Amantadina y rimantadina para la influenza A en niños y personas de edad avanzada
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002745.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 noviembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Márcia G Alves Galvão (MG) selected the trials, extracted data and was responsible of the methodological aspects of the review.
Marilene Augusta Rocha Crispino Santos (MS) selected the trials, extracted data, was responsible of the methodological aspects of the review and supervised the day‐to‐day work of the review.
Antonio Ledo Alves da Cunha (AC) was appointed as an arbitrator to solve disagreements between MG and MS on the selection of the trials. He supervised the work in all phases and provided his experience on the development of the review.
Declarations of interest
Márcia G Alves Galvão: none known.
Marilene Augusta Rocha Crispino Santos: none known.
Antonio Ledo Alves da Cunha: none known.
Acknowledgements
The authors would like to thank Amanda Burls, Rebecca Mears, David Moore, Lisa Gold and Karen Elley for the use of their protocol. We also would like to thank Tom Jefferson and Richard Stubbs for comments provided on the draft protocol. We acknowledge Elizabeth Dooley from the Cochrane Acute Respiratory Infections Group for helping us in all phases of the review process; Ruth Foxlee and Sarah Thorning, for their essential help with the search strategy, the Iberoamerican Cochrane Centre and especially the kindness of Marta Roque, who helped us in the statistical and methodological aspects of the review. We also acknowledge Raimundo Santos, Vladmír Plesnik, Oleg Borisenko and Stuko Nakano for the assessment and translation of the essential topics for this review from the clinical trials published in German, French, Czech, Russian and Japanese. We also thank Jonathan Haliburton for reviewing the English version of this manuscript. The review authors wish to thank Caroline Hall, David Payler and Vladmír Plesnik, who generously provided us with unpublished trial data. Finally, we wish to thank the following referees who gave their permission to be acknowledged for commenting on this review: Maryann Napoli, Nelcy Rodriguez and Tom Jefferson.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Nov 21 | Amantadine and rimantadine for influenza A in children and the elderly | Review | Márcia G Alves Galvão, Marilene Augusta Rocha Crispino Santos, Antonio JL Alves da Cunha | |
| 2012 Jan 18 | Amantadine and rimantadine for influenza A in children and the elderly | Review | Márcia G Alves Galvão, Marilene Augusta Rocha Crispino Santos, Antonio JL Alves da Cunha | |
| 2008 Jan 23 | Amantadine and rimantadine for influenza A in children and the elderly | Review | Márcia G Alves Galvão, Marilene Augusta Rocha Crispino Santos, Antonio JL Alves da Cunha | |
| 2000 Oct 23 | Amantadine and rimantadine for influenza A in children and the elderly | Protocol | Alessandro Buda, A JL Alves de Cunha, Antonio JL Alves da Cunha | |
Differences between protocol and review
Originally in the protocol we planned to study the drug effect on reduction of fever and cough, as they are considered the best predictors of influenza diagnosis. After collecting data, we verified that specific timelines for reduction of signs and symptoms were not reported in the included trials. So, we considered the available data and arbitrarily chose a day of antiviral use to evaluate the response to the treatment. This choice was based on Eccle's study in which clinical manifestations were classified into early and later symptoms (Eccle 2005).
We applied wider age ranges for children than the definition stated in the protocol (participants up to 16 years of age). Trials in older participants who were adolescents by the World Health Organization (WHO) definition were also included (WHO 2007). Data regarding the proportion of the subgroup which strictly fulfilled the age criterion in the protocol were not available in five studies or by contacting the trial authors. The respective age ranges were one to 17 years (Clover 1991), 13 to 19 years (Payler 1984), one to 18 years (Clover 1986; Crawford 1988), and eight to 19 years of age (Finklea 1967).
We planned only to make 12 comparisons. However, whilst analysing data we considered doing an additional comparison and put the two age groups together. As the small samples studied in rimantadine trials for prophylaxis might have influenced the observed results, we tried to overcome this limitation by combining the trials with rimantadine in children and in the elderly. It must be stressed that extraneous characteristics between those groups, other than age or previous immunisations, may have occurred, impairing generalisation of these results.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Aged; Child; Humans; Young Adult;
PICO
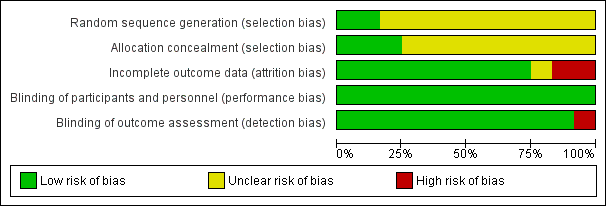
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 1 Fever day 3.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 2 Malaise day 6.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 3 Cough day 7.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 4 Conjunctivitis day 5.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 5 Eye symptoms day 5 (pain on movement and visual distortion).
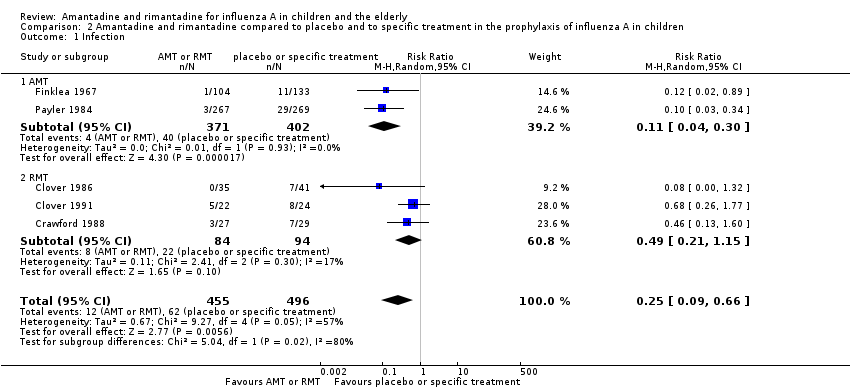
Comparison 2 Amantadine and rimantadine compared to placebo and to specific treatment in the prophylaxis of influenza A in children, Outcome 1 Infection.

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 1 RMT (proved and clinical infection).

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 2 RMT Monto (100 + 200) and Patriarca.
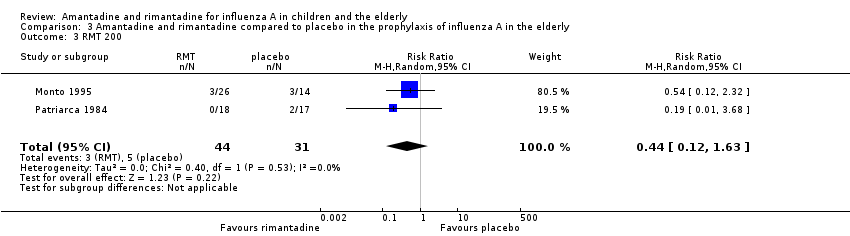
Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 3 RMT 200.

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 4 RMT 100.

Comparison 4 Use of different doses of rimantadine for prophylaxis and treatment of influenza A in the elderly, Outcome 1 Clinical and laboratory infection.
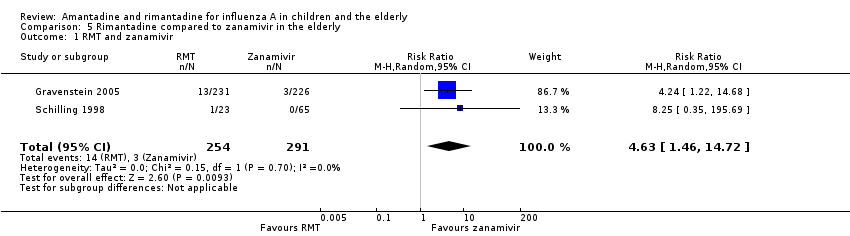
Comparison 5 Rimantadine compared to zanamivir in the elderly, Outcome 1 RMT and zanamivir.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 1 Diarrhoea.
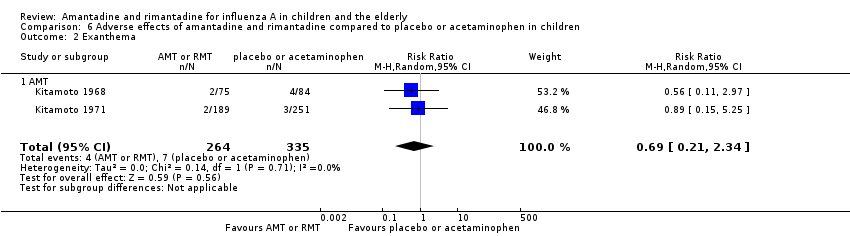
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 2 Exanthema.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 3 Muscular, limb pain.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 4 Headache.
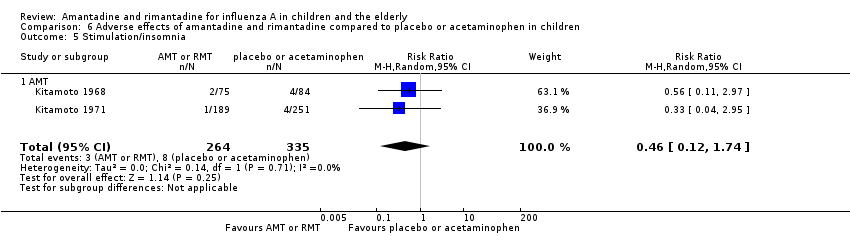
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 5 Stimulation/insomnia.
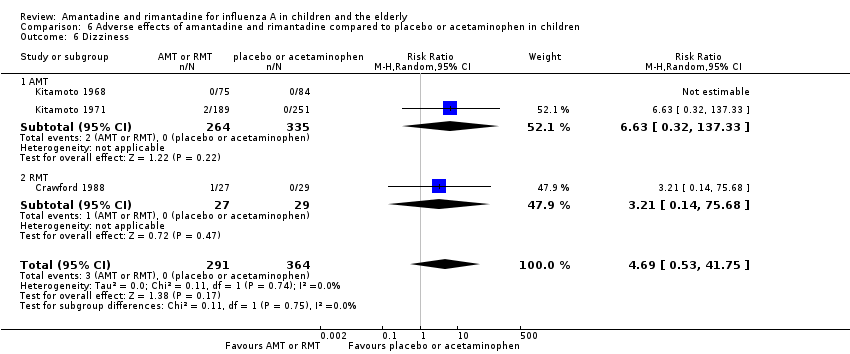
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 6 Dizziness.
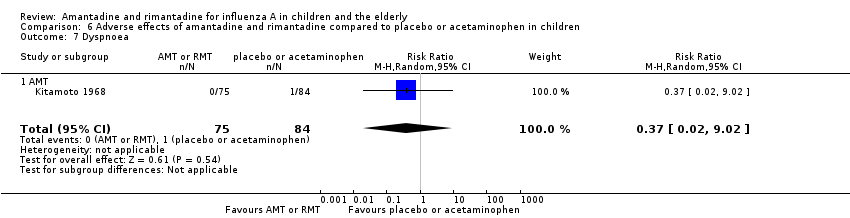
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 7 Dyspnoea.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 8 Central nervous system symptoms.
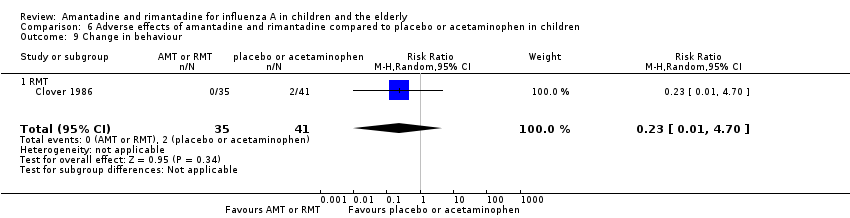
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 9 Change in behaviour.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 10 Gastrointestinal symptoms.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 11 Hyperreactivity.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 12 Tinnitus.
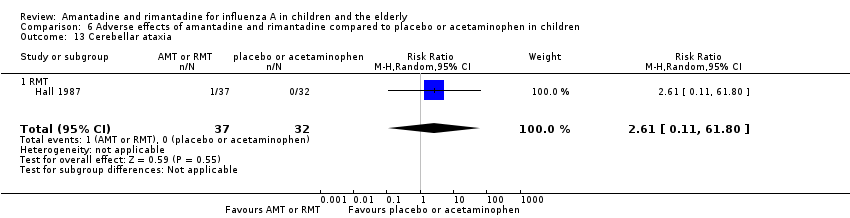
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 13 Cerebellar ataxia.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 14 Malaise.
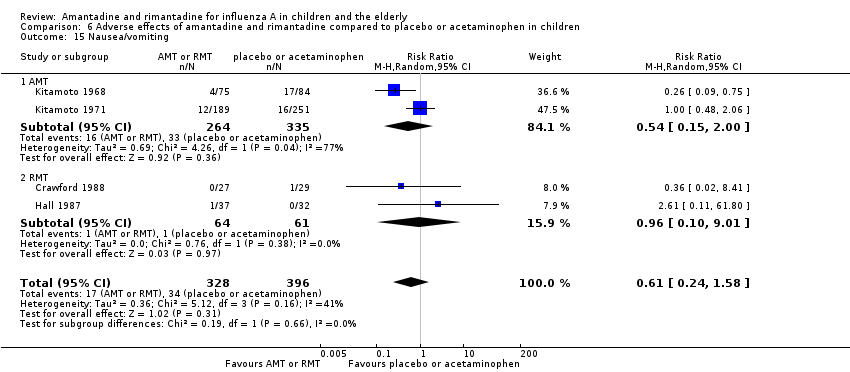
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 15 Nausea/vomiting.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 16 Arrhythmia.
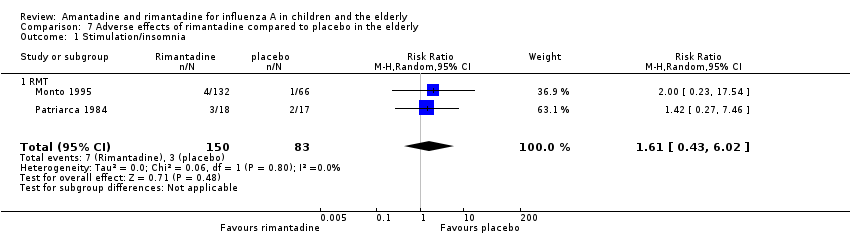
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 1 Stimulation/insomnia.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 2 Confusion.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 3 Fatigue.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 4 Vomiting.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 5 Headache.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 6 Impaired concentration.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 7 Rash or allergic reaction.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 8 Seizures or clonic twitching.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 9 Dry mouth.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 10 Dizziness.
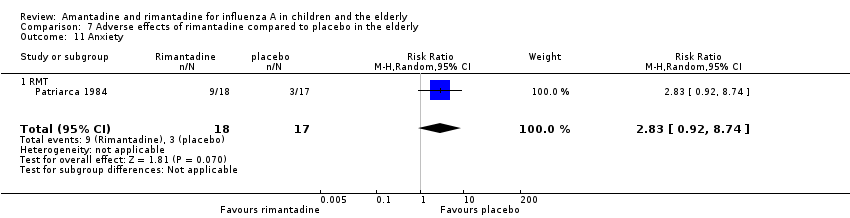
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 11 Anxiety.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 12 Nausea.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 13 Depression.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 14 Loss of appetite.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 1 Confusion.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 2 Depression.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 3 Impaired concentration.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 4 Insomnia or sleeplessness.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 5 Loss of appetite.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 6 Rash or allergic reaction.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 7 Seizure or clonic twitching.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 8 Dry mouth.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 9 Fatigue and drowsiness.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 10 Headache.
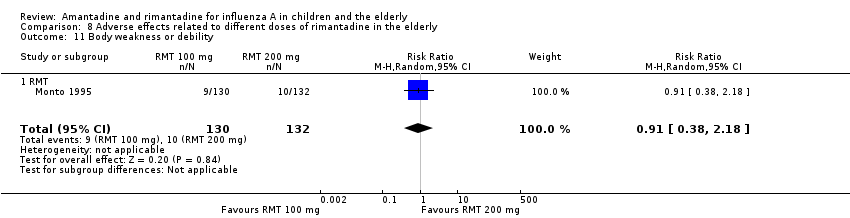
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 11 Body weakness or debility.
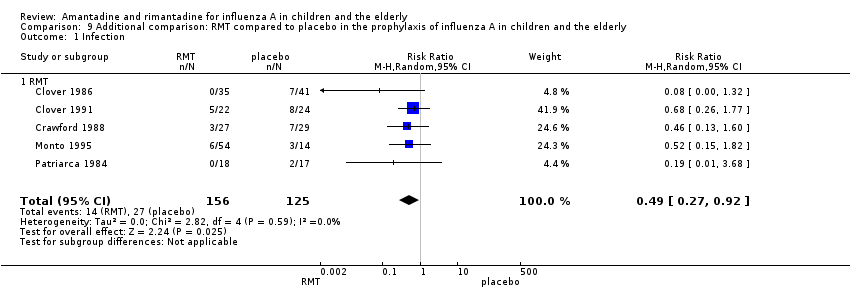
Comparison 9 Additional comparison: RMT compared to placebo in the prophylaxis of influenza A in children and the elderly, Outcome 1 Infection.
| Amantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
| Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: all Intervention: amantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up:14 to 18 weeks) | Medium risk population | RR 0.11 (0.04 to 0.3) | 773 | ⊕⊕⊝⊝ | ||
| 10 per 100 | 1 per 100 | |||||
| Fever after initiation of treatment (follow‐up: 3 days) | Medium risk population | RR 0.37 (0.08 to 1.75) | 104 | ⊕⊕⊝⊝ | ||
| 23 per 100 | 9 per 100 | |||||
| Cough after initiation of treatment | See comment | See comment | Not estimable | 0 (0) | See comment | No selected trial |
| Dizziness (follow‐up: 7 days) | Medium risk population | RR 6.63 (0.32 to 137.33) | 599 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Nausea/vomiting (follow‐up: 7 days) | Medium risk population | RR 0.54 (0.15 to 2) | 599 | ⊕⊝⊝⊝ | ||
| 13 per 100 | 7 per 100 | |||||
| Stimulation/insomnia (follow‐up: 7 days) | Medium risk population | RR 0.46 (0.12 to 1.74) | 599 | ⊕⊕⊝⊝ | ||
| 3 per 100 | 7 per 100 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment not used or unclear. 2Sparse data. 3Allocation concealment unclear. 4Sparse data, confidence intervals do not rule out potential for null effect or harm. 5High heterogeneity unexplained. | ||||||
| Rimantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
| Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: control (placebo or acetaminophen) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up: 1 to 35 days) | Medium risk population | RR 0.49 (0.21 to 1.15) | 178 | ⊕⊕⊝⊝ | ||
| 24 per 100 | 12 per 100 | |||||
| Fever after initiation of treatment (follow‐up: 3 days) | Medium risk population | RR 0.36 (0.14 to 0.91) | 69 | ⊕⊕⊕⊝ | ||
| 38 per 100 | 14 per 100 | |||||
| Cough after initiation of treatment (follow‐up: 7 days) | Medium risk population | RR 0.83 (0.63 to 1.1) | 69 | ⊕⊕⊕⊝ | ||
| 81 per 100 | 67 per 100 | |||||
| Dizziness (follow‐up: 35 days) | Medium risk population | RR 3.21 (0.14 to 75.68) | 56 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Nausea/vomiting (follow‐up: 7 to 35 days) | Medium risk population | RR 0.96 (0.1 to 9.01) | 125 | ⊕⊕⊝⊝ | ||
| 2 per 100 | 2 per 100 | |||||
| Stimulation/insomnia | See comment | See comment | Not estimable | 0 | See comment | No selected trial |
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment unclear. 2Sparse data and confidence intervals do not rule out the potential for no effect or harm | ||||||
| Amantadine compared with placebo for prevention and treatment of influenza A in the elderly | ||||||
| Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: amantadine Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis | See comment | Not estimable | 0 | See comment | No selected trial | |
| Fever after initiation of treatment | See comment | Not estimable | 0 | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | Not estimable | 0 | See comment | No selected trial | |
| Dizziness | See comment | Not estimable | 0 | See comment | No selected trial | |
| Nausea | See comment | Not estimable | 0 | See comment | No selected trial | |
| Vomiting | See comment | Not estimable | 0 | See comment | No selected trial | |
| Stimulation/insomnia | See comment | Not estimable | 0 | See comment | No selected trial | |
| Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis | Medium risk population | RR 0.45 (0.14 to 1.41) | 103 | ⊕⊝⊝⊝ | ||
| 17per 100 | 7 per 100 | |||||
| Fever after initiation of treatment | See comment | 0 | See comment | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | 0 | See comment | See comment | No selected trial | |
| Dizziness (follow‐up: 12 weeks) | Medium risk population | |||||
| 12 per 100 | 11 per 100 (2 to 70) | RR 0.94 | 35 (1) | ⊕⊕⊝⊝ | ||
| Nausea (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.99 (0.45 to 8.75) | 233 | ⊕⊝⊝⊝ | ||
| 8 per 100 | 15 per 100 | |||||
| Vomiting (follow‐up: 8 to 12 weeks) | Medium risk population | RR 0.99 (0.38 to 2.6) | 233 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 7 per 100 | |||||
| Stimulation/insomnia (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.61 (0.43 to 6.02) | 233 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 11 per 100 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment unclear and 1 study had high withdrawal rate. 2Sparse data and confidence interval do not rule out no effect or harm. 3Allocation concealment unclear 4High heterogeneity unexplained. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fever day 3 Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 AMT | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.75] |
| 1.2 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.91] |
| 2 Malaise day 6 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.70] |
| 3 Cough day 7 Show forest plot | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 3.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 4 Conjunctivitis day 5 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.49] |
| 5 Eye symptoms day 5 (pain on movement and visual distortion) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.10, 3.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 5 | 951 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.66] |
| 1.1 AMT | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.30] |
| 1.2 RMT | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RMT (proved and clinical infection) Show forest plot | 3 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.13, 4.07] |
| 2 RMT Monto (100 + 200) and Patriarca Show forest plot | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.14, 1.41] |
| 3 RMT 200 Show forest plot | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.63] |
| 4 RMT 100 Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.10, 21.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical and laboratory infection Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| 1.1 RMT | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RMT and zanamivir Show forest plot | 2 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 4.63 [1.46, 14.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Diarrhoea Show forest plot | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.42, 1.47] |
| 1.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.53] |
| 1.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 2 Exanthema Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 2.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 3 Muscular, limb pain Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 3.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 4 Headache Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 4.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 5 Stimulation/insomnia Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 5.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 6 Dizziness Show forest plot | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.53, 41.75] |
| 6.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [0.32, 137.33] |
| 6.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 7 Dyspnoea Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 7.1 AMT | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 8 Central nervous system symptoms Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 8.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9 Change in behaviour Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 10 Gastrointestinal symptoms Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 10.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 11 Hyperreactivity Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 11.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 12 Tinnitus Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 12.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 13 Cerebellar ataxia Show forest plot | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 13.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 14 Malaise Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 14.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 15 Nausea/vomiting Show forest plot | 4 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.58] |
| 15.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 2.00] |
| 15.2 RMT | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.10, 9.01] |
| 16 Arrhythmia Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stimulation/insomnia Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 1.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 2 Confusion Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 2.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 3 Fatigue Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 3.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 4 Vomiting Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 4.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 5 Headache Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 5.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 6 Impaired concentration Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 6.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 7 Rash or allergic reaction Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 7.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 8 Seizures or clonic twitching Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 8.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 9 Dry mouth Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 9.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 10 Dizziness Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 10.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 11 Anxiety Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 11.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 12 Nausea Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 12.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 13 Depression Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 13.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 14 Loss of appetite Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| 14.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Confusion Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 1.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 2 Depression Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 2.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 3 Impaired concentration Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 3.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 4 Insomnia or sleeplessness Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 4.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 5 Loss of appetite Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 5.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 6 Rash or allergic reaction Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 6.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 7 Seizure or clonic twitching Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 7.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 8 Dry mouth Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 8.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 9 Fatigue and drowsiness Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 9.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 10 Headache Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 10.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 11 Body weakness or debility Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| 11.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |
| 1.1 RMT | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |

