ยาโรคูโรเนียม (rocuronium) เทียบกับ ยาซัคซินิลโคลีน (succinylcholine) สำหรับการใส่ท่อช่วยหายใจในการนำสลบแบบรวดเร็ว
บทคัดย่อ
บทนำ
ผู้ป่วยมักต้องใช้เทคนิคการใส่ท่อช่วยหายใจร่วมกับการนำสลบแบบลำดับเร็ว (rapid sequence induction; RSI) ในกรณีฉุกเฉิน (emergency) หรือกรณีทั่วไป (elective) ที่จะป้องกันการสำลัก ความดันในกะโหลกศีรษะที่เพิ่มขึ้น หรือเพื่อช่วยในการใส่ท่อช่วยหายใจ โดยทั่วไป succinylcholine เป็นยาหย่อนกล้ามเนื้อที่นิยมใช้มากที่สุดสำหรับจุดประสงค์นี้ เนื่องจากเริ่มออกฤทธิ์เร็วและมีระยะเวลาออกฤทธิ์สั้น ข้อเสียคือมีผลข้างเคียงที่รุนแรง Rocuronium ได้รับการแนะนำให้เป็นทางเลือกหนึ่งเช่นเดียวกับ succinylcholine สำหรับใส่ท่อช่วยหายใจ นี่คือการปรับให้เป็นปัจจุบันของการทบทวนวรรณกรรม Cochrane ของเราที่ตีพิมพ์ครั้งแรกในปี 2003 และปรับให้ทันสมัยในปี 2008 และฉบับนี้ในปี 2015
วัตถุประสงค์
เพื่อให้ทราบว่า rocuronium สร้างสภาวะในการใส่ท่อช่วยหายใจเทียบได้กับ succinylcholine ในระหว่างการใส่ท่อช่วยหายใจร่วมกับการนำสลบแบบลำดับเร็ว
วิธีการสืบค้น
ในการทบทวนวรรณกรรมครั้งแรก เราได้สืบค้นฐานข้อมูลทั้งหมดจนถึงเดือนมีนาคม 2000 ตามด้วยการอัปเดตถึงเดือนมิถุนายน 2007 การปรับให้เป็นปัจจุบันล่าสุดนี้รวบรวมการสืบค้นใน Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 2), MEDLINE (1966 ถึง สัปดาห์ที่ 2 ของเดือนกุมภาพันธ์ 2015) และ EMBASE (1988 ถึง วันที่ 14 กุมภาพันธ์ 2015) สำหรับการทดลองแบบสุ่มที่มีกลุ่มควบคุม (Randomised Controlled Trials; RCTs) หรือการทดลองทางคลินิกที่มีกลุ่มควบคุม (Controlled Clinical Trials; CCTs) ที่เกี่ยวข้องกับการใช้ rocuronium และ succinylcholine เรารวบรวมวารสารภาษาต่างประเทศและค้นหาเอกสารอ้างอิงของการศึกษาที่พบสำหรับการหาเอกสารอ้างอิงเพิ่มเติม
เกณฑ์การคัดเลือก
เราได้รวบรวม RCT หรือ CCT ที่รายงานภาวะการใส่ท่อช่วยหายใจโดยเปรียบเทียบการใช้โรคูโรเนียมและซัคซินิลโคลีนสำหรับ RSI หรือ modified RSI ในทุกกลุ่มอายุหรือ ทุกสถานการณ์ทางคลินิก ขนาดของ rocuronium อย่างน้อย 0.6 มก./กก. และ succinylcholine อย่างน้อย 1 มก./กก.
การรวบรวมและวิเคราะห์ข้อมูล
ผู้นิพนธ์สองท่าน (EN และ DT) คัดลอกข้อมูลและประเมินคุณภาพของระเบียบวิธีวิจัยสำหรับตาราง 'Risk of bias' อย่างอิสระต่อกัน เรารวมผลลัพธ์ใน Review Manager 5 โดยใช้อัตราส่วนความเสี่ยง (RR) ด้วย random‐effect model
ผลการวิจัย
การปรับให้ทันสมัยฉบับก่อนหน้านี้ (2008) ได้พบการศึกษาที่เป็นไปได้ 53 รายการและรวมการศึกษา 37 รายการสำหรับ meta‐analysis ในการปรับให้ทันสมัยฉบับล่าสุดนี้เราได้พบการศึกษาเพิ่มเติมอีก 13 รายการและรวมนำเข้า 11 รายการโดยสรุปผลการทดลอง 50 รายการรวมผู้เข้าร่วม 4151 คน โดยรวมแล้ว succinylcholine ดีกว่า rocuronium ในการบรรลุการใส่ท่อช่วยหายใจที่ดีเยี่ยม: RR 0.86 (ช่วงความเชื่อมั่น 95% (CI) 0.81 ถึง 0.92; n = 4151) และการใส่ท่อช่วยหายใจที่ยอมรับได้ทางคลินิก (RR 0.97, 95% CI 0.95 ถึง 0.99; n = 3992, 48 การทดลอง) อุบัติการณ์ของ detection bias ที่สูงของการทดลองร่วมกับความแตกต่างอย่างมีนัยสำคัญ ให้หลักฐานคุณภาพระดับปานกลางสำหรับข้อสรุปเหล่านี้ ซึ่งไม่เปลี่ยนแปลงจากการปรับให้ทันสมัยก่อนหน้านี้ Succinylcholine มีแนวโน้มที่จะทำให้มีภาวะการใส่ท่อช่วยหายใจที่ดีเยี่ยมเมื่อใช้ thiopental เป็นตัวนำสลบ: RR 0.81 (95% CI: 0.73 ถึง 0.88; n = 2302, 28 การทดลอง) ในการปรับก่อนหน้านี้ เราได้ข้อสรุปว่า propofol เป็นสารนำสลบที่เหนือกว่า succinylcholine ไม่มีรายงานอุบัติการณ์ของผลข้างเคียงที่รุนแรง เราพบว่าไม่มีความแตกต่างทางสถิติในภาวะการใส่ท่อช่วยหายใจเมื่อเทียบซัคซินิลโคลีนกับโรโคโรเนียม 1.2 มก./กก. อย่างไรก็ตาม succinylcholine ดีกว่าทางคลินิกเนื่องจากมีระยะเวลาในการออกฤทธิ์ที่สั้นกว่า
ข้อสรุปของผู้วิจัย
Succinylcholine ทำให้เกิดสภาวะการใส่ท่อช่วยหายใจที่เหนือกว่า rocuronium ในการบรรลุการใส่ท่อช่วยหายใจที่ดีเยี่ยมและเป็นที่ยอมรับทางคลินิก
PICO
ข้้อสรุปภาษาธรรมดา
การเปรียบเทียบยาหย่อนกล้ามเนื้อ 2 ชนิดคือยาโรคูโรเนียมและซัคซินิลโคลีนเพื่อช่วยในการใส่ท่อช่วยหายใจในการนำสลบแบบรวดเร็ว (rapid sequence induction intubation; RSI intubation)
คำถามของการทบทวนวรรณกรรม
ยาชนิดใด (โรคูโรเนียมหรือซัคซินิลโคลีน) ที่ดีกว่าในการสร้างสภาวะที่ดีเยี่ยมในการสอดท่อช่วยหายใจแก่ผู้ป่วยทุกกลุ่มอายุได้อย่างรวดเร็วสำหรับสถานการณ์ทั่วไป (elective) และฉุกเฉิน (emergency)
ความเป็นมา
ในสถานการณ์ฉุกเฉินบางรายต้องใช้ยาสลบทั่วไปกับท่อช่วยหายใจ สิ่งสำคัญคือต้องมียาที่ออกฤทธิ์เร็วเพื่อให้แพทย์ทำหัตถการนี้ได้อย่างรวดเร็วและปลอดภัย ปัจจุบันยาที่ใช้บ่อยที่สุดเพื่อหย่อนกล้ามเนื้อคือยาซัคซินิลโคลีน ยาซัคซินิลโคลีนออกฤทธิ์เร็วและคงฤทธิ์อยู่ไม่กี่นาที ซึ่งเป็นที่ต้องการมากในการสถานการณ์นี้ อย่างไรก็ตามบางรายไม่สามารถใช้ยานี้ได้จากการเป็นสาเหตุให้เกลือแร่ในร่างกายเสียสมดุลหรือเกิดปฏิกิริยา ดังนั้นยาที่มีประสิทธิภาพเท่าเทียมกันโดยไม่มีผลข้างเคียงเหล่านี้อาจจะเป็นประโยชน์ ยาทางเลือกตัวหนึ่งที่เป็นไปได้คือโรคูโรเนียม ซึ่งเป็นยาหย่อนกล้ามเนื้อที่มีผลข้างเคียงน้อยกว่า แต่ออกฤทธิ์นานกว่า การทบทวนวรรณกรรมนี้เปรียบเทียบคุณภาพของสภาพการใส่ท่อช่วยหายใจ (ความง่ายที่แพทย์สามารถส่งท่อช่วยหายใจได้อย่างรวดเร็วและปลอดภัย) ระหว่างยาโรคูโรเนียมและซัคซินิลโคลีนในทุกช่วงอายุและสถานการณ์ทางคลินิกที่แตกต่างกัน
ลักษณะของการศึกษา
เรารวบรวมไว้ในการทบทวนการทดลองที่มีการควบคุมตั้งแต่ ปี 1966 ถึงเดือนกุมภาพันธ์ 2015 ซึ่งเกี่ยวข้องกับผู้เข้าร่วมทุกวัยที่ต้องการการใส่ท่อช่วยหายใจแบบเร็วโดยใช้โรคูโรเนียมและซัคซินิลโคลีน ปริมาณที่น้อยที่สุดของยาโรคูโรเนียมที่ให้คือ 0.6 มก./กก. และซัคซินิลโคลีนคือ 1 มก./กก. เราได้รวบรวมผลของการทดลอง 50 รายการที่มีผู้เข้าร่วมทั้งหมด 4151 รายซึ่งเปรียบเทียบประสิทธิผลของยาซัคซินิลโคลีนกับโรคูโรเนียมในการใส่ท่อช่วยหายใจ ไม่มีรายงานผลข้างเคียงที่สำคัญจากการใช้ยา
ผลลัพธ์หลัก
เราพบว่ายาโรคูโรเนียมมีประสิทธิผลน้อยกว่า succinylcholine เล็กน้อยในการสร้างสภาวะในระดับดีเยี่ยมและยอมรับได้เพื่อใส่ท่อช่วยหายใจ ดังนั้นจึงควรใช้ rocuronium เป็นตัวเลือกแทนของ succinylcholine เฉพาะเมื่อทราบว่าไม่ควรใช้ succinylcholine และคาดว่าการใส่ท่อช่วยหายใจใช้เวลานานขึ้น
คุณภาพของหลักฐาน
ระดับของหลักฐานอยู่ในระดับปานกลางเนื่องจากการออกแบบการศึกษาที่ไม่สมบูรณ์และมีการใช้เทคนิคหลากหลายในการทดลอง
Authors' conclusions
Summary of findings
| Rocuronium any dose versus succinylcholine for rapid sequence induction intubation | ||||||
| Patient or population: People requiring rapid sequence induction intubation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Succinylcholine | Rocuronium any dose 2 | |||||
| Excellent versus other intubation conditions | Study population | RR 0.86 | 4151 | ⊕⊕⊕⊝ | Risk of bias: 50% of the studies were at high risk for detection bias because the outcome assessor was not blinded to the fasciculations caused by succinylcholine. Inconsistency: High statistical heterogeneity in the studies could not be explained by subgroup analyses. However we did not downgrade because exclusion of trials contributing to heterogeneity did not significantly change the direction or size of effect. | |
| 76 per 100 | 65 per 100 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk is the average number of excellent intubations with succinylcholine. 2Rocuronium minimum dose 0.6 mg/kg. Succinylcholine minimal dose is 1mg/kg. | ||||||
Background
Description of the condition
Patients who need endotracheal intubation in the emergency department or the operating room often require a rapid sequence induction (RSI) technique to protect against aspiration of gastric contents or to facilitate urgent airway protection in cases of imminent airway closure, haemodynamic instability, failing gas exchange and urgent surgical emergencies (Huizinga 1992; McCourt 1998; Stollings 2014).
Description of the intervention
The RSI technique involves the rapid sequential administration of medications (including a sedative, induction anaesthetic and a muscle relaxant, with or without narcotic) followed by endotracheal intubation within one minute of administering the muscle relaxant. In emergency situations, intubation is often required in unstable situations with the potential of haemodynamic instability. This frequently requires modification of the rapid sequence induction for the individual patient, with the goal of securing a patent airway as safely and quickly as possible.
How the intervention might work
Succinylcholine, a depolarizing muscle relaxant, is the most common agent used for a RSI technique in both the controlled and emergency settings (Weiss 1997). Succinylcholine has been the preferred muscle relaxant because it has a rapid onset of 40 to 60 seconds and a short duration, lasting only six to 10 minutes (Combs 1994). Succinylcholine's depolarizing action can lead to hyperkalaemia, possibly inducing fatal cardiac arrhythmias (Combs 1994; Schreiber 2005; Sullivan 1994). As a result, It is contraindicated in patients with major burns (beyond 48 hours), major crush injuries (beyond 48 hours), severe abdominal sepsis, denervation syndromes (such as amyotrophic lateral sclerosis or Guillain Barré Syndrome), muscular dystrophy and major nerve or spinal cord injuries (Martyn 2006). It is also contraindicated in patients with known hyperkalaemia, a history of malignant hyperthermia or previous allergic reaction to succinylcholine (Lebowitz 1989). Succinylcholine use has also been associated with variable increases in intracranial pressure (Minton 1986) and to a lesser extent intraocular pressure (Vinik 1999), and should be administered with drugs that help mitigate these side effects.
Alternative agents, among others, include pancuronium, vecuronium, atracurium and cisatracurium; however, none achieve acceptable intubating conditions as rapidly as succinylcholine (Mazurek 1998). Rocuronium is a steroid‐based non‐depolarizing muscle relaxant, which has been proposed for creating intubating conditions similar to those of succinylcholine. The duration of action is longer, lasting 37 to 72 minutes with standard doses (Magorian 1993). The only absolute contraindication to rocuronium is allergy. Care must be taken with people who have myasthenia gravis or myasthenic syndrome, hepatic disease, neuromuscular disease, carcinomatosis, or severe cachexia, as the duration of action may be profoundly increased (Stollings 2014).
Why it is important to do this review
There have been many studies looking at the equivalence of rocuronium and succinylcholine, with conflicting outcomes. It has been suggested that inconsistencies in the use of narcotics, the sedative propofol, or the dose of rocuronium administered may have accounted for these differences (Magorian 1993). No previous systematic review comparing the intubation conditions created by rocuronium and succinylcholine had been published prior to our initial review (Perry 2003). This review allows for subgroup analyses to assess for sources of inconsistency between studies. This latest update is important, given that several additional studies have been published since our last update (Perry 2008).
Objectives
To determine whether rocuronium creates intubating conditions comparable to those of succinylcholine during RSI intubation.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized clinical trials (RCTs) and controlled clinical trials (CCTs) meeting the following inclusion criteria:
-
the study reported a score of intubation conditions as one of the main outcomes;
-
the study compared rocuronium to succinylcholine;
-
the dose of rocuronium administered was at least 0.6 mg/kg and the dose of succinylcholine was at least 1 mg/kg (Danzl 2000).
Types of participants
We included in the analysis men, women and children of any age who underwent a rapid sequence induction (RSI), or modified RSI, intubation either electively or emergently. We defined a modified RSI as using both a sedative and a muscle relaxant followed by intubation, with either a delay between the administration of the two drugs or a delay of more than 60 seconds between the administration of the muscle relaxant and the intubation attempt, or both.
Types of interventions
All of the trials we included in this review compared rocuronium to succinylcholine for neuromuscular blockade. The sedative used for induction anaesthesia was thiopental, propofol, benzodiazepines, ketamine or etomidate. We accepted trials with or without narcotic agents. Additional medications allowed in this review were the use of pre‐treatment sedatives (e.g. low‐dose benzodiazepines).
Types of outcome measures
We assessed intubating conditions using the Goldberg scale (see Table 1), (Goldberg 1989; Weiss 1997). This is a widely used scale (although not always attributed to Goldberg et al.) that allocates a score for each of: ease of intubation, vocal cord movement, and patient response to intubation (diaphragmatic movement, coughing or bucking). This scale gives a total point value of 12, in which three represents excellent; four to six represents good; seven to nine represents poor, and 10 to 12 represents impossible or inadequate intubation conditions. Excellent intubation conditions had a score of three which means there must have been good conditions recorded by the operator, open vocal cords that were immobile, and no response by the patient to intubation. We converted trials to this scale if this had not been directly reported, but sufficient detail was available to do so. We compared rocuronium with succinylcholine by comparing the proportions of excellent intubation scores and the proportions of clinically acceptable intubation scores (good or excellent).
| Score | Ease of laryngoscopy | Vocal cords | Intubation response |
| 1. Excellent | Good | Open | None |
| 2. Good | Fair | Open | Diaphragmatic movement |
| 3. Poor | Difficult | Movement | Moderate coughing |
| 4. Impossible | Poor | Closed | Severe coughing or bucking |
Primary outcomes
The primary outcome assessed was excellent intubation conditions created during RSI (or modified RSI) comparing rocuronium with succinylcholine.
Secondary outcomes
The secondary outcome assessed was clinically acceptable (excellent or good) intubation conditions created during RSI (or modified RSI) comparing rocuronium with succinylcholine.
Search methods for identification of studies
Electronic searches
In our initial systematic review (Perry 2003) we searched all databases until March 2000. We reran the search to 2007 in our first update (Perry 2008). For this latest updated version we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 2), MEDLINE (1966 to February 14 2015), and EMBASE (1988 to February 14 2015) to identify all clinical trials relating to the use of rocuronium and succinylcholine during RSI. We used the validated RCT filter for the search (Haynes 1994).
Please refer to Appendix 1 (MEDLINE) , Appendix 2 (EMBASE) and Appendix 3 (CENTRAL) for our search strategies.
The local director of our library services reviewed our search strategy.
Searching other resources
We handsearched the references of included trials to add any citations missed by the electronic searches. We did not apply any language restrictions to the search.
Data collection and analysis
We combined all trials using Review Manager 5 software (RevMan 5.3). We produced the 'Summary of findings' table using GRADEpro software (GRADEpro 2015).
Selection of studies
We retrieved studies by searching by title or abstract. Two independent appraisers (JP, JL, VS, EN or DT) reviewed relevant articles using specific criteria defined in 'Types of studies'. We measured Inter‐rater agreement Kappa statistics. We resolved all disagreements by consensus. If we could not reach consensus, then a third author (GW or JP) was available to give a final decision.
Data extraction and management
Two authors (JP, JL, VS, EN, or DT) independently extracted data using standardized data collection forms. We converted intubation conditions to the Goldberg scale (four levels) if required and if adequate information was provided to do so. Rocuronium was compared to succinylcholine by comparing the proportion of excellent intubation scores to non‐excellent scores and the proportion of clinically acceptable scores (good or excellent) to the proportion of non‐clinically acceptable scores (poor or impossible). We resolved disagreements by consensus, with both extractors referring to the original text together, or by consulting a third author (JP). All data presented were from published literature only. Exact numbers for intubating conditions were provided by the authors for Sluga 2005.
Assessment of risk of bias in included studies
In this update, DT and EN reviewed and assessed all trials included in the review using the 'Risk of bias' tool.
Measures of treatment effect
We calculated dichotomous variables as risk ratios (RRs) for both excellent and acceptable intubation conditions, both with 95% confidence intervals (95% CIs) with a random‐effects model.
Unit of analysis issues
The unit of analysis was the intubation scores provided by each of the included trials. Sometimes the distribution of scores was provided only in graphical format, in which case the authors had to extrapolate from the graphs manually. We converted intubations scores when available to the Goldberg scale.
Dealing with missing data
We only included trials if they reported intubating conditions as a scale or in components which could be converted to the Goldberg scale. We performed analysis on an intention‐to‐treat basis. We conducted subgroup analyses for applicable trials and reported details of excluded information in included trials.
Assessment of heterogeneity
We assessed statistical heterogeneity by using the I statistic with thresholds of 25%, 50% and 75% to indicate mild, moderate and high degrees of heterogeneity respectively (Higgins 2003). Visual inspection was performed of the graphic representation of the trials with their 95% CIs. We explored the causes of significant heterogeneity with subgroup analyses and influence analyses.
Assessment of reporting biases
We performed this by visual inspection of a funnel plot of the included trials, to assess for publication bias.
Data synthesis
We conducted a meta‐analysis for the primary outcome of excellent intubation conditions and the secondary outcome of clinically acceptable conditions (where data were available) using Review Manager 5 software (RevMan 5.3). For trials comparing multiple drugs, we used only data points involving succinylcholine and rocuronium with the same induction agents.
Subgroup analysis and investigation of heterogeneity
A priori subgroup analysis for the outcome of excellent intubation conditions compared the following groups: simulated RSI (i.e. the neuromuscular‐blocking agent is administered immediately following the sedative and conditions evaluated within 60 seconds) versus modified RSI; induction agent; use versus non‐use of a narcotic; doses of rocuronium (0.6, 0.9, or 1.2 mg/kg); adult versus paediatric age groups; and emergency intubations (added in the previous update, Perry 2008).
After we completed the assessment of bias, we conducted subgroup analyses according to categorization of blinding of outcome assessment, to further identify the source of heterogeneity.
Sensitivity analysis
In order to assess their impact on the effect direction, size and precision of the summary estimate,we conducted analyses excluding trials in turn that:
-
contributed most to heterogeneity;
-
were most heavily weighted;
-
showed marked differences in intubation sequence (such as very short time between delivery of muscle relaxant and intubation).
Summary of findings table
We imported data from Review Manager 5 into the online GRADEpro software to produce the 'Summary of findings' table. The assumed risk population was set as the average incidence of excellent intubating conditions in the pooled control group. There is one primary outcome for which we assessed the overall quality of evidence using GRADE methodology by starting at a high level of evidence for RCTs and downgrading for serious deficiencies in the categories of study limitations, indirectness, imprecision, inconsistency and publication bias.
Results
Description of studies
Results of the search
In our previous update (Perry 2008) we identified 53 studies and included 37. For this update we identified 13 new studies. All the included studies are RCTs, with the exception of one CCT identified for this update (De Almeida 2009).
Included studies
We include 11 new trials in this review (Abu‐Halaweh 2007; Ali 2008; Belyamani 2008; De Almeida 2009; Iqbal 2013; Kulkarni 2010; Kwon 2013; Marsch 2011; Tripathi 2010; Singh 2011; Sorensen 2012; ) (see table Characteristics of included studies). Two articles identified from the previous update were translated and the results incorporated in this update (Mencke 2005; Türkmen 2004) (Figure 1). The revised search identified 66 studies, of which 52 met the inclusion criteria. Two of these were duplicate publications (Dubois 1991a; Mirakhur 1994a) and were therefore included as secondary references.
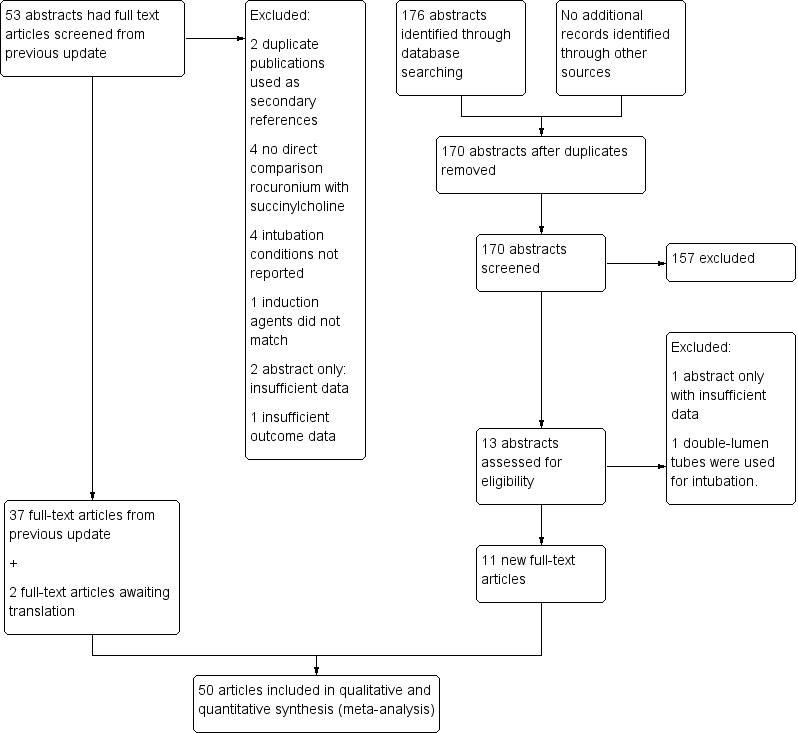
Search flow diagram for this update from July 2007 to February 2015
We now include 50 trials incorporating results from 4151 individuals in this updated review.
Rationale for excluded information from included studies
Andrews 1999 and McCourt 1998 are two of the largest trials conducted to date. Both trials had planned to conduct interim analyses at the halfway mark, and in both cases the steering committees decided to drop the lower dose rocuronium, as it was shown to be inferior to the larger dose (Dubois 1995). Neither trial reported the results of the low‐dose control groups. Hence, the data for the low‐dose rocuronium are not included in this meta‐analysis. In addition, Sparr 1996b used four different treatment groups with only one control group. Only one of the four treatment groups using rocuronium was appropriately controlled for, i.e. the succinylcholine group which used thiopentone without alfentanyl. Hence we have not included the rocuronium groups with propofol or alfentanyl in this meta‐analysis (no control group). Belyamani 2008 performed a trial assessing the benefit of ephedrine on intubating conditions when using either succinylcholine or rocuronium. Of the four treatment groups, we used only the data from the two control groups in this analysis. De Almeida 2009 enrolled morbidly obese participants given different doses of muscle relaxant based on ideal body weight versus total body weight. We have included only data for the two groups dosed for total body weight in this analysis, because the ideal body weight groups would have lower drug levels than those specified in the inclusion criteria. The second trial to involve emergency intubations (Marsch 2011), involved either propofol or etomidate as an induction agent. The authors did not provide separate data for the two groups of participants and we therefore did not include this trial in the induction agent analysis. The figures and tables in Türkmen 2004 were unavailable, and we were therefore able to include only data points for excellent intubation conditions.
Excluded studies
We excluded two of the 13 new studies identified in this update (Misiolek 2009; Stourac 2013).
We have excluded a total of 14 studies, for the reasons detailed in the Characteristics of excluded studies
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
There are no ongoing studies
Risk of bias in included studies
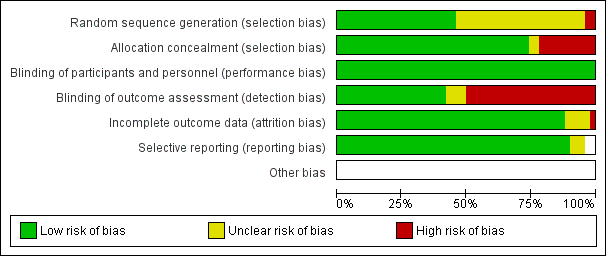
Figure 2 summarizes the findings in the four domains of random sequence generation, allocation concealment, blinding of outcome assessment and completeness of data.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
All but one of the trials (De Almeida 2009) was described as a randomized control trial. However, the exact method of randomization was not always described. We rated two of the 50 included trials at high risk of bias for allocation, due to lack of randomization (De Almeida 2009) and randomization by arrival sequence for surgery (Koroglu 2002).
Blinding
The most prevalent area of high risk of bias was blinding of outcome assessment, resulting in downgrading of the quality of evidence to moderate. Although many investigators blinded the intubator to the medication injected, 50% did not blind the assessor to the obvious effects of the drugs (Figure 3). Succinylcholine causes very discernible fasciculations (muscle twitches) that can be observed by the intubator, unblinding the study drug and bias assessment of the primary outcome. Please refer to individual 'Risk of bias' tables for specific details of each trial .

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
Incomplete outcome data
Completeness of data was almost uniformly low‐risk in the included trials, with the majority of them being complete.
Selective reporting
There were no concerns regarding selective reporting of results, as the outcome data were complete for all randomized participants in all included trials.
Other potential sources of bias
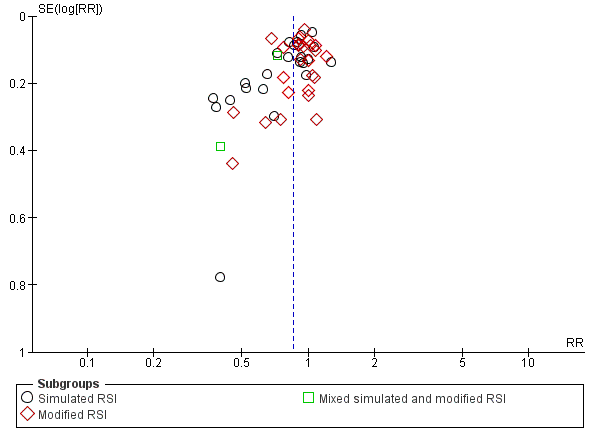
We assessed publication bias with a funnel plot. Visual inspection revealed an equal number of trials on either side of the effect estimate, although there was more scatter to the left indicating a paucity of trials in the lower right quadrant representing small unpublished trials favouring the use of rocuronium (Figure 4).

Funnel plot of comparison: Rocuronium any dose versus succinylcholine, outcome: Excellent versus other intubation conditions.
Effects of interventions
Primary outcome of excellent intubation conditions
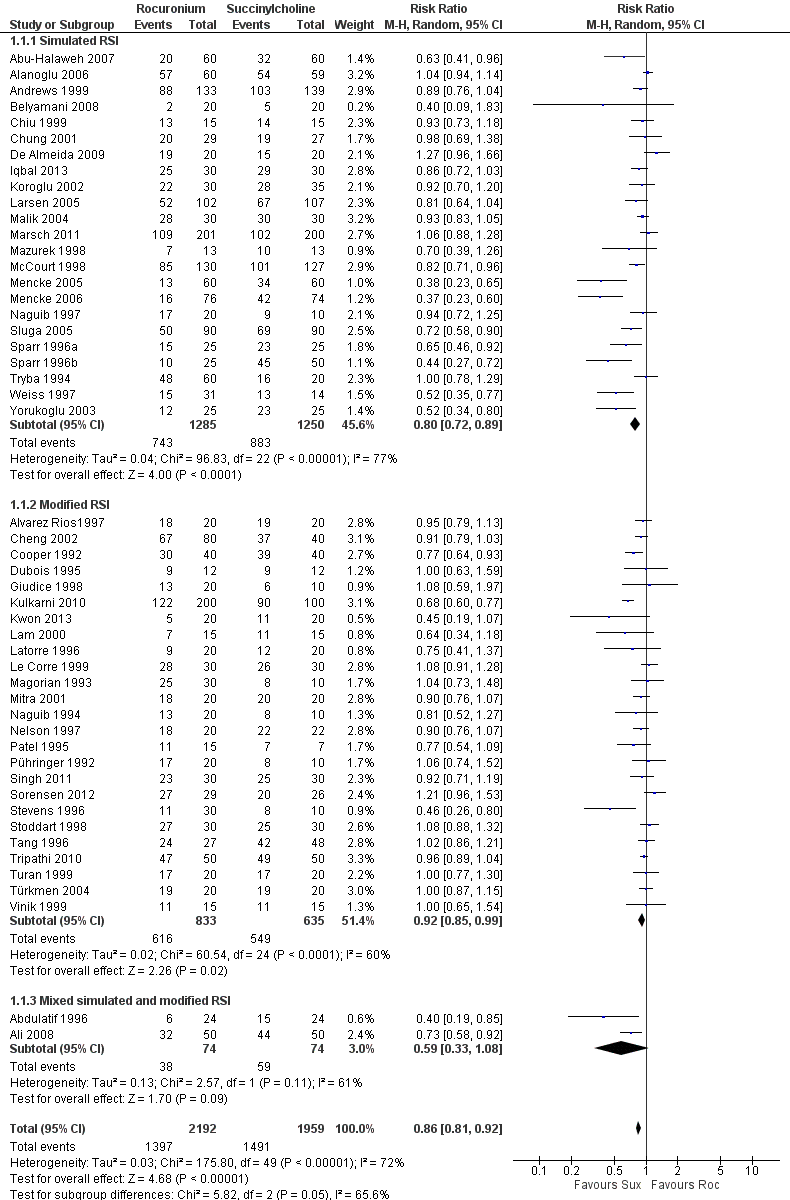
There was a statistically significant risk ratio (RR) favouring succinylcholine in the comparison for the primary outcome of excellent intubating conditions, with a RR 0.86 (95% CI 0.81 to 0.92; participants = 4151; studies = 50; I2 statistic = 72%; Analysis 1.1). The number needed to treat for an additional harmful outcome (NNTH) for this outcome was eight (95% CI 12 to 6). There was heterogeneity present in this comparison, as demonstrated graphically with the 95% CIs for each trial . The Chi² test for heterogeneity was significant (Figure 5). An analysis of the influence on heterogeneity demonstrated that no single trial , regardless of size, significantly altered the I² statistic, with the exception of Kulkarni 2010 for the subgroup of modified RSI. These assessments and the following subgroup analyses were unable to explain the heterogeneity in the trials . However, this did not result in a downgrading of the quality of the evidence because we decided that the sources of heterogeneity were clinical variables which contributed to the generalizability of these results.

Forest plot of comparison: 1 Rocuronium any dose versus succinylcholine, outcome: 1.1 Excellent versus other intubation conditions
Secondary outcome of clinically acceptable intubations
We also found a statistically significant difference using the less stringent endpoint of clinically acceptable conditions (excellent or good, excluding poor or failed) with a RR 0.97 (95% CI 0.95 to 0.99; participants = 3992; studies = 48; I2 statistic = 68%; Analysis 1.2).
Subgroup analysis for the primary outcome of excellent intubation conditions: simulated versus modified RSI
The subgroup which used a simulated RSI technique had a statistically significant RR favouring succinylcholine (RR 0.80, 95% CI 0.72 to 0.89; participants = 2535; studies = 23; I2 statistic = 77%). The NNTH for this outcome was eight (95%CI 12 to 6) and there was significant heterogeneity present. The subgroup using modified RSI also had significantly better intubation conditions in the succinylcholine group (RR 0.92, 95% CI 0.85 to 0.99; participants = 1468; studies = 25; I2 statistic = 60%), and an NNTH of eight (95% CI 11 to 5). There was also significant heterogeneity present for this subgroup. The subgroup using mixed simulated and modified RSI now includes two trials with no statistical difference observed.
Subgroup analysis for the primary outcome of excellent intubation conditions: comparing the dose of rocuronium
The subgroup using a dose of rocuronium of 0.6 to 0.7 mg/kg had a RR favouring succinylcholine for excellent conditions (RR 0.80, 95% CI 0.72 to 0.88; participants = 2808; studies = 39; I2 statistic = 77%). The NNTH for this subgroup is six (95% CI 7 to 5). There was significant heterogeneity between the trials. There were no statistical differences for excellent or acceptable intubation conditions in the group that received 0.9 to 1.0 mg/kg of rocuronium or the group that received 1.2 mg/kg of rocuronium. (Analysis 2.1)
Subgroup analysis for the primary outcome of excellent intubation conditions: induction agents
The thiopental subgroup displayed a statistical difference between succinylcholine and rocuronium for the outcome of excellent intubation conditions (RR 0.81, 95% CI 0.73 to 0.88; participants = 2302; studies = 28; I2 statistic = 81%)(Figure 6). The NNTH for this outcome was six (95% CI 7 to 5). The Chi² test for heterogeneity was significant. Further analysis of the thiopental subgroup compared the effect of thiopental when used with or without a narcotic. Succinylcholine created significantly better outcomes with narcotics ((RR 0.82, 95% CI 0.73 to 0.92; participants = 1300; studies = 17; I2 statistic = 79%; Analysis 4.1) or without narcotics (RR 0.80, 95% CI 0.69 to 0.94; participants = 1002; studies = 12; I2 statistic = 84%; Analysis 5.1) in sequence with thiopental. In a change from our previous update, propofol as an induction agent is no longer associated with better intubating conditions. There were no trials that used benzodiazepines for induction, comparing rocuronium to succinylcholine.
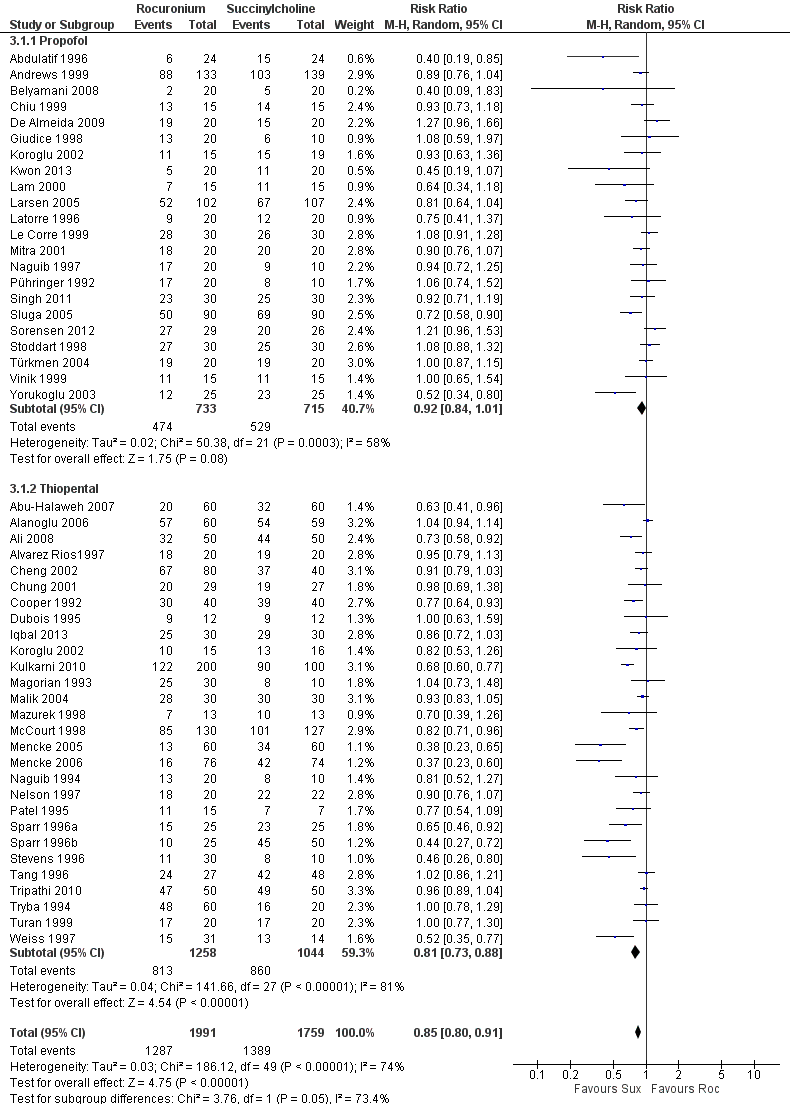
Forest plot of comparison: 3 Rocuronium versus succinylcholine for induction agent, outcome: 3.1 Excellent versus other intubation conditions
Subgroup analysis for the primary outcome of excellent intubation conditions: use of narcotics
Succinylcholine provided better intubating conditions with or without opioid use. The subgroup of trials using a narcotic in the sequence favoured the succinylcholine group (RR 0.85, 95% CI 0.78 to 0.93; participants = 2292; studies = 34; I2 statistic = 74%; Analysis 4.1). The NNTH for the subgroup using narcotics was seven (95% CI 10 to 6). The subgroup without a narcotic in sequence also demonstrated a statistically significant difference (RR 0.85, 95% CI 0.76 to 0.95; participants = 1428; studies = 16; I2 statistic = 76%; Analysis 5.1). The NNTH for this subgroup was six (95% CI 9 to 5). There was significant heterogeneity present for both groups.
Subgroup analysis for the primary outcome of excellent intubation conditions: age groups
The paediatric subgroup demonstrated no statistically significant difference between rocuronium and succinylcholine (RR 0.86, 95% CI 0.70 to 1.06; participants = 536; studies = 5; I2 statistic = 81%). There was significant heterogeneity between the five paediatric trials (Figure 6).
Subgroup analysis for the primary outcome of excellent intubation conditions: emergency intubation
For the subgroup comparing rocuronium and succinylcholine in emergency participants, there was a statistically significant RR favouring succinylcholine (RR 0.84, 95% CI 0.73 to 0.98; participants = 1073; studies = 5; I2 statistic = 53%; Analysis 7.1). The NNTH was 12 (95% CI 38 to 7) for this subgroup, and there was no significant heterogeneity between trials .
Inter‐observer agreement
In the first version of this review (Perry 2003), there was complete agreement between both evaluators regarding article selection (Kappa statistics 1.0). For this most recent update, the Kappa statistic was 0.9 for the articles.
Discussion
Summary of main results
Primary and secondary outcomes
This review summarizes the results of 50 trials in 41521 participants, demonstrating moderate‐quality evidence that succinylcholine creates better intubation conditions than rocuronium for both excellent and clinically acceptable intubation conditions during a rapid sequence induction. This is the same conclusion that we drew in our previous update (Perry 2008). The number of failed intubations was very small, with no clinically or statistically significant difference between rocuronium and succinylcholine.
Subgroup analysis
We have demonstrated that succinylcholine is superior to rocuronium when either a simulated or modified RSI technique is used. There are now two trials (n = 148) with mixed simulated RSI and modified RSI demonstrating no difference between the two muscle relaxants.
An interesting finding in this current update is the conclusion regarding an induction agent used with the muscle relaxant. Thiopental was found to provide superior intubating conditions with or without the use of a narcotic. This is contrary to the conclusions of the last update (Perry 2008). This switch in induction agent of choice was the result of the addition of six trials which used thiopental in this update, representing a total of 800 participants (Abu‐Halaweh 2007; Ali 2008; Iqbal 2013; Kulkarni 2010; Mencke 2005; Tripathi 2010). Unfortunately, this finding will have limited clinical applicability in North America, where the availability of thiopental has become very limited. When propofol was used as an induction agent, we found no significant difference between the two muscle relaxants with or without narcotics. The failure of narcotics to make a difference to the quality of intubation conditions is contrary to research which has reported significantly improved intubation conditions with the addition of a narcotic to the induction sequence (Sparr 1996b). This suggests that narcotics can safely be omitted in patients for whom they are contraindicated.
The dose of rocuronium has been thought to be important in creating intubation conditions equivalent to succinylcholine. This meta‐analysis did not find conclusive evidence that increasing doses of rocuronium led to better intubating conditions. Succinylcholine created significantly more excellent intubation conditions than rocuronium at doses of 0.6 to 0.7 mg/kg. There was no statistically significant difference for the 0.9 to 1.0 mg/kg or 1.2 mg/kg groups, reaffirming the dose of rocuronium used in current practice for RSI when succinylcholine is not clinically indicated. It is difficult to draw conclusions regarding the higher doses of rocuronium, as there are relatively few studies which have examined the higher dose (1.2 mg/kg) of rocuronium (n = 86). It is possible that there may be a benefit to using an increased dose of rocuronium but this meta‐analysis does not support this from the studies conducted to date. However, it should be noted that rocuronium has a longer duration of action compared to succinylcholine, and that increasing the dose of rocuronium increases its duration of action (73 ± 32 minutes for 1.2 mg/kg dose, Magorian 1993) which can result in an increased incidence of adverse outcomes (i.e. increased duration of paralysis in a patient who cannot be successfully intubated).
We include a subgroup analysis for participants undergoing emergency intubation from the last updated version of the review (Perry 2008). We have demonstrated that succinylcholine is superior to rocuronium in creating excellent intubation conditions. This is consistent with our findings in the less than 60‐second time delay subgroup. There was, however, no significant difference between groups for the outcome of clinically acceptable intubation, indicating that in emergency patients for whom succinylcholine is contraindicated, rocuronium can still be used to reliably create acceptable intubating conditions.
The five paediatric trials (Cheng 2002; Kulkarni 2010; Mazurek 1998; Naguib 1997; Stoddart 1998) did not demonstrate a difference in creating excellent intubation conditions between the rocuronium and succinylcholine groups. However, these had very little power to demonstrate any statistically significant difference due to the small sample size (i.e. underpowered for an equivalence trial). In addition, two of the trials (Naguib 1997; Stoddart 1998) used propofol in the sequence, while a third (Mazurek 1998) used a high dose of rocuronium (1.2 mg/kg) which may have confounded the results. This update includes a trial where ketamine was used in addition to a benzodiazepine as a premedication for particularly young children, further confounding the comparison (Kulkarni 2010).
Overall completeness and applicability of evidence
Although the search parameters were designed to identify any articles that could be pertinent to our research question, it is still possible that we have missed research not included in the databases accessible to the English‐speaking community. The inclusion of non‐English articles necessitated translation which, if performed poorly, could be a source of error, especially when assessing the specific domains of risk of bias. For the majority of cases, we pooled data presented in the publications for meta‐analysis. We obtained data from one trial (Sluga 2005) through correspondence with the authors.
This review has identified trials involving participants from a wide age range (one to 77 years) in a variety of clinical settings, including both elective and emergency intubations in the operating room, emergency department and intensive care unit. The funnel plot of the included trials indicates a lack of trials in the right lower quadrant which may represent small unpublished trials favouring the use of rocuronium (Figure 4). However, the reason for such trials not being reported is not evident. Another reason for the asymmetric funnel plot is heterogeneous study effects that can be seen with varying study sizes, intubation sequences and study populations. More effective intubation conditions can be achieved with larger doses of rocuronium, with the drawback of prolonging muscle paralysis and length of intubation. This adverse outcome was not reported in the included trials, although there is a report of tachycardia and coughing. This review is unable to draw conclusions regarding safety.
Quality of the evidence
We found a significant amount of heterogeneity in the analysis of the primary outcome, which we tried to explore with subgroup analyses separating by age, emergencies, doses of rocuronium, timing of muscle relaxant, induction agent and opioid use. The I² statistical value never fell below the 50% thresholds with these sensitivity analyses, nor did the direction or size of the summary estimate. As a result, we did not downgrade the quality of evidence, because unexplored reasons for heterogeneity include:
-
Different populations (varying from simple elective limb surgery to more complex gastric bypass on morbidly obese patients and emergent intensive care intubations);
-
Varying clinical settings;
-
Different medications in induction sequences;
-
Different timing of intubation.
All of these contribute to the generalizability of our results and to reducing concerns about indirectness.
Assessments of the risk of biases demonstrate that the series of trials included in this review are at low risk of selection and attrition bias . All but one trial was described as a randomized controlled trial, with 11% of trials being at high risk for lack of allocation concealment. The area of most concern was the high incidence of detection bias due to lack of blinding of the outcome assessor, which led to a downgrading of the quality of evidence to moderate. Succinylcholine will cause significant fasciculations, and intubators who are not blinded to this effect may assign biased scores to the intubating conditions. We conducted a subgroup analysis based on the blinding of the outcome assessor which failed to explain the source of the heterogeneity in the meta‐analysis (Analysis 8.1). There were no concerns regarding the precision of the estimate, with more than 4000 participants included in the pooled estimate.
Potential biases in the review process
Because the original review was published in 2003 (Perry 2003), this update had to retrospectively formulate 'Risk of bias' tables, a 'Summary of findings' table and GRADE the quality of evidence in accordance with updated Cochrane guidelines. This process may have led to loss of details, now regarded as pertinent, involving inclusions/exclusion decisions made in the previous updates.
With the large number of possible sequences used, multiple testing can result in erroneous conclusions just by chance. This effect was minimized with the use of sensitivity analysis in prespecified subgroups. We conducted an additional subgroup analysis post hoc based on detection bias, to try and account for the heterogeneity observed in the results. At the time of inception of this review, doses of 0 .6 mg/kg of rocuronium were being given for RSI, but higher doses of 1 mg/kg are now favoured, and the subgroup analyses allowed for assessment of these different doses.
Agreements and disagreements with other studies or reviews
A retrospective review of 327 RSI intubations using etomidate with rocuronium or succinylcholine in the emergency department showed equivalent success at first intubation attempts (Patanwala 2011). Median doses for rocuronium were 1.19 mg/kg and 1.5 mg/kg of succinylcholine. Herbstritt 2012 is a short review looking at use of equivalent doses of rocuronium and succinylcholine (1 mg/kg) for RSI. They included seven papers of varying quality (retrospective review, RCT and meta‐analysis), and concluded that there are no differences in intubating conditions between the two. This is consistent with our finding in the 0.9 to 1.0 mg/kg dose range (RR 0.95, 95% CI 0.89 to 1.00; participants = 1458; studies = 16; I2 statistic = 44%). When using doses of 0.6 mg/kg of rocuronium, Larsen 2005 used alfentanil and propofol as their induction agents and found no difference between rocuronium and succinylcholine 1 mg/kg in achieving clinically acceptable intubating conditions. These results are also consistent with those reported in this review for the secondary outcome (RR 0.99, 95% CI 0.96 to 1.02; participants = 952; studies = 16; I2 statistic = 19%).

Search flow diagram for this update from July 2007 to February 2015
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
Funnel plot of comparison: Rocuronium any dose versus succinylcholine, outcome: Excellent versus other intubation conditions.
Forest plot of comparison: 1 Rocuronium any dose versus succinylcholine, outcome: 1.1 Excellent versus other intubation conditions

Forest plot of comparison: 3 Rocuronium versus succinylcholine for induction agent, outcome: 3.1 Excellent versus other intubation conditions
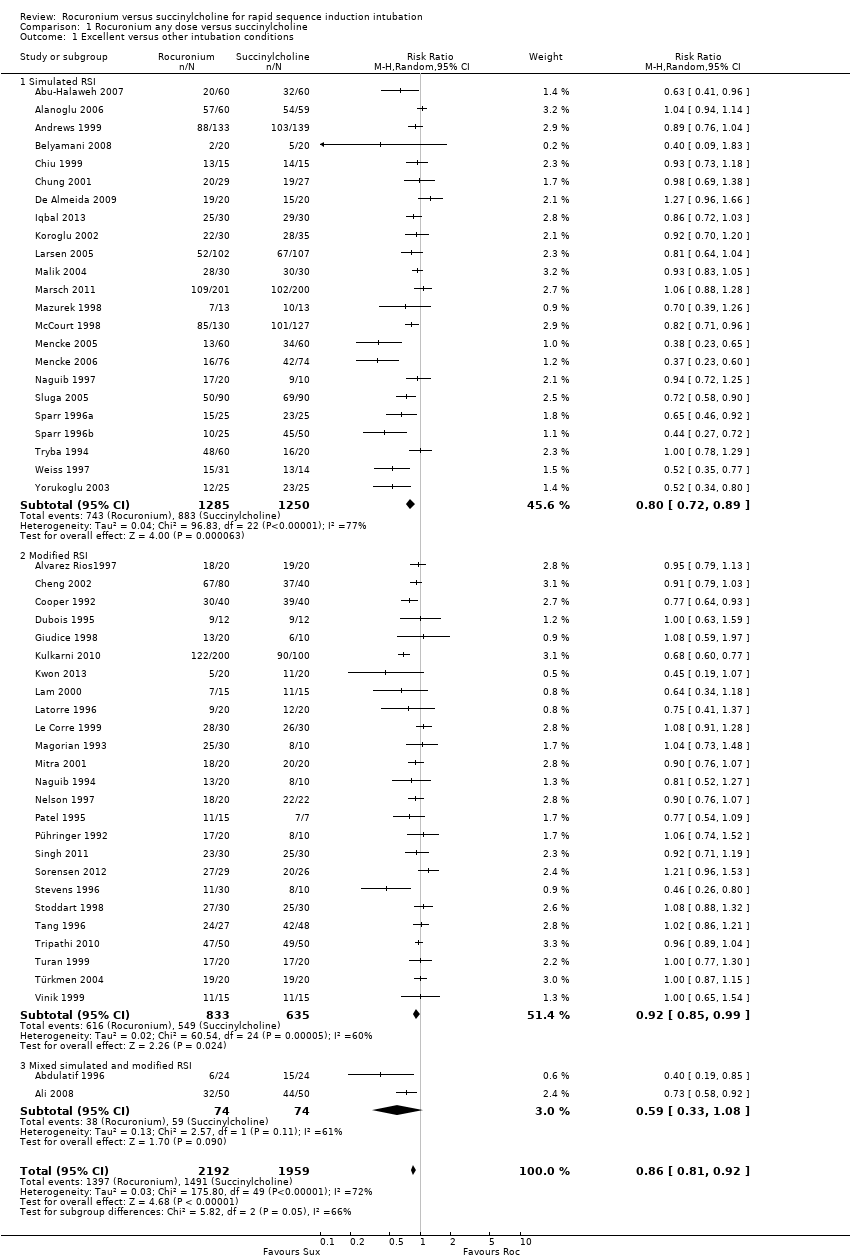
Comparison 1 Rocuronium any dose versus succinylcholine, Outcome 1 Excellent versus other intubation conditions.
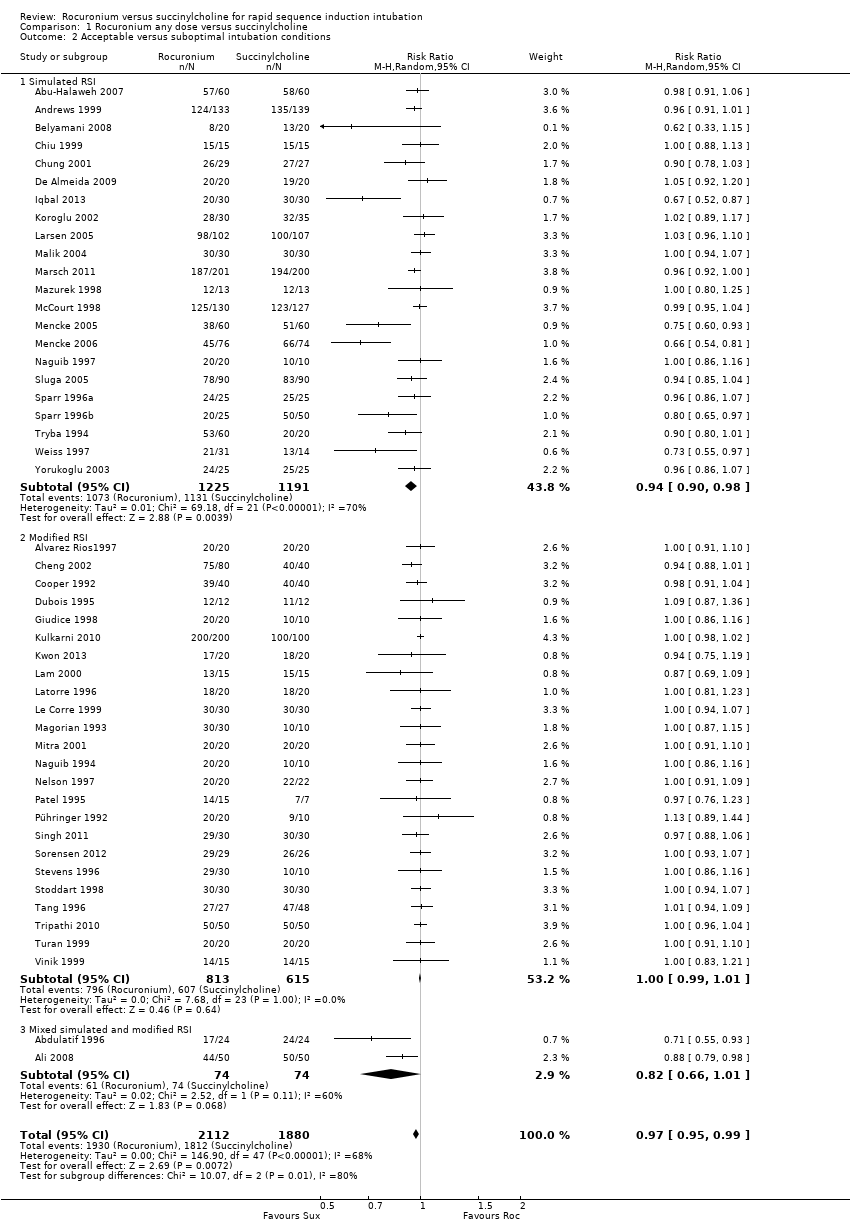
Comparison 1 Rocuronium any dose versus succinylcholine, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 2 Rocuronium specific dose versus succinylcholine, Outcome 1 Excellent versus other intubation conditions.
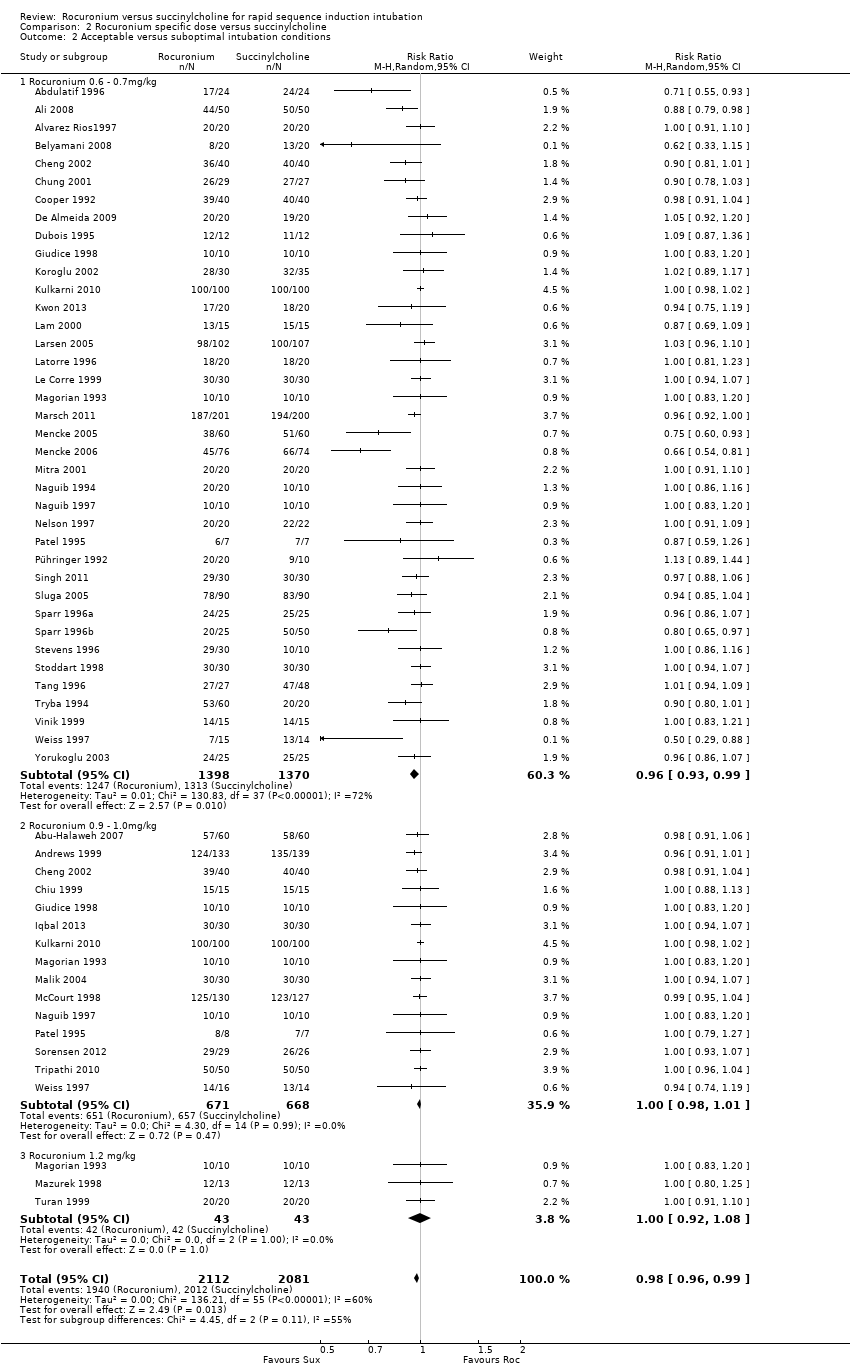
Comparison 2 Rocuronium specific dose versus succinylcholine, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 3 Rocuronium versus succinylcholine for induction agent, Outcome 1 Excellent versus other intubation conditions.
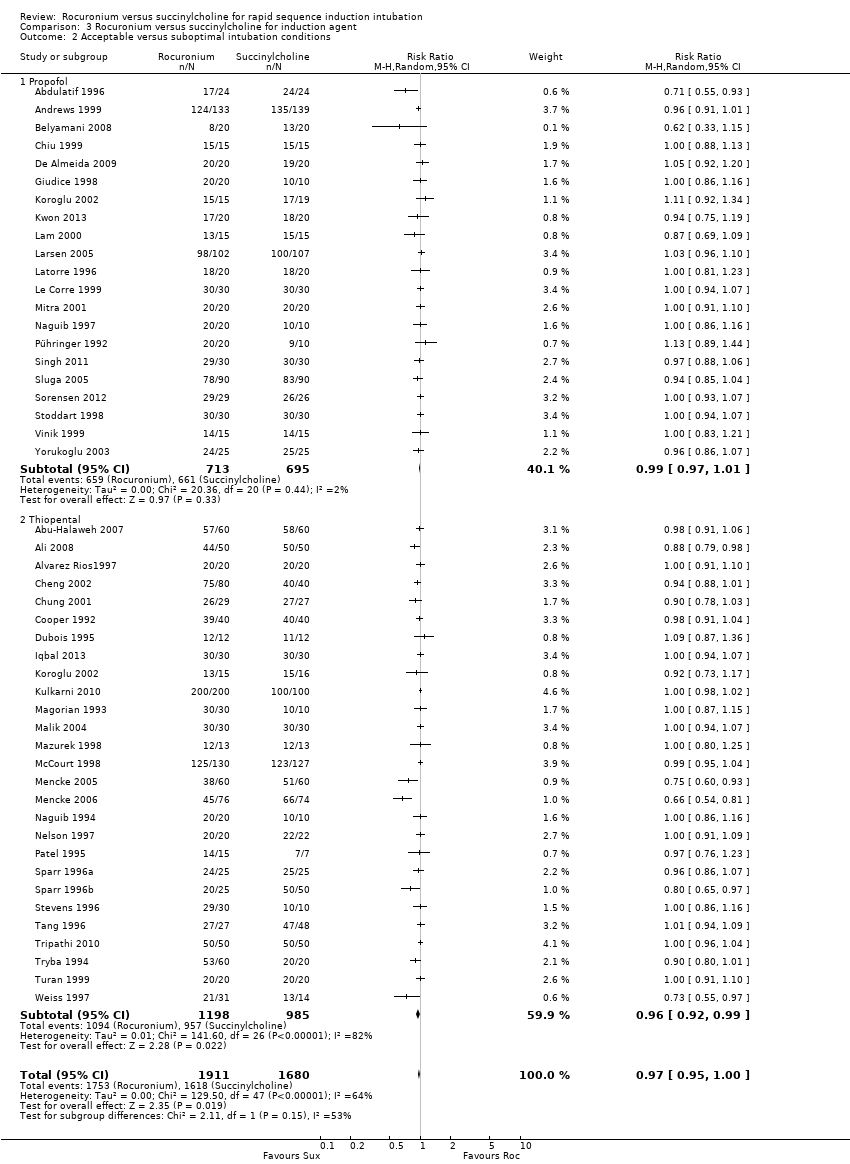
Comparison 3 Rocuronium versus succinylcholine for induction agent, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 4 Rocuronium versus succinylcholine with narcotic, Outcome 1 Excellent versus other intubation outcomes.
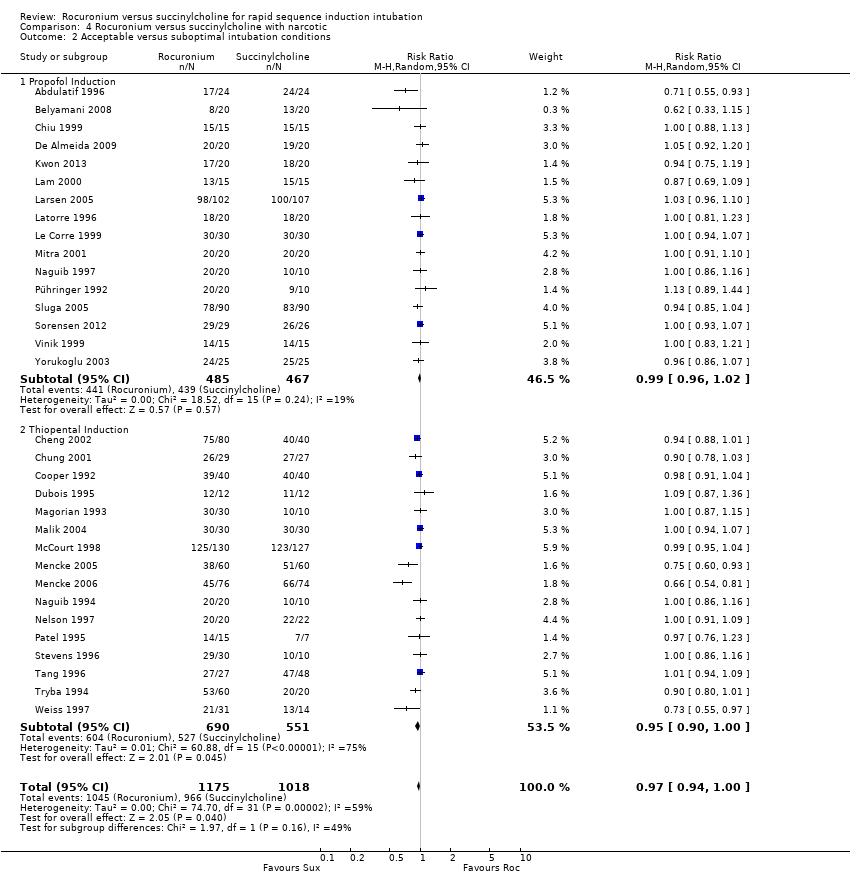
Comparison 4 Rocuronium versus succinylcholine with narcotic, Outcome 2 Acceptable versus suboptimal intubation conditions.
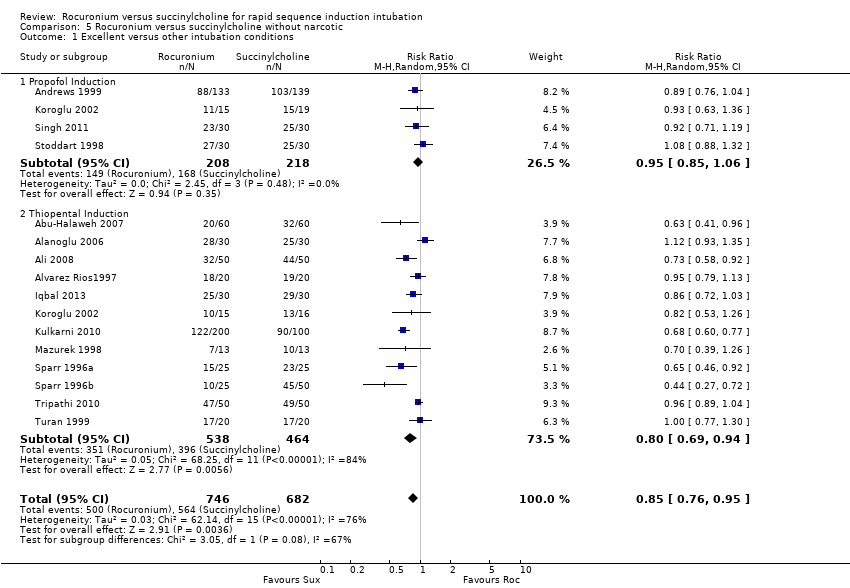
Comparison 5 Rocuronium versus succinylcholine without narcotic, Outcome 1 Excellent versus other intubation conditions.
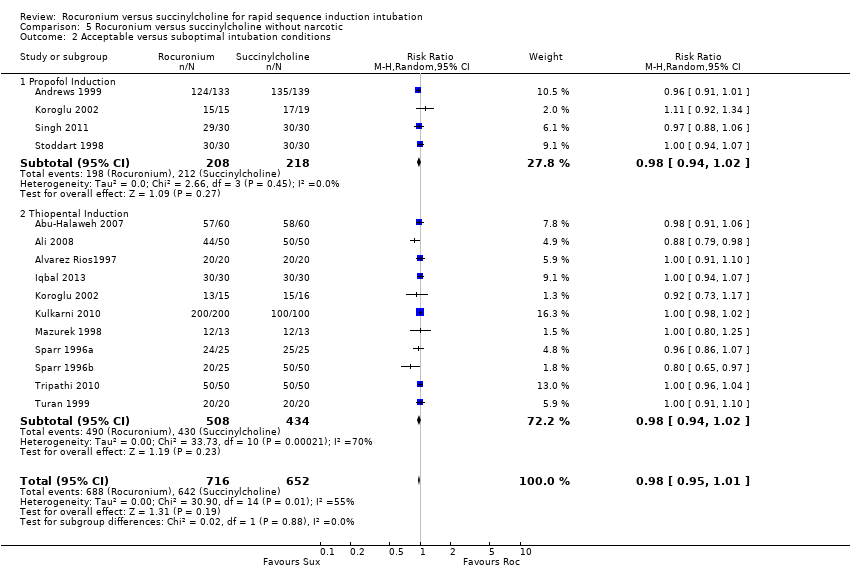
Comparison 5 Rocuronium versus succinylcholine without narcotic, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 6 Comparison of children and adults, Outcome 1 Excellent versus other intubation conditions.

Comparison 6 Comparison of children and adults, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 7 Rocuronium versus succinylcholine in emergency intubation, Outcome 1 Excellent versus other intubation conditions.
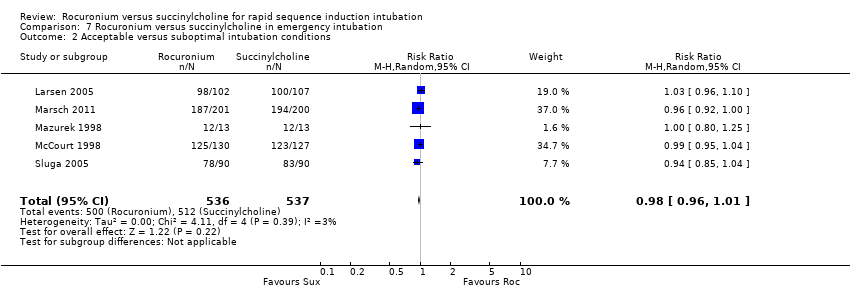
Comparison 7 Rocuronium versus succinylcholine in emergency intubation, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 8 Rocuronium versus succinylcholine by blinding of outcome assessment, Outcome 1 Excellent versus other intubation conditions.

Comparison 8 Rocuronium versus succinylcholine by blinding of outcome assessment, Outcome 2 Acceptable versus suboptimal intubation conditions.
| Rocuronium any dose versus succinylcholine for rapid sequence induction intubation | ||||||
| Patient or population: People requiring rapid sequence induction intubation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Succinylcholine | Rocuronium any dose 2 | |||||
| Excellent versus other intubation conditions | Study population | RR 0.86 | 4151 | ⊕⊕⊕⊝ | Risk of bias: 50% of the studies were at high risk for detection bias because the outcome assessor was not blinded to the fasciculations caused by succinylcholine. Inconsistency: High statistical heterogeneity in the studies could not be explained by subgroup analyses. However we did not downgrade because exclusion of trials contributing to heterogeneity did not significantly change the direction or size of effect. | |
| 76 per 100 | 65 per 100 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk is the average number of excellent intubations with succinylcholine. 2Rocuronium minimum dose 0.6 mg/kg. Succinylcholine minimal dose is 1mg/kg. | ||||||
| Score | Ease of laryngoscopy | Vocal cords | Intubation response |
| 1. Excellent | Good | Open | None |
| 2. Good | Fair | Open | Diaphragmatic movement |
| 3. Poor | Difficult | Movement | Moderate coughing |
| 4. Impossible | Poor | Closed | Severe coughing or bucking |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Simulated RSI | 23 | 2535 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.89] |
| 1.2 Modified RSI | 25 | 1468 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 0.99] |
| 1.3 Mixed simulated and modified RSI | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.08] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Simulated RSI | 22 | 2416 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.90, 0.98] |
| 2.2 Modified RSI | 24 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
| 2.3 Mixed simulated and modified RSI | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.66, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4352 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Rocuronium 0.6 ‐ 0.7mg/kg | 39 | 2808 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.88] |
| 1.2 Rocuronium 0.9 ‐ 1.0mg/kg | 16 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.00] |
| 1.3 Rocuronium 1.2 mg/kg | 3 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 4193 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 0.99] |
| 2.1 Rocuronium 0.6 ‐ 0.7mg/kg | 38 | 2768 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.99] |
| 2.2 Rocuronium 0.9 ‐ 1.0mg/kg | 15 | 1339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.98, 1.01] |
| 2.3 Rocuronium 1.2 mg/kg | 3 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 49 | 3750 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.91] |
| 1.1 Propofol | 22 | 1448 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 1.2 Thiopental | 28 | 2302 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.73, 0.88] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 47 | 3591 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 1.00] |
| 2.1 Propofol | 21 | 1408 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.01] |
| 2.2 Thiopental | 27 | 2183 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.92, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation outcomes Show forest plot | 34 | 2292 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.93] |
| 1.1 Propofol Induction | 17 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.2 Thiopental Induction | 17 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 32 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 2.1 Propofol Induction | 16 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.02] |
| 2.2 Thiopental Induction | 16 | 1241 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 15 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 1.1 Propofol Induction | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 1.2 Thiopental Induction | 12 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.94] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 14 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 2.1 Propofol Induction | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| 2.2 Thiopental Induction | 11 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.91] |
| 1.1 Adults | 45 | 3615 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.92] |
| 1.2 Children | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Adults | 43 | 3456 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.2 Children | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 5 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.98] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 5 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Low Risk | 21 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
| 1.2 Unclear Risk | 4 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.18] |
| 1.3 High Risk | 25 | 2042 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.96] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Low Risk | 23 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 2.2 Unclear Risk | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.07] |
| 2.3 High Risk | 22 | 1912 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |

