Leche maternizada versus leche materna de donante para la alimentación de los lactantes prematuros o de bajo peso al nacer
Resumen
Antecedentes
Cuando no se dispone de suficiente leche materna, las formas alternativas de nutrición enteral para los lactantes prematuros o de bajo peso al nacer (BPN) son la leche materna de donante o la leche artificial. La leche materna de donante puede contener algunos de los efectos beneficiosos no nutricionales de la leche materna para los lactantes prematuros o de BPN. Sin embargo, la alimentación con leche artificial puede asegurar una entrega más consistente de mayores cantidades de nutrientes. Existe incertidumbre acerca del equilibrio entre los riesgos y los efectos beneficiosos de la alimentación con leche maternizada y la leche materna de donante en los lactantes prematuros o de BPN.
Objetivos
Determinar el efecto de la alimentación con leche maternizada en comparación con leche materna de donante sobre el crecimiento y el desarrollo en lactantes prematuros o de bajo peso al nacer (BPN).
Métodos de búsqueda
Se utilizó la estrategia de búsqueda del Grupo Cochrane de Neonatología, que incluye búsquedas electrónicas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2019, número 5), Ovid MEDLINE, Embase y el Cumulative Index to Nursing and Allied Health Literature (3 de mayo de 2019), así como en actas de congresos, revisiones anteriores y ensayos clínicos.
Criterios de selección
Ensayos controlados aleatorizados o cuasialeatorizados (ECA) que compararon la alimentación con leche maternizada versus leche materna de donante en lactantes prematuros o de BPN.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron la elegibilidad de los ensayos y el riesgo de sesgo, y extrajeron los datos de forma independiente. Se analizaron los efectos del tratamiento descritos en los ensayos individuales y se informaron los cocientes de riesgos (CR) y las diferencias de riesgos (DR) para los datos dicotómicos, y las diferencias de medias (DM) para los datos continuos, con los respectivos intervalos de confianza (IC) del 95%. Se utilizó un modelo de efectos fijos en los metanálisis y se exploraron las causas potenciales de la heterogeneidad en análisis de subgrupos. Se evaluó la certeza de la evidencia para la comparación principal a nivel de resultado con el uso de los métodos GRADE.
Resultados principales
Doce ensayos con un total de 1879 lactantes cumplieron los criterios de inclusión. Cuatro ensayos compararon la leche maternizada estándar a término versus la leche materna de donante y ocho compararon la leche maternizada pretérmino fortificada con nutrientes versus la leche materna de donante. Sólo los cinco ensayos más recientes utilizaron leche materna de donante fortificada con nutrientes. Los ensayos tienen varias deficiencias en la calidad metodológica, específicamente problemas relacionados con la ocultación de la asignación en cuatro ensayos y la falta de cegamiento en la mayoría de los ensayos. La mayoría de los ensayos incluidos fueron patrocinados por compañías que hicieron la leche maternizada del estudio.
Los lactantes alimentados con leche maternizada tuvieron tasas más altas de aumento de peso en el hospital (diferencia de medias [DM] 2,51; intervalo de confianza [IC] del 95%: 1,93 a 3,08 g/kg/día), crecimiento lineal (DM 1,21; IC del 95%: 0,77 a 1,65 mm/semana) y crecimiento de la cabeza (DM 0,85; IC del 95%: 0,47 a 1,23 mm/semana). Estos metanálisis tenían altos niveles de heterogeneidad. No se encontró evidencia de un efecto sobre el crecimiento o el neurodesarrollo a largo plazo. La alimentación con leche maternizada aumentó el riesgo de enterocolitis necrosante (cociente de riesgos [CR] típico 1,87; IC del 95%: 1,23 a 2,85; diferencia de riesgos [DR] 0,03; IC del 95%: 0,01 a 0,05; número necesario a tratar para un resultado perjudicial adicional [NNTD] 33; IC del 95%: 20 a 100; nueve estudios, 1675 lactantes).
La certeza de la evidencia según GRADE fue moderada para las tasas de aumento de peso, crecimiento lineal y crecimiento de la cabeza (disminuida por los niveles altos de heterogeneidad) y fue moderada para la discapacidad del neurodesarrollo, la mortalidad por todas las causas y la enterocolitis necrosante (disminuida por imprecisión).
Conclusiones de los autores
En los lactantes prematuros y de BPN, evidencia de certeza moderada indica que la alimentación con leche maternizada en comparación con la leche materna de donante, ya sea como suplemento de la leche materna extraída o como dieta única, da lugar a tasas más altas de aumento de peso, crecimiento lineal y crecimiento de la cabeza y a un mayor riesgo de desarrollar enterocolitis necrosante. Los datos de los ensayos no muestran un efecto sobre la mortalidad por todas las causas, o sobre el crecimiento o el neurodesarrollo a largo plazo.
PICO
Resumen en términos sencillos
Leche maternizada versus leche materna de donante para la alimentación de los lactantes prematuros o de bajo peso al nacer
Pregunta de la revisión
Cuando no se dispone de leche materna propia, ¿la alimentación de los recién nacidos prematuros o de bajo peso al nacer con leche maternizada, en lugar de leche materna de donante, afecta la digestión, el crecimiento y el riesgo de problemas intestinales graves?
Antecedentes
Los recién nacidos prematuros a menudo encuentran que la leche artificial es más difícil de digerir que la leche materna, y existe la preocupación de que la leche maternizada pueda aumentar el riesgo de problemas intestinales graves. Si los recién nacidos prematuros son alimentados con leche materna de donante (cuando la leche materna de la propia madre no es suficiente o no está disponible), en lugar de una leche artificial, se podría reducir el riesgo de estos problemas. Sin embargo, la leche materna de donante es más costosa que muchas leches maternizadas y puede no contener cantidades suficientes de nutrientes clave para asegurar un crecimiento óptimo de los recién nacidos prematuros o de bajo peso al nacer. Debido a estas inquietudes, se revisó todas la evidencia disponible de los ensayos clínicos que compararon leche maternizada versus leche materna de donante para la alimentación de los recién nacidos prematuros o de bajo peso al nacer.
Características de los estudios
Se encontraron 12 ensayos completados (con 1871 recién nacidos). La mayoría de los ensayos, en particular los realizados más recientemente, utilizaron métodos fiables. La evidencia está actualizada hasta el 3 de mayo de 2019.
Resultados clave
El análisis combinado de los datos de estos ensayos muestra que la alimentación con leche maternizada aumenta las tasas de crecimiento durante la estancia hospitalaria, pero se asocia con un mayor riesgo de desarrollar el trastorno intestinal grave llamado "enterocolitis necrosante". No hay evidencia de un efecto sobre la supervivencia o el crecimiento y el desarrollo a más largo plazo.
Conclusiones
La evidencia actualmente disponible indica que la alimentación de los recién nacidos prematuros con leche artificial (en lugar de leche materna de donante cuando no se dispone de leche materna) se asocia con tasas de crecimiento más rápidas, pero con un riesgo casi doble de desarrollar enterocolitis necrosante. Además, ensayos más grandes podrían proporcionar evidencia más sólida y precisa para ayudar a los médicos y las familias a tomar decisiones informadas sobre este tema. Actualmente, cuatro de estos ensayos (con más de 1100 recién nacidos) están en curso a nivel internacional, y se planea incluir los datos de estos ensayos en esta revisión cuando estén disponibles.
Authors' conclusions
Summary of findings
| Formula (term or preterm) compared to donor breast milk (unfortified or fortified) for feeding preterm or low birth weight infants | ||||||
| Patient or population: preterm or low birth weight infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with donated breast milk (unfortified or fortified) | Risk with formula (term or preterm) | |||||
| Weight gain (g/kg/day) | ‐ | MD 2.51 higher | ‐ | 1028 | Moderatea | I² = 90% |
| Linear growth (crown‐heel length mm/week) | ‐ | MD 1.21 higher | ‐ | 820 | Moderatea | I² = 68% |
| Head growth (mm/week) | ‐ | MD 0.85 higher | ‐ | 894 | Moderatea | I² = 74% |
| Neurodevelopmental disability | Study population | RR 1.21 | 400 | Moderateb | ||
| 73 per 1000 | 88 per 1000 (45 to 171) | |||||
| All‐cause mortality | Study population | RR 1.1 | 1527 | Moderateb | ||
| 86 per 1000 | 94 per 1000 | |||||
| Necrotising enterocolitis | Study population | RR 1.87 | 1675 | Moderateb | ||
| 36 per 1000 | 67 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| aDowngraded one level for heterogeneity. | ||||||
| CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
Background
Maternal breast milk is the recommended form of enteral nutrition for preterm or low birth weight (LBW) infants (AAP 2012). Breast milk contains non‐nutrient factors including immunoglobulins and lactoferrin that may promote intestinal adaptation and maturation, improve enteral feed tolerance, and protect against infective and inflammatory disorders (Agostoni 2010; Arslanoglu 2013).
When sufficient maternal breast milk is not available, the two common alternatives available for feeding preterm or LBW infants are artificial formula and donor breast milk (donated by other lactating women). These may be given either as the sole form of enteral feeding or as a supplement to maternal breast milk (Klingenberg 2012).
Description of the condition
Providing appropriate nutrition for preterm or LBW infants is a critical component of neonatal care. Early enteral nutrition strategies may have a substantial impact on clinically important outcomes, such as necrotising enterocolitis and invasive infection. These infectious and inflammatory complications may increase the risk of mortality and other morbidities and adversely affect long‐term growth and neurodevelopmental outcomes.
Description of the intervention
A variety of artificial formulas (usually adapted from cow's milk) are available. These vary in energy, protein and mineral content but can, broadly, be considered as:
-
standard 'term' formula, designed for term infants based on the composition of mature breast milk; the typical energy content is approximately between 67 kCal/100 mL to 70 kCal/100 mL;
-
nutrient‐enriched 'preterm' formula, designed to provide nutrient intakes to match intrauterine accretion rates (Tsang 1993); these are energy‐enriched (typically up to approximately 80 kCal/100 mL) and variably protein‐ and mineral‐enriched (Fewtrell 1999).
The comparison arm for the intervention is donor breast milk. Expressed breast milk from donor mothers, usually mothers who have delivered at term, generally has a lower content of energy and protein than term formula milk (Gross 1980; Gross 1981). The macronutrient content of donor breast milk is not compromised substantially by modern pasteurisation methods but levels of immunoactive components might be reduced (Peila 2016; Castro 2019). Donor human milk also varies with regard to fat, energy and protein content, depending upon the stage of lactation at which it is collected. Milk expressed from the donor's lactating breast usually has a higher energy and protein content than that collected from the contralateral breast ('drip' breast milk) (Lucas 1978).
How the intervention might work
There is concern that the nutritional requirements of preterm or LBW infants, who are born with relatively impoverished nutrient reserves and are subject to additional metabolic stresses compared with term infants, may not be fully met by enteral feeding with donor breast milk (Hay 1994; Schanler 1995). These deficiencies may have adverse consequences for growth and development. However, a major putative benefit of donor breast milk is that the delivery of immunoprotective and growth factors to the immature gut mucosa may prevent serious adverse outcomes, including necrotising enterocolitis and invasive infection (Lucas 1990; Beeby 1992).
Why it is important to do this review
Given the potential for the type of enteral nutrition to affect important outcomes for preterm or LBW infants, and since uncertainty exists about the balance between the putative benefits and harms, an attempt to detect, appraise and synthesise evidence from randomised controlled trials (RCTs) is merited.
Objectives
To determine the effect of feeding with formula compared with donor breast milk on growth and development in preterm or low birth weight (LBW) infants.
Métodos
Obtención y análisis de los datos
Evaluación del riesgo de sesgo de los estudios incluidos
Results
Description of studies
Results of the search
See: Figure 1.

Study flow diagram: 2019 review update.
We included one new trial (12 trials in total) (Costa 2018). We excluded two new full‐text reports (Brandstetter 2018; Castellano 2019) (14 reports in total).
One report is awaiting assessment (Perez 2015).
We identified four ongoing trials (See: Characteristics of ongoing studies).
Included studies
Twelve trials fulfilled the review eligibility criteria (Raiha 1976; Davies 1977; Schultz 1980; Gross 1983; Tyson 1983; Lucas 1984a; Lucas 1984b; Schanler 2005; Cristofalo 2013; Corpeleijn 2016; O'Connor 2016; Costa 2018).
All trials were undertaken in neonatal units in Europe and North America. Seven of the trials were conducted more than 30 years ago (Raiha 1976; Davies 1977; Schultz 1980; Gross 1983; Tyson 1983; Lucas 1984a; Lucas 1984b). Five trials have been undertaken since the year 2000 (Schanler 2005; Cristofalo 2013; Corpeleijn 2016; O'Connor 2016; Costa 2018). For further details see Characteristics of included studies.
Participants
A total of 1879 infants took part in the included trials. Most participants were clinically stable infants of gestational age at birth < 32 weeks' or birth weight < 1800 g. Most trials excluded infants who were small for gestational age at birth and infants with congenital anomalies or gastrointestinal or neurological problems.
Interventions
The trials varied according to type of formula (term or preterm), and whether the intervention was a sole diet or a supplement to mother's own milk.
-
Four trials compared feeding with term formula versus unfortified donor breast milk (Raiha 1976; Davies 1977; Schultz 1980; Gross 1983). In all of these trials, term formula or donor breast milk was the sole diet.
-
Eight trials compared feeding with preterm formula versus donor breast milk, either as the sole diet (Tyson 1983; Lucas 1984a; Cristofalo 2013), or as a supplement to maternal breast milk (Lucas 1984b; Schanler 2005; Corpeleijn 2016; O'Connor 2016; Costa 2018).
The trials varied according to type of donor breast milk, and whether donor breast milk feeds were nutrient‐fortified or not.
-
Five trials used donor breast milk collected from mothers who had delivered an infant at term (Raiha 1976; Davies 1977; Schultz 1980; Lucas 1984a; Lucas 1984b). Two of these trials used 'drip' breast milk (Lucas 1984a; Lucas 1984b). One trial used preterm donor breast milk (Schanler 2005), one trial used both term and preterm donor milk (Gross 1983), and five trials did not specify the type of donor breast milk (Tyson 1983; Cristofalo 2013; Corpeleijn 2016; O'Connor 2016; Costa 2018).
-
In all trials except Tyson 1983, the donor breast milk was pasteurised.
-
Four trials used donor breast milk with multinutrient fortifier added empirically or as indicated (Schanler 2005; Cristofalo 2013; Corpeleijn 2016; O'Connor 2016). Cristofalo 2013 used human milk‐based fortifier, and the other trials used cow's milk‐based fortifier.
In general, feeds were allocated for several weeks, or until participating infants reached a specified body weight (generally > 2 kg). One trial used the allocated feed for only the first 10 days after birth (or earlier if the infant was transferred from the recruiting centre). Infants then received preterm formula if own mother's milk was insufficient (Corpeleijn 2016).
Outcomes
The most commonly reported outcomes were growth parameters during the study period or until hospital discharge. Most reports gave information on adverse outcomes, including feeding intolerance and the incidence of necrotising enterocolitis. Four trials reported growth or neurodevelopmental outcomes assessed during and after infancy following hospital discharge (Gross 1983; Lucas 1984a; Lucas 1984b; O'Connor 2016).
Excluded studies
We excluded 14 studies following full‐text review (Narayanan 1982; Svenningsen 1982; Jarvenpaa 1983; Cooper 1984; Putet 1984; O'Connor 2003; Sullivan 2010; Hair 2014; Colaizy 2015; Marseglia 2015; Perrella 2015; Tewari 2018; Brandstetter 2018; Castellano 2019). The reasons for exclusion are described in the table Characteristics of excluded studies.
Studies awaiting classification
One report is awaiting translation and assessment (Perez 2015).
Ongoing studies
We identified four ongoing trials (see: Characteristics of ongoing studies).
Risk of bias in included studies
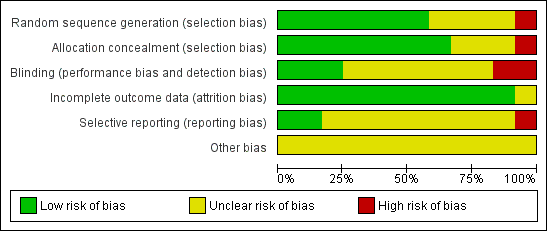
Quality assessments are detailed in the table Characteristics of included studies and are illustrated in Figure 2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Six trials reported adequate allocation concealment methods (sealed, numbered envelopes; central randomisation in blocks) and we assessed these trials as being at low risk of bias (Lucas 1984a; Lucas 1984b; Tyson 1983; Corpeleijn 2016; O'Connor 2016; Costa 2018). The other trials did not report methods of allocation concealment. One quasi‐RCT randomly allocated participants to one of the four formula arms, and allocated every fifth infant to the donor breast milk arm (Raiha 1976); we assessed this trial as being at high risk of selection bias.
Blinding
Four trials blinded the staff or caregivers to the treatments and we assessed them as being at low risk of bias (Schanler 2005; Cristofalo 2013; Corpeleijn 2016; O'Connor 2016). Three trials did not mask the staff and we assessed them as being at high risk of bias (Tyson 1983; Lucas 1984a; Lucas 1984b). The other trial reports did not state whether staff were masked.
Most of the trials did not specify whether the outcome assessors were masked to the feeding arms (unclear risk of bias). In four trials staff were masked to the post‐hospital discharge outcomes and we assessed them as being at low risk of bias (Lucas 1984a; Lucas 1984b; Corpeleijn 2016; O'Connor 2016).
Incomplete outcome data
Most trials reported complete follow‐up for the in‐hospital outcomes assessment and we assessed them as being at low risk of attrition bias. In three trials, infants who developed complications (5% to 10% of the total enrolled) were withdrawn from the study and therefore the in‐hospital growth data for these infants were not presented (Raiha 1976; Gross 1983; Tyson 1983). In the trials that reported data for long‐term outcomes, more than 80% of participants were assessed (low risk of bias) (Gross 1983; Lucas 1984a; Lucas 1984b; O'Connor 2016).
Selective reporting
We assessed Corpeleijn 2016 as being at high risk of reporting bias. Corpeleijn 2016 did not report protocol‐specified outcome data for short‐term growth rate, bone density, Bayley Scores of Infant Development III (at 2 years of age), and growth rate at two years of age. Most of the other trials were at unclear risk of bias as protocols were not available for assessment.
Effects of interventions
Primary outcomes
Growth
Time to regain birth weight
Meta‐analysis of data from Raiha 1976, Gross 1983 and Costa 2018 showed that the formula‐fed group regained birth weight more quickly (mean difference (MD) ‐3.08 days, 95% confidence interval (CI) ‐4.38 to ‐1.77; I² = 37%; 3 trials, 236 participants; Analysis 1.1).
Schultz 1980 did not detect a statistically significant difference, but standard deviations (SDs) were not reported and we could not include the data in the meta‐analysis.
Lucas 1984a reported the median time to regain birth weight as lower in the formula‐fed infants (10 versus 16 days). Lucas 1984b did not find a statistically significant difference (13 versus 15 days). SDs were not reported and we could not include the data in the meta‐analysis.
The other trials did not report time to regain birth weight.
Rate of weight gain
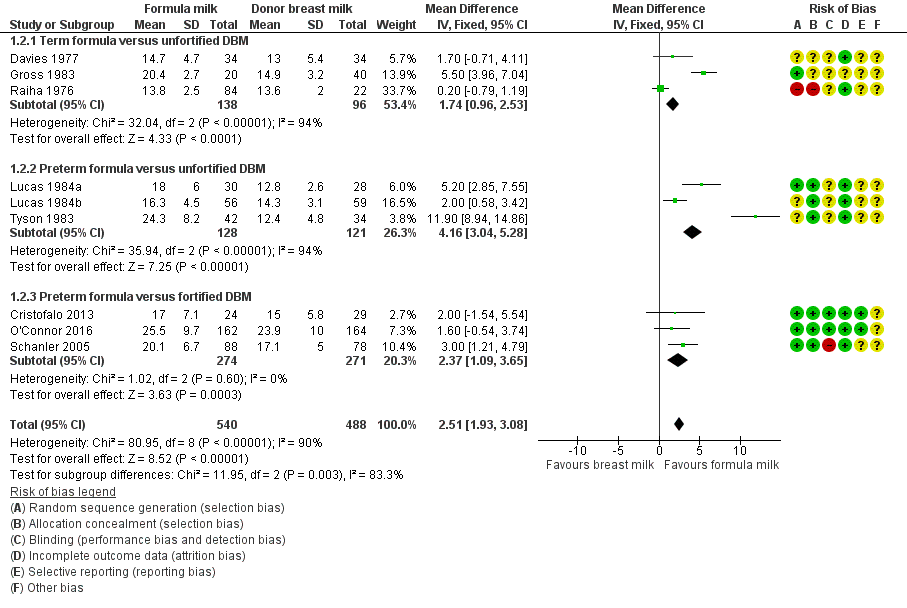
Formula‐fed infants had a higher rate of weight gain but with high heterogeneity in the estimate of this effect (MD 2.51, 95% CI 1.93 to 3.08 g/kg/day; I² = 90%; 9 trials, 1028 participants; moderate‐certainty evidence; summary of findings Table for the main comparison; Analysis 1.2). Significant subgroup differences existed with the largest effect size for the comparison of preterm formula with unfortified donor breast milk (MD 4.16, 95% CI 3.04 to 5.28 g/kg/day) (Figure 3).
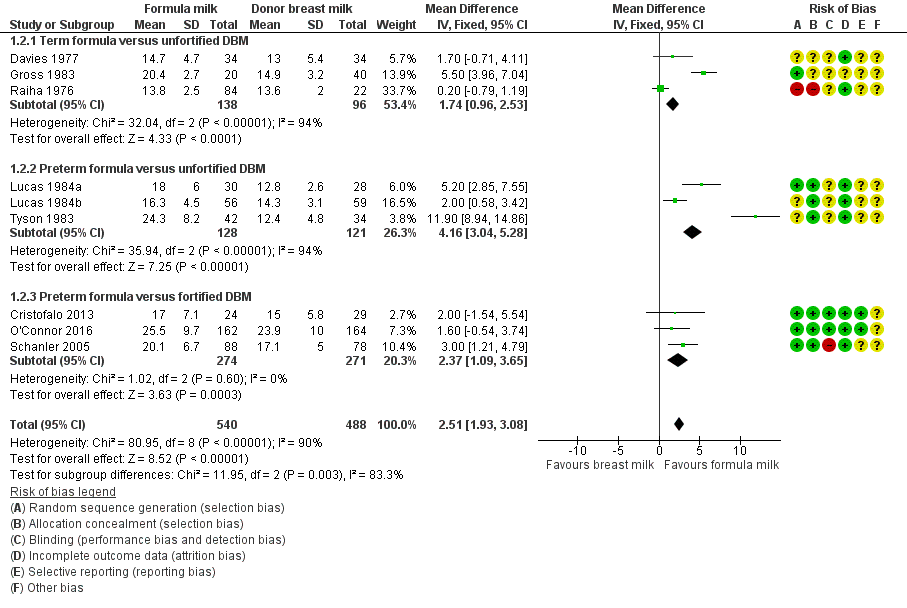
Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.2 Weight gain (g/kg/day).
Schultz 1980 and Corpeleijn 2016 did not report rate of weight gain.
Costa 2018 did not detect a between‐group difference in average weight at 15 days after birth or at 36 weeks' post‐menstrual age.
Linear growth
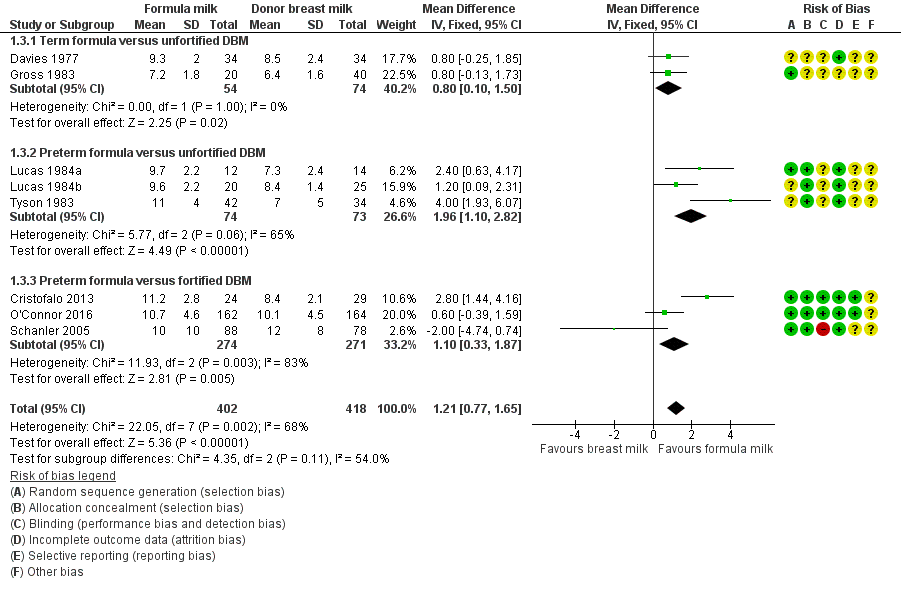
Formula‐fed infants had a higher rate of increase in crown‐heel length but with high heterogeneity in the estimate of this effect (MD 1.21, 95% CI 0.77 to 1.65 mm/week; I² = 68%; 8 trials, 820 participants; moderate‐certainty evidence; summary of findings Table for the main comparison; Analysis 1.3; Analysis 1.4; Analysis 1.5). Significant subgroup differences existed with the largest effect size for the comparison of preterm formula with unfortified donor breast milk (MD 2.01, 95% CI 1.21 to 2.81 mm/week) (Figure 4).
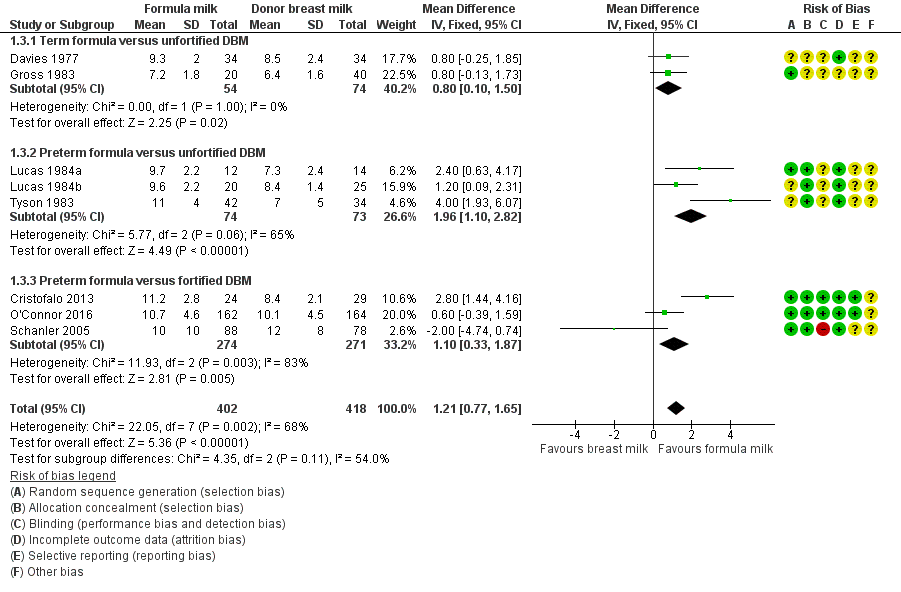
Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.3 Linear growth (crown‐heel length mm/week).
Raiha 1976 reported higher rates of increase in crown‐rump (MD 0.59, 95% CI 0.08 to 1.10 mm/week) and femoral length (MD 0.34, 95% CI 0.13 to 0.55 mm/week) in the formula‐fed group.
Schultz 1980 and Corpeleijn 2016 did not report rate of linear growth.
Costa 2018 did not detect a between‐group difference in average length at 15 days after birth or at 36 weeks' post‐menstrual age.
Head growth
Formula‐fed infants had a higher rate of increase in occipitofrontal head circumference but with high heterogeneity in the estimate of this effect (MD 0.85, 95% CI 0.47 to 1.23 mm/week; I² = 74%; 8 trials, 894 participants; moderate‐certainty evidence; summary of findings Table for the main comparison; Analysis 1.6). Significant subgroup differences existed with the largest effect size for the comparison of preterm formula with unfortified donor breast milk (MD 4.16, 95% CI 3.04 to 5.28 g/kg/day) (Figure 5).
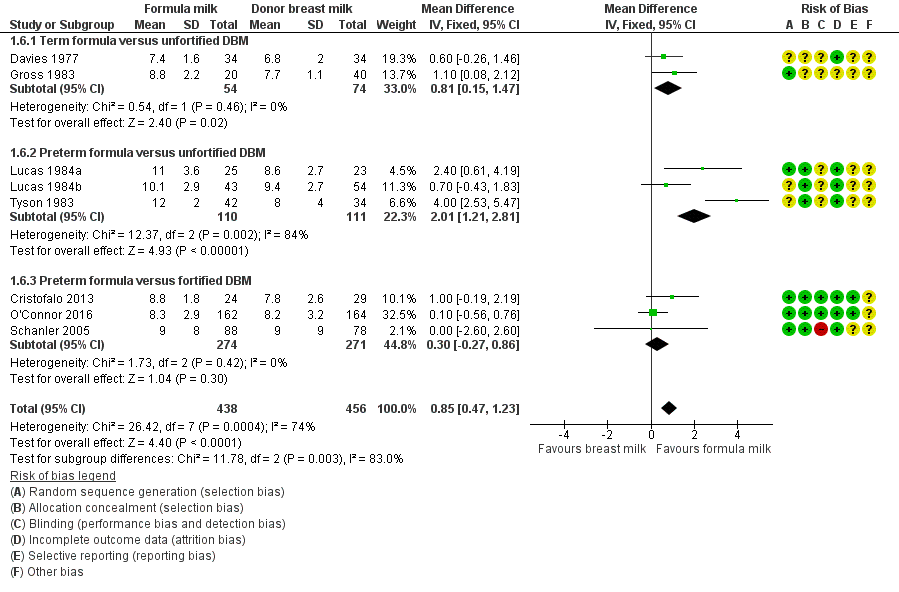
Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.6 Head growth (mm/week).
Raiha 1976, Schultz 1980 and Corpeleijn 2016 did not report rate of head growth.
Costa 2018 did not detect a between‐group difference in average head circumference at 15 days after birth or at 36 weeks' post‐menstrual age.
Long‐term growth
Post‐hospital discharge growth was reported by Lucas 1984a and Lucas 1984b. Neither individual study, nor meta‐analyses of data from both studies, showed differences in the weight, length or head circumference at nine months, 18 months or 7.5 to eight years post‐term; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15.
Neurodevelopment
Death or severe neurodevelopmental disability
These composite data are not yet available from the trials that assessed neurodevelopmental outcomes.
Neurodevelopmental scores
Four trials have reported neurodevelopmental outcomes or assessment scores in children aged at least 12 months, measured using validated assessment tools (Gross 1983; Lucas 1984a; Lucas 1984b; O'Connor 2016):
Gross 1983 stated that there was "no difference" in Bayley Mental or Psychomotor Developmental Indices at 15 months post‐term (numerical data not available).
Lucas 1984a and Lucas 1984b, or a meta‐analysis of data from both, did not show differences in Bayley Psychomotor and Mental Development Indices at 18 months' corrected age.
-
Mental Development Index: MD 1.24, 95% CI ‐2.62 to 5.09 (Analysis 1.16).
-
Psychomotor Development Index: MD ‐0.32, 95% CI ‐3.48 to 2.79 (Analysis 1.17).
"Severe neurodevelopmental disability" (Amiel‐Tison 1986 classification) was assessed in children aged 18 months post‐term in two trials. Neither Lucas 1984a nor Lucas 1984b, or a meta‐analysis of data from both trials, showed a difference: typical RR 1.21 (95% CI 0.62 to 2.35; I² = 17%; 2 trials, 400 participants); RD ‐0.02 (95% CI ‐0.04 to 0.17); moderate‐certainty evidence; summary of findings Table for the main comparison; Analysis 1.18).
O'Connor 2016 did not show any differences in the mean scores on Bayley Scales of Infant and Toddler Development, Third Edition (Bayley‐III) assessments at 18 to 22 months' corrected age.
-
Cognitive: MD 1.60, 95% CI ‐2.71 to 5.91 (Analysis 1.19).
-
Language: MD 3.00, 95% CI ‐2.01 to 8.01 (Analysis 1.19).
-
Motor: MD 2.20, 95% CI ‐2.07 to 6.47 (Analysis 1.19).
There were not any differences in the proportion of children with Bayley‐III scores < 70 in O'Connor 2016.
-
Cognitive: RR 0.82, 95% CI 0.40 to 1.68 (Analysis 1.20); RD ‐0.02, 95% CI ‐0.08 to 0.05.
-
Language: RR 0.78, 95% CI 0.47 to 1.30 (Analysis 1.20); RD ‐0.04 (95% CI ‐0.13 to 0.04).
-
Motor: RR 0.73, 95% CI 0.37 to 1.44 (Analysis 1.20); RD ‐0.03 (95% CI ‐0.10 to 0.04).
There were not any differences in the proportion of children diagnosed with cerebral palsy, or hearing or visual impairment in O'Connor 2016.
-
Cerebral palsy: RR 0.51, 95% CI 0.21 to 1.23 (Analysis 1.21); RD ‐0.05 (95% CI ‐0.10 to 0.01).
-
Hearing impairment: RR 1.02, 95% CI 0.30 to 3.45 (Analysis 1.22); RD 0.00 (95% CI ‐0.04 to 0.04).
-
Visual impairment: RR (not estimable ‐ no events; Analysis 1.23); RD 0.00 (95% CI ‐0.01 to 0.01).
Cognitive and educational outcomes in children aged more than five years old
Lucas 1984a and Lucas 1984b assessed cognitive outcomes (verbal and performance intelligence quotient) in about 20% of participants at ages eight and 16 years. Numerical data were not reported for the individual trials but rather were combined with data from another trial undertaken by the same investigators that compared feeding preterm infants with nutrient‐enriched versus standard formula (Isaacs 2009).
O'Connor 2016 has not yet reported any cognitive and educational outcomes in children aged more than five years old.
Secondary outcomes
All‐cause mortality
Data were available from seven trials. Two trials reported mortality until nine months post‐term (Lucas 1984a; Lucas 1984b). The other trials reported mortality until hospital discharge (Schanler 2005; Cristofalo 2013; Corpeleijn 2016; O'Connor 2016; Costa 2018). None showed a difference between the groups. Since it is likely that most infant mortality in this population occurred before hospital discharge, we combined the data from the trials in a meta‐analysis: RR 1.10, 95% CI 0.80 to 1.50; I² = 0%; 7 trials, 1527 participants; moderate‐certainty evidence; summary of findings Table for the main comparison; Analysis 1.24). There were not any significant subgroup differences (Figure 6).

Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.24 All‐cause mortality.
Necrotising enterocolitis
Meta‐analysis of data available from nine trials showed a higher risk of necrotising enterocolitis in the formula‐fed group: RR 1.87, 95% CI 1.23 to 2.85; I² = 14%; 9 trials, 1675 participants; RD 0.03, 95% CI 0.01 to 0.05; number needed to treat for an additional harmful outcome (NNTH) 33 (95% CI 20 to 100); moderate‐certainty evidence; summary of findings Table for the main comparison; Analysis 1.25). There were not any significant subgroup differences (Figure 7).

Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.25 Necrotising enterocolitis.
Days after birth to establish full enteral feeding
This was reported by three trials. A meta‐analysis of data from Cristofalo 2013 and Costa 2018 did not show a difference (MD 0.33, 95% CI ‐2.57 to 3.23 days; Analysis 1.26). There were not any significant subgroup differences.
Corpeleijn 2016 reported no difference in median time to full feeds independent of parenteral nutrition (12 versus 11 days) but did not provide sufficient data for inclusion in a meta‐analysis.
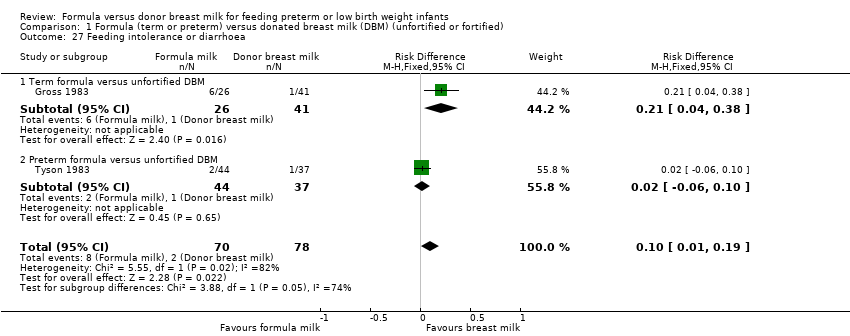
Feeding intolerance
Meta‐analysis of data from Gross 1983 and Tyson 1983 showed a higher incidence of feeding intolerance in the formula‐fed group (RR 4.92, 95% CI 1.17 to 20.70; RD 0.10, 95% CI 0.01 to 0.19; NNTH 10, 95% CI 5 to 100; Analysis 1.27).
Lucas 1984a reported that significantly more infants in the formula‐fed group failed to tolerate full enteral feeds by two weeks after birth (25/76 versus 9/83 in the donor breast milk group) and by three weeks after birth (13/76 versus 4/83).
Incidence of invasive infection
Meta‐analysis of data available from five trials did not show a difference in invasive infection (RR 0.94, 95% CI 0.79 to 1.12; I² = 37%; 5 trials, 1025 infants; Analysis 1.28). There were not any significant subgroup differences.
Subgroup analysis: formula versus donor breast milk as (i) sole diet or (ii) supplement to maternal expressed breast milk
-
Seven trials compared feeding with formula versus donor breast milk as a sole diet (Raiha 1976; Davies 1977; Schultz 1980; Gross 1983; Tyson 1983; Lucas 1984a; Cristofalo 2013).
-
Five trials compared feeding with formula versus donor breast milk as a supplement to maternal expressed breast milk (Lucas 1984b; Schanler 2005; Corpeleijn 2016; O'Connor 2016; Costa 2018).
Growth
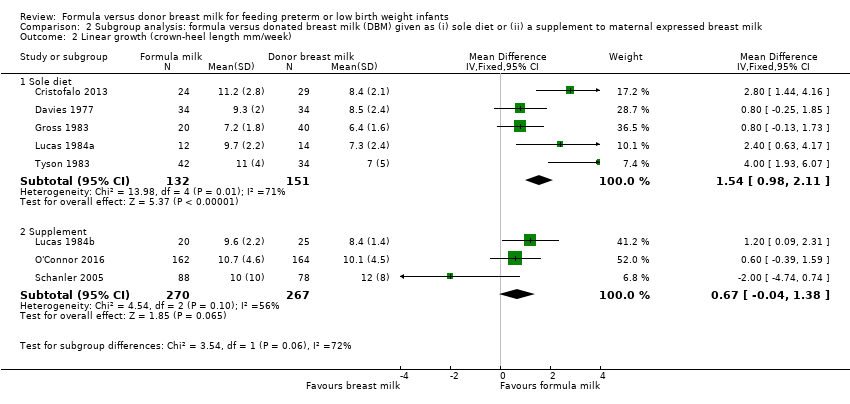
Meta‐analyses did not show subgroup differences for rate of weight gain (Analysis 2.1), or increase in crown‐heel length (Analysis 2.2).
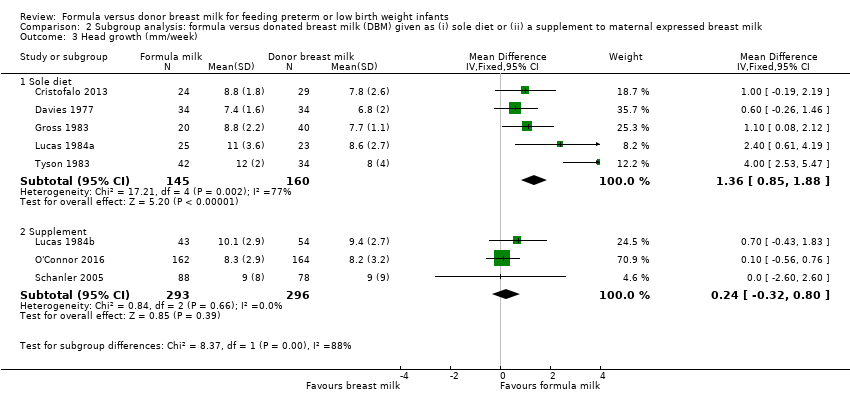
Subgroup comparisons showed significant differences for head growth.
-
Sole diet: MD 1.36, 95% CI 0.85 to 1.88 mm/week.
-
Supplement: MD 0.24, 95% CI ‐0.32 to 0.80 mm/week.
-
Test for subgroup differences: Chi² = 8.37, df = 1 (P = 0.004), I² = 88.1% (Analysis 2.3).
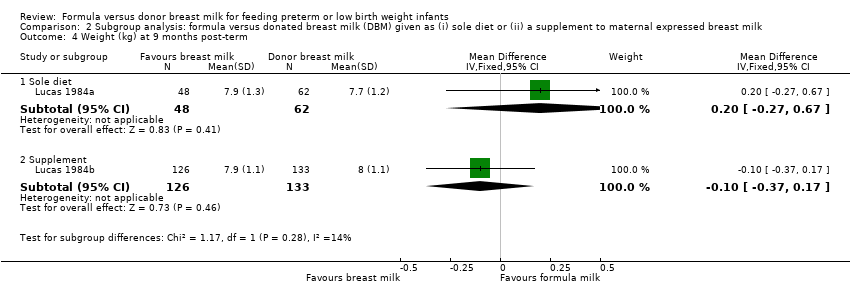
Meta‐analyses of data from Lucas 1984a (sole diet) and Lucas 1984b (supplemental) did not show any subgroup differences for long‐term growth (Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9; Analysis 2.10; Analysis 2.11; Analysis 2.12).
Neurodevelopment
Meta‐analyses of data from Lucas 1984a (sole diet) and Lucas 1984b (supplemental) did not show any subgroup differences for neurodevelopmental outcomes (Analysis 2.13; Analysis 2.14; Analysis 2.15).
Secondary outcomes
Meta‐analyses did not show significant subgroup differences for all‐cause mortality (Analysis 2.16), or necrotising enterocolitis (Analysis 2.17).
Subgroup comparisons showed significant differences for incidence of invasive infection.
-
Sole diet: RR 1.43, 95% CI 0.97 to 2.11; RD 0.24, 95% CI ‐0.00 to 0.48.
-
Supplement: RR 0.89, 95% CI 0.73 to 1.08; RD ‐0.03, 95% CI ‐0.09 to 0.02.
-
Test for subgroup differences: Chi² = 4.70, df = 1 (P = 0.03), I² = 78.7% (Analysis 2.18).
Discussion
Summary of main results
We included 12 randomised controlled trials (RCTs) in which a total of 1879 preterm or low birth weight (LBW) infants participated. Meta‐analyses show that infants who receive formula regain birth weight earlier and have higher in‐hospital rates of weight gain, linear growth, and head growth than infants who receive donor breast milk. These effects on growth parameters are greater in trials that compare feeding with nutrient‐enriched preterm formula rather than standard term formula versus donor breast milk. Follow‐up of the infants who participated in two of the largest trials did not show any effects on long‐term growth. None of the trials that assessed neurodevelopment beyond infancy showed any significant effects.
Meta‐analysis of data from eight trials shows that feeding with formula rather than donor breast milk increases the risk of necrotising enterocolitis in preterm and LBW infants.
Overall completeness and applicability of evidence
These findings should be interpreted with caution. Substantial heterogeneity in the meta‐analyses of weight gain, linear growth, and head growth limits the validity of the pooled estimates of effect size. Many of the trials that contributed data to these meta‐analyses were undertaken more than 20 years ago and the trials used different inclusion criteria and varied with respect to the type of formula and donor breast milk. Five trials have been undertaken in the past 20 years and four of these trials compared feeding with preterm formula versus donor breast milk with added multinutrient fortifier (Schanler 2005; Cristofalo 2013; Corpeleijn 2016; O'Connor 2016). Subgroup analyses of data from these trials, which are more likely to be applicable to current practice in high‐income countries, where nutrient fortification of breast milk is commonly undertaken, shows higher rates of weight gain and linear growth in formula‐fed infants, but no effect on head growth.
The pooled estimate from meta‐analysis of data from nine trials suggests that one extra case of necrotising enterocolitis will occur in every 33 infants who receive formula. This beneficial effect of donor breast milk exists even when donor breast milk is given as a supplement to maternal breast milk, rather than as a sole diet, and when the donor breast milk is nutrient‐fortified. The subgroup meta‐analysis of four trials that compared feeding infants with preterm (nutrient‐enriched) formula versus nutrient‐fortified donor breast milk was moderately heterogeneous (I² = 51%). A possible explanation is that the trials differed in the intensity and duration of exposure to the intervention. Infants participating in Corpeleijn 2016 (the trial that contributed most to the heterogeneity) received the trial interventions for only the first 10 days after birth as maternal (mother's own) breast milk was widely available by this stage. In the other three trials, in contrast, infants received the allocated intervention for up to 90 days or the duration of birth hospitalisation (Schanler 2005; Cristofalo 2013; O'Connor 2016). It is plausible that donor breast milk is less effective in preventing necrotising enterocolitis in settings where formula (rather than maternal breast milk) use is more prevalent.
Most of the trials included in the meta‐analysis did not mask caregivers and assessors to the intervention. This methodological weakness may have resulted in surveillance and ascertainment biases that contributed to the higher rate of detection of necrotising enterocolitis in formula‐fed infants. Caution should be exercised in applying these data to growth‐restricted preterm infants or sick infants since these infants, although at high risk of developing necrotising enterocolitis, were ineligible to participate in many of the included trials.
The data in this review are from trials undertaken in high‐income countries. In low‐ or middle‐incomes countries, the immunoactive properties of breast milk may confer advantages that outweigh the lower rate of short‐term growth. In India, a RCT in LBW infants "at risk of infection" found that serious infections (diarrhoea, pneumonia, septicaemia) were less common in infants allocated to received "expressed human milk" versus formula milk (Narayanan 1982). "Expressed human milk" in this study referred to a mixture of maternal and donor breast milk. As we could not separate these into subgroups, we did not include the data in the review.
Quality of the evidence
The GRADE certainty of evidence was moderate for rates of weight gain, linear growth, and head growth (downgraded for high levels of heterogeneity) and was moderate for neurodevelopmental disability, all‐cause mortality, and necrotising enterocolitis (downgraded for imprecision) (summary of findings Table for the main comparison).
Some of the trials contained various weaknesses in methodological quality, specifically concern about allocation concealment methods in four trials, and about methods to ensure masking in most of the trials. Parents, caregivers, clinicians and investigators were likely to have been aware of the treatment group to which infants had been allocated and this knowledge may have affected some care practices or investigation strategies, including thresholds for screening or diagnosing for necrotising enterocolitis.
Most of the included trials were funded or supported by the manufacturers of the formulas being assessed, but the funders were not involved in trial design or analysis. There remains some concern that formula manufacturers may promote study findings of trials of specialist formulas selectively as part of a marketing strategy that subverts UNICEF Baby Friendly Initiative regulations (Cleminson 2015).
Potential biases in the review process
The main concern with the review process is the possibility that the findings are subject to publication and other reporting biases, including more availability of numerical data for inclusion in meta‐analyses from trials that reported statistically significant or clinically important effects. We attempted to minimise this threat by screening the reference lists of included trials and related reviews and searching the proceedings of the major international perinatal conferences to identify trial reports that are not (or not yet) published in full form in academic journals. However, we cannot be sure whether other trials have been undertaken, but not reported, and the concern remains that such trials are less likely than published trials to have detected statistically significant or clinically important effects. The meta‐analyses that we performed did not contain sufficient trials to explore symmetry of funnel plots as a means of identifying possible publication or reporting bias.
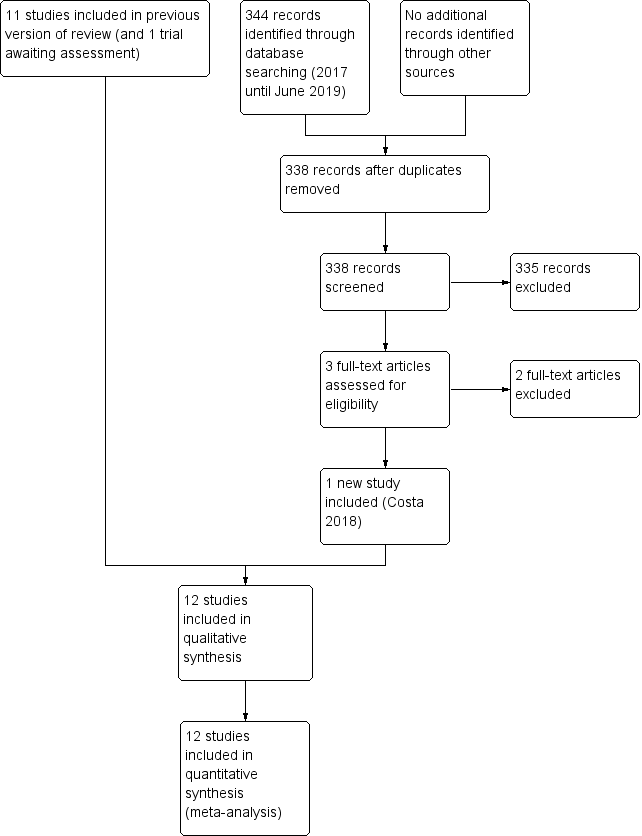
Study flow diagram: 2019 review update.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.2 Weight gain (g/kg/day).
Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.3 Linear growth (crown‐heel length mm/week).

Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.6 Head growth (mm/week).

Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.24 All‐cause mortality.

Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.25 Necrotising enterocolitis.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 1 Time to regain birth weight (days from birth).

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 2 Weight gain (g/kg/day).

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 3 Linear growth (crown‐heel length mm/week).

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 4 Linear growth (crown‐rump length mm/week).

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 5 Linear growth (femoral length mm/week).
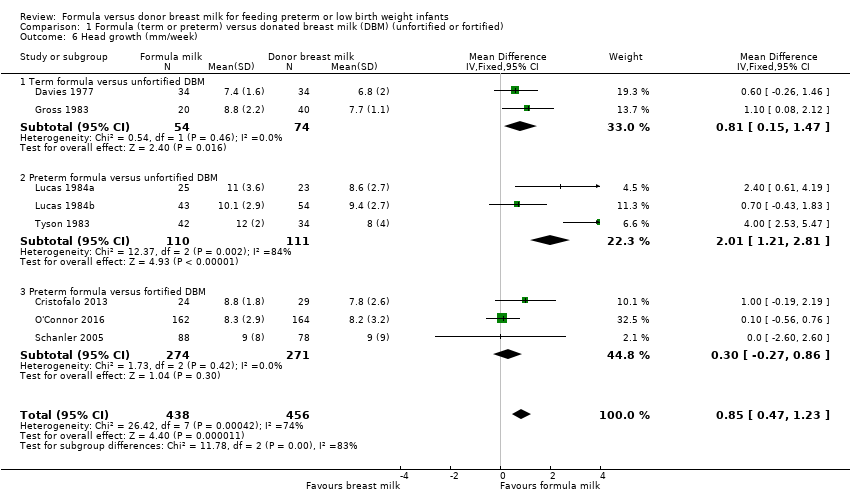
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 6 Head growth (mm/week).

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 7 Weight (kg) at 9 months post‐term.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 8 Length (cm) at 9 months post‐term.
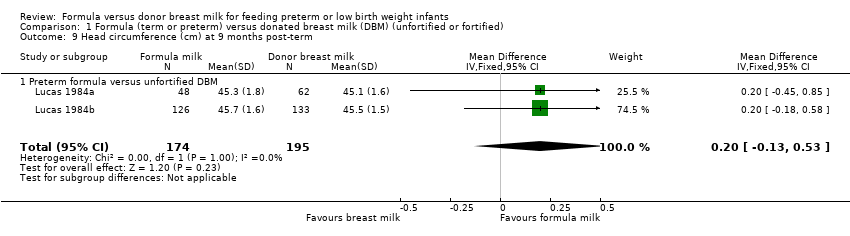
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 9 Head circumference (cm) at 9 months post‐term.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 10 Weight (kg) at 18 months post‐term.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 11 Length (cm) at 18 months post‐term.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 12 Head circumference (cm) at 18 months post‐term.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 13 Weight (kg) at 7.5 to 8 years of age.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 14 Length (cm) at 7.5 to 8 years of age.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 15 Head circumference (cm) at 7.5 to 8 years of age.
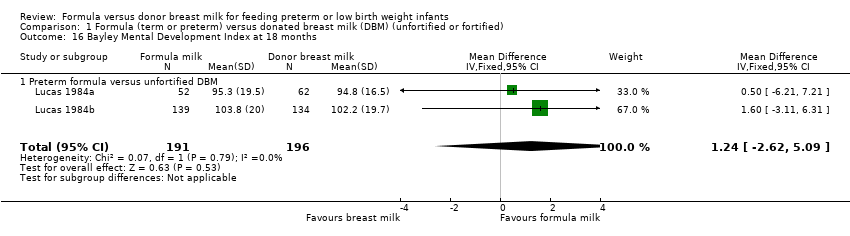
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 16 Bayley Mental Development Index at 18 months.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 17 Bayley Psychomotor Development Index at 18 months.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 18 Neurodevelopmental disability at 18 months.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 19 Bayley‐III.
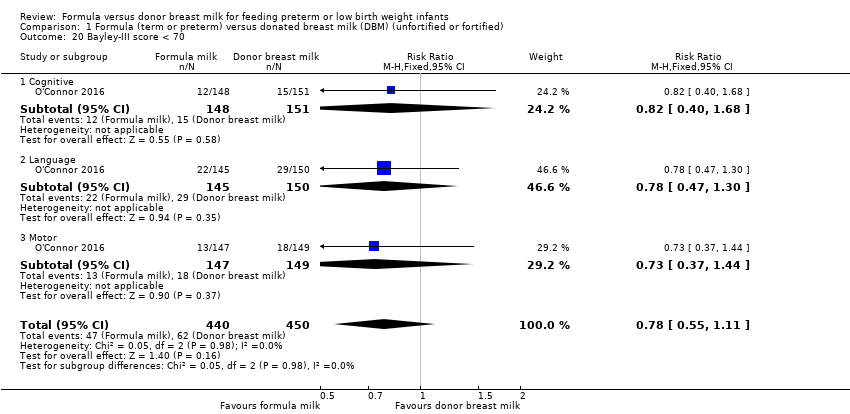
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 20 Bayley‐III score < 70.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 21 Cerebral palsy.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 22 Hearing impairment.
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 23 Visual impairment.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 24 All‐cause mortality.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 25 Necrotising enterocolitis.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 26 Days after birth to establish full enteral feeding.
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 27 Feeding intolerance or diarrhoea.

Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 28 Invasive infection.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 1 Weight gain (g/kg/day).
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 2 Linear growth (crown‐heel length mm/week).
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 3 Head growth (mm/week).
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 4 Weight (kg) at 9 months post‐term.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 5 Length (cm) at 9 months post‐term.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 6 Head circumference (cm) at 9 months post‐term.
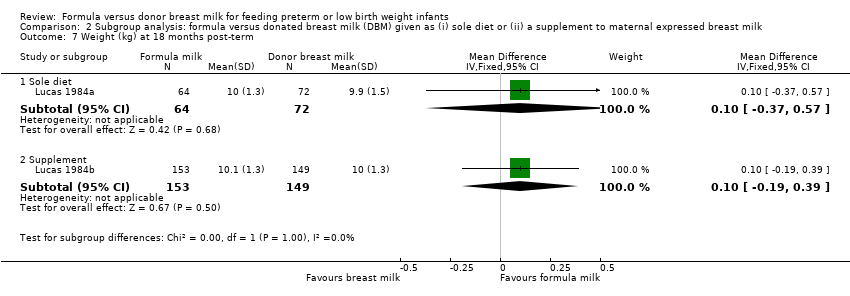
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 7 Weight (kg) at 18 months post‐term.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 8 Length (cm) at 18 months post‐term.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 9 Head circumference (cm) at 18 months post‐term.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 10 Weight (kg) at 7.5 to 8 years of age.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 11 Length (cm) at 7.5 to 8 years of age.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 12 Head circumference (cm) at 7.5 to 8 years of age.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 13 Bayley Mental Development Index at 18 months.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 14 Bayley Psychomotor Development Index at 18 months.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 15 Neurodevelopmental disability at 18 months.
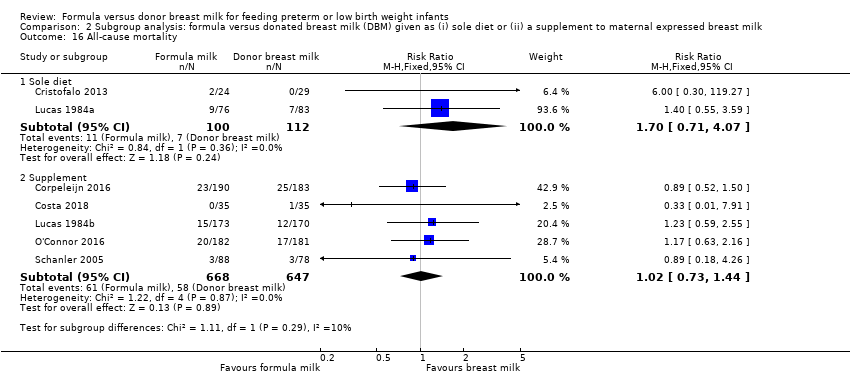
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 16 All‐cause mortality.
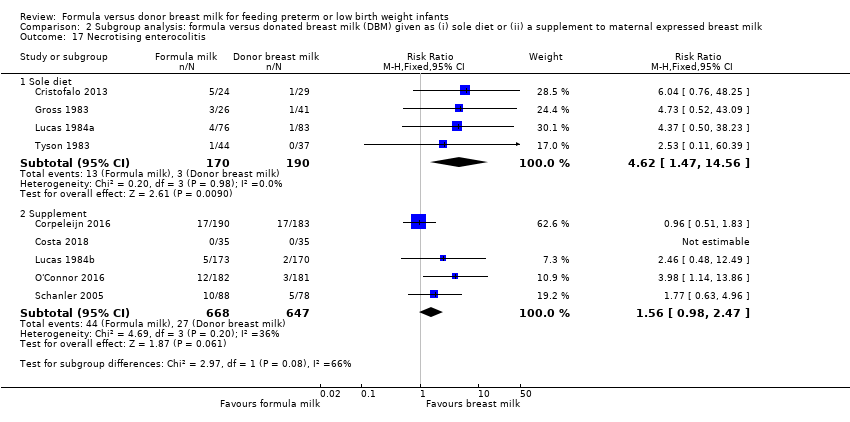
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 17 Necrotising enterocolitis.

Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 18 Incidence of invasive infection.
| Formula (term or preterm) compared to donor breast milk (unfortified or fortified) for feeding preterm or low birth weight infants | ||||||
| Patient or population: preterm or low birth weight infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with donated breast milk (unfortified or fortified) | Risk with formula (term or preterm) | |||||
| Weight gain (g/kg/day) | ‐ | MD 2.51 higher | ‐ | 1028 | Moderatea | I² = 90% |
| Linear growth (crown‐heel length mm/week) | ‐ | MD 1.21 higher | ‐ | 820 | Moderatea | I² = 68% |
| Head growth (mm/week) | ‐ | MD 0.85 higher | ‐ | 894 | Moderatea | I² = 74% |
| Neurodevelopmental disability | Study population | RR 1.21 | 400 | Moderateb | ||
| 73 per 1000 | 88 per 1000 (45 to 171) | |||||
| All‐cause mortality | Study population | RR 1.1 | 1527 | Moderateb | ||
| 86 per 1000 | 94 per 1000 | |||||
| Necrotising enterocolitis | Study population | RR 1.87 | 1675 | Moderateb | ||
| 36 per 1000 | 67 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| aDowngraded one level for heterogeneity. | ||||||
| CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to regain birth weight (days from birth) Show forest plot | 3 | 236 | Mean Difference (IV, Fixed, 95% CI) | ‐3.08 [‐4.38, ‐1.77] |
| 1.1 Term formula versus unfortified DBM | 2 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐5.81, ‐2.18] |
| 1.2 Preterm formula versus unfortified DBM | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐3.97, ‐0.23] |
| 2 Weight gain (g/kg/day) Show forest plot | 9 | 1028 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [1.93, 3.08] |
| 2.1 Term formula versus unfortified DBM | 3 | 234 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [0.96, 2.53] |
| 2.2 Preterm formula versus unfortified DBM | 3 | 249 | Mean Difference (IV, Fixed, 95% CI) | 4.16 [3.04, 5.28] |
| 2.3 Preterm formula versus fortified DBM | 3 | 545 | Mean Difference (IV, Fixed, 95% CI) | 2.37 [1.09, 3.65] |
| 3 Linear growth (crown‐heel length mm/week) Show forest plot | 8 | 820 | Mean Difference (IV, Fixed, 95% CI) | 1.21 [0.77, 1.65] |
| 3.1 Term formula versus unfortified DBM | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.10, 1.50] |
| 3.2 Preterm formula versus unfortified DBM | 3 | 147 | Mean Difference (IV, Fixed, 95% CI) | 1.96 [1.10, 2.82] |
| 3.3 Preterm formula versus fortified DBM | 3 | 545 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.33, 1.87] |
| 4 Linear growth (crown‐rump length mm/week) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Term formula versus unfortified DBM | 1 | 106 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [0.08, 1.10] |
| 5 Linear growth (femoral length mm/week) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Term formula versus unfortified DBM | 1 | 106 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.13, 0.55] |
| 6 Head growth (mm/week) Show forest plot | 8 | 894 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.47, 1.23] |
| 6.1 Term formula versus unfortified DBM | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [0.15, 1.47] |
| 6.2 Preterm formula versus unfortified DBM | 3 | 221 | Mean Difference (IV, Fixed, 95% CI) | 2.01 [1.21, 2.81] |
| 6.3 Preterm formula versus fortified DBM | 3 | 545 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.27, 0.86] |
| 7 Weight (kg) at 9 months post‐term Show forest plot | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.21] |
| 7.1 Preterm formula versus unfortified DBM | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.21] |
| 8 Length (cm) at 9 months post‐term Show forest plot | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.64, 0.70] |
| 8.1 Preterm formula versus unfortified DBM | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.64, 0.70] |
| 9 Head circumference (cm) at 9 months post‐term Show forest plot | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.13, 0.53] |
| 9.1 Preterm formula versus unfortified DBM | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.13, 0.53] |
| 10 Weight (kg) at 18 months post‐term Show forest plot | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.15, 0.35] |
| 10.1 Preterm formula versus unfortified DBM | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.15, 0.35] |
| 11 Length (cm) at 18 months post‐term Show forest plot | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.15, 1.20] |
| 11.1 Preterm formula versus unfortified DBM | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.15, 1.20] |
| 12 Head circumference (cm) at 18 months post‐term Show forest plot | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 12.1 Preterm formula versus unfortified DBM | 2 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 13 Weight (kg) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.42, 0.29] |
| 13.1 Preterm formula versus unfortified DBM | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.42, 0.29] |
| 14 Length (cm) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.12, 1.23] |
| 14.1 Preterm formula versus unfortified DBM | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.12, 1.23] |
| 15 Head circumference (cm) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 15.1 Preterm formula versus unfortified DBM | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 16 Bayley Mental Development Index at 18 months Show forest plot | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [‐2.62, 5.09] |
| 16.1 Preterm formula versus unfortified DBM | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [‐2.62, 5.09] |
| 17 Bayley Psychomotor Development Index at 18 months Show forest plot | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐3.43, 2.79] |
| 17.1 Preterm formula versus unfortified DBM | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐3.43, 2.79] |
| 18 Neurodevelopmental disability at 18 months Show forest plot | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.62, 2.35] |
| 18.1 Preterm formula versus unfortified DBM | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.62, 2.35] |
| 19 Bayley‐III Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 Cognitive | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐2.71, 5.91] |
| 19.2 Language | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐2.01, 8.01] |
| 19.3 Motor | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐2.07, 6.47] |
| 20 Bayley‐III score < 70 Show forest plot | 1 | 890 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.55, 1.11] |
| 20.1 Cognitive | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.68] |
| 20.2 Language | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.47, 1.30] |
| 20.3 Motor | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.37, 1.44] |
| 21 Cerebral palsy Show forest plot | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.21, 1.23] |
| 22 Hearing impairment Show forest plot | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.30, 3.45] |
| 23 Visual impairment Show forest plot | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 All‐cause mortality Show forest plot | 7 | 1527 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.80, 1.50] |
| 24.1 Preterm formula versus unfortified DBM | 3 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.70, 2.14] |
| 24.2 Preterm formula versus fortified DBM | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.52] |
| 25 Necrotising enterocolitis Show forest plot | 9 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.23, 2.85] |
| 25.1 Term formula versus unfortified DBM | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.73 [0.52, 43.09] |
| 25.2 Preterm formula versus unfortified DBM | 4 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.90, 9.87] |
| 25.3 Preterm formula versus fortified DBM | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.03, 2.61] |
| 26 Days after birth to establish full enteral feeding Show forest plot | 2 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐2.57, 3.23] |
| 26.1 Preterm formula versus unfortified DBM | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.66, 2.66] |
| 26.2 Preterm formula versus fortified DBM | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐2.56, 11.96] |
| 27 Feeding intolerance or diarrhoea Show forest plot | 2 | 148 | Risk Difference (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.19] |
| 27.1 Term formula versus unfortified DBM | 1 | 67 | Risk Difference (M‐H, Fixed, 95% CI) | 0.21 [0.04, 0.38] |
| 27.2 Preterm formula versus unfortified DBM | 1 | 81 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.06, 0.10] |
| 28 Invasive infection Show forest plot | 5 | 1025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| 28.1 Preterm formula versus unfortified DBM | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.93] |
| 28.2 Preterm formula versus fortified DBM | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight gain (g/kg/day) Show forest plot | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sole diet | 6 | 421 | Mean Difference (IV, Fixed, 95% CI) | 2.65 [1.94, 3.36] |
| 1.2 Supplement | 3 | 607 | Mean Difference (IV, Fixed, 95% CI) | 2.22 [1.23, 3.21] |
| 2 Linear growth (crown‐heel length mm/week) Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sole diet | 5 | 283 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [0.98, 2.11] |
| 2.2 Supplement | 3 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.67 [‐0.04, 1.38] |
| 3 Head growth (mm/week) Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Sole diet | 5 | 305 | Mean Difference (IV, Fixed, 95% CI) | 1.36 [0.85, 1.88] |
| 3.2 Supplement | 3 | 589 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.32, 0.80] |
| 4 Weight (kg) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Sole diet | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.27, 0.67] |
| 4.2 Supplement | 1 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.37, 0.17] |
| 5 Length (cm) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Sole diet | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.93, 1.73] |
| 5.2 Supplement | 1 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.88, 0.68] |
| 6 Head circumference (cm) at 9 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Sole diet | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.45, 0.85] |
| 6.2 Supplement | 1 | 259 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.18, 0.58] |
| 7 Weight (kg) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Sole diet | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.37, 0.57] |
| 7.2 Supplement | 1 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 8 Length (cm) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Sole diet | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.68, 1.88] |
| 8.2 Supplement | 1 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.29, 1.29] |
| 9 Head circumference (cm) at 18 months post‐term Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Sole diet | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.44, 0.64] |
| 9.2 Supplement | 1 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.25, 0.45] |
| 10 Weight (kg) at 7.5 to 8 years of age Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Sole diet | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.24, 2.24] |
| 10.2 Supplement | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.88, 0.08] |
| 11 Length (cm) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.12, 1.23] |
| 11.1 Sole diet | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.26, 3.26] |
| 11.2 Supplement | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.68, 1.08] |
| 12 Head circumference (cm) at 7.5 to 8 years of age Show forest plot | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 12.1 Sole diet | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.56, 0.76] |
| 12.2 Supplement | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.71, 0.11] |
| 13 Bayley Mental Development Index at 18 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Sole diet | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐6.21, 7.21] |
| 13.2 Supplement | 1 | 273 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐3.11, 6.31] |
| 14 Bayley Psychomotor Development Index at 18 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Sole diet | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐4.38, 6.78] |
| 14.2 Supplement | 1 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.74, 2.74] |
| 15 Neurodevelopmental disability at 18 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Sole diet | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.64, 6.68] |
| 15.2 Supplement | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.40, 2.10] |
| 16 All‐cause mortality Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Sole diet | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.71, 4.07] |
| 16.2 Supplement | 5 | 1315 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.73, 1.44] |
| 17 Necrotising enterocolitis Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Sole diet | 4 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [1.47, 14.56] |
| 17.2 Supplement | 5 | 1315 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.98, 2.47] |
| 18 Incidence of invasive infection Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Sole diet | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.97, 2.11] |
| 18.2 Supplement | 4 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.08] |

