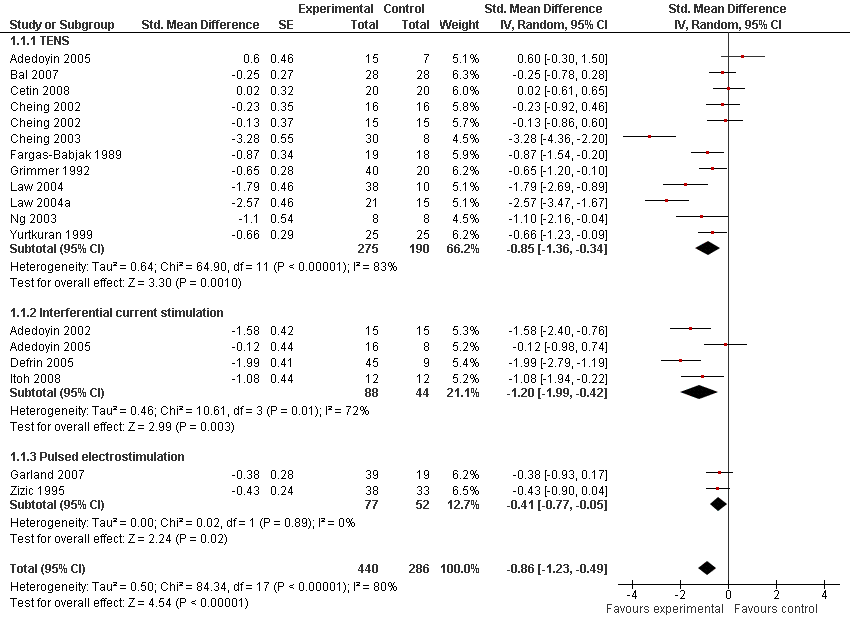
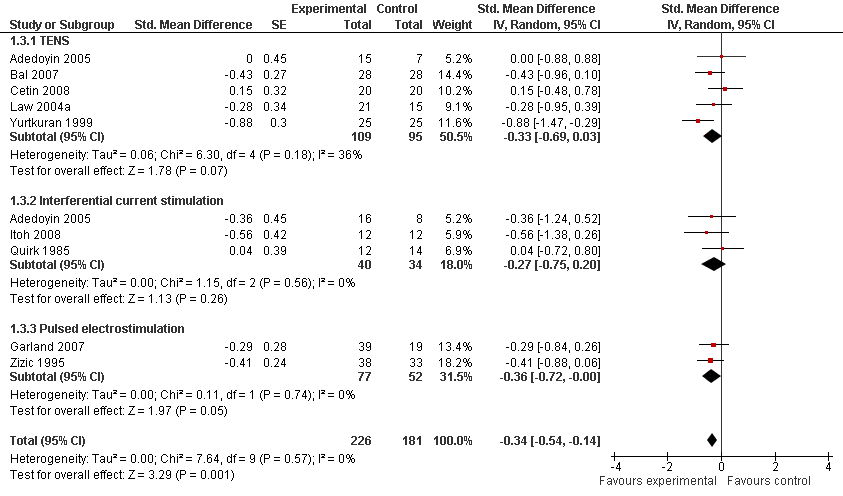
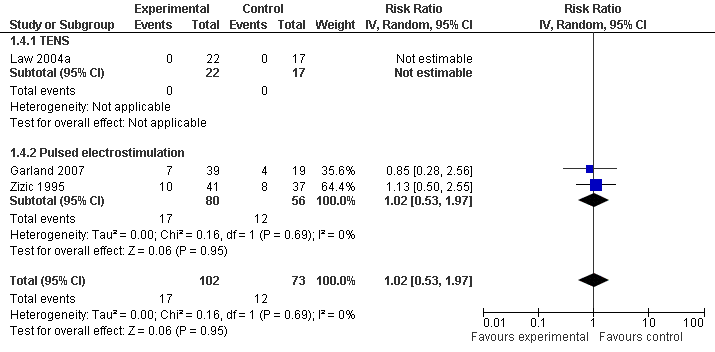
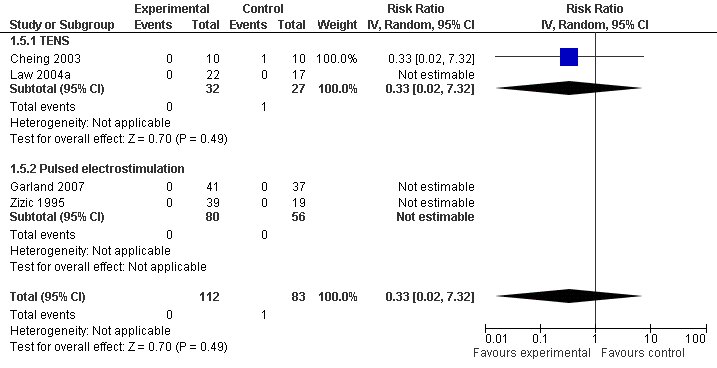
Contenido relacionado
Revisiones y protocolos relacionados
Lucie Brosseau, KA Yonge, Vivian Welch, S Marchand, Maria Judd, George A Wells, Peter Tugwell | 22 abril 2003
Brenda Monaghan, Brian Caulfield, Dónal P O'Mathúna | 20 enero 2010
Lucie Pelland, Lucie Brosseau, Lynn Casimiro, Vivian Welch, Peter Tugwell, George A Wells | 22 abril 2002
Bruno R da Costa, Eveline Nüesch, Stephan Reichenbach, Peter Jüni, Anne WS Rutjes | 14 noviembre 2012
Anne WS Rutjes, Eveline Nüesch, Stephan Reichenbach, Peter Jüni | 7 octubre 2009
Stephan Reichenbach, Anne WS Rutjes, Eveline Nüesch, Sven Trelle, Peter Jüni | 12 mayo 2010
Peter Jüni, Roman Hari, Anne WS Rutjes, Roland Fischer, Maria G Silletta, Stephan Reichenbach, Bruno R da Costa | 22 octubre 2015
Bruno R da Costa, Eveline Nüesch, Rahel Kasteler, Elaine Husni, Vivian Welch, Anne WS Rutjes, Peter Jüni | 17 septiembre 2014
Reinoud W Brouwer, Maarten R Huizinga, Tijs Duivenvoorden, Tom M van Raaij, Arianne P Verhagen, Sita MA Bierma‐Zeinstra, Jan AN Verhaar | 13 diciembre 2014
Else Marie Bartels, Carsten B Juhl, Robin Christensen, Kåre Birger Hagen, Bente Danneskiold‐Samsøe, Hanne Dagfinrud, Hans Lund | 23 marzo 2016