Treadmill training and body weight support for walking after stroke
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel group design. | |
| Participants | 14 participants in the EXP group, and 15 participants in the CTL group. | |
| Interventions | Treated as outpatients for 3 30‐minute sessions per week for 4 weeks. | |
| Outcomes | Assessed at baseline, after treatment phase and 3 month follow up: | |
| Notes | Obtained unpublished data by interview and correspondence with the trialists. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Parallel group design. | |
| Participants | 7 participants in the EXP group, and 8 participants in the CTL group. | |
| Interventions | Treated as inpatients for 5 20‐minute sessions per week for 2‐3 weeks. | |
| Outcomes | Assessed at baseline and after treatment phase: | |
| Notes | The rating of dropouts and the allocation concealment classification were changed based on correspondence from the trialist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Parallel group design. | |
| Participants | 6 participants in the EXP group, and 6 participants in the CTL group. | |
| Interventions | Treated as outpatients for 3 1‐hour sessions per week for 4 weeks. | |
| Outcomes | Assessed at baseline, after treatment phase and 2 months later: | |
| Notes | The CTL group received sham treatment (ie, upper limb training) ‐ at the end of training all but 1 subject indicated that they would recommend the program to others. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Parallel group design. | |
| Participants | 25 participants in the EXP group, and 25 participants in the CTL group. | |
| Interventions | Treated as inpatients for 5 30‐minute sessions per week for 6 weeks. | |
| Outcomes | Assessed at baseline, after treatment phase, and 3 months later: | |
| Notes | Method of randomisation and the allocation concealment classification were changed based on correspondence from the trialist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Parallel group design. | |
| Participants | 11 participants in the EXP group, and 12 participants in the CTL group. | |
| Interventions | Treated as outpatients for 6 1‐hour sessions per week for 2 weeks. | |
| Outcomes | Assessed at baseline, after treatment phase and 2 weeks later: | |
| Notes | Rating of concealed allocation, assessor blinding and dropouts, and the allocation concealment classification were changed based on correspondence from the trialist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Parallel group design. | |
| Participants | 22 participants in the EXP group, and 34 participants in the CTL group. | |
| Interventions | Treated as inpatients for 5 45‐minute sessions per week for an average of 12.5 (SD 4.7) total treatment sessions. | |
| Outcomes | Assessed at baseline and after treatment phase: | |
| Notes | Rating of concealed allocation and the allocation concealment classification were changed based on correspondence from the trialist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Parallel group design. | |
| Participants | 15 participants in the EXP group, and 14 participants in the CTL group. | |
| Interventions | Treated as inpatients for 5 sessions of up to 20 minutes per week for 3 weeks (15 treatment sessions). | |
| Outcomes | Assessed at baseline and after treatment phase: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Cross‐over group design. | |
| Participants | 10 participants allocated to the EXP then CTL order, and 8 participants allocated to the CTL then EXP order. | |
| Interventions | Treated as inpatients or outpatients for 3 1‐hour sessions per week for 4 weeks. | |
| Outcomes | Assessed at baseline, at cross‐over (4 weeks), after treatment phase (at 8 weeks) and 6 weeks after final treatment: | |
| Notes | The rating of dropouts was changed based on correspondence from the trialist. Data treated as a parallel group design for this review by using the first treatment phase only (that is baseline and cross‐over data only). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Parallel group design. | |
| Participants | 32 participants in the EXP group, and 29 participants in the CTL group. | |
| Interventions | Treated as outpatients for 3 40‐minute sessions per week for 6 months. | |
| Outcomes | Assessed at baseline and after treatment phase: | |
| Notes | Method of randomisation and rating of assessor blinding were changed based on correspondence from the trialist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Parallel group design. | |
| Participants | 36 participants in the EXP group, and 37 participants in the CTL group. | |
| Interventions | Treated as inpatients for 5 30‐minute sessions per week for the duration of inpatient rehabilitation. | |
| Outcomes | Assessed at baseline, after treatment phase (when discharged from inpatient rehabilitation) and 10 months after stroke: | |
| Notes | Allocation concealment classification was changed based on correspondence from the trialist. Data divided into two comparisons, see Nilsson 2001a and Nilsson 2001b. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | See Nilsson 2001 | |
| Participants | See Nilsson 2001 | |
| Interventions | See Nilsson 2001 | |
| Outcomes | See Nilsson 2001 | |
| Notes | For Nilsson 2001a, data from the 54 participants who were dependent walkers at the start of treatment were used (26 EXP and 28 CTL). These walking dependency data were obtained through correspondence with the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | See Nilsson 2001 | |
| Participants | See Nilsson 2001 | |
| Interventions | See Nilsson 2001 | |
| Outcomes | See Nilsson 2001 | |
| Notes | For Nilsson 2001b, data from the 19 participants who were independent walkers at the start of treatment were used (10 EXP and 9 CTL). These walking dependency data were obtained through correspondence with the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Parallel group design. | |
| Participants | 22 participants in the EXP 1 group, 22 participants in the EXP 2 group and 25 participants in the CTL group. | |
| Interventions | Treated as inpatients for 3 30‐ (EXP 1 and EXP 2) or 45‐ (CTL) minute sessions per week for 4 weeks. | |
| Outcomes | Assessed at baseline and after treatment phase: | |
| Notes | The rating of concealed allocation and the allocation concealment classification were changed based on correspondence from the trialist. Data divided into two comparisons, see Pohl 2002a and Pohl 2002b. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | See Pohl 2002. | |
| Participants | See Pohl 2002. | |
| Interventions | See Pohl 2002. | |
| Outcomes | See Pohl 2002. | |
| Notes | For Pohl 2002a, EXP 1 group was compared to CTL. Half of the CTL group data were used for this comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | See Pohl 2002. | |
| Participants | See Pohl 2002. | |
| Interventions | See Pohl 2002. | |
| Outcomes | See Pohl 2002. | |
| Notes | For Pohl 2002b, EXP 2 group was compared to CTL. Half of the CTL group data were used for this comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Parallel group design. | |
| Participants | 10 participants in the EXP group, 8 participants in the CTL 1 group, and 9 participants in the CTL 2 group. | |
| Interventions | Treated as inpatients for 6 weeks for a mean of 1.74 (SD 0.15) (EXP), 1.79 (SD 0.10) (CTL 1) and 0.72 (SD 0.10) (CTL 2) hours per day. | |
| Outcomes | Assessed at baseline, after treatment phase and 3 and 6 months later: | |
| Notes | 3 and 6 month follow‐up data not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Cross‐over group design. | |
| Participants | 15 participants allocated to the EXP then CTL order, and 15 participants allocated to the CTL then EXP order. | |
| Interventions | Treated as inpatients for 5 1‐hour sessions per week for 3 weeks. | |
| Outcomes | Assessed at baseline, at cross‐over (3 weeks), and after treatment phase (at 6 weeks): | |
| Notes | Data treated as a parallel group design for this review by using the first treatment phase only (that is baseline and cross‐over data only). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Parallel group design. | |
| Participants | 50 participants in the EXP group, and 50 participants in the CTL group. | |
| Interventions | Treated as inpatients for 4 20‐minute session per week for 6 weeks. | |
| Outcomes | Assessed at baseline, after treatment phase and 3 months later: | |
| Notes | The rating of concealed allocation and the allocation concealment classification were changed based on correspondence from the trialist. Data divided into two comparisons, see Visintin 1998a and Visintin 1998b. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | See Visintin 1998. | |
| Participants | See Visintin 1998. | |
| Interventions | See Visintin 1998. | |
| Outcomes | See Visintin 1998. | |
| Notes | For Visintin 1998a, data from the 59 participants who were dependent walkers at the start of treatment and who did not dropout before the end of the treatment phase were used (33 EXP and 26 CTL). These walking dependency data were obtained through correspondence with the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | See Visintin 1998. | |
| Participants | See Visintin 1998. | |
| Interventions | See Visintin 1998. | |
| Outcomes | See Visintin 1998. | |
| Notes | For Visintin 1998b, data from the 20 participants who were independent walkers at the start of treatment and who did not dropout before the end of the treatment phase were used (10 EXP and 10 CTL). These walking dependency data were obtained through correspondence with the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Cross‐over group design. | |
| Participants | 15 participants allocated to the EXP then CTL order, and 15 participants allocated to the CTL then EXP order. | |
| Interventions | Treated as inpatients for 5 15‐20 minute sessions per week for 2 weeks. | |
| Outcomes | This was an A‐B‐A (or B‐A‐B) design, so participants were assessed at baseline, at first cross‐over (2 weeks), at second cross‐over (4 weeks) and after treatment phase (6 weeks): | |
| Notes | The number of dropouts was changed based on correspondence with the trialists. Data treated as a parallel group design for this review by using the first treatment phase only (that is baseline and first cross‐over data only). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ADL: activities of daily living
CT: computed tomography
CTL: control
EMG: electromyographic activity
EXP: experimental
FAC: Functional Ambulation Category
FIM: Functional Independence Measure
km/hr: kilometres per hour
m/min: metre per minute
m/sec: metre per second
MRI: magnetic resonance imaging
RMAS: Rivermead Motor Assessment Scale
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Correspondence with the author revealed that the trial was abandoned. | |
| Correspondence with the author revealed that the trial was abandoned after the recruitment of only five participants (each allocated to one of three treatment groups). | |
| Both groups received treadmill training. The parameter that was experimentally manipulated was electrical stimulation. | |
| Evaluated a single treatment session, not a full course of treatment. | |
| Correspondence with the author revealed that the trial was abandoned before the commencement of recruitment. | |
| Correspondence with the author revealed that less than 20% of participants in the EXP group participated in treadmill training (that is, only 6 out of 31 participants). | |
| Correspondence with the author revealed that treadmill training (with or without body weight support) was not used in either group. | |
| Correspondence with the author revealed that only one third of participants in the EXP group participated in treadmill training. | |
| All groups received treadmill training with partial body weight support. The parameter that was experimentally manipulated was treadmill speed. | |
| A non‐random process was used to allocate participants to groups in Part II and Part III. Participants chose which treatment they would receive. | |
| All groups received treadmill training (without partial body weight support). The parameters that were experimentally manipulated were walking direction and treadmill slope. | |
| Both groups received treadmill training with body weight support. The parameter that was experimentally manipulated was 'conventional' physiotherapy gait training. |
EXP: experimental
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Efficacy of supported treadmill training in establishing walking in non‐ambulatory patients after stroke. |
| Methods | |
| Participants | 30 participants will be recruited for the EXP group, and 30 participants for the CTL group. |
| Interventions | Treated as inpatients for 5 30‐minute sessions per week. |
| Outcomes | Assessed at baseline and weekly for duration of inpatient rehabilitation: |
| Starting date | 2003 |
| Contact information | Louise Ada |
| Notes |
| Trial name or title | A randomised controlled trial of power and treadmill training to improve walking ability in sub‐acute stroke patients. |
| Methods | |
| Participants | 30 participants will be recruited for the EXP 1 group, 30 participants will be recruited for the EXP 2 group, and 30 participants for the CTL group. |
| Interventions | Treated initially as inpatients and then as outpatients for 3 sessions per week for 10 weeks. |
| Outcomes | Assessed at baseline, after the treatment phase (10 weeks), and 6‐month follow up: |
| Starting date | May 2004 |
| Contact information | Sharon Kilbreath |
| Notes |
| Trial name or title | Treadmill with partial body‐weight support versus conventional gait training after stroke. |
| Methods | |
| Participants | 42 participants will be recruited for the EXP group, and 41 participants for the CTL group. |
| Interventions | Treated as inpatients for 12 30‐minute per day sessions over 3 weeks. |
| Outcomes | Assessed 90 days after stroke: |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | Characteristics derived from conference abstract. |
| Trial name or title | Effects of body‐weight‐supported treadmill training on cardiovascular endurance and recovery of gait early after stroke. |
| Methods | |
| Participants | 30 participants will be recruited for the EXP group, and 30 participants for the CTL group. |
| Interventions | Treated initially as inpatients (60 minutes per day, 5 days per week) and then as outpatients (60 minutes per day, 3 days per week) until discharge from physiotherapy. |
| Outcomes | Assessed at baseline, during the treatment phase (6 weeks), after discharge from physiotherapy, and 6‐ and 12‐month follow up: |
| Starting date | March 2003 |
| Contact information | Marilyn MacKay‐Lyons |
| Notes | Expected completion December 2006. |
| Trial name or title | Exercise training for hemiparetic stroke. |
| Methods | |
| Participants | 40 participants will be recruited for the EXP group, and 40 partipants for the CTL group. |
| Interventions | Treated as outpatients for 3 55‐minute sessions per week for 6 months. |
| Outcomes | Assessed at baseline, during the treatment phase (3 months), after the treatment phase (6 months), and 3‐ and 6‐month follow up (9 and 12 months): |
| Starting date | January 2002 |
| Contact information | Richard F Macko, MD |
| Notes | Expected completion on 1 July 2006. |
| Trial name or title | Walking training with partial body weight support versus conventional walking training of chronic stroke patients. |
| Methods | |
| Participants | 25 participants will be recruited for the EXP group, and 25 participants for the CTL group. |
| Interventions | Treated as inpatients for 5 sessions per week for 3 weeks. |
| Outcomes | Assessed at baseline, and after the treatment phase (3 weeks): |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | Characteristics derived from conference abstract. |
| Trial name or title | Stroke rehabilitation outcomes with supported treadmill ambulation training. |
| Methods | |
| Participants | 24 participants will be recruited for the EXP group, and 24 participants for the CTL group. |
| Interventions | Treated as inpatients for 2 to 3 weeks. |
| Outcomes | Assessed at baseline, and after the treatment phase (discharge from inpatient rehabilitation): |
| Starting date | January 2001 |
| Contact information | Unknown |
| Notes | Expected completion December 2003. |
| Trial name or title | Partial body weight supported treadmill training in early acute stroke rehabilitation. |
| Methods | |
| Participants | 5 participants will be recruited for the EXP group, and 5 participants for the CTL group. |
| Interventions | Treated for 3 sessions per week for 2 weeks. |
| Outcomes | Assessed at baseline, and after the treatment phase (2 weeks): |
| Starting date | February 2002 |
| Contact information | Donna Zielke, PT MPT |
| Notes |
CT: computed tomography
CTL: control
EXP: experimental
FIM: Functional Independence Measure
MRI: magnetic resonance imaging
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 dependence on personal assistance to walk at end of treatment phase Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
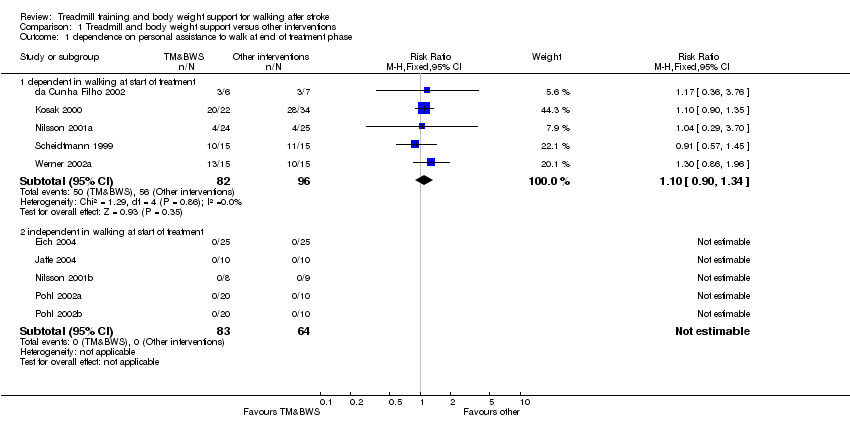
| Analysis 1.1  Comparison 1 Treadmill and body weight support versus other interventions, Outcome 1 dependence on personal assistance to walk at end of treatment phase. | ||||
| 1.1 dependent in walking at start of treatment | 5 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.90, 1.34] |
| 1.2 independent in walking at start of treatment | 5 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 walking speed (m/sec) at end of treatment phase Show forest plot | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Treadmill and body weight support versus other interventions, Outcome 2 walking speed (m/sec) at end of treatment phase. | ||||
| 2.1 dependent in walking at start of treatment | 4 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 2.2 independent in walking at start of treatment | 5 | 147 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.02, 0.20] |
| 3 walking endurance (m) at end of treatment phase Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Treadmill and body weight support versus other interventions, Outcome 3 walking endurance (m) at end of treatment phase. | ||||
| 3.1 dependent in walking at start of treatment | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐2.45 [‐39.43, 34.52] |
| 3.2 independent in walking at start of treatment | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 34.40 [‐7.42, 76.22] |
| 4 dependence on personal assistance to walk at end of scheduled follow up Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
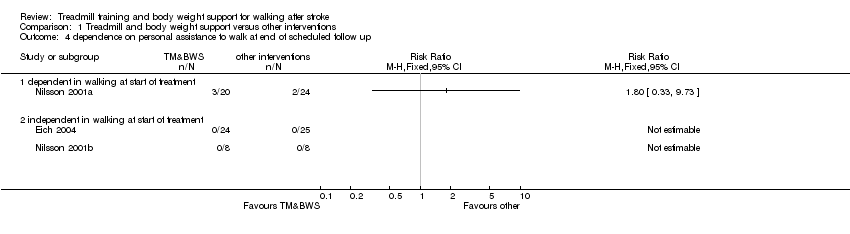
| Analysis 1.4  Comparison 1 Treadmill and body weight support versus other interventions, Outcome 4 dependence on personal assistance to walk at end of scheduled follow up. | ||||
| 4.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 independent in walking at start of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 walking speed (m/sec) at end of scheduled follow up Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Treadmill and body weight support versus other interventions, Outcome 5 walking speed (m/sec) at end of scheduled follow up. | ||||
| 5.1 dependent in walking at start of treatment | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.37, 0.13] |
| 5.2 independent in walking at start of treatment | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.01, 0.24] |
| 6 walking endurance (m) at end of scheduled follow up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Treadmill and body weight support versus other interventions, Outcome 6 walking endurance (m) at end of scheduled follow up. | ||||
| 6.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
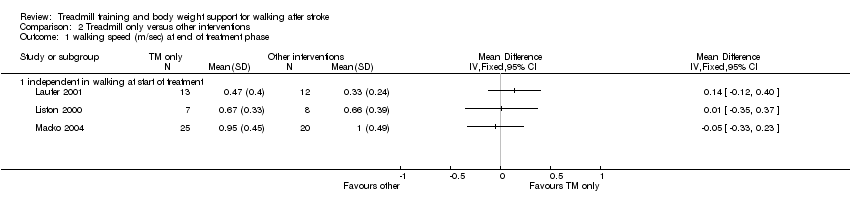
| 1 walking speed (m/sec) at end of treatment phase Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Treadmill only versus other interventions, Outcome 1 walking speed (m/sec) at end of treatment phase. | ||||
| 1.1 independent in walking at start of treatment | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
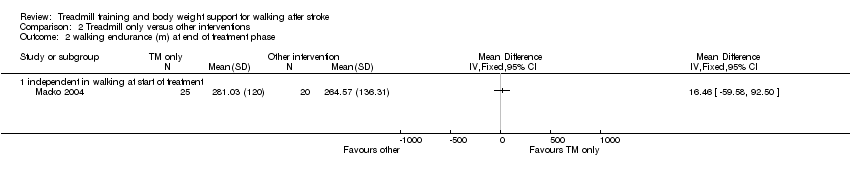
| 2 walking endurance (m) at end of treatment phase Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Treadmill only versus other interventions, Outcome 2 walking endurance (m) at end of treatment phase. | ||||
| 2.1 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 dependence on personal assistance to walk at end of treatment phase Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 1 dependence on personal assistance to walk at end of treatment phase. | ||||
| 1.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 independent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 walking speed (m/sec) at end of treatment phase Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 2 walking speed (m/sec) at end of treatment phase. | ||||
| 2.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 walking endurance (m) at end of treatment phase Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 3 walking endurance (m) at end of treatment phase. | ||||
| 3.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 dependence on personal assistance to walk at end of scheduled follow up Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 4 dependence on personal assistance to walk at end of scheduled follow up. | ||||
| 4.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 independent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 walking speed (m/sec) at end of scheduled follow up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 5 walking speed (m/sec) at end of scheduled follow up. | ||||
| 5.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 walking endurance (m) at end of scheduled follow up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 6 walking endurance (m) at end of scheduled follow up. | ||||
| 6.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 dependence on personal assistance to walk at end of treatment phase Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 1 dependence on personal assistance to walk at end of treatment phase. | ||||
| 1.1 dependent in walking at start of treatment | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 independent in walking at start of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 unknown walking dependency at start of treatment | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 walking speed (m/sec) at end of treatment phase Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 2 walking speed (m/sec) at end of treatment phase. | ||||
| 2.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 unknown walking dependency at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 walking endurance (m) at end of treatment phase Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 3 walking endurance (m) at end of treatment phase. | ||||
| 3.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 unknown walking dependency at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 dependence on personal assistance to walk at end of scheduled follow up Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 4 dependence on personal assistance to walk at end of scheduled follow up. | ||||
| 4.1 dependent in walking at start of treatment | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 independent in walking at start of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 unknown walking dependency at start of treatment | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 walking speed (m/sec) at end of scheduled follow up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.5  Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 5 walking speed (m/sec) at end of scheduled follow up. | ||||
| 5.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 unknown walking dependency at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 walking endurance (m) at end of scheduled follow up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 6 walking endurance (m) at end of scheduled follow up. | ||||
| 6.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 unknown walking dependency at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events during the treatment phase Show forest plot | 15 | 613 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.08] |
| Analysis 5.1  Comparison 5 Adverse events for all included trials, Outcome 1 Adverse events during the treatment phase. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Dropouts for all included trials, Outcome 1 Dropouts. | ||||
| 1.1 by end of treatment phase | 15 | 613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.53, 1.19] |
| 1.2 by end of scheduled follow up (cumulative) | 7 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Walking speed (m/sec) at end of treatment phase Show forest plot | 15 | 428 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.09] |
| Analysis 7.1  Comparison 7 First post‐hoc sensitivity analysis: all trials involving treadmill training, Outcome 1 Walking speed (m/sec) at end of treatment phase. | ||||
| 1.1 dependent in walking at start of treatment | 4 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 1.2 independent in walking at start of treatment | 10 | 266 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.01, 0.17] |
| 1.3 unknown walking dependency at start of treatment | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.75, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 walking speed (m/sec) at end of treatment phase Show forest plot | 11 | 374 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.00, 0.11] |
| Analysis 8.1  Comparison 8 Second post‐hoc sensitivity analysis: all trials involving body weight support, Outcome 1 walking speed (m/sec) at end of treatment phase. | ||||
| 1.1 dependent in walking at start of treatment | 5 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.02, 0.10] |
| 1.2 independent in walking at start of treatment | 6 | 167 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.01, 0.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 walking speed (m/sec) at end of treatment phase Show forest plot | 15 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.1  Comparison 9 Third post‐hoc sensitivity analysis: data reported by trialists, Outcome 1 walking speed (m/sec) at end of treatment phase. | ||||
| 1.1 all trials involving TM training | 14 | 428 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.01, 0.09] |
| 1.2 all trials involving BWS | 9 | 374 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.00, 0.09] |
Comparison 1 Treadmill and body weight support versus other interventions, Outcome 1 dependence on personal assistance to walk at end of treatment phase.

Comparison 1 Treadmill and body weight support versus other interventions, Outcome 2 walking speed (m/sec) at end of treatment phase.

Comparison 1 Treadmill and body weight support versus other interventions, Outcome 3 walking endurance (m) at end of treatment phase.
Comparison 1 Treadmill and body weight support versus other interventions, Outcome 4 dependence on personal assistance to walk at end of scheduled follow up.

Comparison 1 Treadmill and body weight support versus other interventions, Outcome 5 walking speed (m/sec) at end of scheduled follow up.

Comparison 1 Treadmill and body weight support versus other interventions, Outcome 6 walking endurance (m) at end of scheduled follow up.
Comparison 2 Treadmill only versus other interventions, Outcome 1 walking speed (m/sec) at end of treatment phase.
Comparison 2 Treadmill only versus other interventions, Outcome 2 walking endurance (m) at end of treatment phase.

Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 1 dependence on personal assistance to walk at end of treatment phase.
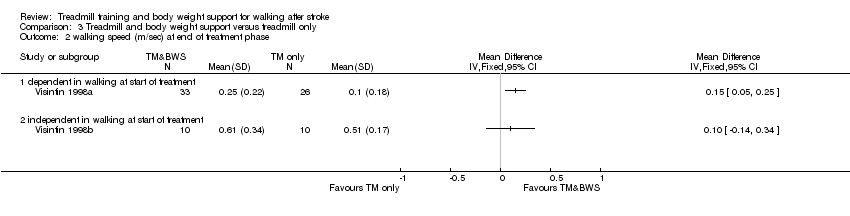
Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 2 walking speed (m/sec) at end of treatment phase.
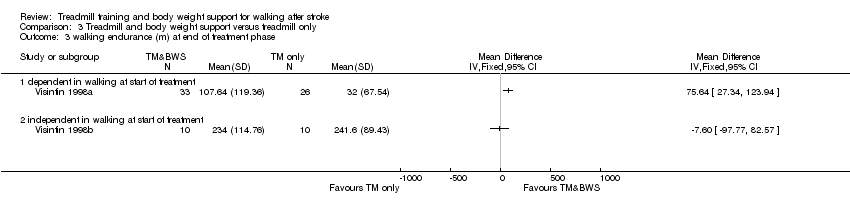
Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 3 walking endurance (m) at end of treatment phase.

Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 4 dependence on personal assistance to walk at end of scheduled follow up.

Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 5 walking speed (m/sec) at end of scheduled follow up.

Comparison 3 Treadmill and body weight support versus treadmill only, Outcome 6 walking endurance (m) at end of scheduled follow up.

Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 1 dependence on personal assistance to walk at end of treatment phase.
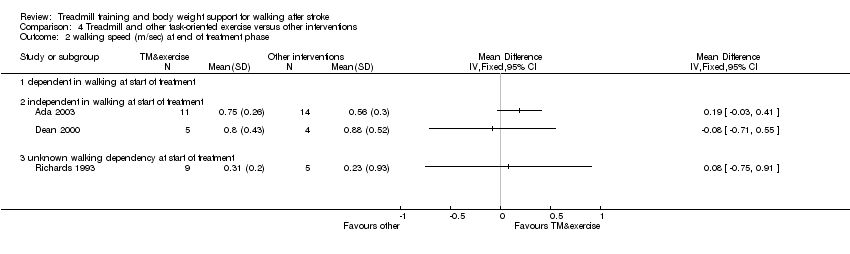
Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 2 walking speed (m/sec) at end of treatment phase.

Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 3 walking endurance (m) at end of treatment phase.

Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 4 dependence on personal assistance to walk at end of scheduled follow up.
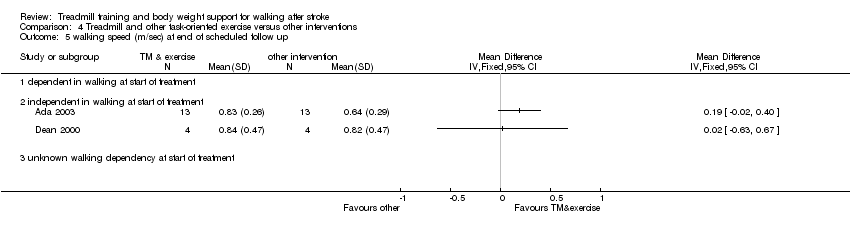
Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 5 walking speed (m/sec) at end of scheduled follow up.
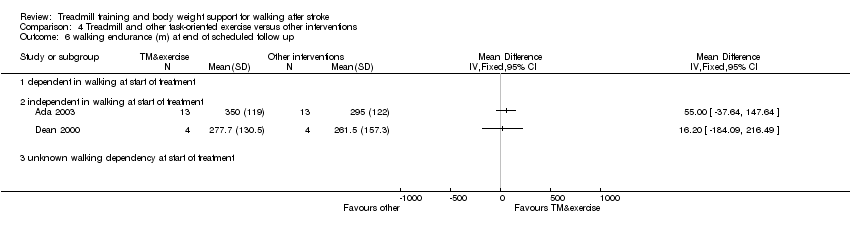
Comparison 4 Treadmill and other task‐oriented exercise versus other interventions, Outcome 6 walking endurance (m) at end of scheduled follow up.

Comparison 5 Adverse events for all included trials, Outcome 1 Adverse events during the treatment phase.
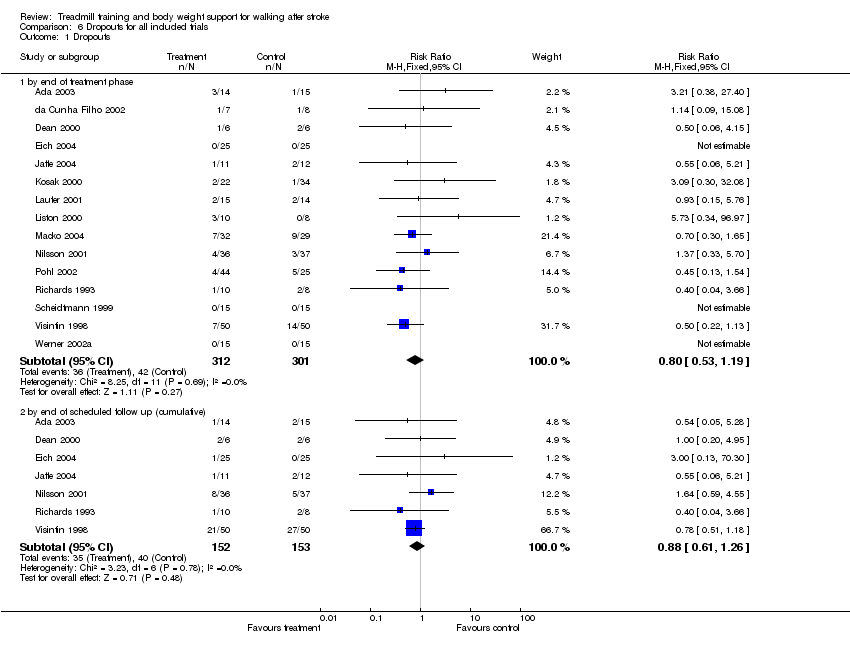
Comparison 6 Dropouts for all included trials, Outcome 1 Dropouts.

Comparison 7 First post‐hoc sensitivity analysis: all trials involving treadmill training, Outcome 1 Walking speed (m/sec) at end of treatment phase.

Comparison 8 Second post‐hoc sensitivity analysis: all trials involving body weight support, Outcome 1 walking speed (m/sec) at end of treatment phase.

Comparison 9 Third post‐hoc sensitivity analysis: data reported by trialists, Outcome 1 walking speed (m/sec) at end of treatment phase.
| STUDY ID | EXP age | CTL age | EXP gender | CTL gender | EXP time post stroke | CTL time post stroke | EXP paresis side | CTL paresis side |
| Ada 2003 | Mean 66 (SD 11) years (excluding 1 dropout) | Mean 66 (SD 11) years (excluding 1 dropout) | Male/female 9/4 | Male/female 10/4 | Mean 28 (SD 17) months | Mean 26 (SD 20) months | Left/right 5/8 | Left/right 8/6 |
| da Cunha Filho 2002 | Mean 57.8 (SD 5.5) years (excluding dropouts) | Mean 58.9 (SD 12.9) years (excluding dropouts) | Male/female 6/0 | Male/female 7/0 | Mean 15.7 (SD 7.7) days | Mean 19.0 (SD 12.7) days | Left/right/bilateral 1/4/1 | Left/right 4/3 |
| Dean 2000 | Mean 66.2 (SD 7.7) years (all participants) | Mean 62.3 (SD 6.6) years | Male/female 3/3 | Male/female 4/2 | Mean 2.3 (SD 0.7) years | Mean 1.3 (SD 0.9) years | Left/right 3/3 | Left/right 2/4 |
| Eich 2004 | Mean 62.4 (SD 4.8) years (all participants) | Mean 64.0 (SD 6.0) years (all participants) | Male/female 17/8 | Male/female 16/9 | Mean 6.1 (SD 2.2) weeks | Mean 6.3 (SD 2.5) weeks | Left/right 14/11 | Left/right 14/11 |
| Jaffe 2004 | Mean 58.2 (SD 11.2) years (excluding dropouts) | Mean 63.2 (SD 8.3) years (excluding dropouts) | Male/female 5/5 (excluding dropouts) | Male/female 7/3 (excluding dropouts) | Mean 3.9 (SD 2.3) years (excluding dropouts) | Mean 3.6 (SD 2.6) years (excluding dropouts) | Left/right 6/4 (excluding dropouts) | Left/right 4/6 (excluding dropouts) |
| Kosak 2000 | Mean 74 (SEM 2) years (all participants) | Mean 70 (SEM 2) years | Male/female 13/9 | Male/female 18/16 | Mean 39 (SEM 3) days | Mean 40 (SEM 4) days | Left/right/bilateral 8/12/2 | Left/right/bilateral 12/16/6 |
| Laufer 2001 | Mean 66.6 (SD 7.2) years (excluding dropouts) | Mean 69.3 (SD 8.1) years (excluding dropouts) | Male/female 7/6 | Male/female 7/5 | Mean 32.6 (SD 21.2) days | Mean 35.8 (SD 17.3) days | Left/right 5/8 | Left/right 5/7 |
| Liston 2000 | Mean 79.1 (SD 6.8) years (all EXP and CTL participants) | Male/female 12/6 | Not reported | Not reported | Not reported | Not reported | ||
| Macko 2004 | Mean 63 (SD 10) years | Mean 64 (SD 8) years | Male/female 22/10 | Male/female 21/8 | Mean 35 (SD 29) months | Mean 39 (SD 59) months | Left/right 18/14 | Left/right 13/16 |
| Nilsson 2001 | Median 54 (range 24‐67) years (all participants) | Median 56 (range 24‐66) years | Male/female 20/16 | Male/female 20/17 | Median 22 (range 10‐56) days | Median 17 (range 8‐53) days | Left/right/bilateral 21/11/4 | Left/right/bilateral 18/14/5 |
| Pohl 2002 | Mean 58.2 (SD 10.5) years for EXP 1 (excluding dropouts) | Mean 61.6 (SD 10.6) years (excluding dropouts) | Male/female 16/4 for EXP 1 | Male/female 13/7 | Mean 16.2 (SD 16.4) weeks for EXP 1 | Mean 16.1 (SD 18.5) weeks | Left/right 15/5 for EXP 1 | Left/right 16/4 |
| Richards 1993 | Mean 69.6 (SD 7.4) years (all participants) | Mean 67.3 (SD 11.2) years (CTL 1) | Male/female 5/5 | Male/female 2/6 | Mean 8.3 (SD 1.4) days | Mean 8.8 (SD 1.5) days | Left/right 8/2 | Left/right 2/6 |
| Scheidtmann 1999 | Mean 57.7 (SD 11.0) years (all participants) | Male/female 16/14 | Mean 52.2 (SD 29.6) days | Left/right 17/13 | ||||
| Visintin 1998 | Mean 66.5 (SD 12.8) years (all participants) | Mean 66.7 (SD 10.1) years | Male/female 31/19 | Male/female 28/22 | Mean 68.1 (SD 26.5) days | Mean 78.4 (SD 30.0) days | Left/right 30/20 | Left/right 21/29 |
| Werner 2002a | Mean 59.7 (SD 10.2) years (all participants) | Mean 60.3 (SD 8.6) years (all participants) | Male/female 8/7 | Male/female 5/10 | Mean 7.4 (SD 2.0) weeks | Mean 6.9 (SD 2.1) weeks | Left/right 7/8 | Left/right 7/8 |
| Study ID | EXP ‐ treadmill | EXP ‐ support | EXP ‐ duration | EXP ‐ frequency | EXP ‐ N weeks | CTL ‐ intervent. | CTL ‐ duration | CTL ‐ frequency | CTL ‐ N weeks |
| Ada 2003 | Gradually increased on an individual basis starting from 0.7 m/sec at the start of the first session and finishing at 1.1 m/sec at the end of the last session, on average | BWS ‐ no; | 30 minutes (24, 21, 18 and 15 minutes in treadmill training in the first, second, third and fourth training weeks, respectively) | 3 times per week | 4 weeks | Sham (task orientated home program with an intensity insufficient to produce an effect, plus telephone follow up once each week) | 30 minutes | 3 times per week (plus encouraged to walk every day) | 4 weeks |
| da Cunha Filho 2002 | Gradually increased in increments of 0.01 m/sec, starting at 0.01 m/sec | BWS ‐ yes, starting at 30% body weight and progressively decreased to 0%; | 20 minutes | 5 times per week | 2 to 3 weeks | Task orientated gait training | 20 minutes | 5 times per week | 2 to 3 weeks |
| Dean 2000 | Gradually increased on an individual basis | BWS ‐ no; | 60 minutes (5 minutes in treadmill training) | 3 times per week | 4 weeks | Sham (task oriented upper limb training) | 60 minutes | 3 times per week | 4 weeks |
| Eich 2004 | Speed and inclination increased on an individual basis to achieve a training heart rate. | BWS ‐ yes, the harness was always secured and body weight was minimally supported (0 to 15%) according to participant need; | 30 minutes | 5 times per week | 6 weeks | Non‐task orientated (neurophysiological) | 30 minutes | 5 times per week | 6 weeks |
| Jaffe 2004 | Comfortable walking speed (speed not reported), speed was not progressed | BWS ‐ yes, harness used to prevent falls only; | 60 minutes | 3 times per week | 2 weeks | Task orientated (overground obstacle training) | 60 minutes | 3 times per week | 2 weeks |
| Kosak 2000 | Gradually increased from 0.22 to 0.89 m/sec, as tolerated | BWS ‐ yes, starting at 30% body weight and progressively decreased to 0% or eliminated; | 45 minutes | 5 times per week | 2 to 3 weeks | Non‐task orientated (orthopaedic) | 45 minutes | 5 times per week | 2 to 3 weeks |
| Laufer 2001 | Comfortable walking speed, speed used and progression not reported | BWS ‐ no; | 8 to 20 minutes | 5 times per week | 3 weeks | Task orientated | 8 to 20 minutes | 5 times per week | 3 weeks |
| Liston 2000 | Speed used and progression not reported | BWS ‐ no; | 60 minutes | 3 times per week | 4 weeks | Task orientated | 60 minutes | 3 times per week | 4 weeks |
| Macko 2004 | Increased from a mean of 0.48 (SE 0.30) m/sec at baseline to 0.75 (SE 0.30) m/sec at treatment end on an individual basis to achieve a target aerobic intensity of 60‐70% heart rate reserve (treadmill slope increased from 0% at baseline to 2.2% (SE 2.2) at treatment end) | BWS ‐ no; | 40 minutes (including 5 minutes warm up and 5 minutes cool down) | 3 times per week | 6 months | Task orientated | 40 minutes | 3 times per week | 6 months |
| Nilsson 2001 | Gradually increased from 0.0 to 2.0 m/sec on an individual basis | BWS ‐ yes, starting at 100% body weight and decreased to 0%; | 30 minutes | 5 times per week | 9 to 10 weeks | Task orientated | 30 minutes | 5 times per week | 9 to 10 weeks |
| Pohl 2002 | Speed dependent treadmill training (EXP 1) ‐ aggressive increase in speed starting from the highest speed the participant could walk at without stumbling and increasing at 10% increments of this speed several times within a session. The average treadmill speed increased from 0.68 m/sec (SD 0.34) at the start of training to 2.05 m/sec (SD 0.71) at the end of training; | Speed dependent treadmill training: | 30 minutes | 3 times per week | 4 weeks | Non‐task orientated (neurophysiological) | 45 minutes | 3 times per week | 4 weeks |
| Richards 1993 | Speed used and progression not reported | BWS ‐ no; | 105 minutes (about 35 minutes in TM training) | 5 times per week | 5 weeks | Non‐task orientated (neurophysiological) | 105 minutes | 5 times per week | 5 weeks |
| Scheidtmann 1999 | Gradually increased from 0.0 to 1.3 m/sec | BWS ‐ yes, amount of body weight support and progression not reported; | 30 minutes | 5 times per week | 3 weeks | Non‐task orientated (neurophysiological) | 30 minutes | 5 times per week | 3 weeks |
| Visintin 1998 | Gradually increased in increments of 0.04 m/sec, from 0.23 to 0.42 m/sec, on average, on an individual basis | BWS ‐ yes, starting at 40% body weight and progressively decreased to 0%; | 20 minutes | 4 times per week | 6 weeks | Task orientated (treadmill only) | 20 minutes | 4 times per week | 6 weeks |
| Werner 2002a | Increased from a mean of 0.32 (SD 0.05) m/sec at baseline on an individual basis | BWS ‐ yes, starting at a mean of 8.93% (SD 1.84) body weight and progressively decreased; | 15‐20 minutes | 5 times per week | 2 weeks | Task orientated | 15‐20 minutes | 5 times per week | 2 weeks |
| Study ID (PED score) | Inclusion criteria | Random allocation | Concealed allocation | Baseline similar | Blinding | Dropouts | Intent‐to‐treat | Statistics | Mean & SD data |
| Ada 2003 | Yes | Yes ‐ coin toss | Yes ‐ by ranking the participants according to independent walking speed at baseline (from fastest to slowest) and then allocating each descending pair of participants by coin toss | Yes | Participants ‐ no | Yes ‐ 14% at end of treatment phase (and 10% at end of follow up) | Yes | Yes | Yes |
| da Cunha Filho 2002 | Yes | Yes ‐ random number table | No | No | Participants ‐ no | Yes ‐ 13% at end of treatment phase (rating of this item was changed based on correspondence from the trialist) | No | Yes | Yes |
| Dean 2000 | Yes | Yes ‐ by drawing a card from a box (there were 6 EXP and 6 CTL cards and they were not replaced after each draw) | Yes ‐ a person independent to the study drew the cards out of the box | Yes | Participants ‐ no | No ‐ 25% at end of treatment phase (33% at 2‐month follow up) | No | Yes | Yes |
| Eich 2000 | Yes | Yes ‐ by an independent person asking the participant to draw an envelope from a box (each envelope contained the group allocation and there were 25 EXP and 25 CTL envelopes) | Yes ‐ sealed, opaque envelopes | Yes | Participants ‐ no | Yes ‐ 0% at end of treatment phase (2% at 3‐month follow up) | Yes | Yes | Yes |
| Jaffe 2004 | Yes | Yes ‐ using an Excel spreadsheet | Yes ‐ using an Excel spreadsheet with group allocation masked using black cells (rating of this item was changed based on correspondence from the trialist) | Yes | Participants ‐ no | Yes ‐ 15% at end of treatment phase (rating of this item was changed based on correspondence from the trialist) | No | Yes | Yes |
| Kosak 2000 | Yes | Yes ‐ random number table | Yes ‐ a person independent to the study allocated participants after they were recruited (rating of this item was changed based on correspondence from the trialist) | Yes | Participants ‐ no | Yes ‐ 5% at end of treatment phase | No | Yes | Yes |
| Laufer 2001 | Yes | No ‐ alternate assignment of participants to groups | No ‐ alternate assignment of participants to groups | Yes | Participants ‐ no | Yes ‐ 14% at end of treatment phase | No | Yes | Yes |
| Liston 2000 | Yes | Yes ‐ toss of a coin | No | No | Participants ‐ no | No ‐ 17% at end of first treatment phase (rating of this item was changed based on correspondence from the trialist) | Yes | Yes | Yes |
| Macko 2004 | Yes | Yes ‐ computer generated randomisation scheme which was stratified by walking speed (<0.44 m/sec and =>0.44 m/sec) and age (<65 years and =>65 years; method of randomisation was changed based on correspondence from the trialist) | No | Yes | Participants ‐ no | No ‐ 26% at end of treatment phase | No | Yes | Yes |
| Nilsson 2001 | Yes | Yes ‐ using a random number computer program by a person not involved in the trial | Yes ‐ sealed, opaque and consecutively numbered envelopes | Yes | Participants ‐ no | Yes ‐ 10% at end of treatment phase (18% at 10 month follow up) | No | Yes | Yes |
| Pohl 2002 | Yes | Yes ‐ stratified into groups of 3 based on walking time over 10m, then randomised to group by drawing an opaque envelope from a group of 3 (each envelope contained a piece of paper marked with one of the 3 experimental conditions) | Yes ‐ sealed, opaque envelopes that were not numbered (rating of this item was changed based on correspondence from the trialist) | Yes | Participants ‐ no | Yes ‐ 13% at end of treatment phase | No | Yes | Yes |
| Richards 1993 | Yes | Yes ‐ using a stratified block randomisation scheme | No | Yes | Participants ‐ no | Yes ‐ 15% at end of treatment phase (number of dropouts not reported for 3 and 6 month follow ups) | No | Yes | Yes |
| Scheidtmann 1999 | Yes | Yes ‐ method of randomisation not stated | No | No | Participants ‐ no | Yes ‐ 0% at end of first treatment phase | No | Yes | Yes |
| Visintin 1998 | Yes | Yes ‐ using a stratified block randomisation scheme. Each block of 8 participants was generated by drawing cards (4 marked experimental and 4 marked control) from a box | Yes ‐ using sealed and numbered envelopes (rating of this item was changed based on correspondence from the trialist) | Yes | Participants ‐ no | No ‐ 21% at end of treatment phase (48% at 3 month follow up) | No | Yes | Yes |
| Werner 2002a | Yes (rating of this item was changed based on correspondence from the trialist) | Yes ‐ by drawing an envelope from a box (there were 15 EXP then CTL order and 15 CTL then EXP order envelopes and they were not replaced after each draw) | Yes ‐ a person independent to the study drew the envelopes out of the box after recruitment | Yes | Participants ‐ no | Yes ‐ 0% at end of first treatment phase (the number of dropouts was changed based on correspondence with the trialists) | No | Yes | Yes |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 dependence on personal assistance to walk at end of treatment phase Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 dependent in walking at start of treatment | 5 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.90, 1.34] |
| 1.2 independent in walking at start of treatment | 5 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 walking speed (m/sec) at end of treatment phase Show forest plot | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 dependent in walking at start of treatment | 4 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 2.2 independent in walking at start of treatment | 5 | 147 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.02, 0.20] |
| 3 walking endurance (m) at end of treatment phase Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 dependent in walking at start of treatment | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐2.45 [‐39.43, 34.52] |
| 3.2 independent in walking at start of treatment | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 34.40 [‐7.42, 76.22] |
| 4 dependence on personal assistance to walk at end of scheduled follow up Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 independent in walking at start of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 walking speed (m/sec) at end of scheduled follow up Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 dependent in walking at start of treatment | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.37, 0.13] |
| 5.2 independent in walking at start of treatment | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.01, 0.24] |
| 6 walking endurance (m) at end of scheduled follow up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 walking speed (m/sec) at end of treatment phase Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 independent in walking at start of treatment | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 walking endurance (m) at end of treatment phase Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 dependence on personal assistance to walk at end of treatment phase Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 independent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 walking speed (m/sec) at end of treatment phase Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 walking endurance (m) at end of treatment phase Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 dependence on personal assistance to walk at end of scheduled follow up Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 independent in walking at start of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 walking speed (m/sec) at end of scheduled follow up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 walking endurance (m) at end of scheduled follow up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 dependence on personal assistance to walk at end of treatment phase Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 dependent in walking at start of treatment | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 independent in walking at start of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 unknown walking dependency at start of treatment | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 walking speed (m/sec) at end of treatment phase Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 unknown walking dependency at start of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 walking endurance (m) at end of treatment phase Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 unknown walking dependency at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 dependence on personal assistance to walk at end of scheduled follow up Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 dependent in walking at start of treatment | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 independent in walking at start of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 unknown walking dependency at start of treatment | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 walking speed (m/sec) at end of scheduled follow up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 unknown walking dependency at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 walking endurance (m) at end of scheduled follow up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 dependent in walking at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 unknown walking dependency at start of treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events during the treatment phase Show forest plot | 15 | 613 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 by end of treatment phase | 15 | 613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.53, 1.19] |
| 1.2 by end of scheduled follow up (cumulative) | 7 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Walking speed (m/sec) at end of treatment phase Show forest plot | 15 | 428 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.09] |
| 1.1 dependent in walking at start of treatment | 4 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 1.2 independent in walking at start of treatment | 10 | 266 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.01, 0.17] |
| 1.3 unknown walking dependency at start of treatment | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.75, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 walking speed (m/sec) at end of treatment phase Show forest plot | 11 | 374 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.00, 0.11] |
| 1.1 dependent in walking at start of treatment | 5 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.02, 0.10] |
| 1.2 independent in walking at start of treatment | 6 | 167 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.01, 0.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 walking speed (m/sec) at end of treatment phase Show forest plot | 15 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 all trials involving TM training | 14 | 428 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.01, 0.09] |
| 1.2 all trials involving BWS | 9 | 374 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.00, 0.09] |

