ยาโรคูโรเนียม (rocuronium) เทียบกับ ยาซัคซินิลโคลีน (succinylcholine) สำหรับการใส่ท่อช่วยหายใจในการนำสลบแบบรวดเร็ว
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT | |
| Participants | ASA I‐II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 24) Sequence with: fentanyl 2 mcg/kg, propofol 2.5 ‐ 3.0 mg/kg | |
| Outcomes | 1. Intubating conditions 60s after muscle relaxant evaluated by blinded observer. Reported as scores (0 ‐ 3) adapted from Fahey et al. Definitions table include vocal cord movement, visualization, participant movement 2. Adductor pollicis response to TOF stimulation | |
| Adverse events | None reported | |
| Time & Place | Study dates not reported. Article accepted November 1995. King Fahad University Hospital, Al‐Khobar, Saudi Arabia. | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated via closed envelope |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Data for all participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Elective and emergency caesarean section N = 120 | |
| Participants | ASA I ‐ II Pregnant women Mean age 32 Mean weight 78 kg | |
| Interventions | 1, Rocuronium 1 mg/kg (n = 60) 2, Succinylcholine 1 mg/kg (n = 60) Sequence with: thiopental 5 mg/kg | |
| Outcomes | 1. Intubating conditions by senior anaesthetist 60s after muscle relaxant. Reported as excellent, good and poor, as modified Viby‐Mogenson Grading system. Features included jaw relaxation, vocal cord position and diaphragmatic activity | |
| Adverse events | Slight increase in heart rate after 5 mins with rocuronium use. | |
| Time & Place | December 2005 to May 2006 Jordan University Hospital, Jordan | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly shuffled envelopes, probably adequate |
| Allocation concealment (selection bias) | Low risk | Randomly shuffled sealed envelopes indicating the type of the muscle relaxant to be used for intubation |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | The intubator who was blinded to the type of administered muscle relaxant was called to the theatre 40s after the relaxant administration |
| Incomplete outcome data (attrition bias) | Low risk | Data for all participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA II ‐ III | |
| Interventions | 1. Succinylcholine 1.0 mg/kg with lidocaine (n = 30) | |
| Outcomes | 1. Intubating conditions 60s after muscle relaxant. Reported as excellent, good, poor based on 6 variables (jaw relaxation, resistance to blade, vocal cord position and movement, movement of limbs and coughing) with table of definitions | |
| Adverse events | Mild muscle rigidity in 6 participants with the use of remifentanil. | |
| Time & Place | Study dates not reported. Article accepted June 2005 Ankara University, Ankara, Turkey | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelope |
| Allocation concealment (selection bias) | Low risk | Allocated to 4 groups at random by sealed envelope technique |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Authors performing the intubation and scoring intubation conditions were blinded to the study medications." Unclear if blinded allocation or drug administration |
| Incomplete outcome data (attrition bias) | Low risk | Adequately described in detail |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Mixed simulated and modified RSI N = 100 | |
| Participants | ASA I ‐ II Age 18 ‐ 60 Elective OR | |
| Interventions | 1. Rocuronium 0.6 mg/kg at 60s (n = 25) 2. Rocuronium 0.6 mg/kg at 90s (n = 25) 3. Succinylcholine 1.5 mg/kg at 60s (n = 25) 4. Succinylcholine 1.5 mg/kg at 90s (n = 25) Sequence with thiopental 5 mg/kg | |
| Outcomes | Intubation conditions at 60 or 90s after muscle relaxant. Reported as score (0 ‐ 3) based on 3 variables (jaw relaxation, vocal cords and response to intubation) from Cooper et al with definitions table | |
| Adverse events | None reported | |
| Time & Place | Study dates not reported. Article published 2008. Sheri Kashmir Institute of Medical Sciences, Soura, Srinagar, India | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Did not provide results of individuals groups. Used aggregate data, classified as modified RSI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" but did not elaborate |
| Allocation concealment (selection bias) | Unclear risk | Used "double‐blind" fashion |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Same fully‐trained anaesthetist (Intubator) performed all the intubations, who was called in the study room 45s after the administration of the neuromuscular blocker in group A participants and after 75s in group B participants (to eliminate possible bias because of fasciculations induced by succinylcholine) and intubation was attempted 15s later |
| Incomplete outcome data (attrition bias) | Unclear risk | Data were not presented for all 4 groups, aggregated into 2 groups |
| Selective reporting (reporting bias) | Unclear risk | Only aggregate data presented |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 20) Sequence with: no opioid | |
| Outcomes | 1. Intubating conditions 90s after muscle relaxant. Reported as excellent, good, poor with definitions described for madibular relaxation, vocal cords and participant movement | |
| Adverse events | None reported. | |
| Time & Place | Study dates were not reported. Article published 1997. Mexico | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Established groups were formed randomly, but does not state how |
| Allocation concealment (selection bias) | High risk | No comment made |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No statement regarding blinding |
| Incomplete outcome data (attrition bias) | Low risk | Data on all participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ V | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 48)* 3. Rocuronium 1.0 mg/kg (n=133) | |
| Outcomes | 1. Intubating conditions 50s after muscle relaxant. Reported as excellent, good, poor based on 6 variables (jaw relaxation, resistance to laryngoscope, vocal cord position and movement, limb movement and diaphragmatic activity) with definitions described | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted September 1998. University of Newcastle‐upon‐Tyne, Turnhout, Belgium | |
| Funding and declarations | Funding source: Organon Teknika Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States randomly without replacement and stratified for centre |
| Allocation concealment (selection bias) | Low risk | Allocation concealed from investigator performing the randomization |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "blinding was achieved by concealing patient from the investigator until immediately before laryngoscopy." |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Simulated RSI N = 80 | |
| Participants | ASA I ‐ II Elective OR Mean age 34 Mean BMI 23.5 | |
| Interventions | 1. Succinylcholine 1 mg/kg + ephedrine (n = 20)* 2. Rocuronium 0.6 mg/kg + ephedrine (n = 20)* 3. Succinylcholine 1 mg/kg + saline (n = 20) 4. Rocuronium 0.6 mg/kg + saline (n = 20) Premedication: Hydroxyzine 1 mg/kg Sequence with: propofol 2.5 mg/kg, fentanyl 3 mcg/kg | |
| Outcomes | 1. Intubation conditions 30s after muscle relaxant. Reported as excellent, good, poor based on criteria from the Copenhagen conference. No definitions provided 2. Heart rate, blood pressure | |
| Adverse events | None reported | |
| Time & Place | Study dates not reported. Article accepted December 2007. Mohammed‐V Military Hospital, Rabat, Maroc | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | In French. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a randomization table |
| Allocation concealment (selection bias) | Low risk | The participant and the anaesthesiologist were not informed of the contents of the syringes (prepared by a separate individual) |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | 30s after injection of the muscle relaxant, another blinded staff anaesthetist performed intubation of the participant |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 40) | |
| Outcomes | 1. Intubating conditions 30s after muscle relaxant. Reported as excellent, good, poor and impossible with table of definitions. Clinical features included: vocal cord movement, participant response to intubation and jaw relaxation | |
| Adverse events | One participant developed bronchospasm during intubation after receiving rocuronium 0.9 mg/kg. This resolved spontaneously. | |
| Time & Place | Study dates not reported. Article published 2002. Prince of Wales Hospital, New Territories, Hong Kong | |
| Funding and declarations | Funding source: Organon Teknika China Ltd provided rocuronium for study. Declarations of interest: none declared | |
| Notes | ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by sealed envelopes |
| Allocation concealment (selection bias) | Low risk | "children were randomly assigned by means of opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "observer had her back turned to the patient during the 30s before attempting to intubate" |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I | |
| Interventions | 1. Rocuronium 0.9 mg/kg (n = 15) Sequence with: fentanyl 2 mcg/kg, propofol 2 mg/kg | |
| Outcomes | 1. Intraocular pressure, mean arterial pressure, heart rate measured before induction, immediately after induction and every minute after intubation for 5 mins | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted January 1999. Univeristy of Malaya, Kuala Lumpur, Malaysia | |
| Funding and declarations | Funding source: Organon Teknika (Malaysia) supplied rocuronium. Kemajuan Abadi Optomedic (Malaysia) supplied Keeler Pulsair air pulse tonometer. Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized, double‐blind, controlled study", but does not elaborate |
| Allocation concealment (selection bias) | Low risk | "drugs were administered ...by one anaesthetist (CYW) who was unaware of the drugs administered" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "the intubating anesthetist were not allowed to observe injection of the neuromuscular blocking drug or the presence of any fasciculations, by standing initially with their back to the patient. They were then asked to turn round to face the patient, 45 s after injection of either succinylcholine or rocuronium; by then the fasciculations had subsided" |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg and then thiopental 5 mg/kg (n = 28)* 3. Thiopental 5 mg/kg and then rocuronium 0.6 mg/kg (n = 27) | |
| Outcomes | 1. Intubating conditions 60s after muscle relaxant. Reported as excellent, good and poor from a score (0 ‐ 9) (from Cooper et al ) based on 3 variables (ease of laryngoscopy, condition of vocal cords, response to intubation) and defined in a table 2. Apnea time before laryngoscopy 3. Intubation time 4. Total apnoea time | |
| Adverse events | 5 participants in Group 1 and 1 in Group 2 had pain in injection. 3 in Group 1 had diminished breathing during induction. 1 in Group 1 had mild desaturation. | |
| Time & Place | Study dates not reported. Article accepted September 2000. Changhua Christian Hospital, Changhau, Taiwan | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly allocated", but did not elaborate |
| Allocation concealment (selection bias) | High risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, 6/90 participants excluded due to "invisible vocal cords after several attempts" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 40) Sequence with: fentanyl 1 ‐ 3 mcg/kg, thiopentone 3 ‐ 5 mg/kg | |
| Outcomes | 1. Intubating conditions 60 and 90s after muscle relaxant. Reported as excellent, good and poor from a score (0 ‐ 9) based on 3 variables (jaw relaxation, vocal cords, response to intubation), defined in table | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted March 1992. Queen's University, Belfast, Britain | |
| Funding and declarations | Funding source: rocuronium supplied by Organon Teknika, Belgium Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were allocated randomly" |
| Allocation concealment (selection bias) | Unclear risk | "patients were allocated randomly" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment on blinding |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | Controlled Trial Simulated RSI N = 80 | |
| Participants | ASA I ‐ III Elective bariatric surgery Morbidly obese participants BMI ≥ 40 18 ‐ 65 yrs Mean age 39 Mean weight 128 kg | |
| Interventions | 1. Succinylcholine 1 mg/kg ideal body weight (n = 20)* 2. Succinylcholine 1 mg/kg total body weight (n = 20) 3. Rocuronium 0.6 mg/kg ideal body weight (n = 20)* 4. Rocuronium 0.6 mg/kg total body weight (n = 20) Premedication: midazolam 7.5 mg Sequence with: propofol 2 mg/kg, fentanyl 2 mcg/kg | |
| Outcomes | Intubation conditions 60s after intubation. Reported as excellent, good, poor based on 5 variables (laryngoscopy, vocal cord position, vocal cord movement, reaction to tube insertion, limb movement with tube insertion) described in a table | |
| Adverse events | None reported. | |
| Time & Place | March 2005 to March 2007. Federal University of Santa Catarina, Santa Catarina, Brazil | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Paper written in Spanish. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No mention of randomization |
| Allocation concealment (selection bias) | High risk | No comment made |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions. |
| Blinding of outcome assessment (detection bias) | High risk | No description or comment on blinding |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 12) Sequence with: fentanyl 1 ‐ 10 mcg/kg, thiopentone 3 ‐ 5 mg/kg | |
| Outcomes | 1. Intubating conditions after 80% first twitch depression of TOF. Reported as excellent good, poor and inadequate based 3 variables (jaw relaxation, vocal cord movement, diaphragm) described in Methods section 2. Heart rate and blood pressure 2. Onset time of muscle relaxant | |
| Adverse events | 5 participants had fasciculations. 2 had skin rash and one experienced hypersalivation. | |
| Time & Place | Study dates not reported. Article accepted March 1994. Georgetown University Medical Center, Washington, DC, USA. | |
| Funding and declarations | Funding source: Support of Clinical Project Director Organon Inc. Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned via computer generation |
| Allocation concealment (selection bias) | Low risk | "either R or S in a coded syringe prepared by the pharmacist was given" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "the investigator intubator was blinded to the muscle relaxant randomization scheme and not in the operating room for drug administration" |
| Incomplete outcome data (attrition bias) | Unclear risk | "The other 6 patients were dropped because of incomplete data retrieval". Did not say why data were missing |
| Selective reporting (reporting bias) | Unclear risk | Outcomes for excluded participants not reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.3 mg/kg (n = 10)* Sequence with: fentanyl prn, propofol 1.5 mg/kg | |
| Outcomes | 1. Intubating conditions when T1 of TOF ≤ 5%. Reported as a score (0 ‐ 6). Variables not presented for score assessment 2. Recovery of T1 to 25% | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted August 1998. Italy | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Italian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly allocated into four groups" |
| Allocation concealment (selection bias) | High risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Simulated RSI N = 60 | |
| Participants | ASA I ‐ II Adult elective surgery Age 20 ‐ 60 yrs | |
| Interventions | 1. Rocuronium 0.9 mg/kg (n = 30) 2. Succinylcholine 1.5 mg/kg (n = 30) Sequence with: thiopental 5 mg/kg No premeds | |
| Outcomes | Intubating conditions 60s after induction drugs. Reported as excellent, good, poor and not possible based on 3 variables (jaw relaxation, vocal cords and response to tube) from modification of Goldberg et and Krieg et al. | |
| Adverse events | None reported. | |
| Time & Place | January to August 2009. Civil Hospital Karachi, Karachi, Pakistan | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", did not elaborate |
| Allocation concealment (selection bias) | Low risk | "double‐blind manner" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "endotracheal intubation was done blinded by standing with the back to the patient." |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg and propofol 2 mg/kg (n = 20) 3. Rocuronium 0.6 mg/kg and thiopentone 5 mg/kg (n = 20) | |
| Outcomes | 1. Intubations conditions. Started intubation 20s after muscle relaxant, intubated according to clinical conditions. Reported as excellent, good, poor based on 3 variables (jaw relaxation, vocal cord movement, reaction to tube) and score (0 ‐ 9) from Cooper et al. 2. Time to intubations | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article published 2002. Dokuz Eylul University, Turkey | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "patients were numbered according to their order of arrival to the surgery" |
| Allocation concealment (selection bias) | High risk | No comment made |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment made |
| Incomplete outcome data (attrition bias) | Unclear risk | All cases were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Modified RSI N = 300 | |
| Participants | ASA I ‐ II Elective cleft palate repair OR Age 1 ‐ 10 Mean age 4 Mean weight 17 kg Mallampati I ‐ II | |
| Interventions | 1. Succinylcholine 1.5 mg/kg (n = 100) 2. Rocuronium 0.6 mg/kg (n = 100) 3. Rocuronium 0.9 mg/kg (n = 100) Premedication: glycopyrrolate 0.004 mg/kg IM, midazolam 0.05 mg/kg IM, ketamine 5 mg/kg IM for younger children, tramadol 1 mg/kg iv Sequence with: thiopental 6 ‐ 8 mg/kg | |
| Outcomes | 1. Intubation conditions at 60s after muscle relaxant. Reported as excellent, good, poor, inadequate according to intubation scoring system as per Mangorian et al. Based on 3 clinical variables: jaw relaxation, vocal cord movement and diaphragmatic movements 2. Intubation time 3. Duration of muscle relaxation with TOF monitoring 4. Clinical recovery | |
| Adverse events | Tachycardia in all three groups (58‐66%) | |
| Time & Place | October 2003 to September 2008. Lotus Hospital & Research Center, Kolhapur, Maharashtra, India | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | used oral RAE tubes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided in three groups" |
| Allocation concealment (selection bias) | High risk | No comment made |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment made |
| Incomplete outcome data (attrition bias) | Low risk | All cases presented |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Modified RSI N = 40 | |
| Participants | ASA I ‐ II Elective OR Mean age 43 Mean weight 61 kg | |
| Interventions | 1. Succinylcholine 1.5 mg/kg (n = 20) 2. Rocuronium 0.6 mg/kg (n = 20) Sequence with: lidocaine 60 mg, fentanyl 1.5 mcg/kg, propofol 1.5 mg/kg | |
| Outcomes | 1. Intubating conditions (with loss of consciousness for rocuronium group and 60s after succinylcholine). Reported as excellent, acceptable and poor based on a score. Variables included: mandibular relaxation, resistance to blade insertion, vocal cord position and movement, limb response, coughing 2. Timing of events 3. Complications of intubation: awareness, respiratory difficulty postoperatively | |
| Adverse events | 3 participants who received rocuronium complained of injection pain. | |
| Time & Place | Study dates were not reported. Article accepted September 2012 Dankook University, Cheonan, Korea | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly assigned" |
| Allocation concealment (selection bias) | Low risk | "tracheal intubation were performed...by an experienced anesthesiologist who was blinded to the anesthetic drug" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding mentioned |
| Incomplete outcome data (attrition bias) | Low risk | All cases were reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 15) Sequence with: fentanyl 2 mcg/kg, propofol 2.5 mg/kg | |
| Outcomes | 1. Intubating conditions 60s after muscle relaxant. Intubation conditions were reported by the same blinded individual as excellent, good, poor and inadequate based on jaw relaxation, vocal cord position and movement, and diaphragm movement | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted August 2000. University of Washington, Seattle, USA | |
| Funding and declarations | Funding source: Organon West Orange, New Jersey Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Intubator unaware of drug |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | "there were no attempts made to blind the individual" |
| Incomplete outcome data (attrition bias) | Low risk | Complete data set |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ III | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 102) Sequence with: alfentanil 10 ‐ 20 ug/kg, propofol 2 ‐ 3 mg/kg | |
| Outcomes | 1. Intubating conditions 60s after muscle relaxant by senior anaesthesiologist. Intubations not achieved in 30s were recorded as failed. Reported as excellent, good, poor and first attempt failed. Based on 5 variables: ease of laryngoscopy, position of vocal cords, movement of vocal cords, movement of limbs and coughing during tracheal intubation | |
| Adverse events | 1 participant in Group 2 had atrial fibrillation requiring treatment verapamil and sotalol. Hypotension requiring treatment with ephedrine occurred in 18 Group2 and 17 Group 1. Five participants in Grp 2 and 2 in Group1 reported postoperative muscle pain. | |
| Time & Place | Study dates not reported. Article accepted June 2005. University of Copenhagen, Glostrup, Denmark | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | The participant was allocated by the concealed envelope method |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "anaesthesiologist (a senior member of the study group) blinded to the muscle relaxant and concealed in a room next to the operation theatre until 40 secs after its administration, hereby preventing him from seeing fasciculations after succinylcholine" |
| Incomplete outcome data (attrition bias) | Low risk | All cases accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ III | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 20) | |
| Outcomes | 1. Intubating conditions 60s after muscle relaxant. Reported as score based on clinical variables: laryngoscopy, vocal cord movement and coughing 5. Heart rate, blood pressure and arterial oxygen saturation | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. University of Johannes‐Gutenberg, Mainz, Germany | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | In German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were allocated randomly" |
| Allocation concealment (selection bias) | Low risk | Examiner did not know which drug was injected |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment |
| Incomplete outcome data (attrition bias) | Low risk | All cases accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 30) 5. Vecuronium 0.08 mg/kg (n = 30)* Sequence with: fentanyl 3 mcg/kg, propofol 2.5 mg/kg | |
| Outcomes | 1. Time to complete disappearance of response to orbicularis oculi after TOF stimulation | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted June 1999. Jean Bernard Hospital, Poitiers, France | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random allocation" |
| Allocation concealment (selection bias) | Low risk | "intubation was performed by another physician unaware of muscle relaxant injected" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "onset time of neuromuscular blockade ...was estimated by a blinded physician who was not involved in the intubating procedure. When the orbicularis oculi was completely blocked, intubation was performed by another physician " |
| Incomplete outcome data (attrition bias) | High risk | Participants were excluded from the final analysis in 2 cases: 1) when the vocal cords were not completely visualized during the laryngoscopy and 2) when onset time was longer than 300s. In participants not fully paralysed after 300s after the administration of the muscle relaxant, intubation was performed after giving a supplemental dose of muscle relaxant |
| Selective reporting (reporting bias) | Unclear risk | due to incomplete outcome data, difficult to assess. |
| Methods | RCT | |
| Participants | ASA I III Mallampati 1 or 2 airway and no contraindication to RSI | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 10) Sequence with: fentanyl (?dose), thiopental 2 ‐ 7 mg/kg | |
| Outcomes | 1. Ablation of T1 (onset) | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted June 1993 University of California, San Franscisco, USA. | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly designated" |
| Allocation concealment (selection bias) | Low risk | "intubation of trachea was attempted by a clinician who was blinded to the muscle relaxant administered" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | "Intubating conditions were judged by each clinician, and the presence or absence of fasciculations was noted" |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.9 mg/kg (n = 30) | |
| Outcomes | 1. Intubation conditions 60s after muscle relaxants. Reported as excellent, adequate and poor as per Abbott and Samuel. Variables included jaw relaxation, vocal cord position and cough reflex | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article published 2004. Rohtak, India | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" |
| Allocation concealment (selection bias) | High risk | No comment made |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment made |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Simulated RSI N = 401 | |
| Participants | Emergency ICU Age ≥ 18 yrs Mean age 62 Mean weight 73 kg | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 201) 2. Succinylcholine 1 mg/kg (n = 200) Sequence with: fentanyl 1 mcg/kg, propofol 1 mg/kg or etomidate 0.2 mg/kg | |
| Outcomes | 1. Incidence of desaturation ≥ 5% by pulse oximetry 2. Duration of intubation sequence 3. Incidence of failed first intubation 4. Intubation conditions after fasciculations stopped or 60s from muscle relaxant injection. Reported as excellent, good and poor based on a score from 6 clinical variables (laryngoscopy, vocal cords position, vocal cord movement and intubation response with regard to coughing and limb movement). 5. Haemodynamic consequences | |
| Adverse events | None reported | |
| Time & Place | August 2006 to June 2010 University Hospital of Basel, Basel, Switzerland. | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomization by gender was used to ensure a similar distribution of gender in both groups |
| Allocation concealment (selection bias) | Low risk | Using sealed envelopes, participants were randomly allocated by the study physician |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessor was unblinded |
| Incomplete outcome data (attrition bias) | Low risk | All cases accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ III | |
| Interventions | 1. Rocuronium 1.2 mg/kg (n = 13) | |
| Outcomes | 1. Onset and quality of muscle paralysis with TOF 3. Onset of apnoea | |
| Adverse events | Precipitation of thiopental and rocuronium during induction in one case. | |
| Time & Place | Study dates not reported. Article accepted for publication September 1998 Chicago, USA. | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using a random numbers table |
| Allocation concealment (selection bias) | Low risk | "all investigators except the one designated to dispense the study drug were blinded to choice of muscle relaxant" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "The investigators performed the laryngoscopies but were blinded to the relaxant by standing with their back to the patient during the induction so that they could not detect fasciculations." |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ IV | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 61) | |
| Outcomes | 1. Intubation conditions 60s after muscle relaxant. Reported as excellent, good and poor after Viby‐Mogensen et al. Based on conditions for laryngoscopy, vocal cords and reaction to intubation presented in a table | |
| Adverse events | Erythema occurred in 6 participants who received succinlycholine and 17 who received rocuronium. Bronchospasm occurred once in Group 2. | |
| Time & Place | The Queen's University of Belfast, the Helsinki University Central Hospital UK | |
| Funding and declarations | Funding source: Organon Teknika Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomizations |
| Allocation concealment (selection bias) | Low risk | Intubator unaware of drug given |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "intubation were carried out by an assessor, blinded to the treatment administered, 50s after the end of injection of the neuro‐muscular blocking drug This assessor was not present in the room until about 45s after the neuromuscular blocking drug had been given." |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete data were accounted for and well explained |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Simulated RSI N = 120 | |
| Participants | ASA I ‐ II Adults Mean age 49.8 Mean weight 75 kg | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 30 men) 2. Rocuronium 0.6 mg/kg (n = 30 women) 3. Succinylcholine 1.0 mg/kg (n = 30 men) 4. Succinylcholine 1.0 mg/kg (n = 30 women) Premed: midazolam 7.5 mg Sequence with: thiopental 5 mg/kg, fentanyl 3 mcg/kg | |
| Outcomes | 1. Intubation conditions 60s after muscle relaxant. Reported as excellent, good and poor based on laryngoscopy, vocal cord position and reaction to tube 2. Intubation times | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. University of Rostock, Rostock, Germany | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | In German. Data aggregated for groups 1 & 2 and groups 3 & 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization |
| Allocation concealment (selection bias) | Low risk | Intubation performed by blind operator |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 76) | |
| Outcomes | 1. Intubation conditions 50s after muscle relaxant by experienced anaesthesiologist. Reported as excellent, good and poor based on laryngoscopy, vocal cord movement and position and reaction to tube insertion or cuff inflation 3. Adverse outcomes: Postoperative hoarseness, sore throat, vocal cord injuries | |
| Adverse events | Thoroughly reported as one of the primary outcomes. | |
| Time & Place | Study dates not reported. Article accepted September 2005. University of Rostock, Rostock, Germany | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number draws |
| Allocation concealment (selection bias) | Low risk | "syringes were prepared by an independent investigator" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "To prevent the anesthesiologist who performed the tracheal intubation from noting succinylcholine‐induced muscle fasciculations, he was called to enter the study room after 40s" |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II Mean weight 59.6 kg Mallampati 1 or 2 airways | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 20) Sequence with: morphine 1 mg/kg, propofol 2.0 mg/kg | |
| Outcomes | 1. Intraocular pressure | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Government Medical College and Hospital, Chandigarh, India | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" |
| Allocation concealment (selection bias) | Low risk | "all drugs administered into ...infusion by one anaesthetist who was unaware of drug administered" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "he intubating anaesthetist were not allowed to observe the injection of the neuromuscular blocking drug or the presence of fasciculation by making them stand with their back to the patient for 45 s after injection of the drug" |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Mivacurium 0.15 mg/kg (n = 10)* 6. Rocuronium 0.06 mg/kg then mivacurium 0.135 mg/kg (n = 10) | |
| Outcomes | 1. Onset time after priming of muscle blockade with TOF 2. Intubation conditions with different priming sequences 30s after thiopentone dose. Reported as excellent, good or poor based on jaw relaxation, vocal cord movement and diaphragm movement. 3. Recovery of twitch height to 10% of control | |
| Adverse events | None reported | |
| Time & Place | Study dates not reported. Article accepted April 1994. King Khalid University Hospital, Riyadh, Sadui Arabia | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly assigned to seven groups" |
| Allocation concealment (selection bias) | Low risk | Tracheal intubation was performed after complete neuromuscular block by an experienced anaesthetist who was not involved in the study and was not aware of the muscle relaxant used |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Unclear risk | No comment on blinding |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I | |
| Interventions | 1. Succinylcholine 1 mg/kg (n = 10) Sequence with: fentanyl 2 mcg/kg, propofol 2 mg/kg | |
| Outcomes | 1. Intubation conditions 60s after muscle relaxant. Reported as excellent, good and poor based on jaw relaxation, vocal cord movement and diaphragm movement. | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted May 1997. King Khalid University Hospital, Riyadh, Sadui Arabia | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly" |
| Allocation concealment (selection bias) | Low risk | To maintain blinding, participants who received a single neuromuscular blocking drug had a simultaneous injection of placebo. 60s after the end of injection the trachea was intubated in all participants by the same anaesthetist who was unaware of the participant’s grouping |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding muscle relaxant used |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 20) Sequence with: fentanyl 2 ‐ 3 mcg/kg, thiopental 4 ‐ 5 mg/kg | |
| Outcomes | 1. Onset time of neuromuscular blocker | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted January 1997. The Bowman Gray School of Medicine, Winston‐Salem, USA | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned, via computer‐generated random numbers table" |
| Allocation concealment (selection bias) | Low risk | Used blinded syringes |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Laryngoscopy and intubation began 60s after the injection of the contents of the final blinded syringe by an anaesthetist unaware of the treatment group. This individual was not allowed to look at or touch the participant during the period of time in which fasciculations would occur, nor was he or she allowed to look at the polygraph tracing |
| Incomplete outcome data (attrition bias) | Unclear risk | Did not explain why 2 participants were excluded from rocuronium group |
| Methods | RCT | |
| Participants | Uncertain ASA | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 7) | |
| Outcomes | 1. Intubation conditions after visual loss of orbicularis oculi TOF or after 90s. Reported as excellent, good, fair based on jaw relaxation, vocal cord position and coughing | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. MetroHealth Medical Center, Cleveland, USA | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" |
| Allocation concealment (selection bias) | Low risk | Anaesthesiologist was blinded to relaxant |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment on blinding effects of drugs |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 20) Sequence with: afentanyl 25 mcg/kg, propofol up to 2.5 mg/kg | |
| Outcomes | 1. Intubation conditions 60s after muscle relaxant. Reported as excellent, good, poor and inadequate based on jaw relaxation, vocal cord position and reaction to intubation | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted February 1992. Univeristy of Innsbruck, Innsbruck, Austria | |
| Funding and declarations | Funding source: grant from Organon Teknika Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization table |
| Allocation concealment (selection bias) | Low risk | "Unaware of the muscle relaxant used" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "This person was unaware of the twitch response at the time of laryngoscopy, unaware of the muscle relaxant used" |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Modified RSI N = 90 | |
| Participants | ASA I ‐ II Major elective surgery in OR 20 ‐ 60 years Mean age 38 Mean weight 53 kg | |
| Interventions | 1. Succinylcholine 1.5 mg/kg (n = 30) 2. Rocuronium 0.6 mg/kg (n = 30) 3. Vecuronium 0.08 mg/kg (n = 30)* Sequence with propofol 2 ‐ 2.5 mg/kg | |
| Outcomes | 1. Intubation conditions were assessed as per Cooper et al. Reported as excellent, good, fair and poor from a score of 0 ‐ 9 2. Intubation time | |
| Adverse events | None reported | |
| Time & Place | Study dates not reported. Regional Institute of Medical Sciences, Imphal, India | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using computer generated randomization" |
| Allocation concealment (selection bias) | High risk | No comment made |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | Once the control response had been noted, the neuromuscular blocking agent was injected and the endotracheal intubation was carried out by the same person (unblinded) |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ IV | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 90) | |
| Outcomes | 1. Intubation conditions. Reported as excellent, good and poor based on a score that was evaluated from laryngoscopy, vocal cords and response to intubation | |
| Adverse events | 5 failure to intubate on first attempt. Desaturations in 5 of 90 in Group 2 and 9 of 90 in Group 1. | |
| Time & Place | Study dates not reported. Article accepted April 2005. Krankenhaus Thusis, Switzerland | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly allocated (sealed envelopes)" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | The staff anaesthesiologist was not blinded to the neuromuscular blocking drug used, and the management of difficulties and complications, if any, was left to his discretion |
| Incomplete outcome data (attrition bias) | Low risk | Adequately described |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Modified RSI N = 55 | |
| Participants | Elective surgery 18 ‐ 60 years Mean age 51 Mean weight 78 kg | |
| Interventions | 1. Succinylcholine 1 mg/kg (n = 26) 2. Rocuronium 1 mg/kg (n = 29) Sequence with alfentanil 0.01 mg/kg, propofol 2 mg/kg | |
| Outcomes | 1. Time from correct placement of endotracheal tube to spontaneous ventilation 2. Duration of action of neuromuscular blocking agent measured on TOF‐WatchSx 3. Intubation difficulty scale 4. Intubation conditions 55s after muscle relaxant administration. Reported as excellent, good and fair | |
| Adverse events | Tachycardia above 100 beats per minute | |
| Time & Place | Study dates not reported. Copenhagen University Hospital, Copenhagen, Denmark | |
| Funding and declarations | Funding source: funding supported by Tryg Foundation, Lyngy Denmark Declarations of interest: none declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐ generated list |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | The investigator (in all cases, an anaesthesiology consultant) was blinded by only being allowed to enter the operating theatre after correct placement of the tracheal tube had been verified. The personnel doing the statistical evaluations were blinded to the allocation by being presented the allocation list without the key. After statistical evaluation, an abstract and a conclusion were written in 2 copies, 1 for each allocation possibility |
| Incomplete outcome data (attrition bias) | Low risk | Adequate description of excluded participants |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 25) Sequence with: thiopentone 6 mg/kg | |
| Outcomes | 1. Intubating conditions 45s after administration of muscle relaxant. Reported as excellent, good, fair and poor according to a scoring condition as per Cooper et al. Clinical variables include ease of laryngoscopy, aspect of vocal cords and response of diaphragm | |
| Adverse events | One case of bronchospasm and 2 cases of ventricular ectopic beast in Group 2. One case of desaturation in Group 1. | |
| Time & Place | Study dates not reported. Article accepted September 1995 University of Innsbruck, Innsbruck, Austria | |
| Funding and declarations | Funding source: supported by Organon Teknika NV, Belgium. Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated |
| Allocation concealment (selection bias) | Low risk | "The intubator was blinded to the muscle relaxant administered" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | " Forty seconds after the administration of the muscle relaxant, the intubator was called to enter the study room...The occurrence of muscle fasciculations or body movements was noted by both the intubator and the anaesthetist" |
| Incomplete outcome data (attrition bias) | Low risk | Accounted for excluded participants |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 + thiopentone 5 mg/kg (n = 25) | |
| Outcomes | 1. Intubating conditions 45s after muscle relaxant administration. Reported as per Cooper et al as excellent, good, fair and poor based on scores evaluating jaw relaxation, vocal cords and response to intubation | |
| Adverse events | Nonre reported | |
| Time & Place | Study dates not reported. Article accepted April 1996 University of Innsbruck, Innsbruck, Austria | |
| Funding and declarations | Funding source: supported by Oganon GmbH, Division of Organon Teknika, Vienna, Austria Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly" |
| Allocation concealment (selection bias) | Low risk | "Intubator was blinded to the treatment each patient received" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions. |
| Blinding of outcome assessment (detection bias) | Low risk | "In order to prevent the intubator from noting …. muscle fasciculations…. called to enter the study room 40s after the administration of the blocker" |
| Incomplete outcome data (attrition bias) | Low risk | All cases accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | Modified RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 30) 3. Mivacurium 0.15mg/kg and rocuronium 0.6mg/kg (n = 30)* Premedication: midazolam 0.02 ‐ 0.05 mg/kg iv Sequence with: fentanyl 3 mcg/kg, thiopental up to 7 mg/kg | |
| Outcomes | 1. Onset time of neuromuscular blocker | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted November 1995. University of Texas Health Science Center, Texas, USA | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes |
| Allocation concealment (selection bias) | Low risk | "previously prepared envelopes containing cards assigning patients to one of the three groups". |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | "Presence or absence of fasciculations was noted. Laryngoscopy was begun with a Miller 2 blade 45 seconds later, and intubation was completed within 15 seconds. The same experienced anesthesiologist, who was unaware of the status of T₁, performed and graded all the intubations in the investigation" |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | Uncertain ASA | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 30) Sequence with: propofol 3 ‐ 4 mg/kg | |
| Outcomes | 1. Intubation conditions 1 min after muscle relaxant. Reported as excellent, good, fair or poor based on scores evaluating jaw relaxation, vocal cords and response to intubation | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Bristol Hospital for Sick Children, Bristol, UK | |
| Funding and declarations | Funding source: rocuronium and TOF guard device was provided by Organon Teknika Ltd, Cambridge UK. Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | Blinded to identity of relaxant but not fasciculations |
| Incomplete outcome data (attrition bias) | Low risk | All cases reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | Uncertain ASA | |
| Interventions | 1. Succinylcholine 1 mg/kg + rocuronium boluses (n = 23) 2. Succinylcholine 1mg/kg + mivacurium boluses(n = 25) Premedication: midazolam 2 mg iv | |
| Outcomes | 1. Intubating conditions 90s after dose of muscle relaxant. Reported using a 3‐point scale: excellent, good and poor based on jaw relaxation and movement of vocal cords | |
| Adverse events | 1 in Group 1 and 6 in Group 4 had upper body erythema not requiring treatment. 16% in Group 1+ 2 developed postoperative myalgias. | |
| Time & Place | Study dates not reported. Article accepted January 1996. University of Texas Southwestern Medical Center, Dallas, USA | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | High risk | No comment made |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "laryngoscopy was performed by an anesthesiologist who was unaware of the twitch response" |
| Incomplete outcome data (attrition bias) | Low risk | Complete data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Modified RSI N = 100 | |
| Participants | ASA I ‐ II Elective OR 20 ‐ 60 yrs Mean age 37 Mean weight 51 kg | |
| Interventions | 1. Rocuronium 0.9 mg/kg (n = 50) 2. Succinylcholine 1.5 mg/kg (n = 50) Premedication: glycopyrrolate 0.004 mg/kg iv, ranitidine 50 mg iv, tramadol 1mg/kg iv, midazolam 0.015 mg/kg iv Sequence with thiopental | |
| Outcomes | 1. Onset time of neuromuscular blockade 2. Intubation conditions reported as excellent, good, fair and poor. Scores were based on jaw relaxation, vocal cord motion and response to intubation 3. Haemodynamics 4. Complications at time of intubation | |
| Adverse events | Both groups demonstrated an increase in blood pressure, heart rate, arrhythmias and laryngospasm. No significant difference between the two groups. | |
| Time & Place | Study dates not reported. Government Medical College, Bhavnagar, Gujarat, India | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "sealed envelope" |
| Allocation concealment (selection bias) | Low risk | "opening sealed envelope" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding to fasciculations |
| Incomplete outcome data (attrition bias) | Low risk | Complete data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ III | |
| Interventions | 1. Rocuronium 0.6 mg/kg prior to induction agent (n = 20) Sequence with: fentanyl 2 mcg/kg, thiopental 6 mg/kg | |
| Outcomes | 1. Intubating conditions 30s after 3rd dose of muscle relaxant. Reported as scores according to scoring system of Damaoal et al and modified by Krieg et al. Factors evaluated include laryngoscopy, severity of coughing and movement of vocal cords | |
| Adverse events | 1 case of severe coughing in Group 1 and 5 in Group 2. | |
| Time & Place | Study dates not reported. University Hospital Bergmannsheil, Bochum, Germany | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective randomized double blind" |
| Allocation concealment (selection bias) | Low risk | "The investigator preparing the syringes was not involved with the induction" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of fasciculations |
| Incomplete outcome data (attrition bias) | Low risk | Complete data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | Uncertain ASA | |
| Interventions | 1. Rocuronium 1.2 mg/kg (n = 20) | |
| Outcomes | 1. Intubation conditions 45s after muscle relaxant reported as excellent, good, poor and inadequate. Evaluated based on Magorian et all and Dubois et all based on jaw relaxation, vocal cords and diaphragm movement | |
| Adverse events | None reported | |
| Time & Place | Study dates not reported. Turkey | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | ITT analysis In Turkish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided into two groups randomly" |
| Allocation concealment (selection bias) | High risk | No comment |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Unclear risk | No comment |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT Modified RSI Adult elective surgery N = 60 | |
| Participants | ASA I ‐ II Age 19 ‐ 73 years (Baseline demographics table unavailable) | |
| Interventions | 1. Mivacurium 0.25 mg/kg (n = 20)* 2. Rocuronium 0.6 mg/kg (n = 20) 3. Succinylcholine 1 mg/kg (n = 20) Premedication: Midazolam 10 mg im Sequence with: fentanyl 2 mg/kg, propofol 2 mg/kg | |
| Outcomes | 1. Intubation conditions after full relaxation as measured by TOF monitoring. Reported as excellent, good and bad 2. Haemodaynamics | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Istanbul Hospital, Istanbul, Turkey | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | In Turksih Only data for excellent intubation conditions were available (paper missing tables) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized'" |
| Allocation concealment (selection bias) | Low risk | "intubation was performed by an anesthesiologist who do not know muscle relaxant used" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No comment made |
| Incomplete outcome data (attrition bias) | Unclear risk | Tables unavailable |
| Methods | RCT | |
| Participants | ASA I ‐ III | |
| Interventions | 1. Rocuronium 0.6 mg/kg (n = 15) | |
| Outcomes | 1. Intraocular pressure | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Article accepted December 1998 Eye Foundation Hospital, Birmingham, USA | |
| Funding and declarations | Funding source: supported by a grant from Organon, Inc. West Orange NJ. Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" |
| Allocation concealment (selection bias) | High risk | "Open‐label" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | High risk | No attempt at blinding made |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II | |
| Interventions | 1. Rocuronium 0.7 mg/kg (n = 15) | |
| Outcomes | 1. Intubating conditions 60s after muscle relaxation. Reported as excellent, good, poor or impossible based on ease of laryngoscopy, vocal cords and response to intubation | |
| Adverse events | None reported. | |
| Time & Place | Study dates not reported. Accepted March 1997. Robert Wood Johnson Medical School at Camden, Camden, USA | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomly assigned, via computer‐generated random numbers table" |
| Allocation concealment (selection bias) | Low risk | "Both the patient and the anesthesiologist intubating the patient were blinded to the muscle relaxant used" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | "anesthesiologist performing the intubation was out of the OR during the induction to avoid witnessing the fasciculations from succinylcholine." |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | RCT | |
| Participants | ASA I ‐ II Excluded expected difficult intubations | |
| Interventions | 1. Succinylcholine 1 mg/kg intubated 60s(n = 25) 2. Rocuronium 0.6 mg/kg intubated 60s (n = 25) 3. Rocuronium 0.6 mg/kg intubated 60s with lidocaine 1.5mg/kg (n = 25)* 4. Rocuronium 0.6 mg/kg intubated 90s (n = 25)* 5. Rocuronium 0.6 mg/kg intubated 90s with lidocaine 1.5mg/kg (n = 25)* Premedication: atropine 0.5 mg/kg, pethidine 50 mg im Sequence with: alfentanyl 10 mcg/kg, propofol 2 mg/kg | |
| Outcomes | 1. Intubating conditions 60 or 90s after end of muscle relaxant injection. Reported as excellent, good, poor and inadequate as per Goldberg et al, based on vocal cords and coughing | |
| Adverse events | None reported | |
| Time & Place | Study dates not reported. Article accepted November 2002 University of Ankara, Ankara, Turkey | |
| Funding and declarations | Funding source: none declared Declarations of interest: none declared | |
| Notes | Efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated into five groups using a computer‐generated table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "patients and the intubating anesthetist were blinded to the study solutions administered" |
| Blinding of participants and personnel (performance bias) | Low risk | Participant asleep and personnel performance does not affect intubating conditions |
| Blinding of outcome assessment (detection bias) | Low risk | 60 or 90secs after the end of the muscle relaxant injection, the intubating anaesthesist was called to enter the study room and the intubating anaesthesist was instructed by an assistant to start laryngoscopy |
| Incomplete outcome data (attrition bias) | Low risk | 125 participants were enrolled and all completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
* Not used in analysis
ASA status: American Society of Anesthesia score I ‐ IV, determined by health (decreased health as score increases)
BMI: Body mass index, kg/m²
EMG: electromyogram
i.m: intramuscular
IOP: Intraocular pressure
ITT: Intention‐to‐treat
iv: intravenous
N: number
OR: operating room
po: per os
R: rocuronium
RAE: name of endotracheal tube
RCT: randomized controlled trial
RSI: rapid sequence induction
S: succinylcholine
s: seconds
SBP: systolic blood pressure
T₁: first twitch of train of four
TOF: train‐of‐four
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Only looked at rocuronium with propofol versus rocuronium with thiopental without comparing to succinylcholine | |
| No comparison with succinylcholine | |
| No outcome of intubation conditions | |
| The control group used not only succinylcholine but also gallamine in the sequence which cannot be controlled for when combining studies | |
| Abstract data only. Unclear what intubation scores were based on results given | |
| No comparison of single intubating dose of rocuronium versus succinylcholine. Study looks at priming dose of non‐depolarizing muscle relaxants with succinylcholine only | |
| Used double lumen tubes | |
| No comparison with succinylcholine | |
| RCT but intubation condition data is presented in graphic form only and cannot be reliably extracted | |
| No outcome of intubation conditions | |
| Conference abstract only, no data could be abstracted | |
| Does not document intubation scores in paper | |
| Abstract only. Unable to obtain document from North American source. Will reconsider if able to obtain in future | |
| Did not record intubating conditions, measures other parameters only |
RCT = randomized controlled trial
RSI: rapid sequence intubation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| Analysis 1.1  Comparison 1 Rocuronium any dose versus succinylcholine, Outcome 1 Excellent versus other intubation conditions. | ||||
| 1.1 Simulated RSI | 23 | 2535 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.89] |
| 1.2 Modified RSI | 25 | 1468 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 0.99] |
| 1.3 Mixed simulated and modified RSI | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.08] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| Analysis 1.2 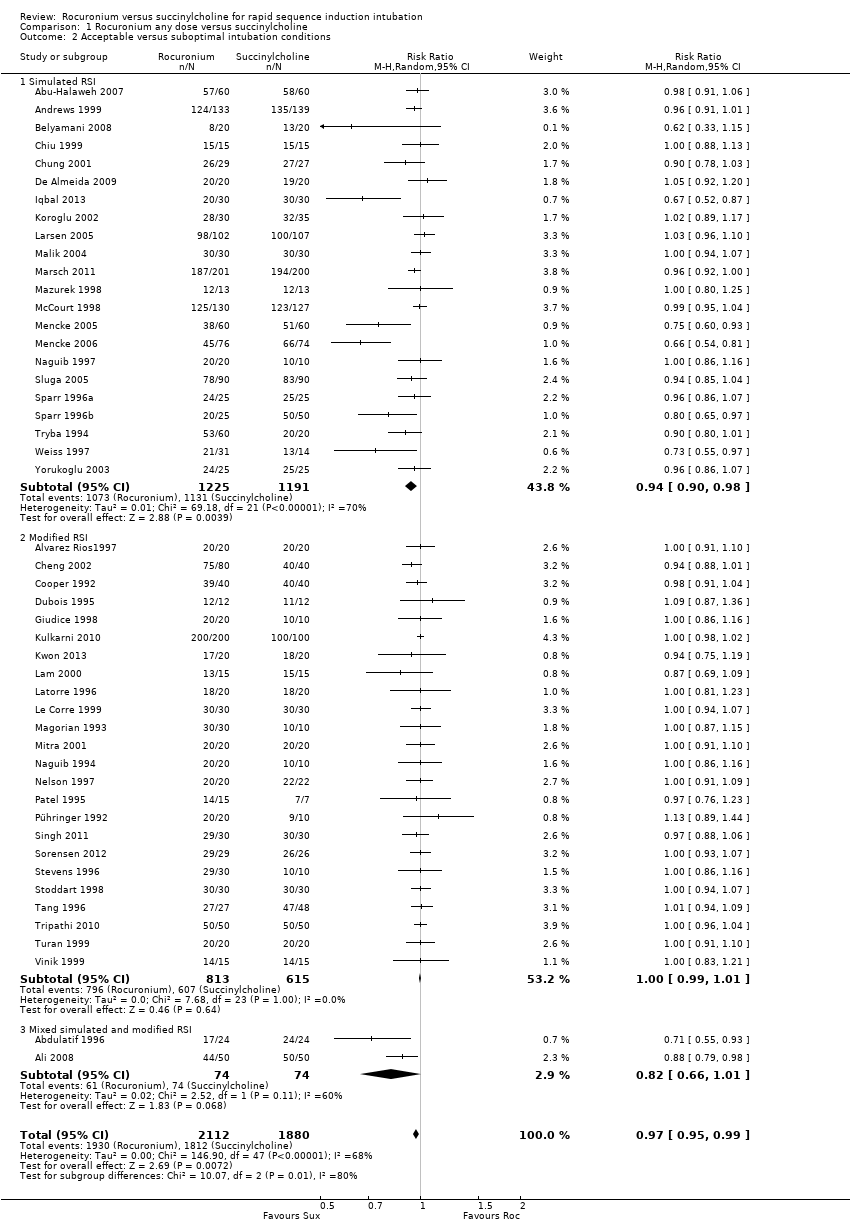 Comparison 1 Rocuronium any dose versus succinylcholine, Outcome 2 Acceptable versus suboptimal intubation conditions. | ||||
| 2.1 Simulated RSI | 22 | 2416 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.90, 0.98] |
| 2.2 Modified RSI | 24 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
| 2.3 Mixed simulated and modified RSI | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.66, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4352 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| Analysis 2.1 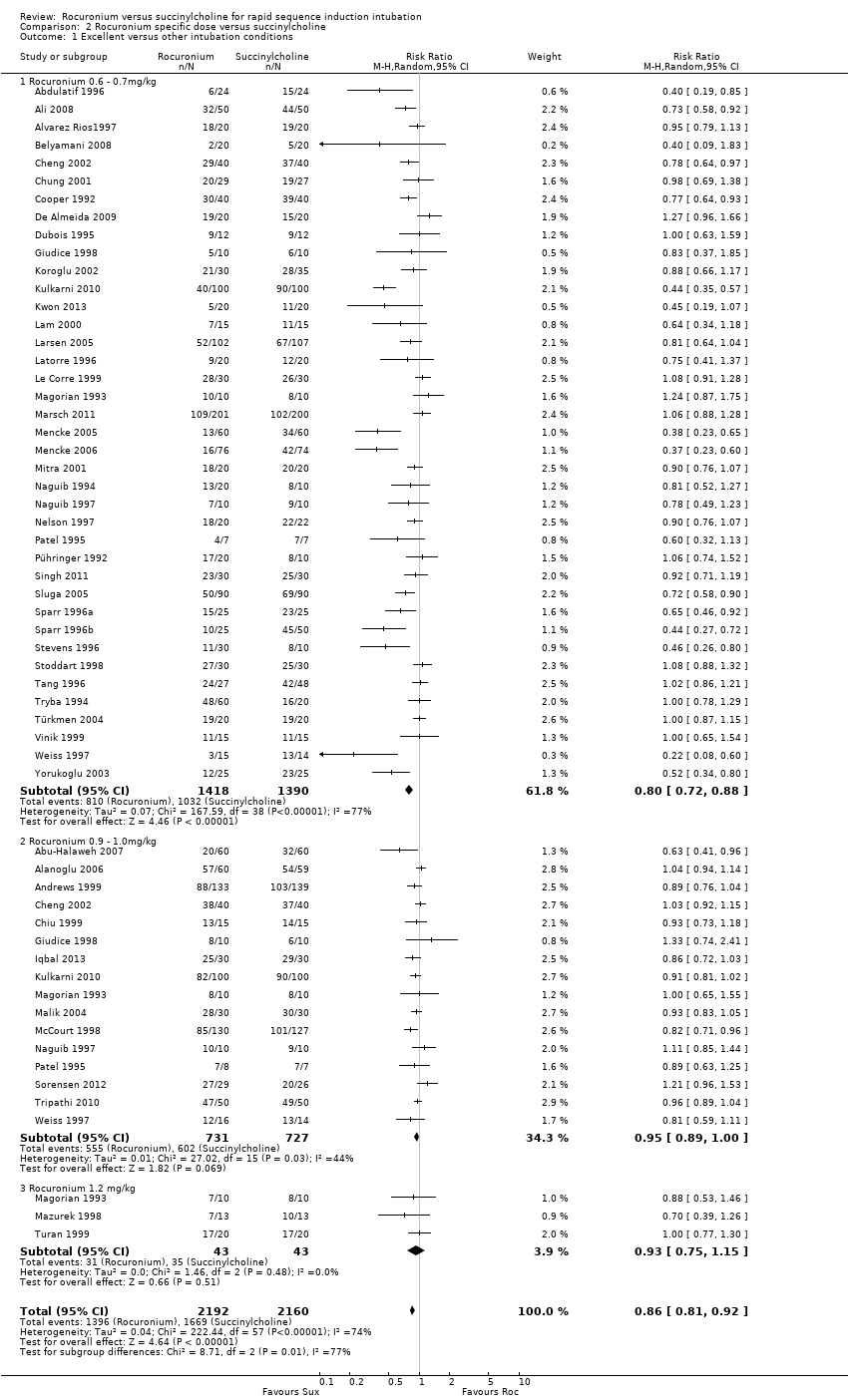 Comparison 2 Rocuronium specific dose versus succinylcholine, Outcome 1 Excellent versus other intubation conditions. | ||||
| 1.1 Rocuronium 0.6 ‐ 0.7mg/kg | 39 | 2808 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.88] |
| 1.2 Rocuronium 0.9 ‐ 1.0mg/kg | 16 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.00] |
| 1.3 Rocuronium 1.2 mg/kg | 3 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 4193 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 0.99] |
| Analysis 2.2 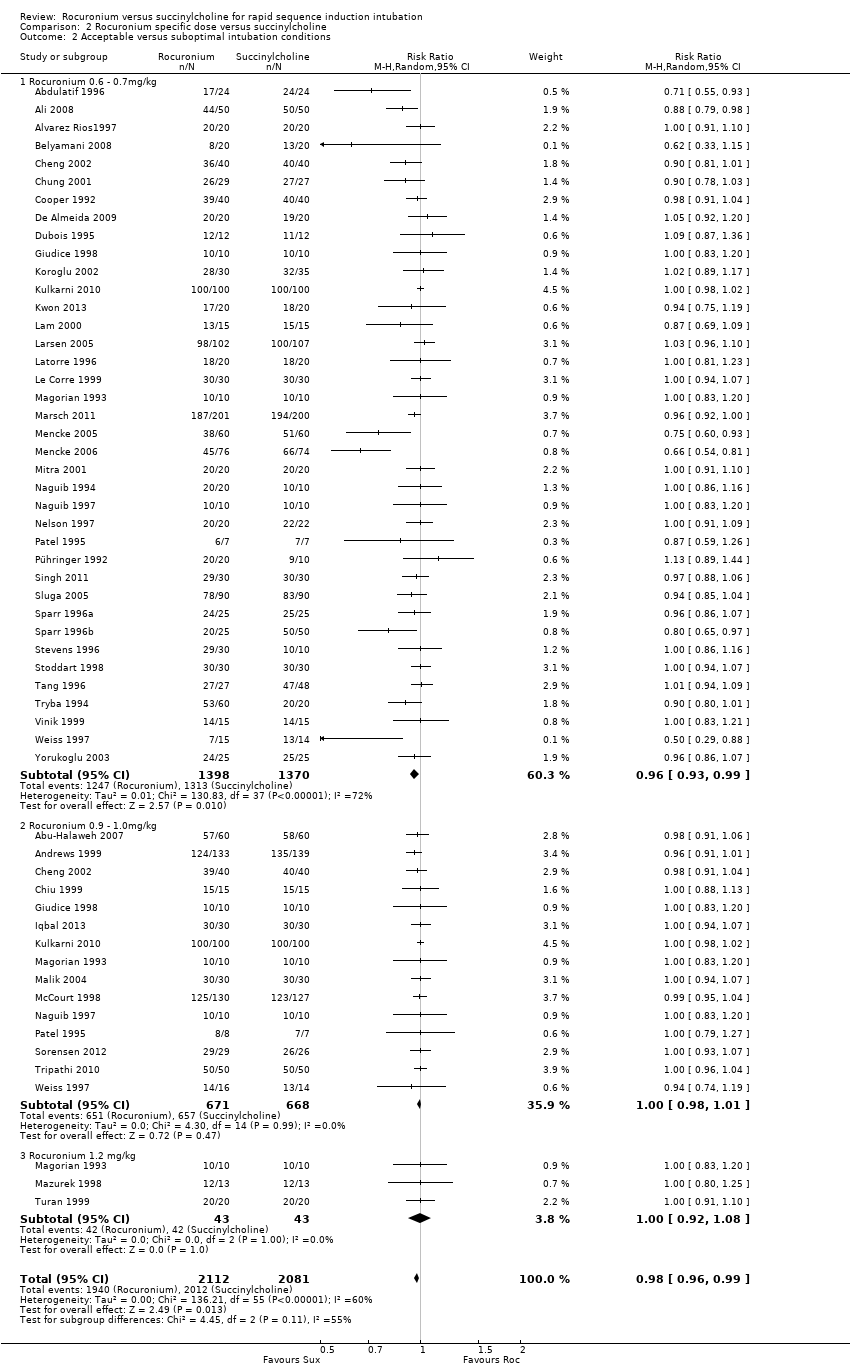 Comparison 2 Rocuronium specific dose versus succinylcholine, Outcome 2 Acceptable versus suboptimal intubation conditions. | ||||
| 2.1 Rocuronium 0.6 ‐ 0.7mg/kg | 38 | 2768 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.99] |
| 2.2 Rocuronium 0.9 ‐ 1.0mg/kg | 15 | 1339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.98, 1.01] |
| 2.3 Rocuronium 1.2 mg/kg | 3 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 49 | 3750 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.91] |
| Analysis 3.1 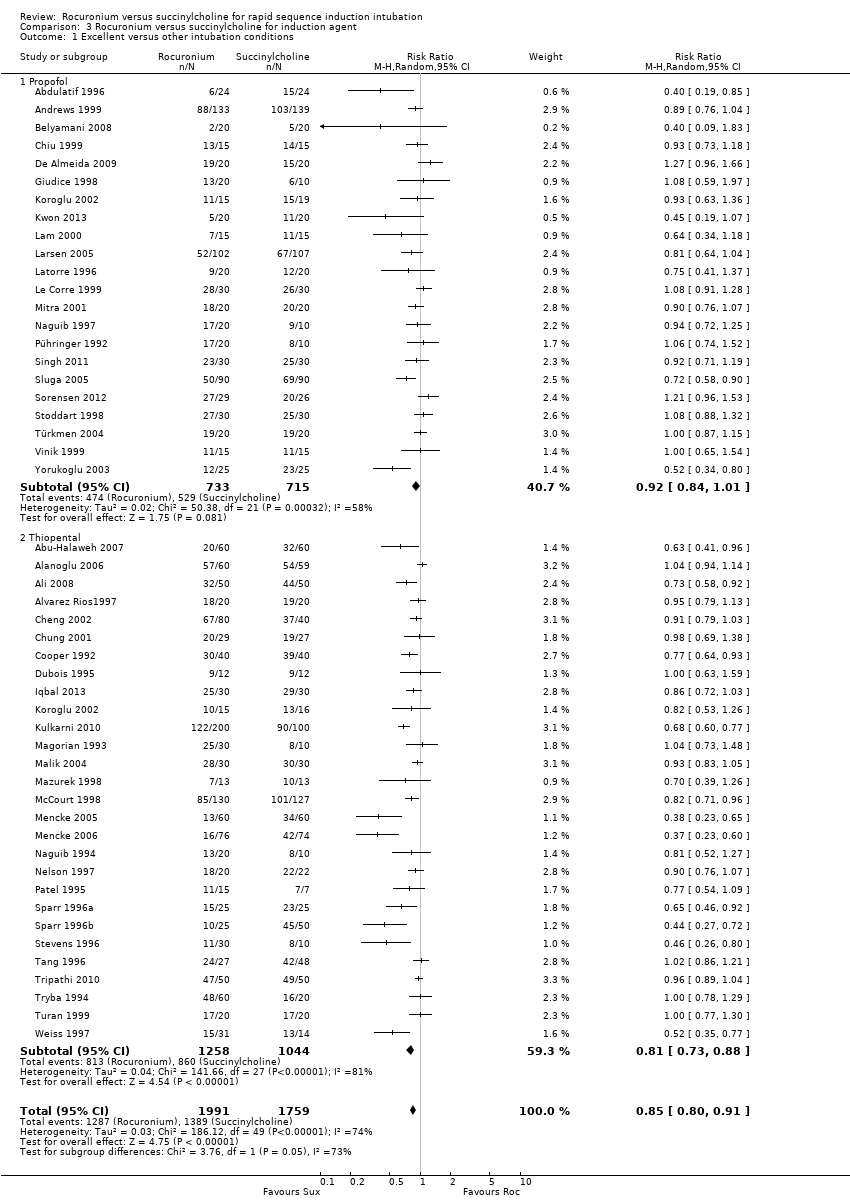 Comparison 3 Rocuronium versus succinylcholine for induction agent, Outcome 1 Excellent versus other intubation conditions. | ||||
| 1.1 Propofol | 22 | 1448 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 1.2 Thiopental | 28 | 2302 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.73, 0.88] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 47 | 3591 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 1.00] |
| Analysis 3.2 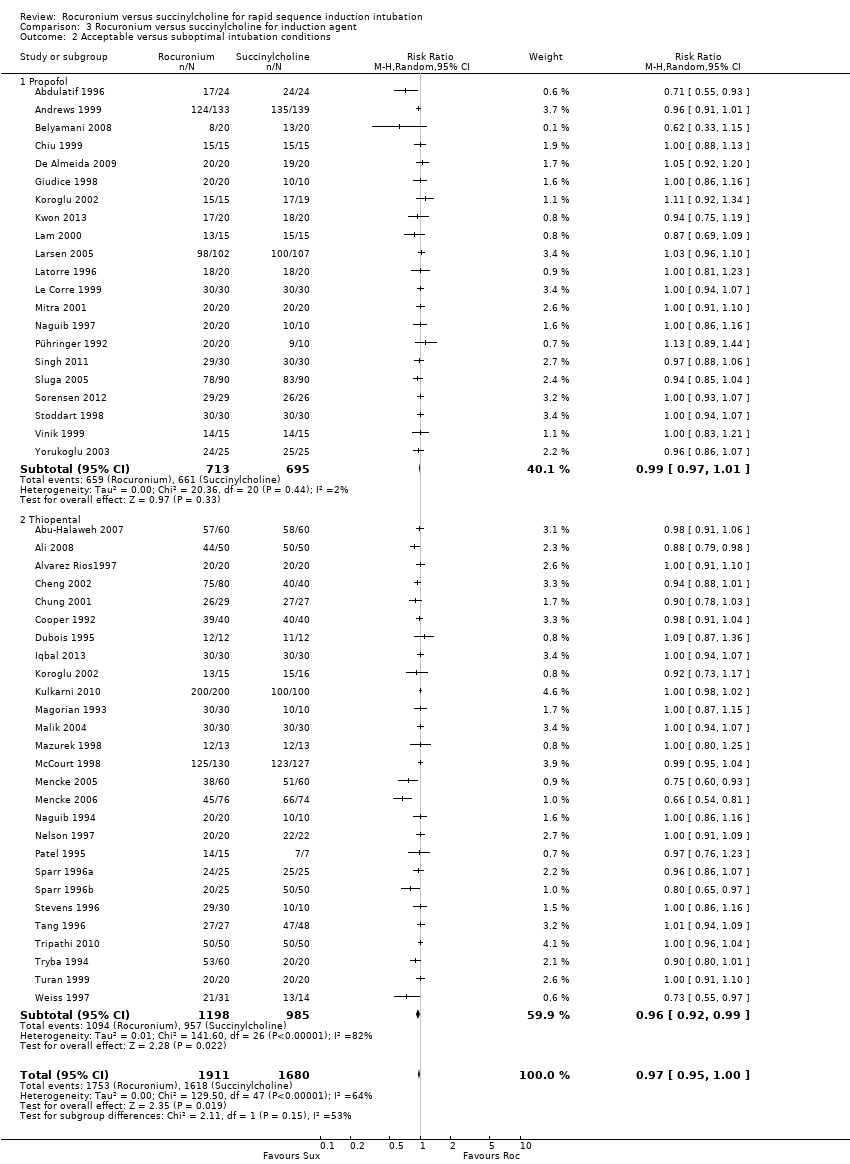 Comparison 3 Rocuronium versus succinylcholine for induction agent, Outcome 2 Acceptable versus suboptimal intubation conditions. | ||||
| 2.1 Propofol | 21 | 1408 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.01] |
| 2.2 Thiopental | 27 | 2183 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.92, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation outcomes Show forest plot | 34 | 2292 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.93] |
| Analysis 4.1  Comparison 4 Rocuronium versus succinylcholine with narcotic, Outcome 1 Excellent versus other intubation outcomes. | ||||
| 1.1 Propofol Induction | 17 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.2 Thiopental Induction | 17 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 32 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| Analysis 4.2  Comparison 4 Rocuronium versus succinylcholine with narcotic, Outcome 2 Acceptable versus suboptimal intubation conditions. | ||||
| 2.1 Propofol Induction | 16 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.02] |
| 2.2 Thiopental Induction | 16 | 1241 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 15 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| Analysis 5.1  Comparison 5 Rocuronium versus succinylcholine without narcotic, Outcome 1 Excellent versus other intubation conditions. | ||||
| 1.1 Propofol Induction | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 1.2 Thiopental Induction | 12 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.94] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 14 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| Analysis 5.2  Comparison 5 Rocuronium versus succinylcholine without narcotic, Outcome 2 Acceptable versus suboptimal intubation conditions. | ||||
| 2.1 Propofol Induction | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| 2.2 Thiopental Induction | 11 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.91] |
| Analysis 6.1 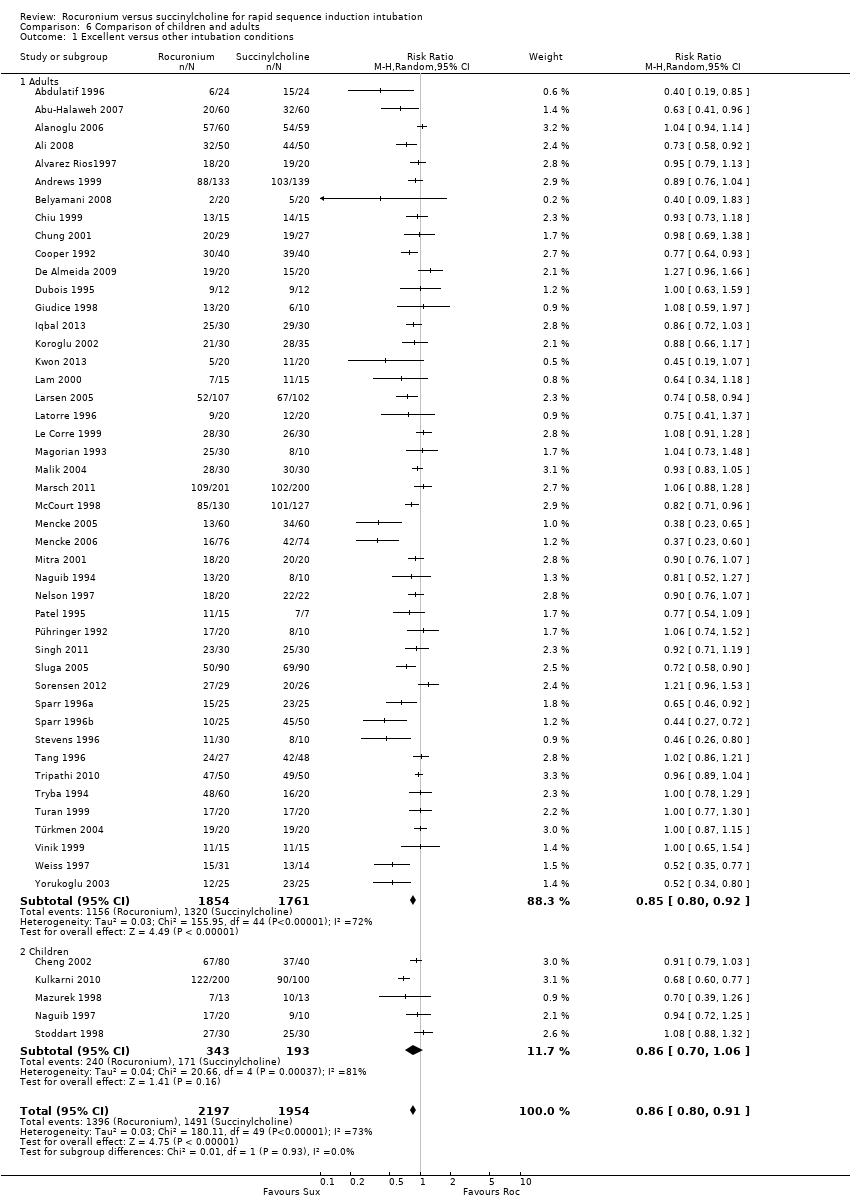 Comparison 6 Comparison of children and adults, Outcome 1 Excellent versus other intubation conditions. | ||||
| 1.1 Adults | 45 | 3615 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.92] |
| 1.2 Children | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| Analysis 6.2  Comparison 6 Comparison of children and adults, Outcome 2 Acceptable versus suboptimal intubation conditions. | ||||
| 2.1 Adults | 43 | 3456 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.2 Children | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 5 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.98] |
| Analysis 7.1 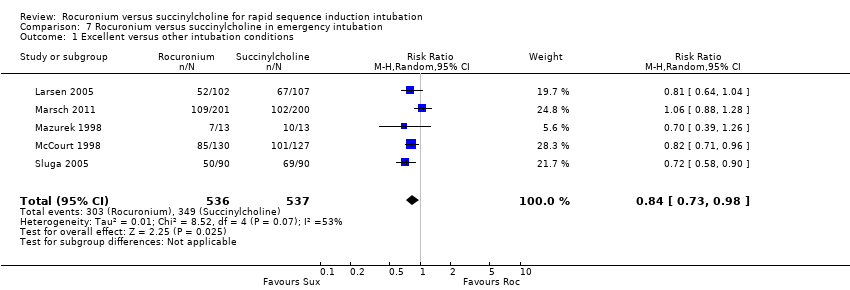 Comparison 7 Rocuronium versus succinylcholine in emergency intubation, Outcome 1 Excellent versus other intubation conditions. | ||||
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 5 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 1.01] |
| Analysis 7.2  Comparison 7 Rocuronium versus succinylcholine in emergency intubation, Outcome 2 Acceptable versus suboptimal intubation conditions. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| Analysis 8.1  Comparison 8 Rocuronium versus succinylcholine by blinding of outcome assessment, Outcome 1 Excellent versus other intubation conditions. | ||||
| 1.1 Low Risk | 21 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
| 1.2 Unclear Risk | 4 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.18] |
| 1.3 High Risk | 25 | 2042 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.96] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| Analysis 8.2  Comparison 8 Rocuronium versus succinylcholine by blinding of outcome assessment, Outcome 2 Acceptable versus suboptimal intubation conditions. | ||||
| 2.1 Low Risk | 23 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 2.2 Unclear Risk | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.07] |
| 2.3 High Risk | 22 | 1912 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |

Search flow diagram for this update from July 2007 to February 2015
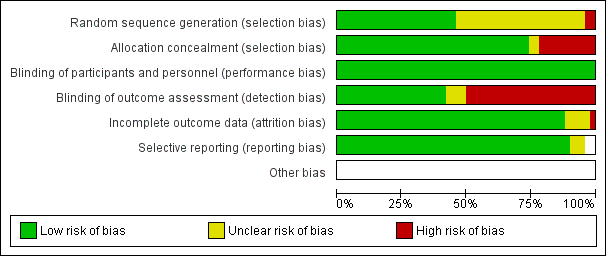
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
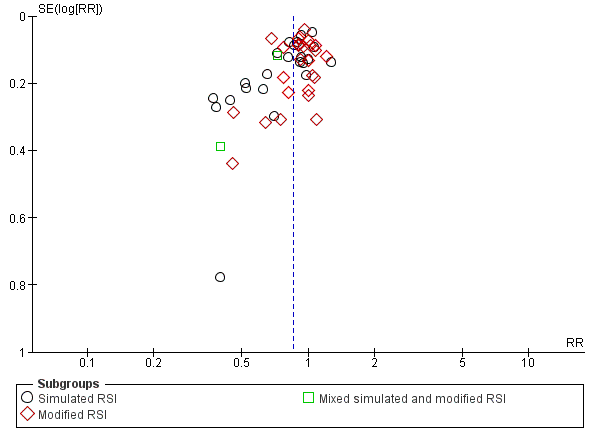
Funnel plot of comparison: Rocuronium any dose versus succinylcholine, outcome: Excellent versus other intubation conditions.
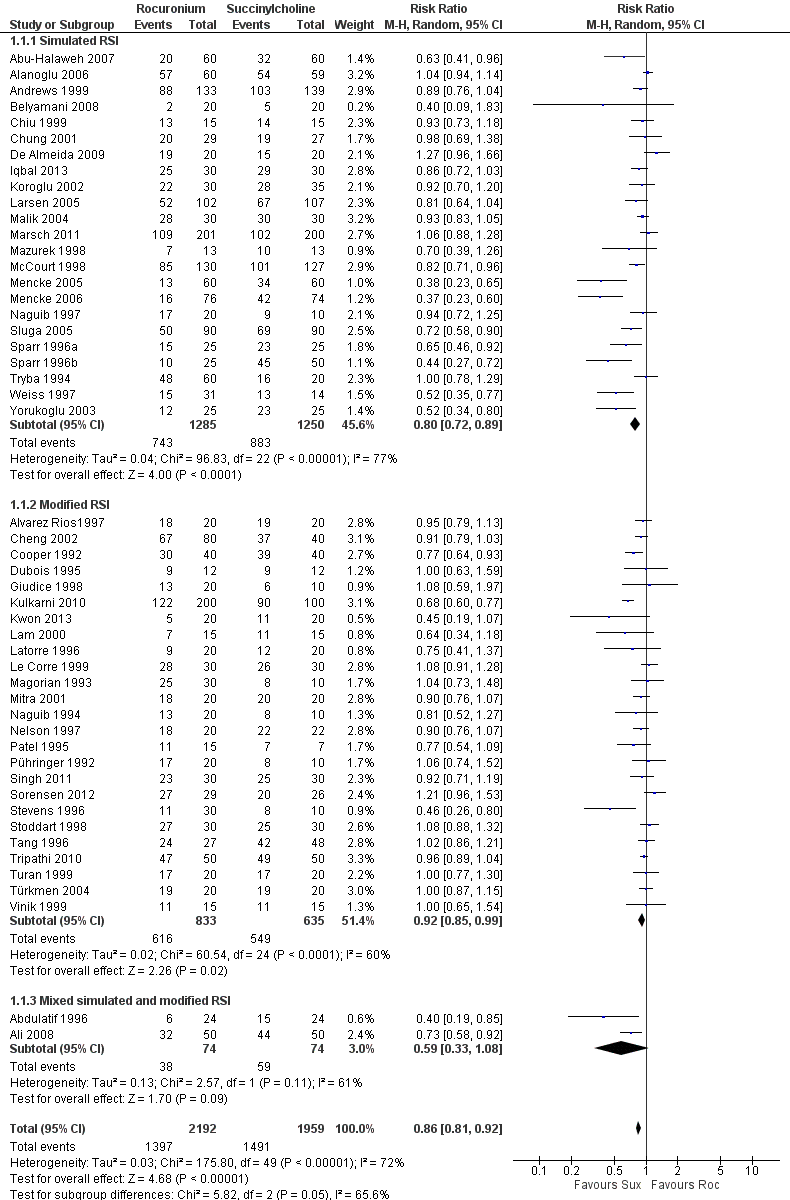
Forest plot of comparison: 1 Rocuronium any dose versus succinylcholine, outcome: 1.1 Excellent versus other intubation conditions

Forest plot of comparison: 3 Rocuronium versus succinylcholine for induction agent, outcome: 3.1 Excellent versus other intubation conditions
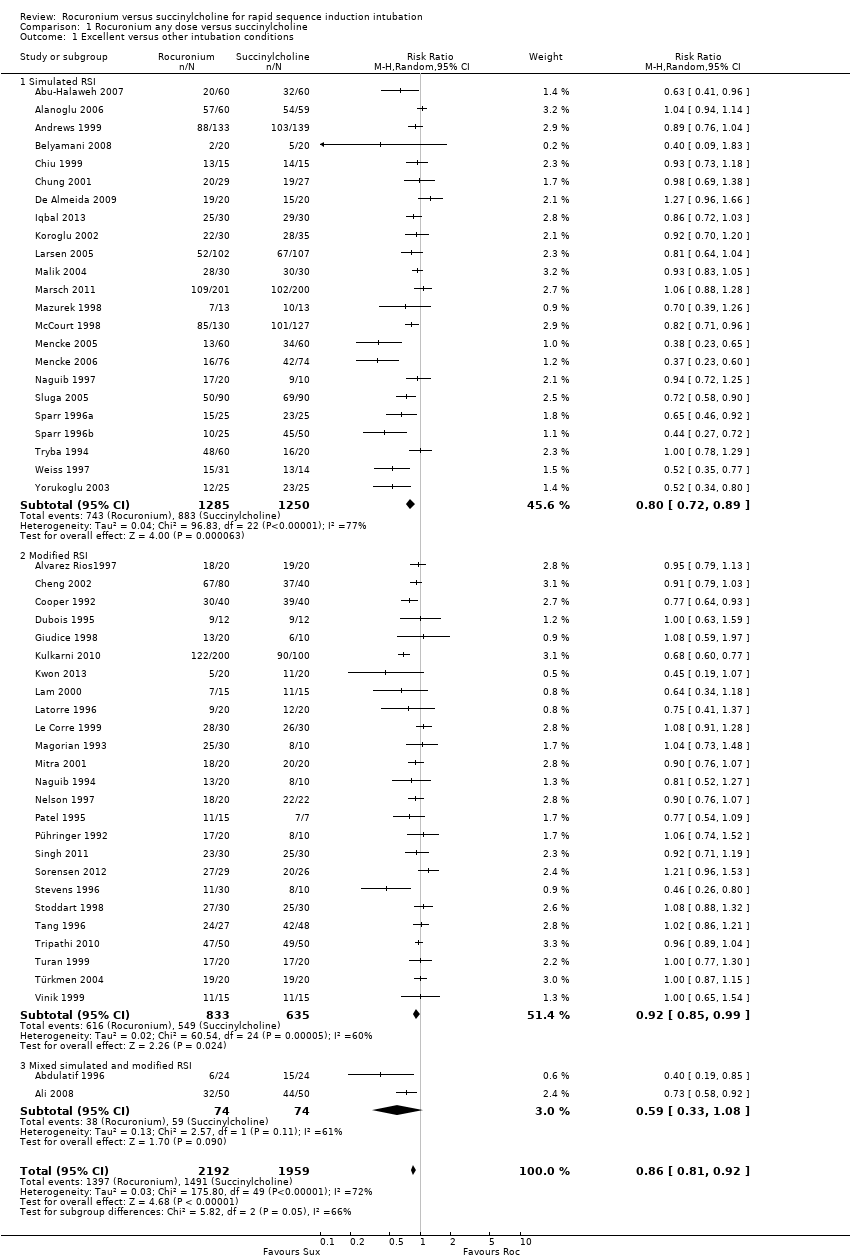
Comparison 1 Rocuronium any dose versus succinylcholine, Outcome 1 Excellent versus other intubation conditions.
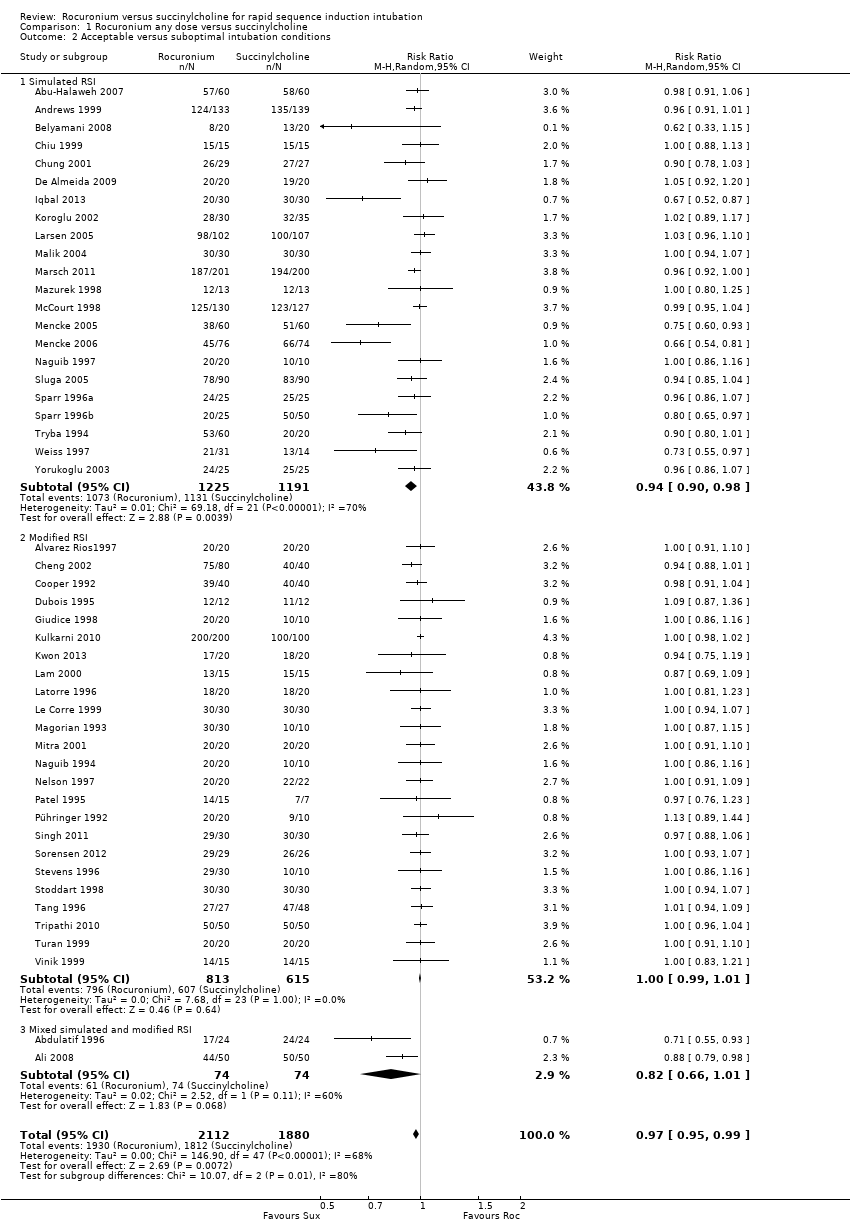
Comparison 1 Rocuronium any dose versus succinylcholine, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 2 Rocuronium specific dose versus succinylcholine, Outcome 1 Excellent versus other intubation conditions.
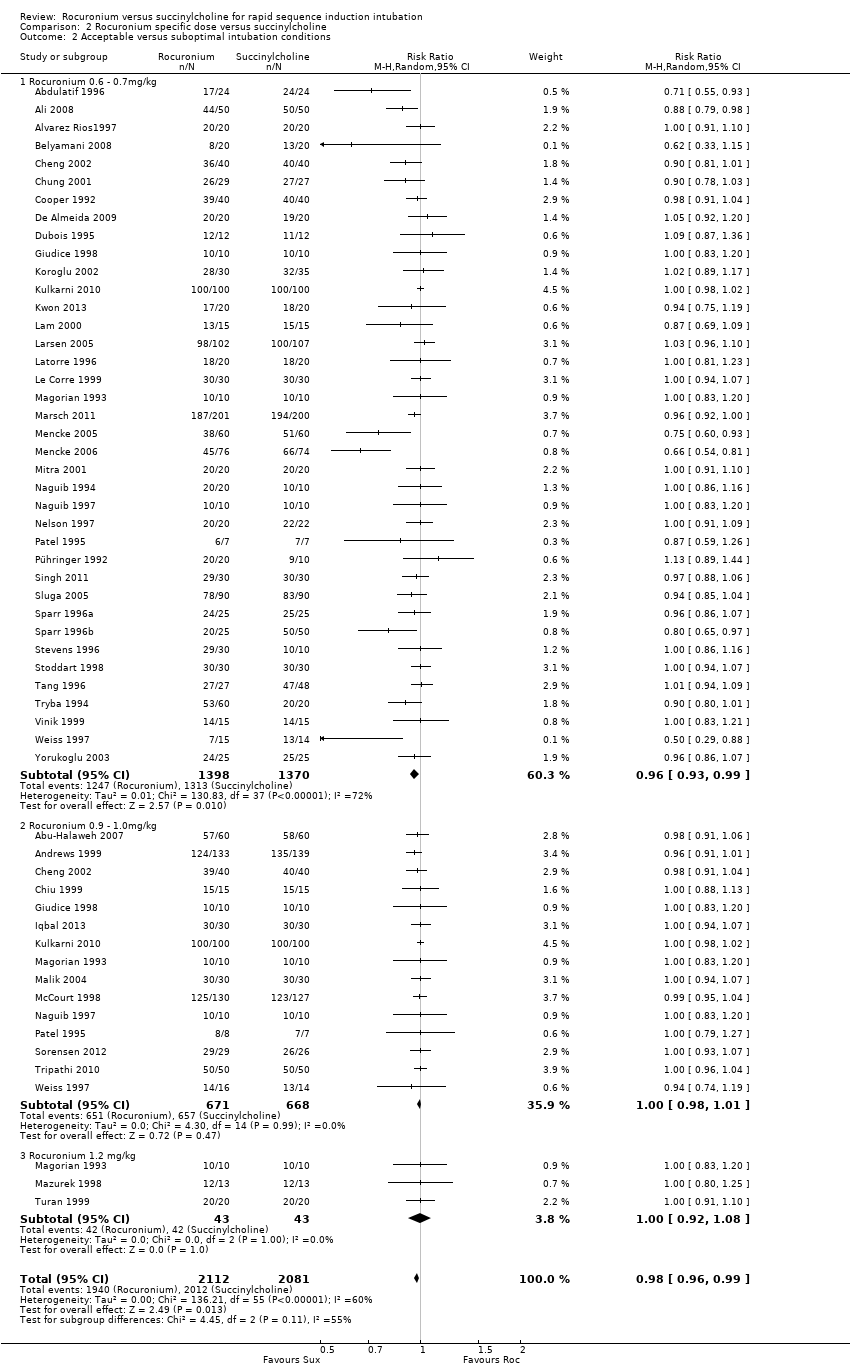
Comparison 2 Rocuronium specific dose versus succinylcholine, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 3 Rocuronium versus succinylcholine for induction agent, Outcome 1 Excellent versus other intubation conditions.
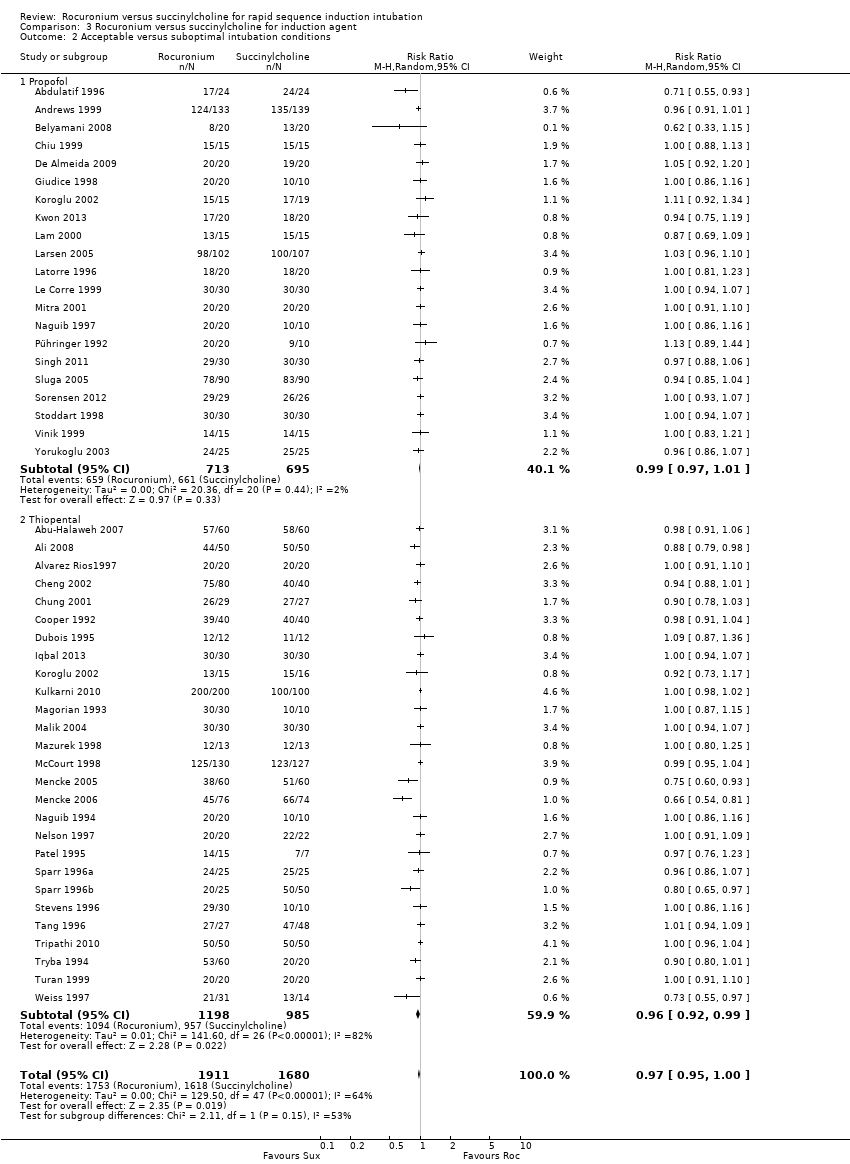
Comparison 3 Rocuronium versus succinylcholine for induction agent, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 4 Rocuronium versus succinylcholine with narcotic, Outcome 1 Excellent versus other intubation outcomes.
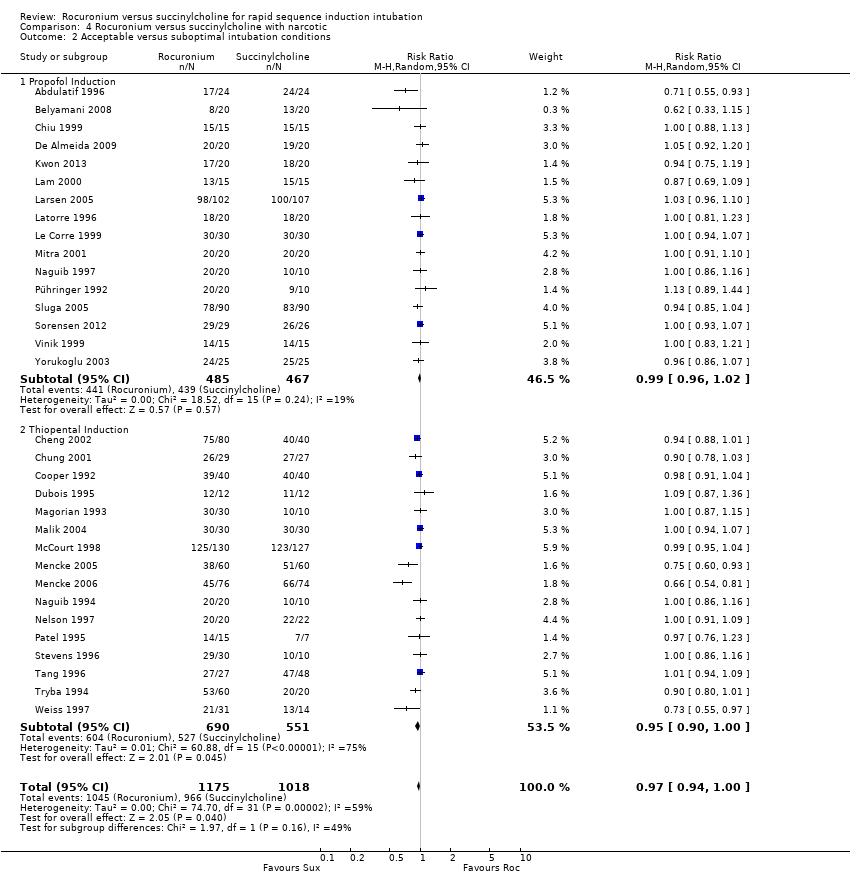
Comparison 4 Rocuronium versus succinylcholine with narcotic, Outcome 2 Acceptable versus suboptimal intubation conditions.
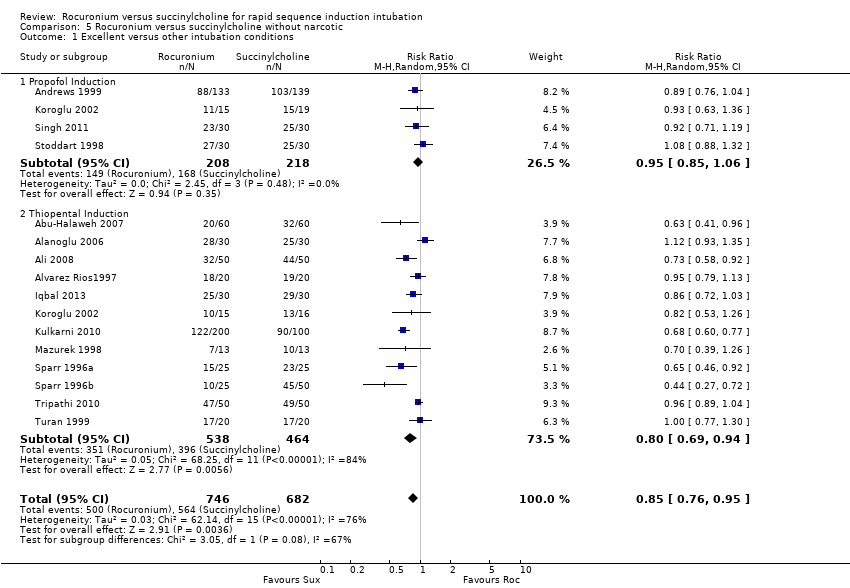
Comparison 5 Rocuronium versus succinylcholine without narcotic, Outcome 1 Excellent versus other intubation conditions.
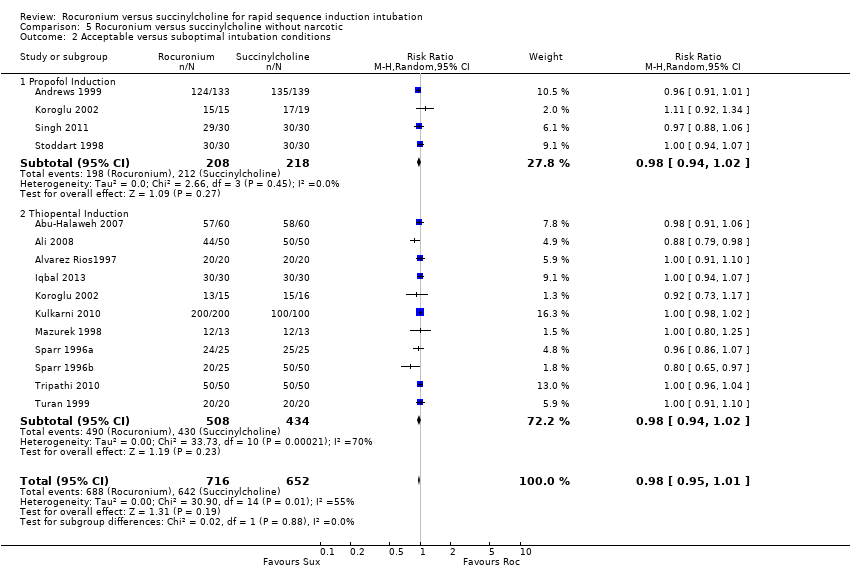
Comparison 5 Rocuronium versus succinylcholine without narcotic, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 6 Comparison of children and adults, Outcome 1 Excellent versus other intubation conditions.

Comparison 6 Comparison of children and adults, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 7 Rocuronium versus succinylcholine in emergency intubation, Outcome 1 Excellent versus other intubation conditions.
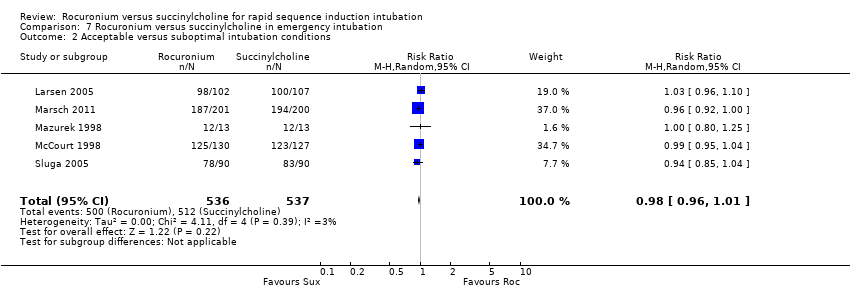
Comparison 7 Rocuronium versus succinylcholine in emergency intubation, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 8 Rocuronium versus succinylcholine by blinding of outcome assessment, Outcome 1 Excellent versus other intubation conditions.

Comparison 8 Rocuronium versus succinylcholine by blinding of outcome assessment, Outcome 2 Acceptable versus suboptimal intubation conditions.
| Rocuronium any dose versus succinylcholine for rapid sequence induction intubation | ||||||
| Patient or population: People requiring rapid sequence induction intubation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Succinylcholine | Rocuronium any dose 2 | |||||
| Excellent versus other intubation conditions | Study population | RR 0.86 | 4151 | ⊕⊕⊕⊝ | Risk of bias: 50% of the studies were at high risk for detection bias because the outcome assessor was not blinded to the fasciculations caused by succinylcholine. Inconsistency: High statistical heterogeneity in the studies could not be explained by subgroup analyses. However we did not downgrade because exclusion of trials contributing to heterogeneity did not significantly change the direction or size of effect. | |
| 76 per 100 | 65 per 100 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk is the average number of excellent intubations with succinylcholine. 2Rocuronium minimum dose 0.6 mg/kg. Succinylcholine minimal dose is 1mg/kg. | ||||||
| Score | Ease of laryngoscopy | Vocal cords | Intubation response |
| 1. Excellent | Good | Open | None |
| 2. Good | Fair | Open | Diaphragmatic movement |
| 3. Poor | Difficult | Movement | Moderate coughing |
| 4. Impossible | Poor | Closed | Severe coughing or bucking |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Simulated RSI | 23 | 2535 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.89] |
| 1.2 Modified RSI | 25 | 1468 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 0.99] |
| 1.3 Mixed simulated and modified RSI | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.08] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Simulated RSI | 22 | 2416 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.90, 0.98] |
| 2.2 Modified RSI | 24 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
| 2.3 Mixed simulated and modified RSI | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.66, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4352 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Rocuronium 0.6 ‐ 0.7mg/kg | 39 | 2808 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.88] |
| 1.2 Rocuronium 0.9 ‐ 1.0mg/kg | 16 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.00] |
| 1.3 Rocuronium 1.2 mg/kg | 3 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 4193 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 0.99] |
| 2.1 Rocuronium 0.6 ‐ 0.7mg/kg | 38 | 2768 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.99] |
| 2.2 Rocuronium 0.9 ‐ 1.0mg/kg | 15 | 1339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.98, 1.01] |
| 2.3 Rocuronium 1.2 mg/kg | 3 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 49 | 3750 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.91] |
| 1.1 Propofol | 22 | 1448 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 1.2 Thiopental | 28 | 2302 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.73, 0.88] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 47 | 3591 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 1.00] |
| 2.1 Propofol | 21 | 1408 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.01] |
| 2.2 Thiopental | 27 | 2183 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.92, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation outcomes Show forest plot | 34 | 2292 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.93] |
| 1.1 Propofol Induction | 17 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.2 Thiopental Induction | 17 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 32 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 2.1 Propofol Induction | 16 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.02] |
| 2.2 Thiopental Induction | 16 | 1241 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 15 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 1.1 Propofol Induction | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 1.2 Thiopental Induction | 12 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.94] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 14 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 2.1 Propofol Induction | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| 2.2 Thiopental Induction | 11 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.91] |
| 1.1 Adults | 45 | 3615 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.92] |
| 1.2 Children | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Adults | 43 | 3456 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.2 Children | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 5 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.98] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 5 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Low Risk | 21 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
| 1.2 Unclear Risk | 4 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.18] |
| 1.3 High Risk | 25 | 2042 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.96] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Low Risk | 23 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 2.2 Unclear Risk | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.07] |
| 2.3 High Risk | 22 | 1912 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |

