Electroestimulación transcutánea para la osteoartritis de la rodilla
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Quasi‐randomised trial using alternation for the allocation of patients | |
| Participants | 30 patients randomised | |
| Interventions | Experimental intervention: interferential current stimulation, dietary advice and exercise, twice per week Device: Enraf‐Nonius Endomed 5921 (4 pole) | |
| Outcomes | Extracted pain outcome: global pain after 4 weeks, described as "Pain perception (VAS)" Primary outcome: global pain (VAS) | |
| Notes | All subjects from black Nigerian population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternation |
| Allocation concealment? | High risk | Alternation |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Low risk | Sham device: identical in appearance, not increasing intensity, flash light on, patient in position unable to read level of intensity |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Low risk | — |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable, no function outcome reported |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 51 patients randomised | |
| Interventions | Comparison 1 Comparison 2 Duration of treatment period: 4 weeks TENS Device: Endomed 5921D Interferential Current Stimulation Device: Endomed 5921D (2 pole) | |
| Outcomes | Extracted pain outcome: pain on activities other than walking after 4 weeks, described as "Pain recorded while standing (10‐point pain rating scale with 0 “no pain”, 5 “moderate pain” and 10 “worst pain imaginable”)" No primary outcome reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | 15 out of 15 (100%) in TENS group, 16 out of 19 (84%) in interferential current stimulation group, 15 out of 17 (88%) in control group analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Quasi‐randomised single centre controlled trial with allocation according to hospital registration number | |
| Participants | 56 patients randomised | |
| Interventions | Experimental intervention: TENS and infra‐red therapy, 5 times per week Device: PlusMED 1‐904 Pulse frequency: 80 Hz | |
| Outcomes | Extracted pain outcome: WOMAC pain subscore after 13 weeks (Likert) No primary outcome reported | |
| Notes | Article in Turkish, outcome assessment done by AR and RS assisted by a native Turkish researcher. Serpil Bal verified all extracted data. *as indicated by Serpil Bal in personal communication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | The published report only stated that there was a random allocation of patients to comparison groups. In personal communication, investigator Serpil Bal stated that the patients were allocated according to last digit of their hospital registration number. Patients with even numbers were assigned to TENS group, patients with odd numbers to a sham intervention. |
| Allocation concealment? | High risk | No, the same investigator responsible of randomisation was giving interventions, as indicated by Serpil Bal in personal communication |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes, we have been unable to sort out this item with investigator Serpil Bal |
| Adequate blinding of patients? | Low risk | Trial is described as single blind study using sham device PlusMED 1‐904, indistinguishable from real TENS unit. Sham device had broken leads, no current passed but flashing light was on. None of the patients had prior experience with TENS. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Low risk | All subjects were available for end of treatment measurements, as indicated by Serpil Bal in personal communication |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Low risk | All subjects were available for end of treatment measurements, as indicated by Serpil Bal in personal communication |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 100 patients randomised | |
| Interventions | Experimental intervention: TENS + hot packs + isokinetic exercise, 3 times per week Device: MED911 | |
| Outcomes | Extracted pain outcome: pain on walking after 8 weeks, described as "Knee pain severity after a 50‐m walk (VAS)" No primary outcome reported | |
| Notes | Only 2 arms qualified for inclusion in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | No information provided |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | No information provided |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 66 patients randomised | |
| Interventions | Comparison 1 Comparison 2 Duration of treatment period: 4 weeks Device: MAXIMA III (dual channel) | |
| Outcomes | Extracted pain outcome: global pain after 8 weeks, described as "Intensity of subjective pain sensation (Baseline score on 0‐10 cm VAS was standardised to be 100% in each of the groups. Follow up values were expressed as mean decrease in % from baseline)". No primary outcome reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Low risk | Comparison 1: Yes, sham device identical in appearance to real TENS unit, no current passed but indicator light was lit up Comparison 2: No, no sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | Comparison 1: 16 out of 16 (100%) randomised to experimental and 16 out of 18 (89%) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 40 patients randomised | |
| Interventions | Experimental intervention: 20 min TENS in group 1, 40 min TENS in group 2, 60 min TENS in group 4, 5 times per week Device: ITO 120Z TENS (dual channel) | |
| Outcomes | Extracted pain outcome: pain on walking after 4 weeks, described as "pain during walking (VAS)" No primary outcome reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Low risk | Sham device: electronic circuit disconnected, no current passed, but indicator light on |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | 30 out of 30 (100%) randomised to experimental and 8 out of 10 (80%) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 62 patients randomised | |
| Interventions | Experimental intervention: noxious adjusted interferential current stimulation in group 1, noxious unadjusted interferential current stimulation in group 2, innocuous adjusted interferential current stimulation in group 3, innocuous unadjusted interferential current stimulation in group 4, 3 times per week Device: Uniphy: Phyaction electrical stimulator | |
| Outcomes | Extracted pain outcome: global pain after 4 weeks, described as "chronic pain intensity (VAS)" No primary outcome reported | |
| Notes | 1 out of 6 trial arms, the no‐intervention control group was excluded in the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Unclear risk | Use of sham device: Uniphy‐Phyaction electrical stimulator, however the device described as shut‐off |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | No information provided |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 56 patients randomised | |
| Interventions | Experimental intervention: burst TENS, twice per day Device: Codetron | |
| Outcomes | Extracted pain outcome: global pain after 13 weeks described as "Pain improvement (percentage pain improvement based on VAS)" No primary outcome reported | |
| Notes | *Investigators named their intervention AL‐TENS, but we coded it burst TENS in the analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | High risk | Quote: "Full details of this (Percent Improvement Pain Scale) are reported elsewhere". Investigators however failed to provide reference. |
| Adequate blinding of patients? | Low risk | Use of sham device: Codetron, identical in appearance, set at frequency of 0.2 Hz with a threshold electrical stimulus of 0.5 mA, which caused a sensation on the skin but failed causing the deep muscle afferent stimulation |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | 56 patients randomised but only 19 analysed in the experimental, and 18 analysed in the control group |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | High risk | Sponsor: Electronic Health Machines |
| Funding by non‐profit organisation? | Low risk | NRC grant no: 689 |
| Methods | Randomised multicentre controlled trial | |
| Participants | 100 patients randomised | |
| Interventions | Experimental intervention: pulsed electrical stimulation Device: BIO‐1000 | |
| Outcomes | Extracted pain outcome: global pain after 12 weeks, described as "Considering your pain and symptoms in your study joint how are you doing today? (VAS)" No primary outcome reported | |
| Notes | *Due to major protocol violations, all 42 randomised patient of one site were excluded by Garland et al | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Low risk | Central randomisation |
| Free of selective reporting? | Unclear risk | Quote: "Total WOMAC scores were not a defined outcome in the protocol, but are shown in Tables II(a)‐(d)." |
| Adequate blinding of patients? | Low risk | Use of sham device: BIO‐1000, indistinguishable from active device, with automatic shut‐off as soon as amplitude is reduced (all patients were instructed to reduce intensity just below perception level). Further adjustments required all devices to be restarted. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | Due to major protocol violations, all 42 randomised patient of 1 site were excluded by original authors. From the other site, all patients randomised were included in the analysis. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | See above |
| Funding by commercial organisation avoided? | High risk | Sponsor: BioniCare Medical Technologies |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 60 patients randomised | |
| Interventions | Experimental intervention: high frequency TENS, once only in group 1, burst TENS, once only in group 2 Device: Medtronic Neuromed Selectra (dual channel) | |
| Outcomes | Extracted pain outcome: global pain immediately after first and only application, described as "Immediate pain relief (VAS)" No primary outcome reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "randomly allocated (by dice) into three groups of 20" |
| Allocation concealment? | Low risk | By a person independent of the study |
| Free of selective reporting? | Unclear risk | Insufficient information provided; no access to study protocol |
| Adequate blinding of patients? | Low risk | Sham device: Medtronic Neuromed Selectra, with non‐functioning leads. Patient were told that a very high frequency current was being tested and that no skin sensation would be felt. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Low risk | Degrees of freedom reported indicate that all randomised patients were included in the analysis |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 32 patients randomised | |
| Interventions | Experimental intervention: interferential current stimulation*, once per week 16 out of 32 patients (50%) allocated to acupuncture using a factorial design; no evidence for an interaction between treatments Duration of treatment period: 5 weeks Device: HV‐F3000 (single channel, 2 pole) | |
| Outcomes | Extracted pain outcome: global pain after 10 weeks, described as "Pain intensity (VAS)" Primary outcomes: pain intensity, WOMAC global scale | |
| Notes | *The investigators used the label TENS in their report, but from their description of the intervention it was clear that interferential current stimulation was applied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated block randomisation. Quote "According to a block randomised allocation table (generated by Sample Size, version 2.0, Int), the enrolled patients were allocated to (1) the control (CT) group, (2) the acupuncture (ACP) group, (3) the transcutaneous electrical nerve stimulation (TENS) group or (4) the acupuncture and TENS (A&T) group." |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Insufficient information provided, no access to study protocol |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | 12 out of 16 (75%) randomised to experimental and 12 out of 16 (75%) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 36 patients randomised | |
| Interventions | Experimental intervention: 2 Hz TENS in group 1, 100 Hz TENS in group 2, modulation TENS with alternations between 2 to 100 Hz in group 3, 5 times per week in all groups Device: Han Acupoint Nerve Stimulation LH204H | |
| Outcomes | Extracted pain outcome: pain on walking after 4 weeks, described as "intensity of pain felt while walking (VAS)" No primary outcome reported | |
| Notes | Outcome data were reported on knee level. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomization was carried out by drawing lots from the randomization envelope." |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Insufficient information provided; no access to study protocol |
| Adequate blinding of patients? | Low risk | Use of sham device: identical in appearance, internal circuit disconnected, no current passed, indicator light on, digital display of intensity control functioned normally. Quote: "Only therapists who administered treatment to the subjects knew the group allocation, while the subjects and the assessor were not given this information." |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | In total, 3 patients dropped out and were excluded from analysis, as indicated by Gladys Cheing and Pearl Law in personal communication |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 39 patients randomised | |
| Interventions | Experimental intervention: TENS, 5 times per week Device: ITO model 120Z (dual channel) | |
| Outcomes | Extracted pain outcome: pain on walking after 2 weeks, described as "intensity of pain felt while walking (VAS)"** No primary outcome reported | |
| Notes | **Only baseline values reported in the report. Contact established with investigators Law and Cheing, who provided end of treatment and follow‐up data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "'by drawing lots from the randomization envelope without replacement" |
| Allocation concealment? | Unclear risk | Quote : "(...) carried out by physiotherapists who performed the treatment" |
| Free of selective reporting? | High risk | No results reported for some outcomes mentioned in the methods section, including pain intensity on VAS |
| Adequate blinding of patients? | Low risk | Use of sham device: ITO model 120Z, no current delivered but flashing light on. Quote: "The assessors and subjects were blind to the group allocation. All subjects were told that when the indicator light of the TENS was blinking, it meant the machine was working properly. They might or might not feel any tingling sensation during treatment because the intensity of the current was small." |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | In total, 3 patients dropped out and were excluded from analysis, as indicated by Gladys Cheing and Pearl Law in personal communication |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 24 patients randomised | |
| Interventions | Experimental intervention: TENS, 4 times per week, with a total of 8 applications and educational pamphlet Device: ITO model F‐2 (dual channel) | |
| Outcomes | Extracted pain outcome: global pain after 4 weeks, described as "pain (Numeric rating scale (NRS))" No primary outcome reported | |
| Notes | 2 out of 3 trial arms qualified for inclusion in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Drawing lots. Quote: "Subjects were randomly assigned by drawing a piece of paper that designated each person to the EA, TENS, and control groups" |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Low risk | Quote: "In each evaluation session, three outcome measures were collected." The authors present results of all these 3 outcomes. |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | No information provided |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 38 patients randomised | |
| Interventions | Experimental intervention: interferential current + exercise, interferential current stimulation: 3 times per week, exercise twice daily Device: Endomed 433 and Vacutron 423 (unclear whether 2 or 4 pole) | |
| Outcomes | Extracted pain outcome: other after 26 weeks, described as "Pain composite score with items rest, post‐exercise and night pain (approach unclear; either VAS or verbal scoring technique modified after Newland)"** No primary outcome reported | |
| Notes | *1 trial arm, in which shortwave diathermy was given, was excluded, **only baseline values with standard error and P values for change from baseline per group reported. No contact could be established with the investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | High risk | No results reported for some outcomes mentioned in the methods section, including maximum knee girth |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Low risk | Quote: "All patients completed their therapy and the first two assessments (baseline and end of treatment), while 92% completed the final assessment (3‐6 months after treatment)" |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Low risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised sham controlled trial | |
| Participants | 32 patients randomised | |
| Interventions | Experimental intervention: TENS, twice per week* Device: RDG Tiger Pulse | |
| Outcomes | Extracted pain outcome: global pain after 8 weeks, described as "Weekly pain score derived from daily pain recording (linear 7‐point scale)"** No primary outcome reported | |
| Notes | *Preceded by 1 'standard' week without any treatment, **No pain outcome data presented, investigators were contacted, but we did not receive any reply. This study only contributed in safety analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated. Quote: "(...) assigned by random computer programme and effected by using sealed envelopes containing cards which defined the treatment (...)". |
| Allocation concealment? | Unclear risk | Sealed assignment envelopes, but unclear whether these were opaque and sequential |
| Free of selective reporting? | High risk | No results reported for some outcomes mentioned in the methods section, including sleep disturbance |
| Adequate blinding of patients? | Low risk | Use of sham device: RDG Tiger Pulse with broken electrode connection at jack point, no current passed but flashing light on. Quote: "Exactly the same procedure were followed for both the treatment and control groups". |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | 15 out of 16 (0.94) randomised to experimental and 15 out of 16 (0.94) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 100 patients randomised, 25 per group | |
| Interventions | Experimental intervention: TENS, 5 times per week Device: MEA‐TENS (dual channel) | |
| Outcomes | Extracted pain outcome: global pain after 2 weeks described as "Overall present pain intensity at rest (Likert)" No primary outcome reported | |
| Notes | Two out of 4 groups, the electroacupuncture and ice massage groups, were excluded in this review. *Investigators named their intervention AL‐TENS, but we coded it low frequency TENS in our analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Low risk | Sham device: MEA‐TENS with broken lead at jack plug, no current passed but red indicator light on. Quote: "(...) treatment appeared to be done in the same way as the other groups without the subjects suspecting the nature of the stimulation". |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | Investigators reported that "no subject was withdrawn either active or placebo groups". However, the reported degrees of freedom indicate that 5 out of 100 patients were not included. It remained unclear to which of the 4 groups the excluded patients belonged. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
| Methods | Randomised controlled trial | |
| Participants | 78 patients randomised | |
| Interventions | Experimental intervention: pulsed electrostimulation stimulation, daily application Device: Bionicare Stimulator BIO‐1000 | |
| Outcomes | Extracted pain outcome: global pain after 34 weeks described as "Patient evaluation of pain of treated knee (Baseline based on 0‐10 VAS, follow‐up based on % change from baseline)" More than 2 primary outcomes reported (1 physician global evaluation; 2) VAS pain; 3) VAS function) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | High risk | No results reported for some outcomes mentioned in the methods, including walking time, tenderness and swelling |
| Adequate blinding of patients? | Low risk | Sham device: BIO‐1000, identical in appearance to active device, with automatic shut‐off as soon as amplitude is reduced (all patients were instructed to reduce intensity just below perception level) |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | 38 out of 41 (0.93) randomised to experimental and 33 out of 37 (0.89) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? | High risk | See above |
| Funding by commercial organisation avoided? | High risk | Sponsor: Murray Electronics |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
BMI = body mass index
min = minutes
OA = osteoarthritis
VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Less than 50% of patients diagnosed with osteoarthritis of the knee | |
| Not a randomised controlled trial, use of active control groups. Additional description: comparing diadynamic electrostimulation df, diadynamic electrostimulation cf and galvanic current | |
| Use of active control group. Additional description: randomised controlled trial comparing interferential current stimulation followed by patterned muscle stimulation and low‐current transcutaneous electrical nerve stimulation (TENS). | |
| Not concerning osteoarthritis | |
| No randomised controlled trial (review) | |
| Not transcutaneous but subcutaneous application | |
| Not transcutaneous but subcutaneous application. Abstract referring to same RCT as described in Cottingham 1985a. | |
| Use of active control group (exercise) | |
| Neuromuscular electrostimulation primarily aiming at muscle strengthening | |
| Neuromuscular electrostimulation primarily aiming at muscle strengthening | |
| Most likely not a randomised controlled trial; percutaneous electrostimulation primarily aiming at muscle strengthening | |
| Faradic electrostimulation with parameters set to increase muscle strength and use of active control (exercise plus low intensity (sham) faradic electrostimulation) | |
| No relevant pain or function outcomes | |
| High voltage galvanic electrostimulation for muscle strengthening | |
| Only 34% of patients suffered OA; use of active controls. Additional description: cross‐over design evaluating faradic electrostimulation. | |
| TENS as part of a combined experimental intervention. Additional description design: 3 groups, Group A receiving auricular acupuncture, diet control and aerobic exercise, Group B like A with addition of TENS and ultrasound, Group C receiving TENS and ultrasound; unclear whether allocation was at random. | |
| Use of active control: high frequency TENS versus low frequency TENS | |
| Percutaneous electrostimulation | |
| Electrode placement not involving knee innervation: transcranial electrostimulation | |
| Electrode placement not involving knee innervation: transcranial electrostimulation | |
| Cross‐over RCT reporting pooled results after completion of all phases. Contact established with Daniel and Beverly Lewis, who were unable to provide results for the first phase (before cross‐over) | |
| RCT reporting P values of effect only. Contact established with Daniel and Beverly Lewis, who could not provide any additional outcome data, nor could they indicate whether the design concerned a cross‐over or a parallel RCT | |
| Published abstract addressing the same cross‐over RCT reported by Lewis 1994 | |
| Cross‐over RCT reporting pooled results after completion of all phases. Contact established with Daniel and Beverly Lewis, who were unable to provide results for the first phase (before cross‐over) | |
| Not a randomised controlled study. Additional description: before‐after study design that was incorrectly labelled as randomised study by original authors. | |
| Not concerning osteoarthritis | |
| Not a randomised controlled trial (review) | |
| Not concerning osteoarthritis, not a randomised clinical trial. Tetanus‐like faradisation electrostimulation with exercise after surgical removal of meniscus, primarily aiming at muscle enhancement. Active control with 10 Hz sinusoidal current application and exercise. | |
| Electrical muscle stimulation using sport400 (Complex), primarily aiming at muscle strengthening | |
| Not a randomised clinical trial. Description: comparative study with historical control evaluating pulsed electrostimulation. | |
| Neuromuscular electrostimulation primarily aiming at muscle strengthening | |
| Electrostimulation primarily aiming at muscle strengthening | |
| Mixed population, only 4 out of 163 patients reported to have knee, hip or ankle OA | |
| Not concerning osteoarthritis (healthy volunteers) | |
| Not concerning osteoarthritis and not a randomised controlled trial | |
| Not a randomised controlled trial, combined multiple interventions in both interventions and control group | |
| Not a randomised controlled trial (review) | |
| Animal study | |
| Concerns chronic knee pain. First author was contacted by email to verify how many patients had osteoarthritis. No response received. Additional description: article in Korean, using a TENS device, abstract however suggests that parameters were set to strengthen muscles. | |
| Use of active control groups. Additional description: controlled trial with groups receiving either galvanic electrostimulation or YES ultrasound or pulsed shortwaves. Within these groups, half of the patients received ibuprofen, half received placebo ibuprofen. It was unclear whether allocation was at random. | |
| See Svarcova 1988a. Double publication of the same study, including the same number of patient and outcome data. | |
| Use of active control group. Additional description: galvanic electrostimulation versus electroacupuncture. | |
| Neuromuscular electrostimulation primarily aiming at muscle strengthening | |
| No relevant pain or function outcomes used | |
| Incomplete presentation of data. Additional description: cross‐over randomised clinical trial presenting pooled results only. Contact established with Mark Hallett, who was unable to provide data concerning the first phase, before cross‐over. We were unable to contact the other authors. | |
| Not concerning osteoarthritis | |
| Use of active control group. Additional description: random allocation of patients to 4 different types of diadynamic current. | |
| Not transcutaneous but periosteal (needle) application | |
| Use of active control group. Additional description: the combination of low‐energy laser, pulsed electromagnetic field and kinesitherapy was compared to the combination of electrotherapy, pulsed electromagnetic field and kinesitherapy. |
OA = osteoarthritis
RCT = randomised controlled trial
TENS = transcutaneous electrical nerve stimulation
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | ACTRNI2607000492459 |
| Methods | Double‐blind, randomised placebo‐controlled trial Randomisation method: computer‐generated block randomisation with stratification for gender, age and intensity of pain Blinding: patients, those administering treatment/s, those assessing outcomes, those analysing results/data Sample size calculation: reported Analyses based on intention‐to‐treat principle Trial duration: 26 weeks Sponsored by: non‐profit organisation Arthritis Australia and Physiotherapy Research Foundation |
| Participants | 70 patients with primary knee OA to be randomised |
| Interventions | Experimental intervention: pulsed electrostimulation, daily Device: Metron Digi‐10s, adapted by engineer Sham device: identical in appearance |
| Outcomes | Primary outcomes: conflicting information reported in Australian/New Zealand clinical trial register (ANZCTR) and subsequent publication in BMC. In ANZCR reported as pain on VAS, in BMC more than 2 primary outcomes are reported; pain (VAS and WOMAC), function (WOMAC), and patient global assessment (VAS). Main time points of interest are reported consistently as baseline, 4, 16 and 26 weeks. Secondary outcomes: in ANZCTR reported as function (WOMAC) and patient global assessment (VAS); in BMC reported as stiffness (WOMAC 3.1), quality of life (SF‐36), global perceived effect scale (GPES), physical activity (Human Activity Profile (HAP) questionnaire plus accelerometers Safety outcomes: in BMC, the recording of adverse events was reported |
| Starting date | 26th of September 2007 |
| Contact information | Robyn E Fary |
| Notes | Status at 17 July 2009: open to recruitment |
| Trial name or title | ISRCTN12912789 |
| Methods | A randomised, sham‐controlled trial with 3 parallel arms Randomisation method: not reported Blinding: not reported Sample size calculation: not reported Analyses: not reported whether is based on intention‐to‐treat principle Trial duration: 6 weeks Sponsored by: not reported |
| Participants | 261 (87 in each arm) patients with primary knee OA to be randomised |
| Interventions | Experimental intervention: TENS, as much as needed and group education including self‐efficacy and exercise training, once per week Device: not reported Sham device: identical in appearance, displays are active but there is no current output |
| Outcomes | Primary outcome: WOMAC function subscale (at baseline, 3, 6, 12 and 24 weeks) Secondary outcomes: |
| Starting date | 1 October 2007 |
| Contact information | Dr Shea Palmer |
| Notes | Status at 17 July 2009: completed at 30 June 2009 |
OA = osteoarthritis
TENS = transcutaneous electrical nerve stimulation
VAS = visual analogue scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 16 | 726 | Std. Mean Difference (Random, 95% CI) | ‐0.86 [‐1.23, ‐0.49] |
| Analysis 1.1  Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 1 Pain. | ||||
| 1.1 TENS | 11 | 465 | Std. Mean Difference (Random, 95% CI) | ‐0.85 [‐1.36, ‐0.34] |
| 1.2 Interferential current stimulation | 4 | 132 | Std. Mean Difference (Random, 95% CI) | ‐1.20 [‐1.99, ‐0.42] |
| 1.3 Pulsed electrostimulation | 2 | 129 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.77, ‐0.05] |
| 2 Number of patients withdrawn or dropped out because of adverse events Show forest plot | 8 | 363 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.16, 6.00] |
| Analysis 1.2  Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 2 Number of patients withdrawn or dropped out because of adverse events. | ||||
| 2.1 TENS | 6 | 255 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.03, 14.15] |
| 2.2 Interferential current stimulation | 1 | 30 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Pulsed electrostimulation | 1 | 78 | Risk Ratio (IV, Random, 95% CI) | 1.80 [0.17, 19.10] |
| 3 Function Show forest plot | 9 | 407 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.54, ‐0.14] |
| Analysis 1.3  Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 3 Function. | ||||
| 3.1 TENS | 5 | 204 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.69, 0.03] |
| 3.2 Interferential current stimulation | 3 | 74 | Std. Mean Difference (Random, 95% CI) | ‐0.27 [‐0.75, 0.20] |
| 3.3 Pulsed electrostimulation | 2 | 129 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.72, ‐0.00] |
| 4 Number of patients experiencing any adverse event Show forest plot | 3 | 175 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| Analysis 1.4  Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 4 Number of patients experiencing any adverse event. | ||||
| 4.1 TENS | 1 | 39 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Pulsed electrostimulation | 2 | 136 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| 5 Number of patients experiencing any serious adverse event Show forest plot | 4 | 195 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.02, 7.32] |
| Analysis 1.5  Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 5 Number of patients experiencing any serious adverse event. | ||||
| 5.1 TENS | 2 | 59 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.02, 7.32] |
| 5.2 Pulsed electrostimulation | 2 | 136 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |

Methodological characteristics and source of funding of included trials. (+) indicates low risk of bias, (?) unclear and (‐) a high risk of bias on a specific item.
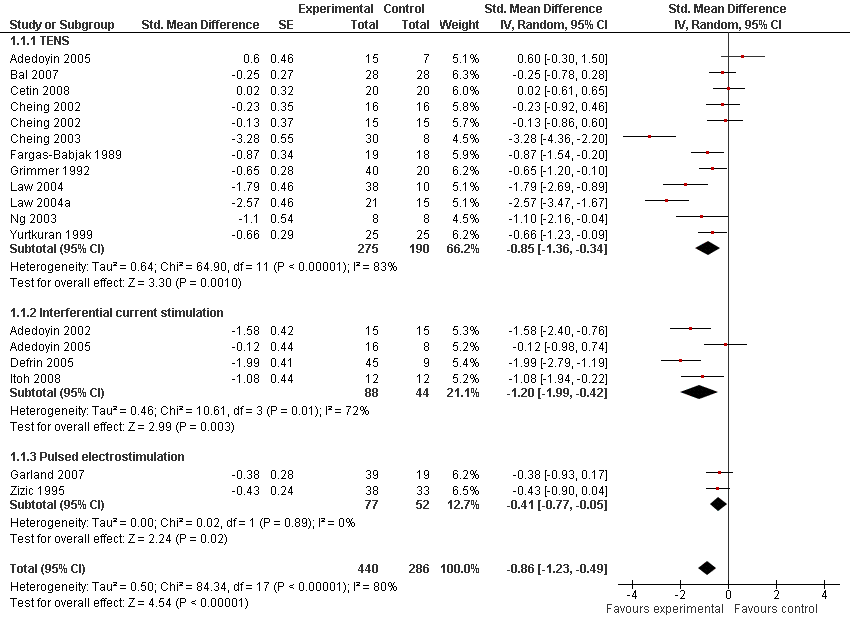
Forest plot of 16 trials comparing the effects of any type of transcutaneous electrostimulation and control (sham or no intervention) on knee pain. Values on x‐axis denote standardised mean differences. The plot is stratified according to type of electrostimulation. Law 2004 reported on knee level, we inflated the standard error with sqrt(number knees)/sqrt(number patients) to correct for clustering of knees within patients. Adedoyin 2005 and Cheing 2002 contributed with two comparisons each. In Adedoyin 2005, the standard error was inflated and the number of patients in the control group was halved to avoid duplicate counting of patients when including 2 both comparisons in the overall meta‐analysis. Data relating to the 3, 2, 3 and 4 active intervention arms in Cheing 2003, Grimmer 1992, Law 2004 and Defrin 2005, respectively, were pooled.

Funnel plot for effects on knee pain.
Numbers on x‐axis refer to standardised mean differences (SMDs), on y‐axis to standard errors of SMDs.

Forest plot of 8 trials comparing patients withdrawn or dropped out because of adverse events between any transcutaneous electrostimulation and control (sham or no intervention). Values on x‐axis denote risk ratios. Risk ratios could not be estimated in 5 trials, because no drop‐out occurred in either group. The plot is stratified according to type of electrostimulation. Data relating to the 3 and 2 active intervention arms in Cheing 2003 and Grimmer 1992, respectively, were pooled.
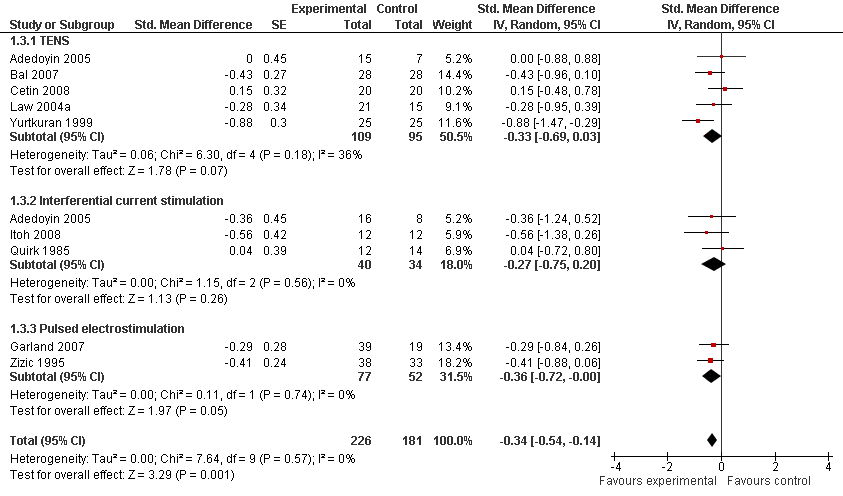
Forest plot of 9 trials comparing the effects of any type of transcutaneous electrostimulation and control (sham or no intervention) on function. Values on x‐axis denote standardised mean differences. The plot is stratified according to type of electrostimulation. In Adedoyin 2005, the standard error was inflated and the number of patients in the control group was halved to avoid duplicate counting of patients when including both comparisons in the overall meta‐analysis.

Funnel plot for effects on functioning of the knee.
Numbers on x‐axis refer to standardised mean differences (SMDs), on y‐axis to standard errors of SMDs.
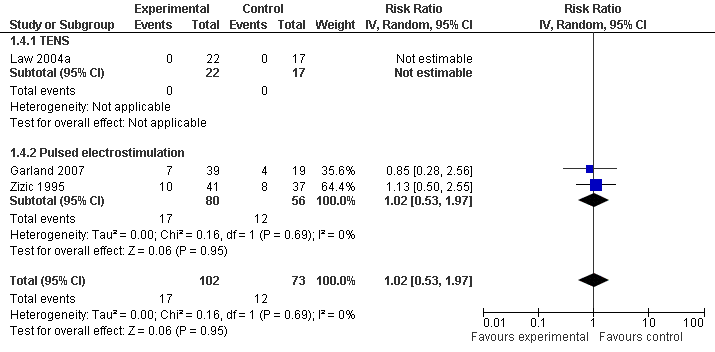
Forest plot of 3 trials comparing patients experiencing any adverse event between any transcutaneous electrostimulation and control (sham or no intervention). Values on x‐axis denote risks ratios. The risk ratio in one TENS trial could not be estimated because no adverse event occurred in either group. The plot is stratified according to type of electrostimulation.
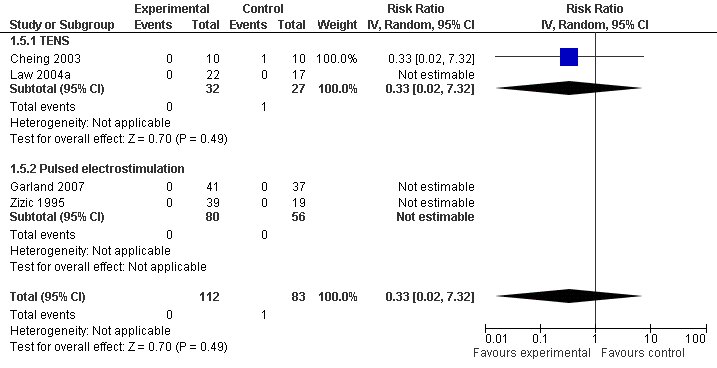
Forest plot of 4 trials comparing patients experiencing any serious adverse event between any transcutaneous electrostimulation and control (sham or no intervention). Values on x‐axis denote risk ratios. Risk ratios could not be estimated in 3 trials, because no serious adverse event occurred in either group. The plot is stratified according to type of electrostimulation. Data relating to the 3 active intervention arms in Cheing 2003 were pooled.
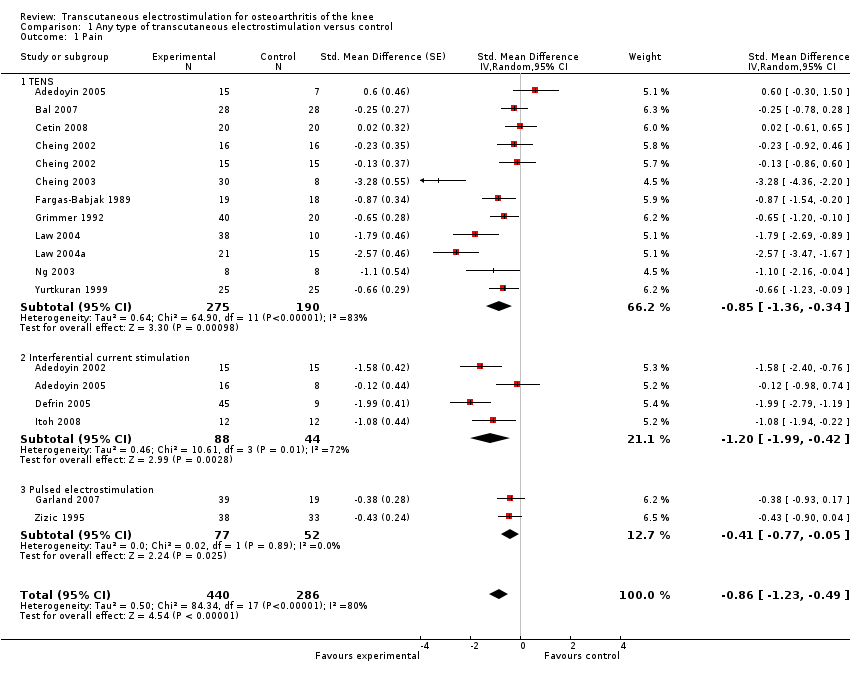
Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 1 Pain.
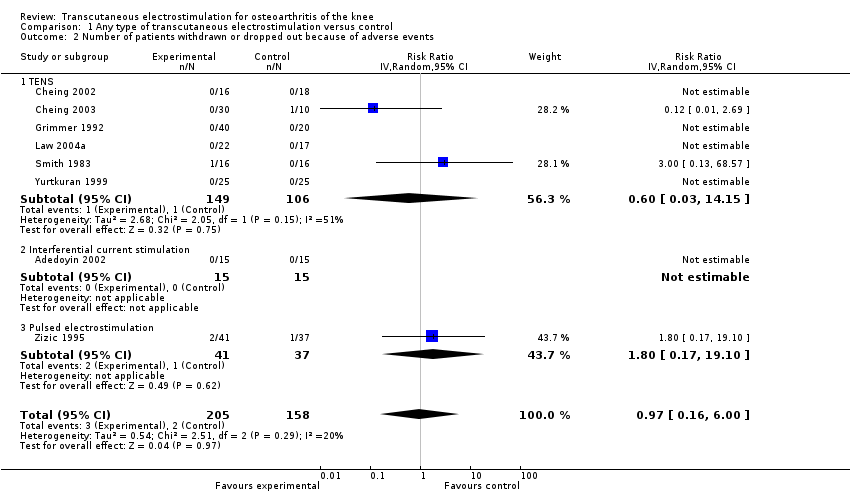
Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 2 Number of patients withdrawn or dropped out because of adverse events.

Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 3 Function.
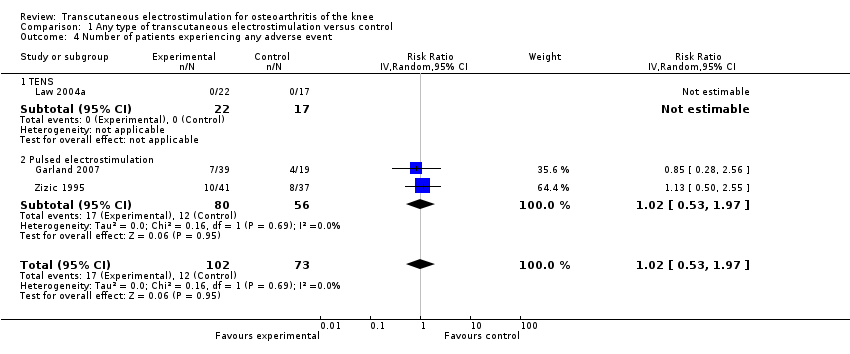
Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 4 Number of patients experiencing any adverse event.

Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 5 Number of patients experiencing any serious adverse event.
| Any type of transcutaneous electrostimulation compared with sham or no intervention for osteoarthritis of the knee | ||||||
| Patient or population: patients with osteoarthritis Settings: physical therapy practice of outpatient clinic Intervention: any type of transcutaneous applied electrostimulation Comparison: sham or no specific intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk* | Corresponding risk | |||||
| Sham or no specific intervention | Any type of transcutaneous electrostimulation | |||||
| Pain Various pain scales Median follow‐up: 4 weeks | ‐1.8 cm change on 10 cm VAS1 29% improvement | ‐2.0 cm change 33% improvement | SMD ‐0.07 (‐0.46 to 0.32) | 726 | +OOO | Little evidence of beneficial effect (NNT: not statistically significant) The estimated pain in the intervention group of large trials was derived from meta‐regression using the standard error as independent variable |
| Function Various validated function scales Median follow‐up: 4 weeks | ‐1.2 units on WOMAC 21% improvement | ‐2.3 units on WOMAC 41% improvement | SMD ‐0.34 | 407 | +OOO | NNT: 10 (95% CI 7 to 22)8 |
| Number of patients experiencing any adverse event Median follow‐up: 4 weeks | 150 per 1000 patient‐years1 | 153 per 1000 patient‐years | RR 1.02 (0.53 to 1.97) | 175 | ++OO | No evidence of harmful effect (NNH: not statistically significant) |
| Number of patients withdrawn or dropped out because of adverse events Median follow‐up: 4 weeks | 17 per 1000 patient‐years1 | 16 per 1000 patient‐years | RR 0.97 (0.16 to 6.00) | 363 | +++O | No evidence of harmful effect (NNH: not statistically significant) |
| Number of patients experiencing any serious adverse event Median follow ‐up: 4 weeks | 4 per 1000 patient‐years1 | 1 per 1000 patient‐years | RR 0.33 (0.02 to 7.32) | 195 | ++OO | No evidence of harmful effect (NNH: not statistically significant) |
| *The basis for the assumed risk in the safety outcomes (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence 1 Median reduction as observed across control groups in large osteoarthritis trials (Nuesch 2009). | ||||||
| Variable | N of trials | N of patients | N of patients | Pain intensity | Heterogeneity | P for interaction |
| n | n | n | SMD (95% CI) | I2 (%) |
| |
| All trials | 16 | 440 | 286 | ‐0.86 (‐1.23 to ‐0.49) | 80% | |
| Allocation concealment | 0.47 | |||||
| Adequate | 2 | 79 | 39 | ‐0.52 (‐0.91 to ‐0.13) | 0% | |
| Inadequate or unclear | 14 | 361 | 247 | ‐1.03 (‐1.49 to ‐0.57) | 84% | |
| Type of control intervention* | 0.12 | |||||
| Sham intervention | 12 | 354 | 216 | ‐1.13 (‐1.59 to ‐0.67) | 82% | |
| No control intervention | 5 | 86 | 70 | ‐0.31 (‐0.80 to 0.19) | 58% | |
| Blinding of patients | 0.37 | |||||
| Adequate | 11 | 309 | 205 | ‐1.05 (‐1.52 to ‐0.59) | 82% | |
| Inadequate or unclear | 6 | 131 | 79 | ‐0.63 (‐1.31 to 0.05) | 81% | |
| Use of analgesic cointerventions | 0.36 | |||||
| Similar between groups | 4 | 124 | 83 | ‐0.57 (‐1.16 to 0.02) | 74% | |
| Not similar or unclear | 12 | 316 | 23 | ‐1.10 (‐1.60 to ‐0.59) | 84% | |
| Intention‐to‐treat analysis | 0.73 | |||||
| Yes | 3 | 83 | 63 | ‐0.76 (‐1.43 to ‐0.09) | 72% | |
| No or unclear | 13 | 357 | 223 | ‐1.00 (‐1.48 to ‐0.53) | 84% | |
| Type of ES** | 0.94 | |||||
| High frequency TENS | 8 | 177 | 139 | ‐0.82 (‐1.51 to ‐0.12) | 86% | |
| Burst TENS | 2 | 39 | 38 | ‐0.85 (‐1.32 to ‐0.38) | 0% | |
| Modulation TENS | 1 | 13 | 3 | ‐1.41 (‐2.92 to 0.10) | N/A | |
| Low frequency TENS | 3 | 46 | 40 | ‐0.82 (‐1.29 to ‐0.34) | 0% | |
| Interferential current stimulation | 4 | 88 | 44 | ‐1.20 (‐1.99 to ‐0.42) | 71% | |
| Pulsed ES | 2 | 77 | 52 | ‐0.41 (‐0.77 to ‐0.05) | 0% | |
| Duration of ES per session† | 0.69‡ | |||||
| ≤ 20 minutes | 8 | 166 | 112 | ‐0.95 (‐1.55 to ‐0.35) | 78% | |
| 30 to 40 minutes | 6 | 156 | 99 | ‐1.45 (‐2.28 to ‐0.62) | 85% | |
| ≥ 60 minutes | 4 | 118 | 91 | ‐0.47 (‐0.96 to 0.02) | 58% | |
| Number of sessions per week | 0.90‡ | |||||
| ≤ 3 | 6 | 163 | 91 | ‐0.81 (‐1.48 to ‐0.14) | 82% | |
| 4 to 6 | 7 | 182 | 125 | ‐1.33 (‐2.11 to ‐0.54) | 88% | |
| ≥ 7 | 3 | 96 | 70 | ‐0.51 (‐0.83 to ‐0.19) | 0% | |
| Duration of ES per week*** | 0.74‡ | |||||
| ≤1 hour | 5 | 123 | 71 | ‐0.85 (‐1.72 to 0.01) | 86% | |
| > 1 to 5 hours | 8 | 180 | 122 | ‐1.42 (‐2.11 to ‐0.74) | 81% | |
| > 5 hours | 5 | 137 | 109 | ‐0.53 (‐0.96 to ‐0.11) | 55% | |
| Duration of treatment period | 0.14 | |||||
| < 4 weeks | 7 | 190 | 114 | ‐1.39 (‐2.13 to ‐0.66) | 86% | |
| ≥ 4 weeks | 9 | 250 | 172 | ‐0.64 (‐1.06 to ‐0.22) | 75% | |
| ES: electrostimulation; *In Cheing 2002, two independent comparisons contributed in the two different strata. **Adedoyin 2005, Grimmer 1992 and Law 2004 contributed to two, two and three different strata: high‐frequency TENS and interferential current stimulation, high‐frequency TENS and burst, and high‐, low‐frequency and modulation TENS, respectively. † = Cheing 2003 contributed to all three different strata, with the same 8 control patients displayed in each stratum. ‡ = P values from test for trend. | ||||||
| Variable | N of trials | N of patients | N of patients | Function | Heterogeneity | P for interaction |
|
| SMD (95% CI) | I2 (%) |
| |||
| All trials | 9 | 226 | 181 | ‐0.34 (‐0.54 to ‐0.14) | 0% | |
| Allocation concealment | 0.88 | |||||
| Adequate | 1 | 39 | 19 | ‐0.29 (‐0.85 to 0.26) | N/A | |
| Inadequate or unclear | 8 | 187 | 162 | ‐0.34 (‐0.56 to ‐0.12) | 5% | |
| Type of control intervention | 0.14 | |||||
| Sham intervention | 5 | 151 | 120 | ‐0.46 (‐0.70 to ‐0.21) | 0% | |
| No control intervention | 4 | 75 | 61 | ‐0.10 (‐0.45 to 0.24) | 0% | |
| Blinding of patients | 0.14 | |||||
| Adequate | 5 | 151 | 120 | ‐0.46 (‐0.70 to ‐0.21) | 0% | |
| Inadequate or unclear | 4 | 75 | 61 | ‐0.10 (‐0.45 to 0.24) | 0% | |
| Use of analgesic cointerventions | 0.95 | |||||
| Similar between groups | 2 | 69 | 48 | ‐0.33 (‐0.70 to 0.05) | 0% | |
| Not similar or unclear | 7 | 157 | 133 | ‐0.34 (‐0.60 to ‐0.08) | 15% | |
| Intention‐to‐treat analysis | 0.76 | |||||
| Yes | 2 | 40 | 42 | ‐0.28 (‐0.71 to 0.16) | 0% | |
| No or unclear | 7 | 186 | 139 | ‐0.35 (‐0.58 to ‐0.12) | 5% | |
| Type of ES** | 0.32 | |||||
| High frequency TENS | 4 | 84 | 70 | ‐0.18 (‐0.50 to 0.14) | 0% | |
| Burst TENS | 0 | |||||
| Modulation TENS | 0 | |||||
| Low frequency TENS | 1 | 25 | 25 | ‐0.88 (‐1.46 to ‐0.30) | N/A | |
| Interferential current stimulation | 3 | 40 | 34 | ‐0.27 (‐0.75 to 0.20) | 0% | |
| Pulsed ES | 2 | 77 | 52 | ‐0.36 (‐0.72 to ‐0.00) | 0% | |
| Duration of ES per session | 0.80‡ | |||||
| ≤ 20 minutes | 5 | 100 | 86 | ‐0.29 (‐0.69 to 0.11) | 44% | |
| 30 to 40 minutes | 2 | 49 | 43 | ‐0.37 (‐0.79 to 0.04) | 0% | |
| ≥ 60 minutes | 2 | 77 | 52 | ‐0.36 (‐0.72 to ‐0.00) | 0% | |
| Number of sessions per week | 0.32‡ | |||||
| ≤ 3 | 4 | 75 | 61 | ‐0.10 (‐0.45 to 0.24) | 0% | |
| 4 to 6 | 3 | 74 | 68 | ‐0.54 (‐0.88 to ‐0.20) | 2% | |
| ≥ 7 | 2 | 77 | 52 | ‐0.36 (‐0.72 to ‐0.00) | 0% | |
| Duration of ES per week | 0.32‡ | |||||
| ≤ 1 hour | 4 | 75 | 61 | ‐0.10 (‐0.45 to 0.24) | 0% | |
| > 1 to 5 hours | 3 | 74 | 68 | ‐0.54 (‐0.88 to ‐0.20) | 2% | |
| > 5 hours | 2 | 77 | 52 | ‐0.36 (‐0.72 to ‐0.00) | 0% | |
| Duration of treatment period | 0.18 | |||||
| < 4 weeks | 3 | 74 | 68 | ‐0.54 (‐0.88 to ‐0.20) | 2% | |
| ≥ 4 weeks | 6 | 152 | 113 | ‐0.23 (‐0.47 to 0.02) | 0% | |
| ES: electrostimulation; **Adedoyin 2005 contributed to two different strata: high‐frequency TENS and interferential current stimulation; ‡ = P values from test for trend. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 16 | 726 | Std. Mean Difference (Random, 95% CI) | ‐0.86 [‐1.23, ‐0.49] |
| 1.1 TENS | 11 | 465 | Std. Mean Difference (Random, 95% CI) | ‐0.85 [‐1.36, ‐0.34] |
| 1.2 Interferential current stimulation | 4 | 132 | Std. Mean Difference (Random, 95% CI) | ‐1.20 [‐1.99, ‐0.42] |
| 1.3 Pulsed electrostimulation | 2 | 129 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.77, ‐0.05] |
| 2 Number of patients withdrawn or dropped out because of adverse events Show forest plot | 8 | 363 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.16, 6.00] |
| 2.1 TENS | 6 | 255 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.03, 14.15] |
| 2.2 Interferential current stimulation | 1 | 30 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Pulsed electrostimulation | 1 | 78 | Risk Ratio (IV, Random, 95% CI) | 1.80 [0.17, 19.10] |
| 3 Function Show forest plot | 9 | 407 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.54, ‐0.14] |
| 3.1 TENS | 5 | 204 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.69, 0.03] |
| 3.2 Interferential current stimulation | 3 | 74 | Std. Mean Difference (Random, 95% CI) | ‐0.27 [‐0.75, 0.20] |
| 3.3 Pulsed electrostimulation | 2 | 129 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.72, ‐0.00] |
| 4 Number of patients experiencing any adverse event Show forest plot | 3 | 175 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| 4.1 TENS | 1 | 39 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Pulsed electrostimulation | 2 | 136 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| 5 Number of patients experiencing any serious adverse event Show forest plot | 4 | 195 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.02, 7.32] |
| 5.1 TENS | 2 | 59 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.02, 7.32] |
| 5.2 Pulsed electrostimulation | 2 | 136 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |


