Coasting疗法(抑制促性腺激素)预防卵巢过度刺激综合征
摘要
研究背景
卵巢过度刺激综合征(ovarian hyperstimulation syndrome, OHSS)是一种因卵巢过度刺激而导致的医源性且可能危及生命的疾病。据报告,中度至重度OHSS的发病率为体外受精(in vitro fertilization, IVF)周期的0.6%至5%。OHSS的病因尚未得到完全解释。在人绒毛膜促性腺激素(human chorionic gonadotrophin, hCG)刺激下,卵巢分泌的血管活性物质的释放可能在引发该综合征中发挥关键作用。这种情况的特征是液体从血管内室大量转移到第三空间,导致严重的血管内耗竭和血液浓缩。
研究目的
评估在辅助生殖周期中Coasting疗法(抑制促性腺激素)对预防卵巢过度刺激综合征的效果。
检索策略
为了更新本综述,我们检索了截至2016年7月6日的Cochrane妇科和生育小组试验注册库(Cochrane Gynaecology and Fertility Group Trials Register)、Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL)、MEDLINE(PubMed)、CINHAL、PsycINFO、Embase、Google和美国临床试验注册平台( ClinicalTrials.gov )。
纳入排除标准
我们仅纳入了随机对照试验(randomized controlled trials, RCT),其中使用Coasting疗法来预防OHSS。
资料收集与分析
两名综述作者独立筛选试验和提取资料。通过讨论解决分歧。并联系研究作者以获取更多信息。干预比较包括Coasting疗法与无Coasting疗法、Coasting疗法与早期单侧卵泡抽吸(early unilateral follicular aspiration, EUFA)、Coasting疗法与促性腺激素释放激素拮抗剂(拮抗剂)、Coasting疗法与hCG触发时卵泡刺激素给药(卵泡刺激素共同触发)以及Coasting疗法与卡麦角林。我们根据Cochrane指南进行统计分析。我们的主要结局是中度或重度OHSS和活产。
主要结果
我们纳入了八项随机对照试验(702名处于发生OHSS高风险的女性)。证据质量为低或极低。主要限制是未能报告活产、由于缺乏研究方法相关信息而导致的偏倚风险以及由于事件发生率低和缺乏数据而导致的不精确性。其中四项研究仅作为摘要发表,并提供有限的数据。
Coasting疗法与无Coasting疗法
Coasting疗法组的OHSS发生率较低(比值比(odds ratio, OR)为0.11,95%置信区间(confidence interval, CI)0.05至0.24;I²=0%,两项随机对照试验;207名女性;低质量证据),这表明如果45%的女性在未接受Coasting疗法的情况下发展为中度或重度OHSS,那么4%到17%接受Coasting疗法的女性会进一步发展。数据太少,无法确定各组之间的活产率(OR=0.48,95%CI [0.14, 1.62];一项随机对照试验;68名女性;证据质量极低)、临床妊娠(OR=0.82, 95% CI [0.46, 1.44]; I²=0%;两项随机对照试验;207名女性;低质量证据),多胎妊娠(OR=0.31, 95% CI [0.12, 0.81];一项随机对照试验;139名女性;低质量证据),或流产(OR=0.85, 95% CI [0.25, 2.86]; I²=0%;两项随机对照试验;207名女性;证据质量极低)。
Coasting疗法与与EUFA
数据太少,无法确定各组间OHSS发生率(OR=0.98,95% CI [0.34, 2.85]; I²=0%; 2项随机对照试验;83名女性;证据质量极低)或临床妊娠(OR=0.67,95% CI [0.25, 1.79];I²=0%;2项随机对照试验;83名女性;证据质量极低)是否存在差异;没有研究报告活产、多胎妊娠和流产。
Coasting疗法与与拮抗剂
一项随机对照试验(190名女性)报告了这一组对照,组均未发生OHSS事件。数据太少,无法确定组间在临床妊娠率(OR=0.74, 95% CI [0.42, 1.31]; 1项RCT;190名女性;低质量证据)和多胎妊娠率(OR=1.00, 95% CI [0.43, 2.32]; 1项RCT;98名女性;极低质量证据)方面是否存在差异;本研究未报告活产或流产情况。
Coasting疗法与hCG触发时卵泡刺激素给药(卵泡刺激素共同触发)
Coasting疗法组的OHSS发生率较高(OR=43.74, 95% CI [2.54, 754.58];一项随机对照试验;102名女性;证据质量极低),Coasting疗法组中有15例OHSS,hCG触发时卵泡刺激素给药(卵泡刺激素共同触发)组中没有发生OHSS事件。数据太少,无法确定各组之间的临床妊娠率是否存在差异(OR=0.92, 95% CI [0.43, 2.10];一项随机对照试验;102名女性;低质量证据)。这项研究没有报告适合分析活产、多胎妊娠或流产的数据,但指出各组之间没有显著差异。
Coasting疗法与卡麦角林
数据太少,无法确定各组之间的OHSS发生率是否存在差异(OR=1.98, 95% CI [0.09, 5.68]; P=0.20; I²=72%;两项随机对照试验;120名女性;证据质量极低),Coasting疗法组有11例OHSS,卡麦角林组有6例。有证据表明,Coasting疗法与较低的临床妊娠率相关(OR=0.38,95% CI [0.16, 0.88]; P=0.02; I²=0%;两项随机对照试验;120名女性;证据质量极低),但总共只有33例。这些研究没有报告适合分析活产、多胎妊娠或流产的数据。
作者结论
低质量证据表明,Coasting疗法比无Coasting疗法更能降低中度或重度OHSS的发生率。没有证据表明Coasting疗法比其他干预措施更有益,只是有一项小型研究的极低质量证据表明在HCG给药时使用FSH共同触发可能比Coasting疗法能更好地降低OHSS的风险。数据太少,无法明确定各组之间在其他结局方面是否存在差异。
PICO
简语概要
Coasting疗法(抑制促性腺激素)预防卵巢过度刺激综合征
研究问题
评估在辅助生殖周期中Coasting疗法(抑制促性腺激素)对预防卵巢过度刺激综合征的效果。
背景
卵巢过度刺激综合征(ovarian hyperstimulation syndrome, OHSS)是体外受精(in vitro fertilization, IVF)中使用激素诱导排卵(卵子释放)的并发症。激素有时会过度刺激卵巢。严重的OHSS可能会危及生命。“coasting”疗法或长期“coasting”疗法可用来降低OHSS的风险。这涉及在排卵前抑制一种激素(促性腺激素)。
检索日期
我们纳入了截至2016年7月6日的研究。
研究特征
确定了八项随机对照试验(702名女性),将抑制促性腺激素(coasting疗法)与另一种预防卵巢过度刺激综合征的干预进行比较。其他干预措施包括无coasting疗法、早期单侧卵泡抽吸(在注射人绒毛膜促性腺激素(human chorionic gonadotrophin, hCG)后10到12小时,从一侧卵巢取卵泡)、促性腺激素释放激素(gonadotrophin‐releasing hormone, GnRH)拮抗剂(阻断黄体生成素(luteinising hormone, LH)释放的药物)、卵泡刺激素(follicle‐stimulating hormone, FSH)、FSH共同触发(在hCG注射的同时给予额外剂量的FSH)。
主要结果
低质量证据表明,Coasting疗法比无Coasting疗法更能降低中度或重度OHSS的发生率。没有证据表明Coasting疗法比其他干预措施更有益,只是有一项小型研究的极低质量证据表明在HCG给药时使用FSH共同触发可能比Coasting疗法能更好地降低OHSS的风险。数据太少,无法明确定各组之间在其他结局方面是否存在差异。
证据质量
证据质量为低或极低。主要限制是未能报告活产、由于缺乏研究方法相关信息而导致的偏倚风险以及由于事件发生率低和缺乏数据而导致的不精确性。其中四项研究仅作为摘要发表,并提供有限的数据。
Authors' conclusions
Summary of findings
| Coasting versus no coasting for prevention of ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no coasting | Risk with coasting | |||||
| OHSS | 457 per 1000 | 85 per 1000 | OR 0.11 | 207 | ⊕⊕⊝⊝ | |
| Live birth | 265 per 1000 | 147 per 1000 | OR 0.48 | 68 | ⊕⊝⊝⊝ | |
| Clinical pregnancy | 390 per 1000 | 344 per 1000 | OR 0.82 | 207 | ⊕⊕⊝⊝ | |
| Multiple pregnancy | 268 per 1000 | 102 per 1000 | OR 0.31 | 139 | ⊕⊝⊝⊝ | |
| Miscarriage | 57 per 1,000 | 49 per 1,000 (15 to 148) | OR 0.85 (0.25 to 2.86) | 207 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias: one study did not clearly describe the methods used, studies not blinded 2 Downgraded one level for serious imprecision: few events, wide confidence intervals, or both 3 Downgraded two levels for very serious imprecision: very few events, very wide confidence intervals, or both | ||||||
| Coasting versus early unilateral follicular aspiration for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with early unilateral follicular aspiration (EUFA) | Risk with coasting | |||||
| OHSS | 244 per 1000 | 240 per 1000 | OR 0.98 | 83 | ⊕⊝⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 317 per 1000 | 237 per 1000 | OR 0.67 | 83 | ⊕⊝⊝⊝ | |
| Multiple pregnancy | No data available | |||||
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias: one study did not clearly describe methods, lack of blinding 2 Downgraded two levels for very serious imprecision: very few events and very wide confidence intervals | ||||||
| Coasting versus gonadotrophin‐releasing hormone antagonist for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with gonadotrophin‐releasing hormone antagonist | Risk with coasting | |||||
| OHSS | Not estimable | Not estimable | not estimable | 190 | ⊕⊕⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 553 per 1000 | 478 per 1000 | OR 0.74 | 190 | ⊕⊕⊝⊝ | |
| Multiple pregnancy | 181 per 1000 | 156 per 1000 | OR 0.84 | 98 | ⊕⊕⊝⊝ | |
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: method of sequence generation not reported, lack of blinding 2 Downgraded two levels due to very serious imprecision: no OHSS occurred in either group. Few events for multiple pregnancy. 3 Downgraded one level due to serious imprecision. Wide confidence intervals, few events | ||||||
| Coasting versus follicle stimulating hormone (FSH) administration at time of hCG trigger in preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with FSH co‐trigger with hCG administration | Risk with Coasting | |||||
| OHSS | Not estimable | Not estimable | OR 43.74 | 102 | ⊕⊝⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 510 per 1000 | 489 per 1000 | OR 0.92 | 102 | ⊕⊕⊝⊝1,3 | |
| Multiple pregnancy | No data available | |||||
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: method of sequence generation not reported, lack of blinding 2 Downgraded two levels due to very serious imprecision: only 15 events, all in one arm. 3 Downgraded one level due to serious imprecision: very wide confidence intervals | ||||||
| Coasting compared to cabergoline for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with cabergoline | Risk with Coasting | |||||
| OHSS | 100 per 1000 | 180 per 1000 | OR 1.98 | 120 | ⊕⊝⊝⊝1,2 | |
| Live birth | Not reported | |||||
| Clinical pregnancy rate | 367 per 1000 | 180 per 1000 | OR 0.38 (0.16 to 0.88) | 120 | ⊕⊕⊝⊝1 | |
| Multiple pregnancy | Not reported | |||||
| Miscarriage | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious risk of bias: one study did not clearly define method, method of sequence generation not reported, lack of blinding 2 Downgraded one level due to serious imprecision: very few events and/or wide confidence interval. | ||||||
Background
Description of the condition
Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, potentially life threatening condition resulting from excessive ovarian stimulation. The American Society for Reproductive Medicine (ASRM) noted that the incidence of OHSS is difficult to determine, as there is no consensus definition. Estimates of the incidence of moderate to severe OHSS range from 0.6% to 5% of assisted reproduction cycles (ASRM 2016; Calhaz‐Jorge 2016; Kawwass 2015). Studies of risk factors for OHSS have identified younger age, black race, ovulation, tubal factor, unexplained infertility, younger age, and possibly a lower body mass index (ASRM 2016).
In addition, polycystic ovarian syndrome (PCOS) or an ultrasonographic ovarian appearance of polycystic ovaries (presence of multiple, small follicles at the periphery of the ovary with echogenic stroma); establishment of pregnancy during assisted reproduction treatment (ART); human chorionic gonadotrophin (hCG) supplementation of the luteal phase; and high serum estradiol (E2; higher than 2500 pg/ml) were reported to be associated with OHSS.
Rabau and colleagues originally classified OHSS as mild, moderate or severe (Rabau 1967; Schenker 1978). Golan 1989 subsequently modified it by incorporating ultrasonographic measurement of the stimulated ovaries (Table 1). Navot 1992 introduced further modification by differentiating between the severe and the life threatening form of OHSS (Table 2). There was further classification from Rizk 1999 (Table 3).
| Classification | Size of ovaries | Grade | Symptoms |
| Mild | 5 to 10 cm | grade 1 | abdominal tension and discomfort |
| grade 2 | grade 1 signs plus nausea, vomiting, diarrhoea, or a combination | ||
| Moderate | > 10 cm | grade 3 | grade 2 signs plus ultrasound evidence of ascites |
| Severe | > 12 cm | grade 4 | grade 3 signs plus clinical evidence of ascites, pleural effusion and dyspnoea, or a combination |
| grade 5 | grade 4 signs plus haemoconcentration increased blood viscosity, hypovolaemia, decreased renal perfusion, oliguria |
| Severe | Critical |
| Variably enlarged ovary | Variably enlarged ovary |
| Massive ascites ± hydrothorax | Tense ascites ± hydrothorax |
| Hct > 45% (> 30% increment over baseline value) | Hct > 55% |
| WBC > 15,000 | WBC > 35,000 |
| Oliguria | |
| Creatinine 1.0 to 1.5 | Creatinine > 1.6 |
| Creatinine clearance > 50 ml/min | Creatinine clearance < 50ml/min |
| Liver dysfunction | Renal failure |
| Anasarca | Thromboembolic phenomena |
| Adult respiratory distress syndrome (ARDS) |
| Moderate | Severe Grade A | Severe Grade B | Severe Grade C |
| Discomfort, pain, nausea, distension, ultrasonic evidence of ascites and enlarged ovaries, normal haematological and biological profile | Dyspnoea, oliguria, nausea, vomiting, diarrhoea, abdominal pain, clinical evidence of ascites, marked distension of abdomen or hydro‐thorax, US showing large ovaries and marked ascites, normal biochemical profile | Grade A plus massive tension ascites, markedly enlarged ovaries, severe dyspnoea and marked oliguria, increased haematocrit, elevated serum creatinine and liver dysfunction | Complications such as respiratory distress syndrome, renal shut‐down, or venous thrombosis |
The factors leading to this syndrome have not been completely elucidated. It seems likely that the release of vasoactive substances, for example vascular endothelium growth factor (VEGF) secreted by the ovaries under hCG stimulation, play a key role in triggering this syndrome (Goldsman 1995; Tsirigotis 1994). As more follicles are recruited in response to gonadotrophin stimulation, the mass of the granulosa cells increases, while at the same time, the cells gain functional maturation. These two factors, acting synergistically, cause a concomitant increase in serum estradiol levels and vasoactive substances (as yet poorly defined (Agrawal 1999; Al‐Shawaf 2001)). This condition is characterised by a massive shift of fluid from the intra‐vascular compartment to the third space, resulting in profound intra‐vascular depletion and haemoconcentration (Rabau 1967; Schenker 1978).
Alternative strategies (embryo freezing, antagonist cycles, intravenous albumin infusion, agonist trigger, cabergoline) have been proposed for women undergoing ART who are at high risk of OHSS. Each of these strategies may reduce, but not eliminate, the risk.
Description of the intervention
Coasting, or prolonged coasting, may be defined as a process whereby: (1) gonadotrophin therapy is discontinued while administration of gonadotrophin‐releasing hormone agonist (GnRHa) is continued; (2) there is a delay, by a variable number of days, in administering hCG injection to trigger oocyte maturation prior to oocyte retrieval, until safe estradiol levels are attained (Sher 1993). Coasting should not be initiated too early, or too late. The effective duration of coasting is still to be determined. Many authors believe that each IVF centre should identify its own cut‐off limit of serum estradiol, follicle size, or both, at the onset of coasting, and hCG administration (Al‐Shawaf 2001; Waldenstrom 1999; Table 4).
| Authors | E2 at coasting | E2 at hCG | Number and follicle size | Coasting time |
| > 3000 pg/mL or > 11,000 pmol/L* | < 3000 pg/mL or < 11,000 pmol/L* | > 29 follicles at least 30% > 15 mm | 3 to 11 days (mean 6.1) | |
| ≥ 3000 pg/ml or ≥ 11,000 pmol/l* | < 3000 pg/ml or < 11,000 pmol/l* | at least 3 follicles of 15.6 ± 1.4 mm | 1.9 ± 0.9 days | |
| > 3000 pg/ml or > 11,000 pmol/l* | < 3000 pg/mL or < 11,000 pmol/L* | 5 follicles at least 16 mm, two of which are at least 19 mm | 1 to 5 days | |
| > 2500 pg/ml or > 9000 pmol/l* | < 2500 pg/ml or < 9000 pmol/l* | ≥ 20 follicles > 15 mm | 1 to 6 days (mean 1.94) | |
| > 2700 pg/ml or > 10,000 pmol/l* | no values given | many immature follicles < 3 at 18 mm | 3 days | |
| > 2500 pg/ml or > 9000 pmol/l* | < 2500 pg/ml or < 9000 pmol/l* | ≥ 20 follicles > 14 mm | 1 to 6 days (mean 1.94) | |
| > 6000 pg/ml or > 22,000 pmol/l* | < 3000 pg/ml or 11,000 pmol/l* | > 15 follicles, each of > 18 mm in each ovary | 4.9 ± 1.6 days | |
| > 2700 pg/ml or > 10,000 pmol/l* | < 2700 pg/ml or < 10,000 pmol/l* | > 25 follicles, at least 3 follicles > 17 mm | 3 to 6 days (mean 4.3) | |
| > 3000 pg/ml or > 11,000 pmol/l* | 25% decline < 2250 pg/ml or 8250 pmol/l* | > 3 follicles of > 18 mm | 3 to 5 days (mean 3.4 ± 0.1) | |
| > 3600 pg/ml or > 13,000 pmol/l* | < 2700 pg/ml or < 10,000 pmol/l* | at least 25% of the follicles > 15 mm | 2 to 9 days (mean 3.4 ± 1.6) | |
| > 3000 pg/ml or > 11,000 pmol/l* | < 5500 pg/ml or < 20,000 pmol/l* | > 20 follicles at least 15 mm | 2.8 days | |
| > 4000 pg/ml or > 14,684 pmol/l* | < 4000 pg/ml or < 14,684 pmol/l* | > 20 follicles, at least 30% of them >15 mm | 2.9 ± 0.33 days | |
| * conversion factor to SI unit, 3.671 |
It has also been postulated that follicles of varying size have a different threshold to gonadotrophins, and smaller follicles appear to be more susceptible to gonadotrophin deprivation than larger follicles (Fluker 1999). When coasting is initiated prior to 30% of follicles having attained a mean diameter of 15 mm, an abrupt arrest in follicular development and a rapid decline in plasma estradiol usually compromise the oocyte quality. But the quality of the oocytes is also compromised, and large cystic follicles are commonly encountered, if most of the follicles are larger than 15 mm in mean diameter when coasting is implemented (Sher 1995).
How the intervention might work
It was suggested that this approach prevents severe OHSS by removing the follicle stimulating hormone (FSH) stimulation of granulosa cells, thereby inhibiting their proliferation and reducing the number of granulosa cells available for luteinization (Sher 1995). This would allow continued follicular growth and maturation, while reducing the risk of OHSS (Fluker 1999). Tortoriello 1998 also suggested that the falling FSH concentration induces an increased apoptosis of granulosa cells, which results in a reduction of chemical mediators or precursors that augment fluid extravasation.
Why it is important to do this review
Ovarian hyperstimulation syndrome is a potentially life threatening condition associated with super‐ovulation in ART. Coasting is a widespread practise, for which evidence is currently lacking. Therefore, it is important to establish, from the scientific literature, whether coasting can reduce the incidence of this condition.
Objectives
To assess the effect of withholding gonadotrophins (coasting) in the prevention of ovarian hyperstimulation syndrome (OHSS) in assisted reproduction cycles.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), in which coasting was used as a preventive strategy to reduce OHSS. We excluded cross‐over trials, and trials that explored ovulation induction treatment without in vitro fertilisation (IVF) or intra‐cytoplasmic sperm injection (ICSI).
Types of participants
-
Women of reproductive age, undergoing super‐ovulation in IVF or ICSI cycle
Types of interventions
Coasting is the withholding of gonadotrophins. A definition is provided under the Description of the intervention section of this report. Early unilateral follicular aspiration involves the aspiration of every visible follicle from one ovary between 10 to 12 hours after the administration of human chorionic gonadotrophin (hCG). We considered these comparisons:
-
Coasting versus no coasting;
-
Coasting versus early unilateral follicular aspiration;
-
Coasting versus gonadotrophin‐releasing hormone antagonist
-
Coasting versus pure FSH co‐trigger;
-
Coasting versus cabergoline.
Types of outcome measures
Primary outcomes
The primary outcomes of this systematic review were:
-
incidence of moderate or severe OHSS, as defined in the included studies;
-
live birth rate.
Secondary outcomes
The secondary outcomes were:
-
clinical pregnancy rate per woman randomised, as defined in the included studies;
-
multiple pregnancy rate;
-
miscarriage rate;
-
number of oocytes retrieved.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases and trial registers (last searched 6 July 2016):
-
Cochrane Gynaecology and Fertility Group Specialised Register (6 July 2016), Appendix 1
-
CENTRAL (inception to 6 July 2016), Appendix 2;
-
MEDLINE (2001 to 6 July 2016), Appendix 3;
-
Embase (2001 to 6 July 2016), Appendix 4.
-
CINAHL (2001 to 6 July 2016), Appendix 5
-
PsycINFO (2001 to 6 July 2016), Appendix 6
-
PubMed (www.ncbi.nlm.nih.gov/pubmed/; searched 6 July 2016)
-
Google (searched 6 July 2016)
-
ClinicalTrials.gov (www.clinicaltrials.gov; searched 6 July 2016)
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, from chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We combined the Embase search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html#random).
Searching other resources
We handsearched the reference list of articles retrieved by the search, and contacted the trial authors if we required additional data. We handsearched relevant journals not included in the Cochrane register.
Data collection and analysis
Selection of studies
Two review authors (NNA and ADA in the original review; ADA and JB in the 2011 update, and ADA and RH in the current (2017) update) scanned the titles and abstracts of the identified studies. They removed studies that were clearly irrelevant. For this update, ADA and RH retrieved the full texts of potentially relevant articles, and independently assessed them for inclusion. Disagreement was resolved by consensus.
Data extraction and management
The review authors (ADA and RH for this update) independently extracted data using forms designed according to Cochrane guidelines. Where studies had multiple publications, the main trial report was used as the reference, and additional details supplemented from the additional papers. We contacted authors of the original papers for additional information, if necessary. Disagreements were resolved by consensus.
Assessment of risk of bias in included studies
The review authors independently assessed the included studies for risk of bias, using the Cochrane 'Risk of bias' assessment tool to assess: sequence generation; allocation concealment; blinding of participants, providers, and outcome assessors; completeness of outcome data, and selective outcome reporting. They resolved disagreements by consensus.
Measures of treatment effect
For dichotomous data, we used the number of events in the control and intervention groups to calculate Mantel Haenszel odds ratios (OR), in a fixed‐effect model. For continuous data, we calculated mean differences (MD) between groups, in a fixed‐effect model. We reported the 95% confidence intervals (CI) with all treatment effects.
Unit of analysis issues
We presented data per woman randomised and not per cycle. We analyzed multiple pregnancy and miscarriage rate per woman (primary analysis) and also per pregnancy. We analyzed multiple pregnancy as one live birth.
Dealing with missing data
Where more than 20% of participants were lost to follow‐up or dropped out, we did not include the data in the meta‐analysis. Where data were missing, we contacted the original authors for further information.
Assessment of heterogeneity
Statistical heterogeneity was assessed using the I² statistic (Higgins 2011). If the I² exceeded 50%, we deemed that there was substantial heterogeneity.
Assessment of reporting biases
If eight or more original papers were identified, we had planned to produce a funnel plot, in an attempt to identify publication bias.
Data synthesis
For dichotomous data, we used the Mantel Haenszel method for the meta‐analysis, with a fixed‐effect model. For continuous data, we used the inverse variance method, with a fixed‐effect model.
We combined data in the following comparisons:
-
Coasting versus no treatment (no coasting)
-
Coasting versus other treatments (e.g. early unilateral follicular aspiration, antagonist, GnRHa, FSH co‐trigger, and cabergoline), each as a separate comparison.
Subgroup analysis and investigation of heterogeneity
We did not do any subgroup analyses. If heterogeneity had exceeded 50%, we would have conducted subgroup or sensitivity analysis, exploring clinical or methodological differences between the studies that might explain the data. When we were unable to satisfactorily explain heterogeneity, we deemed the data unsuitable to include in the meta‐analysis.
Sensitivity analysis
When heterogeneity was high (over 50%), we had planned to conduct sensitivity analysis, based on the quality of the included studies as indicated in the 'Risk of bias' table, and whether the data were from a full paper or conference proceedings. Had there been sufficient studies, we would have conducted sensitivity analyses, even if heterogeneity was not high.
Overall quality of the body of evidence: Summary of findings table
We prepared 'Summary of findings' tables using GRADEpro software and Cochrane methods (GRADEpro GDT 2014; Higgins 2011). These tables summarized the overall quality of the body of evidence for the main review outcomes (OHSS, live birth, clinical pregnancy, adverse effects), using five GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias). For each outcome, we justified, documented, and incorporated into the reporting of the results, our judgements about the quality of the evidence quality (high, moderate, low, or very low).
Results
Description of studies
Results of the search
In the search conducted in 2016, we identified and assessed the full text of six potentially eligible studies. We excluded two studies and included four. The updated review now includes eight studies; 14 studies have been excluded. See the Characteristics of included studies and Characteristics of excluded studies tables and the PRISMA study‐flow chart Figure 1 for details.

Study flow diagram: July 2016 search for 2017 review update
Included studies
Design
Eight RCTs, with 702 women, met the inclusion criteria (Aboulgar 2007; Aflatoonian 2006; Dalal 2014; Egbase 1999; Egbase 2011; Kamthane 2007; Lukaszuk 2011; Sohrabvand 2009). They were conducted in Egypt, Iran, India, Kuwait, Poland, and the UK. Aboulgar 2007, Egbase 1999, Lukaszuk 2011, and Sohrabvand 2009 were full publications, the other four were only reported in conference abstracts.
Participants
All eight trials included women at high risk of developing OHSS. Egbase 2011 defined this as 15 or more follicles in each ovary, E2 of at least 3500 pg/ml, and two leading follicles with at least an 18 mm diameter. Lukaszuk 2011 defined this as women who had unsuccessfully undergone a previous long protocol and ICSI procedure, complicated by moderate or severe OHSS. Aboulgar 2007 defined it as at least 20 follicles in both ovaries, 90% of which had less than a 14 mm mean diameter, and E2 of at least 3000 pg/ml. Aflatoonian 2006 used the same criteria, although they did not define the mean diameter of follicles. Kamthane 2007 defined women at risk of OHSS as having follicles larger than 10 mm in diameter, or E2 higher than 700 pg/ml on day 7 or day 8. Egbase 1999 defined risk for OHSS as an E2 threshold of 6000 pg/ml. Sohrabvand 2009 included women with at least 20 follicles in both ovaries, the majority of which had diameters less than14 mm, and a serum E2 level of 3000 pg/ml. Dalal 2014 included women with at least 20 follicles of more than 11 mm, E2 levels higher than 300ng/ml on day 9 of stimulation, or both.
Interventions
Kamthane 2007 and Lukaszuk 2011 compared coasting with no coasting; Aflatoonian 2006 and Egbase 1999 compared coasting with early unilateral follicular aspiration; Aboulgar 2007 compared coasting with antagonist administration; and Egbase 2011 compared coasting with FSH administration at the time of hCG trigger (FSH co‐trigger). Sohrabvand 2009 and Dalal 2014 compared coasting with cabergoline.
Outcomes
For our primary outcomes, all the included studies reported OHSS rates, but only one reported data suitable for analysis on live birth (Kamthane 2007). For our secondary outcomes, all trials reported clinical pregnancy, three reported multiple pregnancy (Aboulgar 2007; Egbase 2011; Lukaszuk 2011), three reported miscarriage (Egbase 2011; Kamthane 2007; Lukaszuk 2011), and all reported the number of oocytes retrieved.
Excluded studies
Fourteen studies were excluded. One was randomised but the control was matched retrospectively (Aboulghar 1998) . The other 13 studies were excluded because they were not randomised. Four were prospective observational studies (Al‐Shawaf 2001; Lee 1998; Sher 1995; Waldenstrom 1999). Six were retrospective observational studies (Benadiva 1997; Esinler 2013; Fluker 1999; Herrero 2011; Tortoriello 1998; Tortoriello 1999; Ulug 2002). Two studies were retrospective case‐control studies (Dhont 1998; VanderStraeten 1998).
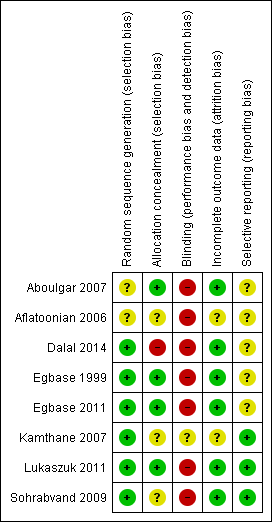
Risk of bias in included studies
See the 'Characteristics of included studies' table for details, and Figure 2 and Figure 3 for summaries.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Egbase 1999 and Egbase 2011 reported using computer generated random allocation to the intervention groups. Sohrabvand 2009 reported using block randomization. Dalal 2014 reported using a randomization software (www.randomizer.org). There were no details on the method of randomization in the other studies.
Dalal 2014, Aboulgar 2007, and Egbase 1999 provided adequate information on allocation concealment. No details were provided by Aflatoonian 2006, Kamthane 2007, Sohrabvand 2009. or Egbase 2011.
Blinding
None of the included studies reported on blinding. Blinding of investigators and women is not possible in these interventions, which are based on timing. Outcome assessors could have been blinded to allocation of intervention.
Incomplete outcome data
All randomised participants were included in the data analyses reported by Dalal 2014, Egbase 2011, Lukaszuk 2011, Sohrabvand 2009, Aboulgar 2007, and Egbase 1999. There were no details provided by Aflatoonian 2006 or Kamthane 2007.
Selective reporting
Dalal 2014 and Aflatoonian 2006 were reported in conference abstracts, and did not report outcomes a priori. Kamthane 2007 and Egbase 2011 summarized the a priori outcomes in their conference abstracts. There was no evidence in the literature of a full paper publication for these four studies. None of the studies except Kamthane 2007 reported live birth, but they were rated as at unclear risk of selective reporting.
Other potential sources of bias
There were no other sources of bias that were identified.
Effects of interventions
See: Summary of findings for the main comparison Coasting versus no coasting for prevention of ovarian hyperstimulation syndrome; Summary of findings 2 Coasting versus early unilateral follicular aspiration for preventing ovarian hyperstimulation syndrome; Summary of findings 3 Coasting versus gonadotrophin‐releasing hormone antagonist for preventing ovarian hyperstimulation syndrome; Summary of findings 4 Coasting versus follicle stimulating hormone administration at time of hCG for preventing ovarian hyperstimulation syndrome; Summary of findings 5 Coasting compared to cabergoline for preventing ovarian hyperstimulation syndrome
'Summary of findings' tables for comparisons with usable data can be referred to in summary of findings Table for the main comparison, summary of findings Table 3, summary of findings Table 2, and summary of findings Table 4.
1. Coasting versus no coasting
Two trials compared coasting (N = 102) with no coasting (N = 105 (Kamthane 2007; Lukaszuk 2011)).
Primary outcomes
1.1 Moderate or severe ovarian hyperstimulation syndrome (OHSS)
There were fewer cases of moderate or severe OHSS in the coasting group than in the no coasting group (OR 0.11, 95% CI 0.05 to 0.24; 2 RCTs; 207 women; I2 = 0%; low quality evidence; P < 0.00001; Analysis 1.1). Refer to Figure 4.

Forest plot of comparison: 3 Coasting versus no coasting, outcome: 3.1 OHSS.
1.2 Live birth
One trial reported on live birth (Kamthane 2007). It was unclear whether there was any difference between coasting and no coasting for this outcome (OR 0.48, 95% CI 0.14 to 1.62; 68 women; very low quality evidence; P = 0.24; Analysis 1.2). Refer to Figure 5.
Secondary outcomes
1.3 Clinical pregnancy
Two trials reported on the rate of clinical pregnancy (Kamthane 2007; Lukaszuk 2011). It was unclear whether there was any difference between the groups (OR 0.82, 95% CI 0.46 to 1.44; 2 RCTs; 207 women; I2 = 0%; low quality evidence; P = 0.49; Analysis 1.3).
1.4 Multiple pregnancy
One trial reported on the rate of multiple pregnancy (Lukaszuk 2011). The evidence suggested fewer multiple pregnancies per woman in the coasting group (OR 0.31, 95% CI 0.12 to 0.81, 139 women, low quality evidence, P = 0.02; Analysis 1.4). This finding persisted when the data were analyzed per pregnancy (OR 0.18, 95% CI 0.06 to 0.58; 56 women).
1.5 Miscarriage
Both trials reported this outcome. It was unclear whether there was any difference between the groups in the rate of miscarriage per woman (OR 0.85, 95% CI 0.25 to 2.86, 2 RCTs, 207 women, very low quality evidence, Analysis 1.5). This finding persisted when the data were analyzed per pregnancy (OR 1.05, 95% CI 0.28 to 3.88.; 2 RCTs; 207 women; I2 = 0%).
1.6 Number of oocytes retrieved
Both trials reported on the number of oocytes retrieved. The evidence suggested that fewer oocytes were retrieved in women undergoing coasting than no coasting (MD ‐3.86, 95% CI ‐4.38 to ‐333; 2 RCTs; 76 women; I2 = 0%; low quality evidence; P < 0.00001; Analysis 1.6).
2. Coasting versus early unilateral follicular aspiration
Two studies compared coasting (N = 42) with early unilateral follicular aspiration (N = 41 (Aflatoonian 2006; Egbase 1999)).
Primary outcomes
2.1 Moderate or severe ovarian hyperstimulation syndrome (OHSS)
It was unclear whether there was any difference between the groups (OR 0.98, 95% CI 0.34 to 2.85; 2 RCTs; 83 women; I2 = 0%; very low quality evidence; P = 0.97; I² = 0%; Analysis 2.1). Refer Figure 5.

Forest plot of comparison: 1 Coasting versus EUFA, outcome: 1.1 OHSS.
2.2 Live birth
No data suitable for analysis were reported by either trial. The authors of one trial stated that there was no significant difference between the groups (Egbase 2011).
Secondary outcomes
2.3 Clinical pregnancy
It was unclear whether there was any difference between the groups (OR 0.67, 95% CI 0.25 to 1.79; 2 RCTs; 83 women; I2 = 0%; very low quality evidence; P = 0.42; I² = 0%; Analysis 2.2).
2.4 Multiple pregnancy
This outcome was not reported by either trial.
2.5 Miscarriage
This outcome was not reported by either trial.
2.6 Number of oocytes retrieved
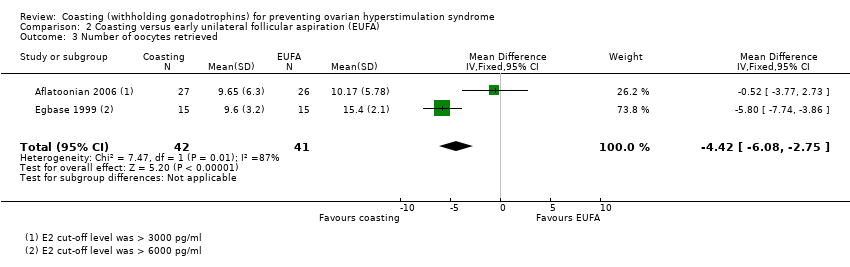
Both trials reported this outcome. Heterogeneity was evident (I² = 87%). The heterogeneity may be due to the differences in the cut‐off for oestrogen levels in the definition of high risk for OHSS. Aflatoonian 2006 used a cut‐off level of > 3000 pg/ml E2 and Egbase 1999 used a cut‐off level of > 6000 pg/ml E2.
3. Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist)
One trial compared coasting with antagonist (Aboulgar 2007).
Primary outcomes
3.1 Moderate or severe ovarian hyperstimulation syndrome (OHSS)
There were no instances of OHSS in either group (N = 190).
3.2 Live birth
Live birth was not reported in this trial.
Secondary outcomes
3.3 Clinical pregnancy
It was unclear whether there was any difference between the groups (OR 0.74, 95% CI 0.42 to 1.31; 190 women; low quality evidence; P = 0.31; Analysis 3.2).
3.4 Multiple pregnancy
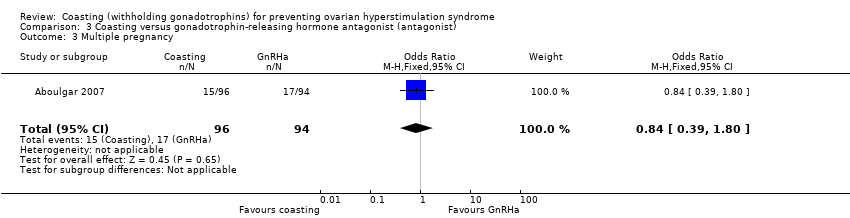
It was unclear whether there was any difference between the groups in the rate of multiple pregnancy per woman (OR 0.84, 95% CI 0.39 to 1.80; 190 women; very low quality evidence; P = 0.65; Analysis 3.3). This finding persisted when the data were analyzed per pregnancy (OR 1.0; 95% CI 0.43 to 2.32; 98 women).
3.5 Miscarriage
Miscarriage was not reported in this trial.
3.6 Number of oocytes retrieved
The evidence suggested that there were fewer oocytes retrieved in the group receiving coasting (MD ‐2.44, 95% CI 4.30 to ‐0.58; 190 women; P = 0.01; Analysis 2.3).
4. Coasting versus follicle stimulating hormone co‐trigger
One trial compared coasting versus FSH administration at the time of hCG trigger (N = 102; Egbase 2011).
Primary outcomes
4.1 Moderate or severe ovarian hyperstimulation syndrome (OHSS)
Fifteen cases of moderate or severe OHSS occurred, all in the coasting group (OR 43.74, 95% CI 2.54 to 754.58; 102 women; very low quality evidence; P = 0.009; Analysis 4.1).
4.2 Live Birth
The authors reported that there was no evidence of a difference between the groups for this outcome (data not available).
Secondary outcomes
4.3 Clinical Pregnancy
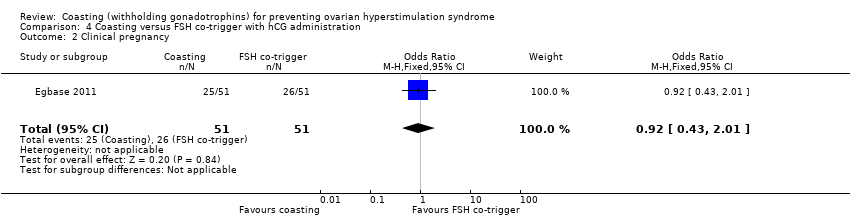
It was unclear whether there was a difference between the groups (OR 0.92, 95% CI 0.43 to 2.01; 102 women; low quality evidence; P = 0.84; Analysis 4.2).
4.4 Multiple Pregnancy
The trial authors reported that there was no evidence of a difference between the groups for this outcome (data not available).
4.5 Miscarriage
The trial authors reported that there was no evidence of a difference between the groups for this outcome (data not available).
4.6 Number of oocytes retrieved
The evidence suggested that fewer oocytes were retrieved in women undergoing coasting than FSH co‐trigger (MD ‐17.80, 95% CI ‐19.05 to ‐16.55; 102 women; P < 0.00001; Analysis 3.4).
5. Coasting versus cabergoline
Two trials compared coasting versus cabergoline (N = 120 (Dalal 2014; Sohrabvand 2009)).
Primary outcomes
5.1 Moderate or severe ovarian hyperstimulation syndrome (OHSS)
There was insufficient evidence to determine whether there was a difference between the two groups (OR 1.98, 95% CI 0.09 to 5.68; 120 women; I² = 72%; very low quality evidence; P=0.20, Analysis 5.1). Refer to Figure 6. Heterogeneity was high (72%) and there is no reason to explain this heterogeneity.

Forest plot of comparison: 5 Coasting versus cabergoline, outcome: 5.1 OHSS.
5.2 Live Birth
Live birth rates were not reported in these trials.
Secondary outcomes
5.3 Clinical Pregnancy
There was evidence of a difference in clinical pregnancy favouring the cabergoline group compared to the coasting group (OR 0.38, 95% CI 0.16 to 0.88; 2 RCTs; 120 women; very low quality evidence; P = 0.02; I² = 0%; Analysis 5.2).
5.4 Multiple Pregnancy
Multiple pregnancy rates were not reported in these trials.
5.5 Miscarriage
Miscarriage rates were not reported in these trials.
5.6 Number of oocytes retrieved
In one trial (Sohrabvand 2009) the evidence suggested that fewer oocytes were retrieved in women undergoing coasting than cabergoline (MD ‐4.3, 95% CI 7.88 to ‐0.72; 60 women; P 0.02; Analysis 5.4). The authors of one trial (Dalal 2014) stated that there was significant difference between the groups but no data were provided.
Other analyses
There were insufficient data to conduct planned sensitivity analyses or to construct a funnel plot.
Discussion
Summary of main results
There was low‐quality evidence to suggest that coasting reduced moderate or severe OHSS more than no coasting. There was very low‐quality evidence suggesting that using FSH co‐trigger prevented moderate and severe OHSS better than coasting, but the evidence was derived from only one small trial. There was no clear evidence of any difference in the rate of moderate or severe OHSS or in the achievement of clinical pregnancy when coasting was compared with early unilateral follicular aspiration (very low‐quality evidence), GnRH antagonist (low or very low quality evidence), or cabergoline (low or very low‐quality evidence). Nor was there evidence to suggest a benefit in live birth from coasting compared with no coasting, although the data were from a single trial only. It was difficult to draw any conclusions about the number of oocytes obtained due to the statistical heterogeneity of the studies. Nor was there any clear evidence of differences between the groups in clinical pregnancy, multiple pregnancies, live births or miscarriage rates.
Overall completeness and applicability of evidence
Only eight randomised trials were identified, and four of these were conference abstracts that never became full publications. The completeness and applicability of the data were substantially limited by this factor. Authors were contacted, but did not respond directly, and some missing data were obtained from another Cochrane review. Only one of the trials reported data suitable for analysis on the outcome of live birth. There may be additional interventions that have not been compared yet with coasting, in a randomised controlled trial.
Quality of the evidence
The overall quality of the evidence was low or very low. The main limitations were failure to report live birth, risk of bias due to lack of information about study methods, and imprecision due to low event rates and lack of data. Four of the studies were published only as abstracts, with limited data and no evidence of full publication (Aflatoonian 2006; Dalal 2014; Egbase 2011; Kamthane 2007). Three to four years is longer than expected to publish a full paper. With the exception of the number of oocytes retrieved, the statistical heterogeneity between studies was acceptable. The inclusion criteria and OHSS classification used varied between the studies.
Potential biases in the review process
The authors attempted to identify all potential studies using a variety of methods. One of the main biases, as mentioned above, is that several of the trials were only published as conference abstracts. There may be other relevant data which were not reported. Therefore, the results of the review must be interpreted with caution.
Agreements and disagreements with other studies or reviews
In some excluded studies, the higher the oestrogen level at the beginning of coasting, the less effective the intervention was at preventing OHSS, regardless of the duration of the coasting period (see Aboulghar 1998; Dhont 1998; Fluker 1999; Lee 1998; Sher 1995; Waldenstrom 1999). The inclusion criterion for this review was oestrogen levels higher than 2500 pg/ml. The four included trials used a cut‐off of 3000 pg/ml, and this may, in part, explain the lack of effect.

Study flow diagram: July 2016 search for 2017 review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 3 Coasting versus no coasting, outcome: 3.1 OHSS.

Forest plot of comparison: 1 Coasting versus EUFA, outcome: 1.1 OHSS.

Forest plot of comparison: 5 Coasting versus cabergoline, outcome: 5.1 OHSS.

Comparison 1 Coasting versus no coasting, Outcome 1 OHSS.

Comparison 1 Coasting versus no coasting, Outcome 2 Live birth.

Comparison 1 Coasting versus no coasting, Outcome 3 Clinical pregnancy.

Comparison 1 Coasting versus no coasting, Outcome 4 Multiple pregnancy.

Comparison 1 Coasting versus no coasting, Outcome 5 Miscarriage.

Comparison 1 Coasting versus no coasting, Outcome 6 Number of oocytes retrieved.

Comparison 2 Coasting versus early unilateral follicular aspiration (EUFA), Outcome 1 OHSS.

Comparison 2 Coasting versus early unilateral follicular aspiration (EUFA), Outcome 2 Clinical Pregnancy.
Comparison 2 Coasting versus early unilateral follicular aspiration (EUFA), Outcome 3 Number of oocytes retrieved.

Comparison 3 Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist), Outcome 1 OHSS.

Comparison 3 Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist), Outcome 2 Clinical Pregnancy.
Comparison 3 Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist), Outcome 3 Multiple pregnancy.

Comparison 3 Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist), Outcome 4 Number of oocytes retrieved.

Comparison 4 Coasting versus FSH co‐trigger with hCG administration, Outcome 1 OHSS.
Comparison 4 Coasting versus FSH co‐trigger with hCG administration, Outcome 2 Clinical pregnancy.
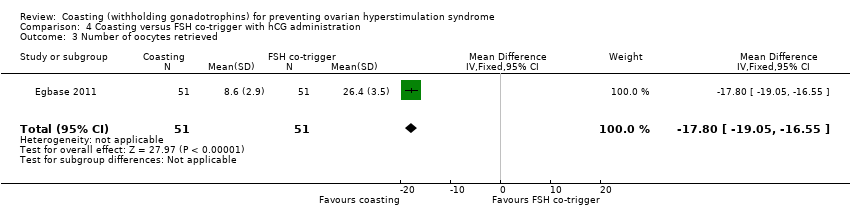
Comparison 4 Coasting versus FSH co‐trigger with hCG administration, Outcome 3 Number of oocytes retrieved.

Comparison 5 Coasting versus cabergoline, Outcome 1 OHSS.
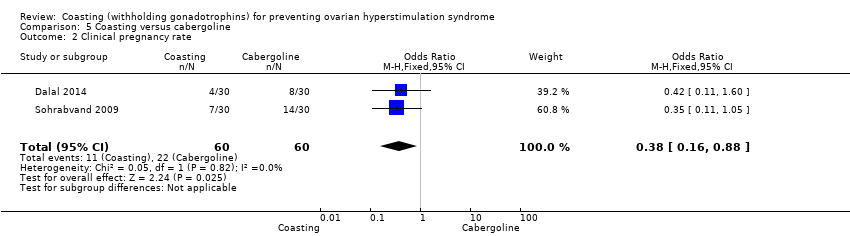
Comparison 5 Coasting versus cabergoline, Outcome 2 Clinical pregnancy rate.
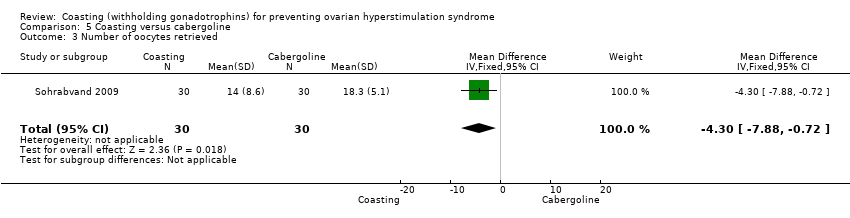
Comparison 5 Coasting versus cabergoline, Outcome 3 Number of oocytes retrieved.
| Coasting versus no coasting for prevention of ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no coasting | Risk with coasting | |||||
| OHSS | 457 per 1000 | 85 per 1000 | OR 0.11 | 207 | ⊕⊕⊝⊝ | |
| Live birth | 265 per 1000 | 147 per 1000 | OR 0.48 | 68 | ⊕⊝⊝⊝ | |
| Clinical pregnancy | 390 per 1000 | 344 per 1000 | OR 0.82 | 207 | ⊕⊕⊝⊝ | |
| Multiple pregnancy | 268 per 1000 | 102 per 1000 | OR 0.31 | 139 | ⊕⊝⊝⊝ | |
| Miscarriage | 57 per 1,000 | 49 per 1,000 (15 to 148) | OR 0.85 (0.25 to 2.86) | 207 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias: one study did not clearly describe the methods used, studies not blinded 2 Downgraded one level for serious imprecision: few events, wide confidence intervals, or both 3 Downgraded two levels for very serious imprecision: very few events, very wide confidence intervals, or both | ||||||
| Coasting versus early unilateral follicular aspiration for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with early unilateral follicular aspiration (EUFA) | Risk with coasting | |||||
| OHSS | 244 per 1000 | 240 per 1000 | OR 0.98 | 83 | ⊕⊝⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 317 per 1000 | 237 per 1000 | OR 0.67 | 83 | ⊕⊝⊝⊝ | |
| Multiple pregnancy | No data available | |||||
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias: one study did not clearly describe methods, lack of blinding 2 Downgraded two levels for very serious imprecision: very few events and very wide confidence intervals | ||||||
| Coasting versus gonadotrophin‐releasing hormone antagonist for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with gonadotrophin‐releasing hormone antagonist | Risk with coasting | |||||
| OHSS | Not estimable | Not estimable | not estimable | 190 | ⊕⊕⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 553 per 1000 | 478 per 1000 | OR 0.74 | 190 | ⊕⊕⊝⊝ | |
| Multiple pregnancy | 181 per 1000 | 156 per 1000 | OR 0.84 | 98 | ⊕⊕⊝⊝ | |
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: method of sequence generation not reported, lack of blinding 2 Downgraded two levels due to very serious imprecision: no OHSS occurred in either group. Few events for multiple pregnancy. 3 Downgraded one level due to serious imprecision. Wide confidence intervals, few events | ||||||
| Coasting versus follicle stimulating hormone (FSH) administration at time of hCG trigger in preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with FSH co‐trigger with hCG administration | Risk with Coasting | |||||
| OHSS | Not estimable | Not estimable | OR 43.74 | 102 | ⊕⊝⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 510 per 1000 | 489 per 1000 | OR 0.92 | 102 | ⊕⊕⊝⊝1,3 | |
| Multiple pregnancy | No data available | |||||
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: method of sequence generation not reported, lack of blinding 2 Downgraded two levels due to very serious imprecision: only 15 events, all in one arm. 3 Downgraded one level due to serious imprecision: very wide confidence intervals | ||||||
| Coasting compared to cabergoline for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with cabergoline | Risk with Coasting | |||||
| OHSS | 100 per 1000 | 180 per 1000 | OR 1.98 | 120 | ⊕⊝⊝⊝1,2 | |
| Live birth | Not reported | |||||
| Clinical pregnancy rate | 367 per 1000 | 180 per 1000 | OR 0.38 (0.16 to 0.88) | 120 | ⊕⊕⊝⊝1 | |
| Multiple pregnancy | Not reported | |||||
| Miscarriage | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious risk of bias: one study did not clearly define method, method of sequence generation not reported, lack of blinding 2 Downgraded one level due to serious imprecision: very few events and/or wide confidence interval. | ||||||
| Classification | Size of ovaries | Grade | Symptoms |
| Mild | 5 to 10 cm | grade 1 | abdominal tension and discomfort |
| grade 2 | grade 1 signs plus nausea, vomiting, diarrhoea, or a combination | ||
| Moderate | > 10 cm | grade 3 | grade 2 signs plus ultrasound evidence of ascites |
| Severe | > 12 cm | grade 4 | grade 3 signs plus clinical evidence of ascites, pleural effusion and dyspnoea, or a combination |
| grade 5 | grade 4 signs plus haemoconcentration increased blood viscosity, hypovolaemia, decreased renal perfusion, oliguria | ||
| Severe | Critical |
| Variably enlarged ovary | Variably enlarged ovary |
| Massive ascites ± hydrothorax | Tense ascites ± hydrothorax |
| Hct > 45% (> 30% increment over baseline value) | Hct > 55% |
| WBC > 15,000 | WBC > 35,000 |
| Oliguria | |
| Creatinine 1.0 to 1.5 | Creatinine > 1.6 |
| Creatinine clearance > 50 ml/min | Creatinine clearance < 50ml/min |
| Liver dysfunction | Renal failure |
| Anasarca | Thromboembolic phenomena |
| Adult respiratory distress syndrome (ARDS) | |
| Moderate | Severe Grade A | Severe Grade B | Severe Grade C |
| Discomfort, pain, nausea, distension, ultrasonic evidence of ascites and enlarged ovaries, normal haematological and biological profile | Dyspnoea, oliguria, nausea, vomiting, diarrhoea, abdominal pain, clinical evidence of ascites, marked distension of abdomen or hydro‐thorax, US showing large ovaries and marked ascites, normal biochemical profile | Grade A plus massive tension ascites, markedly enlarged ovaries, severe dyspnoea and marked oliguria, increased haematocrit, elevated serum creatinine and liver dysfunction | Complications such as respiratory distress syndrome, renal shut‐down, or venous thrombosis |
| Authors | E2 at coasting | E2 at hCG | Number and follicle size | Coasting time |
| > 3000 pg/mL or > 11,000 pmol/L* | < 3000 pg/mL or < 11,000 pmol/L* | > 29 follicles at least 30% > 15 mm | 3 to 11 days (mean 6.1) | |
| ≥ 3000 pg/ml or ≥ 11,000 pmol/l* | < 3000 pg/ml or < 11,000 pmol/l* | at least 3 follicles of 15.6 ± 1.4 mm | 1.9 ± 0.9 days | |
| > 3000 pg/ml or > 11,000 pmol/l* | < 3000 pg/mL or < 11,000 pmol/L* | 5 follicles at least 16 mm, two of which are at least 19 mm | 1 to 5 days | |
| > 2500 pg/ml or > 9000 pmol/l* | < 2500 pg/ml or < 9000 pmol/l* | ≥ 20 follicles > 15 mm | 1 to 6 days (mean 1.94) | |
| > 2700 pg/ml or > 10,000 pmol/l* | no values given | many immature follicles < 3 at 18 mm | 3 days | |
| > 2500 pg/ml or > 9000 pmol/l* | < 2500 pg/ml or < 9000 pmol/l* | ≥ 20 follicles > 14 mm | 1 to 6 days (mean 1.94) | |
| > 6000 pg/ml or > 22,000 pmol/l* | < 3000 pg/ml or 11,000 pmol/l* | > 15 follicles, each of > 18 mm in each ovary | 4.9 ± 1.6 days | |
| > 2700 pg/ml or > 10,000 pmol/l* | < 2700 pg/ml or < 10,000 pmol/l* | > 25 follicles, at least 3 follicles > 17 mm | 3 to 6 days (mean 4.3) | |
| > 3000 pg/ml or > 11,000 pmol/l* | 25% decline < 2250 pg/ml or 8250 pmol/l* | > 3 follicles of > 18 mm | 3 to 5 days (mean 3.4 ± 0.1) | |
| > 3600 pg/ml or > 13,000 pmol/l* | < 2700 pg/ml or < 10,000 pmol/l* | at least 25% of the follicles > 15 mm | 2 to 9 days (mean 3.4 ± 1.6) | |
| > 3000 pg/ml or > 11,000 pmol/l* | < 5500 pg/ml or < 20,000 pmol/l* | > 20 follicles at least 15 mm | 2.8 days | |
| > 4000 pg/ml or > 14,684 pmol/l* | < 4000 pg/ml or < 14,684 pmol/l* | > 20 follicles, at least 30% of them >15 mm | 2.9 ± 0.33 days | |
| * conversion factor to SI unit, 3.671 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 2 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.24] |
| 2 Live birth Show forest plot | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.14, 1.62] |
| 3 Clinical pregnancy Show forest plot | 2 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.44] |
| 4 Multiple pregnancy Show forest plot | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.12, 0.81] |
| 5 Miscarriage Show forest plot | 2 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.25, 2.86] |
| 6 Number of oocytes retrieved Show forest plot | 2 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐3.86 [‐4.38, ‐3.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.34, 2.85] |
| 2 Clinical Pregnancy Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.79] |
| 3 Number of oocytes retrieved Show forest plot | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐4.42 [‐6.08, ‐2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical Pregnancy Show forest plot | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.31] |
| 3 Multiple pregnancy Show forest plot | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.39, 1.80] |
| 4 Number of oocytes retrieved Show forest plot | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | ‐2.44 [‐4.30, ‐0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 43.74 [2.54, 754.58] |
| 2 Clinical pregnancy Show forest plot | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.43, 2.01] |
| 3 Number of oocytes retrieved Show forest plot | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐17.80 [‐19.05, ‐16.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.69, 5.68] |
| 2 Clinical pregnancy rate Show forest plot | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.88] |
| 3 Number of oocytes retrieved Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐7.88, ‐0.72] |

