NNTB) 25 (95% CI 14 to 50). There was no statistically significant heterogeneity (I² = 0%) (Figure 6).

Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.20 Necrotising enterocolitis.
Days after birth to establish full enteral feeding
See Analysis 1.21
This was reported by only one of the included trials. Cristofalo 2013 did not detect a statistically significant difference: MD 4.70 (95% CI ‐2.56 to 11.96).
Feeding intolerance
See Analysis 1.22.
Meta‐analysis of data from Gross 1983 and Tyson 1983 found a statistically significant higher incidence of feeding intolerance in the formula‐fed group: typical RR 4.92 (95% CI 1.17 to 20.70); RD 0.10 (95% CI 0.01, 0.19); NNTH 10 (95% CI 5 to 100).
Lucas 1984a reported that significantly more infants in the formula‐fed group failed to tolerate full enteral feeds by two weeks after birth (25/76 versus 9/83 in the donor breast milk group) and by three weeks after birth (13/76 versus 4/83).
Incidence of invasive infection
Meta‐analysis of data from Schanler 2005 and Cristofalo 2013 did not find a statistically significant difference: RR 1.12 (95% CI 0.84 to 1.49).
Subgroup analysis: formula versus donor breast milk as (i) sole diet or (ii) supplement to maternal expressed breast milk
Seven trials compared feeding with preterm formula versus donor breast milk as a sole diet (Raiha 1976; Davies 1977; Schultz 1980; Gross 1983; Tyson 1983; Lucas 1984a; Cristofalo 2013).
Two trials compared feeding with preterm formula versus donor breast milk as a supplement to maternal expressed breast milk (Lucas 1984b; Schanler 2005).
Growth
Analysis 2.1 to Analysis 2.12.
Time to regain birth weight
Sole diet
Meta‐analysis of data from Gross 1983 and Raiha 1976 found that the formula‐fed group regained birth weight more quickly: mean difference (MD) ‐4.0 days (95% confidence interval (CI) ‐5.8 to ‐2.2) (Analysis 1.1; Figure 2).
Schultz 1980 did not detect a statistically significant difference, but standard deviations were not reported and the data could not be included in the meta‐analysis.
Lucas 1984a reported the median time to regain birth weight as statistically significantly lower in the formula‐fed infants (10 versus 16 days). Standard deviations were not reported.
Supplement to maternal expressed breast milk
Lucas 1984b did not find a statistically significant difference (13 versus 15 days). Standard deviations were not reported.
Weight gain
Sole diet
Meta‐analysis demonstrated a statistically significant higher rate of weight gain in the formula‐fed group: MD 2.65 (95% CI 1.94 to 3.36) g/kg/day (Analysis 2.1).
Supplement to maternal expressed breast milk
Meta‐analysis demonstrated a statistically significant higher rate of weight gain in the formula‐fed group: MD 2.39 (95% CI 1.28 to 3.50) g/kg/day (Analysis 2.1).
Linear growth
Sole diet
Meta‐analysis demonstrated a statistically significant higher rate of increase in crown‐heel length: MD 1.54 (95% CI 0.98 to 2.11) mm/week (Analysis 2.2).
Supplement to maternal expressed breast milk
Meta‐analysis did not detect a statistically significant difference: MD 0.75 (95% CI ‐0.28 to 1.78) mm/week (Analysis 2.2).
Head growth
Sole diet
Meta‐analysis demonstrated a statistically significant higher rate of increase in occipito‐frontal head circumference: MD 1.36 (95% CI 0.85 to 1.88) mm/week (Analysis 2.3).
Supplement to maternal expressed breast milk
Meta‐analysis did not detect a statistically significant difference: MD 0.59 (95% CI ‐0.44 to 1.62) mm/week (Analysis 2.3).
Long‐term growth
See Analysis 2.4 to Analysis 2.12.
Sole diet
Lucas 1984a did not find any statistically significant differences in the weight, length or head circumference at nine months, 18 months or 7.5 to eight years post‐term.
Supplement to maternal expressed breast milk
Lucas 1984b did not find any statistically significant differences in the weight, length or head circumference at nine months, 18 months or 7.5 to eight years post‐term.
Neurodevelopment
See Analysis 2.13 to Analysis 2.15.
Sole diet
Lucas 1984a did not find statistically significant differences in Bayley Psychomotor and Mental Development Indices or in the incidence of neurological impairment at 18 months post‐term. Numerical data for cognitive and educational outcomes were not reported.
Supplement to maternal expressed breast milk
Lucas 1984b did not find statistically significant differences in Bayley Psychomotor and Mental Development Indices or in the incidence of neurological impairment at 18 months post‐term. Numerical data for cognitive and educational outcomes were not reported.
Secondary outcomes
All‐cause mortality
See Analysis 2.16.
Sole diet
Meta‐analysis did not find a statistically significant difference [MD: 1.70 (95% CI 0.71 to 4.07)]
Supplement to maternal expressed breast milk
Meta‐analysis did not detect a statistically significant difference [MD: 1.16 (95% CI 0.60 to 2.24)].
Necrotising enterocolitis
See Analysis 2.17.
Sole diet
Meta‐analysis demonstrated a statistically significant higher incidence of necrotising enterocolitis in the formula‐fed group: typical RR 4.62, 95% CI 1.47 to 14.56 (Analysis 2.17).
Supplement to maternal expressed breast milk
Meta‐analysis did not detect a statistically significant difference (typical RR 1.96, 95% CI 0.82 to 4.67) (Analysis 2.17).
Feeding intolerance
See Analysis 2.18.
Sole diet
Meta‐analysis demonstrated a statistically significant higher incidence of feeding intolerance in the formula‐fed group: typical RR 4.92, 95% CI 1.17 to 20.70 (Analysis 2.18).
Supplement to maternal expressed breast milk
Feeding intolerance was not reported by Lucas 1984b or Schanler 2005.
Incidence of invasive infection
See Analysis 2.19.
Sole diet
Cristofalo 2013 did not detect a statistically significant difference: RR 3.09 (95% CI 0.90 to 10.53).
Supplement to maternal expressed breast milk
Schanler 2005 did not detect a statistically significant difference: RR 0.97 (95% CI 0.66 to 1.44).
Subgroup analysis: formula versus donor breast milk that is (i) unfortified or (ii) fortified
Seven trials compared feeding with preterm formula versus unfortified donor breast milk (Raiha 1976; Davies 1977; Schultz 1980; Gross 1983; Tyson 1983; Lucas 1984a; Lucas 1984b).
Two trials compared feeding with preterm formula versus donor breast milk with multi‐nutrient fortifier (Schanler 2005; Cristofalo 2013).
Growth
See Analysis 3.1 to Analysis 3.12.
Time to regain birth weight
Unfortified donor breast milk
Meta‐analysis of data from Gross 1983 and Raiha 1976 found that the formula‐fed group regained birth weight more quickly: mean difference (MD) ‐4.0 days (95% confidence interval (CI) ‐5.8 to ‐2.2) (Analysis 1.1; Figure 2).
Schultz 1980 did not detect a statistically significant difference, but standard deviations were not reported and the data could not be included in the meta‐analysis.
Lucas 1984a reported the median time to regain birth weight as statistically significantly lower in the formula‐fed infants (10 versus 16 days). Lucas 1984b did not find a statistically significant difference (13 versus 15 days). Standard deviations were not reported.
Fortified donor breast milk
Time to regain birth weight was not reported by Schanler 2005 or Cristofalo 2013.
Weight gain
Unfortified donor breast milk
Meta‐analysis demonstrated a statistically significant higher rate of weight gain in the formula‐fed group: MD 2.54 (95% CI 1.89 to 3.19) g/kg/day. There was statistically significant heterogeneity in this meta‐analysis (I² = 94%) (Analysis 3.1).
Fortified donor breast milk
Meta‐analysis of data from Schanler 2005 and Cristofalo 2013 found a statistically significant higher rate of weight gain in the formula‐fed group: MD 2.80 (95% CI 1.20 to 4.39) g/kg/day (Analysis 3.1).
Linear growth
Unfortified donor breast milk
Meta‐analysis demonstrated a statistically significant higher rate of increase in crown‐heel length: MD 1.26 (95% CI 0.72 to 1.80) mm/week. There was statistically significant heterogeneity in this meta‐analysis (I² = 60%) (Analysis 3.2).
Fortified donor breast milk
Meta‐analysis of data from Schanler 2005 and Cristofalo 2013 found a statistically significant higher rate of linear growth in the formula‐fed group:: MD 1.86 (95% CI 0.64 to 3.07) mm/week (Analysis 3.2).
Head growth
Unfortified donor breast milk
Meta‐analysis demonstrated a statistically significant higher rate of increase in occipitofrontal head circumference: MD 1.29 (95% CI 0.79 to 1.80) mm/week. There was statistically significant heterogeneity in this meta‐analysis (I² = 78%) (Analysis 3.3).
Fortified donor breast milk
Meta‐analysis did not detect a statistically significant difference: MD 0.83 (95% CI ‐0.25 to 1.91) mm/week (Analysis 3.3).
Long‐term growth
See Analysis 3.4 to Analysis 3.12.
Unfortified donor breast milk
Post‐hospital discharge growth was reported by Lucas 1984a and Lucas 1984b. Neither individual study, nor meta‐analyses of data from both studies, found any statistically significant differences in the weight, length or head circumference at nine months, 18 months or 7.5 to eight years post‐term.
Fortified donor breast milk
Long‐term growth was not reported by Schanler 2005 or Cristofalo 2013.
Neurodevelopment
See Analysis 3.13 to Analysis 3.15.
Unfortified donor breast milk
Neither Lucas 1984a nor Lucas 1984b, nor a meta‐analysis of data from both, found statistically significant differences in Bayley Psychomotor and Mental Development Indices at 18 months corrected age. Numerical data for cognitive and educational outcomes were not reported.
Supplement to maternal expressed breast milk
Neurodevelopmental outcomes were not reported by Schanler 2005 or Cristofalo 2013.
Secondary outcomes
All‐cause mortality
See Analysis 3.16.
Unfortified donor breast milk
Meta‐analysis did not detect a statistically significant difference in mortality (typical RR 1.29, 95% CI 0.73 to 2.29) (Analysis 3.16).
Fortified donor breast milk
Meta‐analysis did not detect a statistically significant difference (RR 1.53, 95% CI 0.42 to 5.51) (Analysis 3.16).
Necrotising enterocolitis
See Analysis 3.17.
Unfortified donor breast milk
Meta‐analysis demonstrated a statistically significant difference (typical RR 3.30, 95% CI 1.16 to 9.41) (Analysis 3.17).
Fortified donor breast milk
Meta‐analysis of data from Schanler 2005 and Cristofalo 2013 did not detect a statistically significant difference (typical RR 2.40, 95% CI 0.98 to 5.87) (Analysis 3.17).
Feeding intolerance
See Analysis 3.18.
Unfortified donor breast milk
Meta‐analysis detected a statistically significant higher rate in the formula‐fed group (RR 4.92, 95% CI 1.17 to 20.70) (Analysis 3.18).
Fortified donor breast milk
Feeding intolerance was not reported by either trial.
Incidence of invasive infection
See Analysis 3.19.
Unfortified donor breast milk
Incidence of invasive infection was not reported by any of the trials.
Fortified donor breast milk
Meta‐analysis of data from Schanler 2005 and Cristofalo 2013 did not detect a statistically significant difference (RR 1.12, 95% CI 0.84 to 1.49) (Analysis 3.19).
Discussion
Summary of main results
We found nine randomised controlled trials in which 1070 preterm or low birth weight infants participated. Preterm or low birth weight infants who receive formula regain birth weight earlier and have higher short‐term rates of weight gain, linear growth and head growth than infants who receive donor breast milk. These effects on growth parameters are greater in trials that compare feeding with nutrient‐enriched preterm formula compared to standard term formula versus donor breast milk. Follow‐up of the infants who participated in two of the largest trials did not find any statistically significant effects on long‐term growth parameters or neurodevelopmental outcomes (Lucas 1984a; Lucas 1984b).
Meta‐analysis of data from six trials suggests that feeding with formula significantly increases the risk of feeding intolerance and necrotising enterocolitis in preterm and low birth weight infants. The pooled estimate suggests that one extra case of necrotising enterocolitis will occur in every 25 infants who receive formula.
Overall completeness and applicability of evidence
These findings should be interpreted with caution. Substantial heterogeneity in the meta‐analyses of the effect on growth parameters limits the validity of the pooled estimates of effect size. Most of these trials were undertaken more than 20 years ago and the trials used different inclusion criteria and varied with respect to the type of formula and donor breast milk used. Only two trials were undertaken in the past 15 years and only these trials compared feeding with formula versus donor breast milk with added multi‐nutrient fortifier (Schanler 2005; Cristofalo 2013). This limits the applicability of the findings to current practice, where nutrient fortification of breast milk is commonly undertaken (Klingenberg 2012).
Meta‐analysis of data from six trials suggests that feeding with formula more than doubles the risk of necrotising enterocolitis. The observed effect sizes were similar across the trials and there was no statistical evidence of heterogeneity. The pooled estimate suggests that one extra case of necrotising enterocolitis will occur in every 25 infants who receive formula. This beneficial effect of donor breast milk exists even when donor breast milk is given as a supplement to maternal breast milk rather than as a sole diet and also when the donor breast milk is fortified. However, only one of the trials was able to blind caregivers and assessors to the intervention. This methodological weakness may have resulted in surveillance and ascertainment biases that contributed to the higher rate of detection of necrotising enterocolitis in formula‐fed infants. Finally, caution should be exercised in applying these data to growth‐restricted preterm infants or sick infants, since these infants, although at high risk of developing necrotising enterocolitis, were generally excluded from the included trials.
The data in this review are from trials undertaken in high‐income countries. In low‐ or middle‐incomes countries, the anti‐infective properties of breast milk may confer advantages that outweigh the lower rate of short‐term growth. In India, a randomised trial in low birth weight infants "at risk of infection" found that serious infections (diarrhoea, pneumonia, septicaemia) were statistically significantly less common in infants allocated to received "expressed human milk" versus formula milk (Narayanan 1982). "Expressed human milk" in this study referred to a mixture of maternal and donor breast milk. As these could not be separated into subgroups, the data were not included in the review.
Quality of the evidence
The trials contain various methodological quality weaknesses, specifically uncertainty about adequate allocation concealment methods in three trials and lack of blinding in most of the trials. Parents, caregivers, clinicians and investigators were likely to have been aware of the treatment group to which infants had been allocated and this knowledge may have affected some care practices or investigation strategies including thresholds for screening or diagnosing for necrotising enterocolitis, which may have affected the outcomes assessed.
Potential biases in the review process
The main concern with the review process is the possibility that the findings are subject to publication and other reporting biases, including more availability of numerical data for inclusion in meta‐analyses from trials that reported statistically significant or clinically important effects. We attempted to minimise this threat by screening the reference lists of included trials and related reviews and searching the proceedings of the major international perinatal conferences to identify trial reports that are not (or not yet) published in full form in academic journals. However, we cannot be sure whether other trials have been undertaken but not reported and the concern remains that such trials are less likely than published trials to have detected statistically significant or clinically important effects. The meta‐analyses that we performed did not contain sufficient trials to explore symmetry of funnel plots as a means of identifying possible publication or reporting bias.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.1 Time to regain birth weight (days from birth).

Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.2 Short‐term weight change (g/kg/day).

Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.3 Short‐term change in crown‐heel length (mm/week).
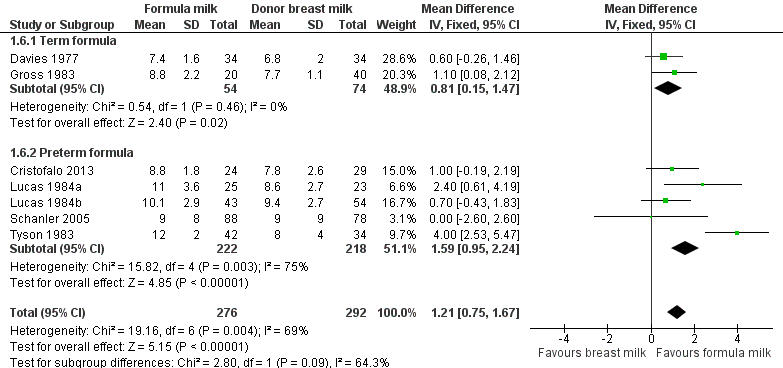
Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.6 Short‐term change in head circumference (mm/week).
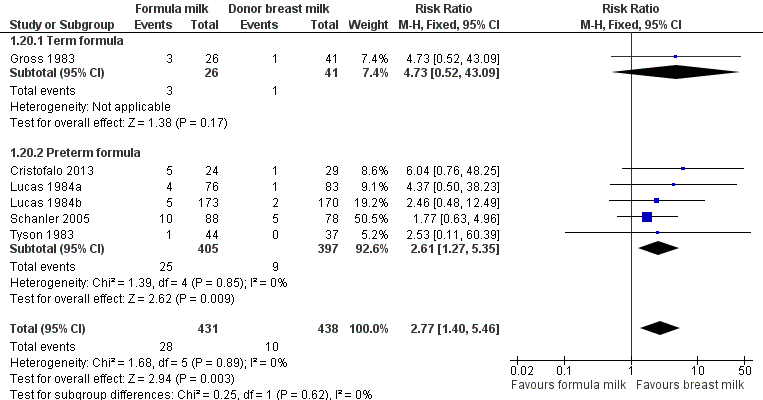
Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.20 Necrotising enterocolitis.
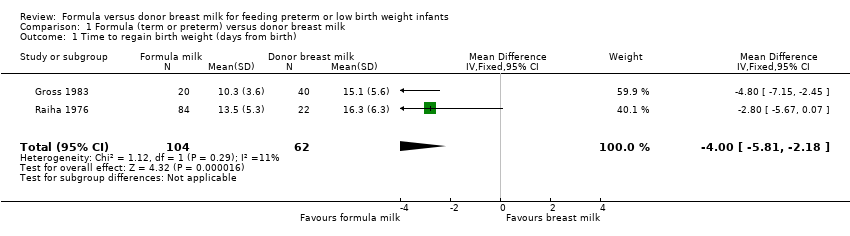
Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 1 Time to regain birth weight (days from birth).

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 2 Short term weight change (g/kg/day).

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 3 Short‐term change in crown‐heel length (mm/week).

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 4 Short‐term change in crown‐rump length (mm/week).

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 5 Short‐term change in femoral length (mm/week).
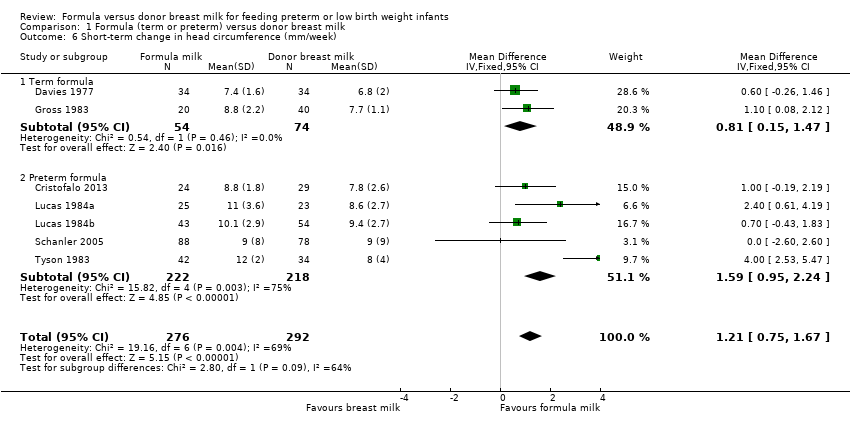
Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 6 Short‐term change in head circumference (mm/week).
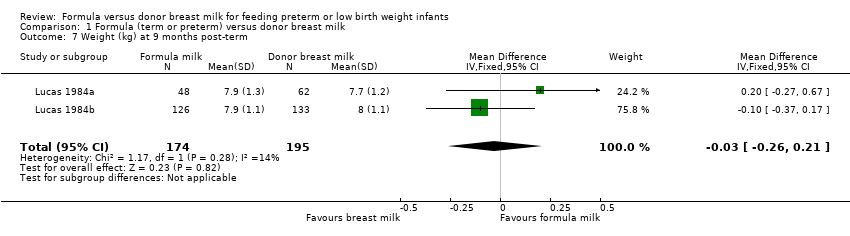
Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 7 Weight (kg) at 9 months post‐term.
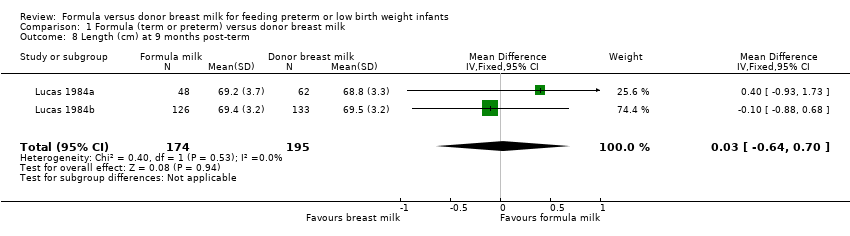
Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 8 Length (cm) at 9 months post‐term.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 9 Head circumference (cm) at 9 months post‐term.
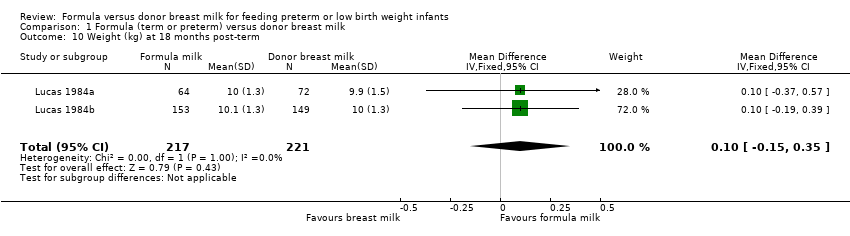
Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 10 Weight (kg) at 18 months post‐term.
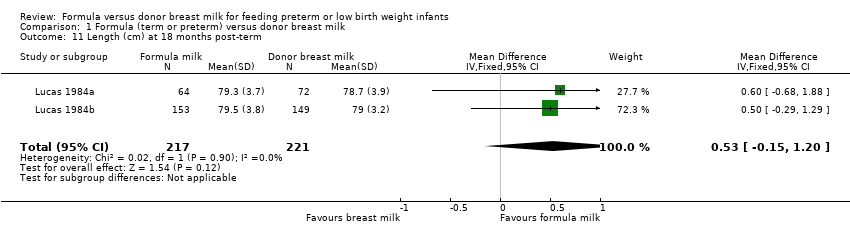
Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 11 Length (cm) at 18 months post‐term.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 12 Head circumference (cm) at 18 months post‐term.
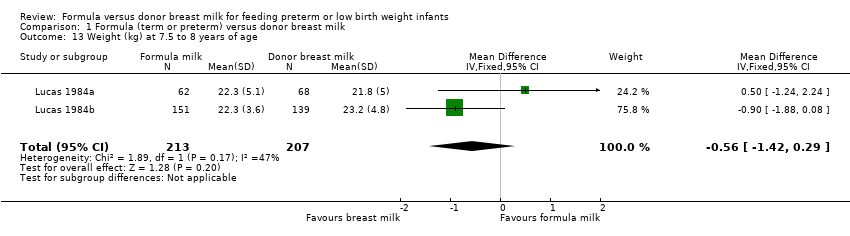
Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 13 Weight (kg) at 7.5 to 8 years of age.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 14 Length (cm) at 7.5 to 8 years of age.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 15 Head circumference (cm) at 7.5 to 8 years of age.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 16 Bayley Mental Development Index at 18 months.
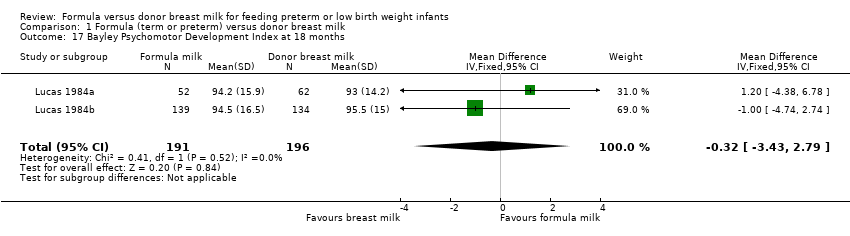
Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 17 Bayley Psychomotor Development Index at 18 months.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 18 Neurological impairment at 18 months.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 19 All‐cause mortality.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 20 Necrotising enterocolitis.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 21 Days after birth to establish full enteral feeding.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 22 Feeding intolerance or diarrhoea.

Comparison 1 Formula (term or preterm) versus donor breast milk, Outcome 23 Incidence of invasive infection.
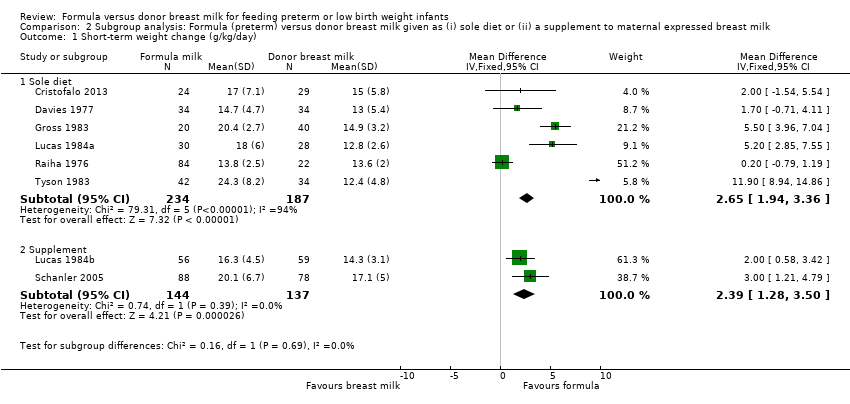
Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 1 Short‐term weight change (g/kg/day).

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 2 Short‐term change in crown‐heel length (mm/week).

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 3 Short‐term change in head circumference (mm/week).
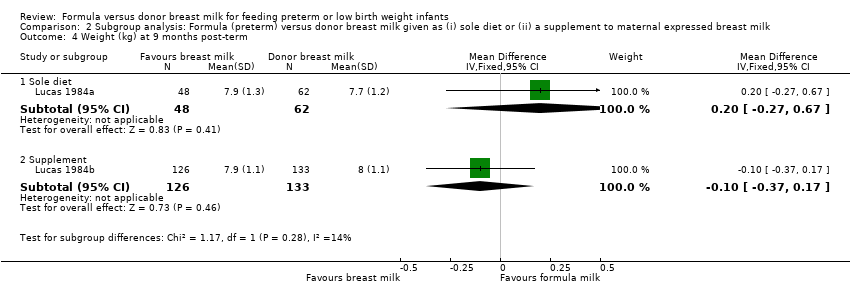
Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 4 Weight (kg) at 9 months post‐term.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 5 Length (cm) at 9 months post‐term.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 6 Head circumference (cm) at 9 months post‐term.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 7 Weight (kg) at 18 months post‐term.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 8 Length (cm) at 18 months post‐term.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 9 Head circumference (cm) at 18 months post‐term.
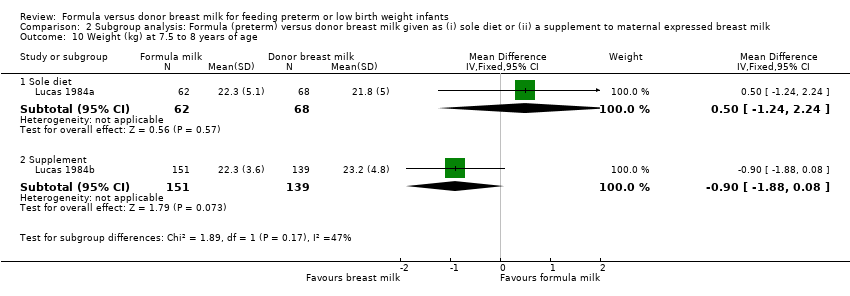
Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 10 Weight (kg) at 7.5 to 8 years of age.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 11 Length (cm) at 7.5 to 8 years of age.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 12 Head circumference (cm) at 7.5 to 8 years of age.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 13 Bayley Mental Development Index at 18 months.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 14 Bayley Psychomotor Development Index at 18 months.
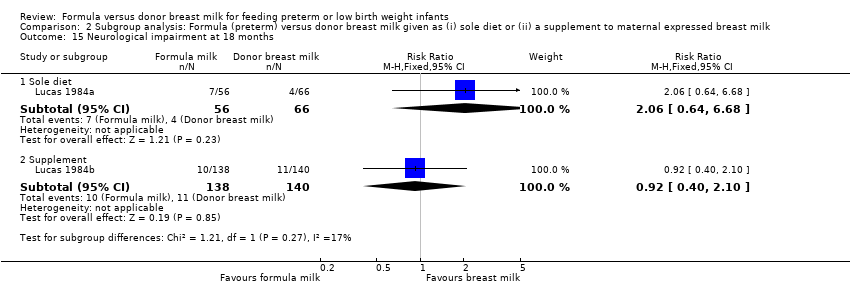
Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 15 Neurological impairment at 18 months.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 16 All‐cause mortality.

Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 17 Necrotising enterocolitis.
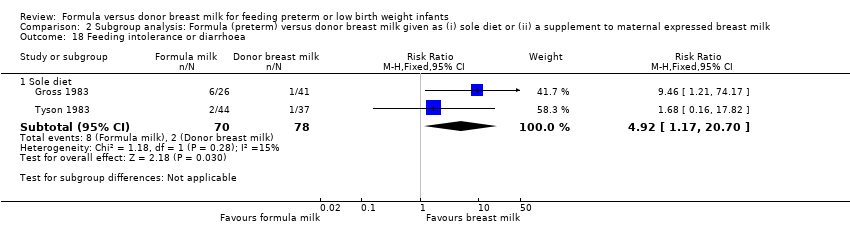
Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 18 Feeding intolerance or diarrhoea.
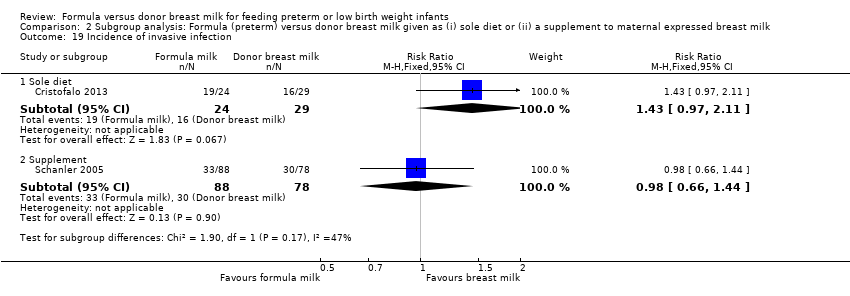
Comparison 2 Subgroup analysis: Formula (preterm) versus donor breast milk given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 19 Incidence of invasive infection.
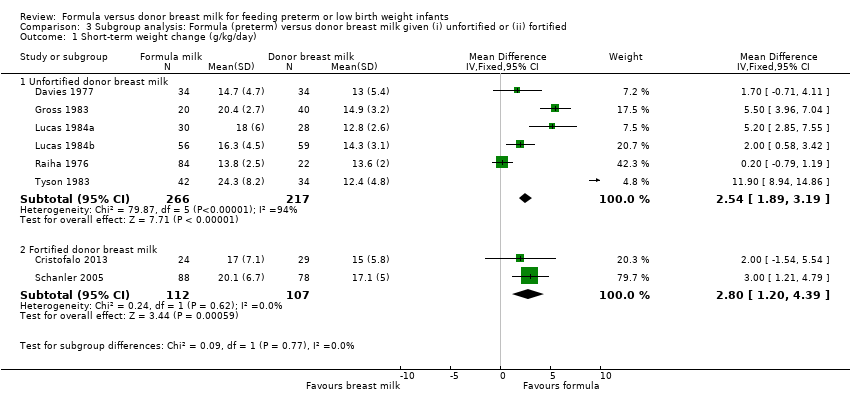
Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 1 Short‐term weight change (g/kg/day).

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 2 Short‐term change in crown‐heel length (mm/week).
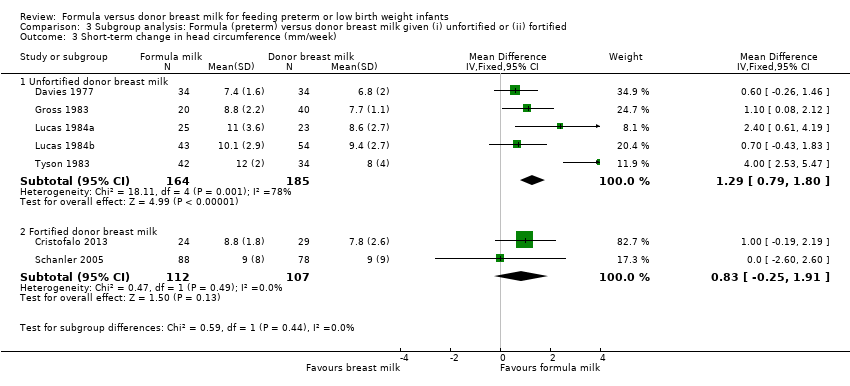
Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 3 Short‐term change in head circumference (mm/week).

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 4 Weight (kg) at 9 months post‐term.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 5 Length (cm) at 9 months post‐term.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 6 Head circumference (cm) at 9 months post‐term.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 7 Weight (kg) at 18 months post‐term.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 8 Length (cm) at 18 months post‐term.
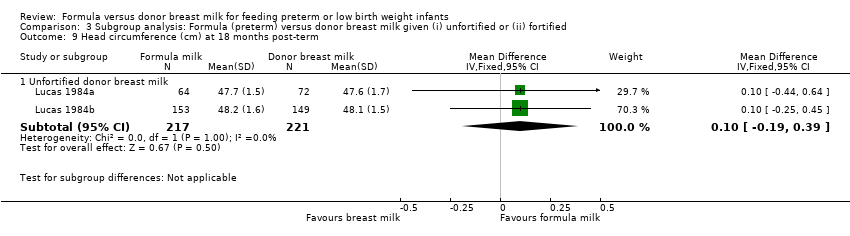
Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 9 Head circumference (cm) at 18 months post‐term.
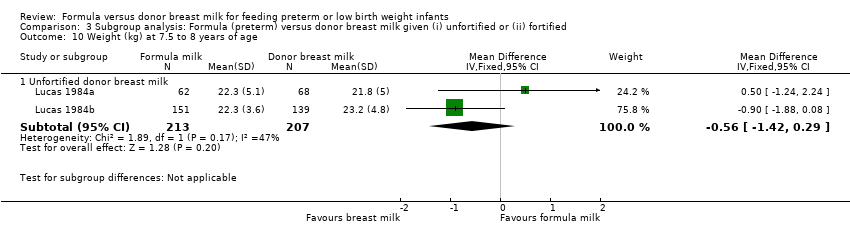
Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 10 Weight (kg) at 7.5 to 8 years of age.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 11 Length (cm) at 7.5 to 8 years of age.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 12 Head circumference (cm) at 7.5 to 8 years of age.
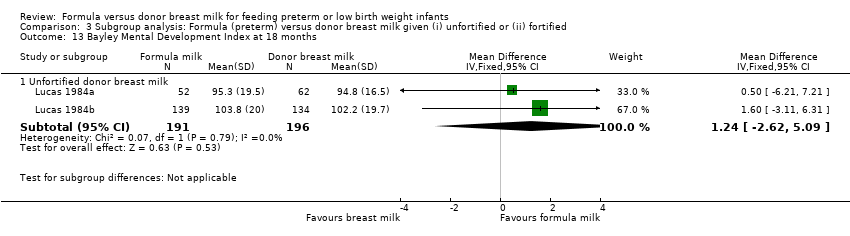
Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 13 Bayley Mental Development Index at 18 months.
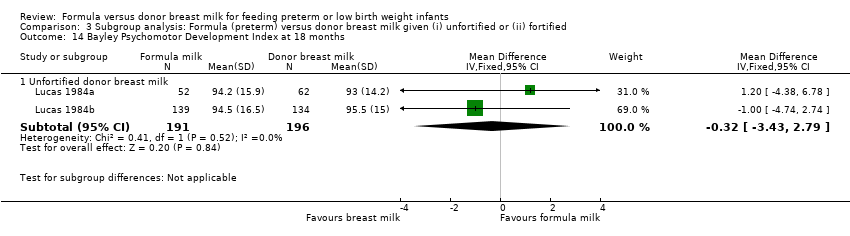
Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 14 Bayley Psychomotor Development Index at 18 months.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 15 Neurological impairment at 18 months.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 16 All‐cause mortality.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 17 Necrotising enterocolitis.
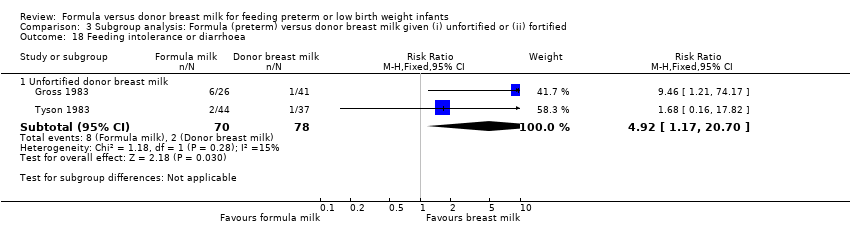
Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 18 Feeding intolerance or diarrhoea.

Comparison 3 Subgroup analysis: Formula (preterm) versus donor breast milk given (i) unfortified or (ii) fortified, Outcome 19 Incidence of invasive infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to regain birth weight (days from birth) Show forest plot | 2 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐5.81, ‐2.18] |
| 2 Short term weight change (g/kg/day) Show forest plot | 8 | 702 | Mean Difference (IV, Fixed, 95% CI) | 2.58 [1.98, 3.17] |
| 2.1 Term formula | 3 | 234 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [0.96, 2.53] |
| 2.2 Preterm formula | 5 | 468 | Mean Difference (IV, Fixed, 95% CI) | 3.71 [2.79, 4.63] |
| 3 Short‐term change in crown‐heel length (mm/week) Show forest plot | 7 | 494 | Mean Difference (IV, Fixed, 95% CI) | 1.36 [0.87, 1.85] |
| 3.1 Term formula | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.10, 1.50] |
| 3.2 Preterm formula | 5 | 366 | Mean Difference (IV, Fixed, 95% CI) | 1.93 [1.23, 2.62] |
| 4 Short‐term change in crown‐rump length (mm/week) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Short‐term change in femoral length (mm/week) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Short‐term change in head circumference (mm/week) Show forest plot | 7 | 568 | Mean Difference (IV, Fixed, 95% CI) | 1.21 [0.75, 1.67] |
| 6.1 Term formula | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [0.15, 1.47] |
| 6.2 Preterm formula | 5 | 440 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [0.95, 2.24] |
| 7 Weight (kg) at 9 months post‐term Show forest plot | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.21] |
| 8 Length (cm) at 9 months post‐term Show forest plot | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.64, 0.70] |
| 9 Head circumference (cm) at 9 months post‐term Show forest plot | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.13, 0.53] |
| 10 Weight (kg) at 18 months post‐term Show forest plot | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.15, 0.35] |
| 11 Length (cm) at 18 months post‐term Show forest plot | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.15, 1.20] |
| 12 Head circumference (cm) at 18 months post‐term Show forest plot | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 13 Weight (kg) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.42, 0.29] |
| 14 Length (cm) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.12, 1.23] |
| 15 Head circumference (cm) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 16 Bayley Mental Development Index at 18 months Show forest plot | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [‐2.62, 5.09] |
| 17 Bayley Psychomotor Development Index at 18 months Show forest plot | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐3.43, 2.79] |
| 18 Neurological impairment at 18 months Show forest plot | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.62, 2.35] |
| 19 All‐cause mortality Show forest plot | 4 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.25] |
| 20 Necrotising enterocolitis Show forest plot | 6 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.40, 5.46] |
| 20.1 Term formula | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.73 [0.52, 43.09] |
| 20.2 Preterm formula | 5 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.61 [1.27, 5.35] |
| 21 Days after birth to establish full enteral feeding Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐2.56, 11.96] |
| 22 Feeding intolerance or diarrhoea Show forest plot | 2 | 148 | Risk Difference (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.19] |
| 22.1 Term formula | 1 | 67 | Risk Difference (M‐H, Fixed, 95% CI) | 0.21 [0.04, 0.38] |
| 22.2 Preterm formula | 1 | 81 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.06, 0.10] |
| 23 Incidence of invasive infection Show forest plot | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.84, 1.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term weight change (g/kg/day) Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sole diet | 6 | 421 | Mean Difference (IV, Fixed, 95% CI) | 2.65 [1.94, 3.36] |
| 1.2 Supplement | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 2.39 [1.28, 3.50] |
| 2 Short‐term change in crown‐heel length (mm/week) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sole diet | 5 | 283 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [0.98, 2.11] |
| 2.2 Supplement | 2 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐0.28, 1.78] |
| 3 Short‐term change in head circumference (mm/week) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Sole diet | 5 | 305 | Mean Difference (IV, Fixed, 95% CI) | 1.36 [0.85, 1.88] |
| 3.2 Supplement | 2 | 263 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [‐0.44, 1.62] |
| 4 Weight (kg) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Sole diet | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.27, 0.67] |
| 4.2 Supplement | 1 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.37, 0.17] |
| 5 Length (cm) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Sole diet | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.93, 1.73] |
| 5.2 Supplement | 1 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.88, 0.68] |
| 6 Head circumference (cm) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Sole diet | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.45, 0.85] |
| 6.2 Supplement | 1 | 259 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.18, 0.58] |
| 7 Weight (kg) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Sole diet | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.37, 0.57] |
| 7.2 Supplement | 1 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 8 Length (cm) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Sole diet | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.68, 1.88] |
| 8.2 Supplement | 1 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.29, 1.29] |
| 9 Head circumference (cm) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Sole diet | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.44, 0.64] |
| 9.2 Supplement | 1 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.25, 0.45] |
| 10 Weight (kg) at 7.5 to 8 years of age Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Sole diet | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.24, 2.24] |
| 10.2 Supplement | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.88, 0.08] |
| 11 Length (cm) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.12, 1.23] |
| 11.1 Sole diet | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.26, 3.26] |
| 11.2 Supplement | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.68, 1.08] |
| 12 Head circumference (cm) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 12.1 Sole diet | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.56, 0.76] |
| 12.2 Supplement | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.71, 0.11] |
| 13 Bayley Mental Development Index at 18 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Sole diet | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐6.21, 7.21] |
| 13.2 Supplement | 1 | 273 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐3.11, 6.31] |
| 14 Bayley Psychomotor Development Index at 18 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Sole diet | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐4.38, 6.78] |
| 14.2 Supplement | 1 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.74, 2.74] |
| 15 Neurological impairment at 18 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Sole diet | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.64, 6.68] |
| 15.2 Supplement | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.40, 2.10] |
| 16 All‐cause mortality Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Sole diet | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.71, 4.07] |
| 16.2 Supplement | 2 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.60, 2.24] |
| 17 Necrotising enterocolitis Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Sole diet | 4 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [1.47, 14.56] |
| 17.2 Supplement | 2 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.82, 4.67] |
| 18 Feeding intolerance or diarrhoea Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Sole diet | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.92 [1.17, 20.70] |
| 19 Incidence of invasive infection Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Sole diet | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.97, 2.11] |
| 19.2 Supplement | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.66, 1.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term weight change (g/kg/day) Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Unfortified donor breast milk | 6 | 483 | Mean Difference (IV, Fixed, 95% CI) | 2.54 [1.89, 3.19] |
| 1.2 Fortified donor breast milk | 2 | 219 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [1.20, 4.39] |
| 2 Short‐term change in crown‐heel length (mm/week) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Unfortified donor breast milk | 5 | 275 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [0.72, 1.80] |
| 2.2 Fortified donor breast milk | 2 | 219 | Mean Difference (IV, Fixed, 95% CI) | 1.86 [0.64, 3.07] |
| 3 Short‐term change in head circumference (mm/week) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Unfortified donor breast milk | 5 | 349 | Mean Difference (IV, Fixed, 95% CI) | 1.29 [0.79, 1.80] |
| 3.2 Fortified donor breast milk | 2 | 219 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [‐0.25, 1.91] |
| 4 Weight (kg) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Unfortified donor breast milk | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.21] |
| 5 Length (cm) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Unfortified donor breast milk | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.64, 0.70] |
| 6 Head circumference (cm) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Unfortified donor breast milk | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.13, 0.53] |
| 7 Weight (kg) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Unfortified donor breast milk | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.15, 0.35] |
| 8 Length (cm) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Unfortified donor breast milk | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.15, 1.20] |
| 9 Head circumference (cm) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Unfortified donor breast milk | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 10 Weight (kg) at 7.5 to 8 years of age Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Unfortified donor breast milk | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.42, 0.29] |
| 11 Length (cm) at 7.5 to 8 years of age Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Unfortified donor breast milk | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.12, 1.23] |
| 12 Head circumference (cm) at 7.5 to 8 years of age Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Unfortified donor breast milk | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 13 Bayley Mental Development Index at 18 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Unfortified donor breast milk | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [‐2.62, 5.09] |
| 14 Bayley Psychomotor Development Index at 18 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Unfortified donor breast milk | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐3.43, 2.79] |
| 15 Neurological impairment at 18 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Unfortified donor breast milk | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.62, 2.35] |
| 16 All‐cause mortality Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Unfortified donor breast milk | 2 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.73, 2.29] |
| 16.2 Fortified donor breast milk | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.42, 5.51] |
| 17 Necrotising enterocolitis Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Unfortified donor breast milk | 4 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.16, 9.41] |
| 17.2 Fortified donor breast milk | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.98, 5.87] |
| 18 Feeding intolerance or diarrhoea Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Unfortified donor breast milk | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.92 [1.17, 20.70] |
| 19 Incidence of invasive infection Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Fortified donor breast milk | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.84, 1.49] |

