Agentes antileucotrienos comparados con corticosteroides inhalados para el tratamiento del asma recurrente y crónica en pacientes adultos y niños
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | DESIGN: Randomised clinical study Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 24 WITHDRAWALS: Not mentioned AGE in years ± SD: 35.65 ± 10.75 GENDER (male %): 20.83% ASTHMA SEVERITY: Mild persistent asthma ASTHMA DURATION:
MEAN ( ± SD) β2‐AGONIST USE (puffs/day): Not described DOSE OF inhaled corticosteroids AT STUDY ENTRY AND AT RUN‐IN: Not mentioned ATOPY (% of patients): 54.16% ELIGIBILITY CRITERIA: A history of recurrent symptoms of wheezing, shortness of breath, cough; Demonstration of objective signs of reversible airway obstruction by means of at least 12 % increase in FEV1 after 15 minutes with an inhalation of 200 μg salbutamol; A PC20 methacholine < 8 mg/mLas stated by the American Thoracic Society and International Asthma Guidelines. Asthma severity was determined by the frequency of asthma symptoms, pulmonary function tests. EXCLUSION CRITERIA: On inhaled corticosteroids, leukotriene receptor antagonists, theophylline and long acting β2‐agonists within the preceding 12 months of the study; airway infection for at least 6 weeks before investigation. | |
| Interventions | PROTOCOL DURATION
TEST GROUP: Montelukast CONTROL GROUP: Fluticasone propionate DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL FROM STUDY: Not mentioned | |
| Outcomes | ANALYSIS: Not by intention‐to‐treat analysis OUTCOMES: Reported at 4 weeks; Report outcomes are reported as pre‐ and post‐values (not change from baseline) PULMONARY FUNCTION TESTS: Only pretreatment FEV1 was reported as not difference was observed after treatment FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS: Eosinophils count; Apoptotic eosinophils; Apoptotic ratio ADVERSE EVENTS: Not mentioned WITHDRAWALS: Not mentioned | |
| ICS dose in HFA beclomethasone ‐ equivalent | 250 μg | |
| Notes | Full paper (2005) Funding not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | There is no report as to whether some patients withdrew from the study after randomisation |
| Selective reporting (reporting bias) | Low risk |
All primary and secondary data are presented |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Randomised clinical study Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: Not mentioned AGE in years (± SD): 35.65 ± 10.75 GENDER (male%): 20.83% ASTHMA SEVERITY: Mild persistent asthma ASTHMA DURATION:
MEAN ( ± SD) β2‐AGONIST USE (puffs per day): Not described DOSE OF inhaled corticosteroids AT STUDY ENTRY AND AT RUN‐IN: Not mentioned ATOPY (% of patients): 54.16% ELIGIBILITY CRITERIA:
EXCLUSION CRITERIA: Not mentioned | |
| Interventions | PROTOCOL DURATION:
TEST GROUP: Zafirlukast TEST GROUP 2: Theophylline CONTROL GROUP: Budesonide DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL FROM STUDY: Not mentioned | |
| Outcomes | ANALYSIS (ITT) OUTCOMES: PULMONARY FUNCTION TESTS: Spirometry FUNCTIONAL STATUS: Not mentioned INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not mentioned WITHDRAWALS: Not mentioned | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full paper (2004) Funding not available Confirmation of methodology and data extraction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | All data presented, withdrawal rate was not observed |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined but no bias is observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE in years ± SD: M: 35.9 ± 14.9 GENDER (% male): ASTHMA SEVERITY: Not described ASTHMA DURATION: Not reported % Pred. FEV1 % ± SD: MEAN ± SD β2‐AGONIST USE (puffs/day): ATOPY: ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration
TEST GROUP: Montelukast 10 mg per day CONTROL GROUP 1: Beclomethasone 200 ug twice a day CONTROL GROUP 2: Placebo DEVICE: MDI (actuation inhaler) CO‐INTERVENTION: Not specified CRITERIA FOR WITHRAWAL FROM STUDY: Not described | |
| Outcomes | ANALYSIS BY MODIFIED INTENTION‐TO‐TREAT OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 SYMPTOM SCORES: Change in mean asthma score (6‐point) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Change from baseline in serum eosinophils ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Merck Research Labratories Confirmation of methodology and data extraction received from Dr Theodore Reiss | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented with reasons for withdrawal by group and adverse effects by group, analysis wad done with intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary endpoint was described |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group Confirmation of methodology obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE ( ± SD): GENDER (% male): ASTHMA SEVERITY: % mean pred. FEV1: Mean ( ± SD) β2‐agonist use (puffs per day): Not reported ATOPY: ASTHMA DURATION (years): 10 years ELIGIBILITY CRITERIA
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 12 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Inhaled Fluticasone 100 μg twice a day DEVICE: Not described CO‐INTERVENTION: None allowed | |
| Outcomes | ANALYSIS ( ITT ) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Change in mean symptom score (average/week) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data extraction received from Gerry Hogan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation of block of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data reported with intention‐to‐treat analysis, adverse events were reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, placebo controlled, clinical trial | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE (years±SD): GENDER (% male): ASTHMA SEVERITY: Not described % Pred. FEV1 (mean ± SD) Mean ( ± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION (years): Z: 20.9 ± 13.1 ELIGIBILITY CRITERIA
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 52 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Inhaled Budesonide 200 μg twice a day DEVICE: Turbuhaler CO‐INTERVENTION: None allowed | |
| Outcomes | ANALYSIS: Not reported OUTCOMES reported at 52 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptom score FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by National Institute of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: stratified adaptive randomisation to balance the effect of PC20, age, racial or ethnicity |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Unclear risk | The withdrawal rate was unbalanced between groups: 8.2% and 18.4% in budesonide and zafirlukast group, respectively; the reasons for withdrawal are reported but not by group. There was no report of intention‐to‐treat analysis; although this was appropriate for the main outcome (time to event), it may have biased the interpretation of the some secondary outcomes |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE: years ± SD: GENDER: n (% male): ASTHMA SEVERITY: Mild persistent asthma as defined by Global Initiative for Asthma (GINA) guidelines ASTHMA DURATION: Not described % Pred. FEV1 ± SD β2‐AGONIST USE (puffs per day): ATOPY: n (%) M: 42 (12.9%) ELIGIBILITY CRITERIA
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 10 mg per day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Metered dose inhaler (MDI) | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Days with symptoms (%) FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: Eosinophil count (103/μL) ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Merck & Co., Inc. Confirmation of methodology and data extraction: not obtained USER‐DEFINED ORDER: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented; intention‐to‐treat analysis, reasons for withdrawal by group and adverse effects by group presented, |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre , randomised, double‐blind, double‐dummy, clinical trial Confirmation of methodology not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE (± SD): GENDER (% male): ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described % Pred. FEV1 (± SD): MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF inhaled corticosteroids AT STUDT ENTRY AND AT RUN‐IN: ATOPY: Not described ELIGIBILITY CRITERIA
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Zafirlukast 20 mg twice daily CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Metered‐dose inhaler CRITERIA FOR WITHDRAWAL FROM STUDY
| |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo SmithKline Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Details were not provided |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data reported including side effects; withdrawal rate with reasons by group |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial Confirmation of methodology obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE (± SD): 12‐75 years GENDER (% male): 50% ASTHMA SEVERITY: % moderate: % Pred. FEV1 (mean ± SD): 66‐67% Mean (± SD) β2‐agonist use (puffs/day) ATOPY: ASTHMA DURATION (years): at least 10 years ELIGIBILITY CRITERIA
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Zafirlukast 20 mg twice a day po CONTROL GROUP: Inhaled Fluticasone 100 μg twice a day (2 puffs of 50 μg twice a day) DEVICE: Metered dose inhaler (no spacer) CO‐INTERVENTION: None allowed other than rescue β2‐agonist and systemic corticosteroids | |
| Outcomes | ANALYSIS ( ITT ) OUTCOMES reported at 6 and 12 weeks (also documented at 2, 4, 8 weeks) PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Weekly average symptom score (6‐point scale) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data extraction obtained from Shailesh Patel and Rob Pearson, Nov 2, 2001 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated in block of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial Confirmation of methodology obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE (± SD) years: M: 34.4 (15‐67) GENDER (% male): ASTHMA SEVERITY: % Pred. FEV1 (mean ± SD): Mean (± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 24 weeks TEST GROUP: Montelukat 10 mg per day CONTROL GROUP: Inhaled Fluticasone 100 μg twice a day DEVICE: Metered dose inhaler CO‐INTERVENTION: None allowed | |
| Outcomes | ANALYSIS ( ITT ) OUTCOMES reported at 4, 8, 12, 16, 24 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptom score/week (6‐point scale) FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data extraction and additional unpublished data obtained from and Shailesh Patel and Rob Pearson | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated in block of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented including reasons for withdrawals and adverse effects, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Three‐way cross‐over, placebo controlled, randomised, clinical trial | |
| Participants | MILD TO MODREATE PERSISTENT ASTHMA MEETING NAEPP CRITERIAS RANDOMISED: N = 24 WITHDRAWALS: 16.67% AGE: years(range): 8.92 (5‐12) GENDER (% male): Not reported ASTHMA SEVERITY: Mild to moderate as per NAEPP criteria % Pred. FEV1: mean(range)%: 88 (47‐111) % ATOPY: Not reported ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 4 weeks TEST GROUP: Montelukat 10 mg qd CONTROL GROUP: Inhaled Fluticasone 50 μg twice a day DEVICE: Aerochamber CO‐INTERVENTION: None allowed | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 2, 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Daily PM asthma symptom score (6‐point scale) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: *Fractional exhaled nitric oxide (FeNO) ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full‐text publication Funded by GlaxoSmithKline and by National Health Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, reasons for withdrawal by group and adverse effects by group presented, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were described |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Cross‐over, randomised, clinical trial Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 21 WITHDRAWALS: Not mentioned AGE (years): N = 33.5 GENDER (% male): Not mentioned ASTHMA SEVERITY: Mild persistent atopic asthma ASTHMA DURATION: Not described % Pred. FEV1: N = 96.3% MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF inhaled corticosteroids AT STUDY ENTRY AND AT RUN‐IN: Not mentioned ATOPY: Not described ELIGIBILITY CRITERIA: Mild persistent asthma EXCLUSION: Not mentioned | |
| Interventions | PROTOCOL Duration CONTROL GROUP: Hfa‐triamcinolone acetonide 450 μg od TEST GROUP: Montelukast 10 mg od DEVICE: Not mentioned Criteria for withdrawal from study: Not mentioned | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptoms FUNCTIONAL STATUS: Change from baseline in night‐time use of rescue β2‐agonist use (puffs/day) INFLAMMATORY MEDIATORS
ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 112.5 μg | |
| Notes | Abs 2001 Funding not mentioned Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Single blind |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | PARTICIPANTS WHO ARE RECEIVING β2‐AGONISTS ALONE RANDOMISED: WITHDRAWALS: AGE (± SD): GENDER (% male): ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described % Pred. FEV1 (±SD): Not described MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Not described ELIGIBILITY CRITERIA
EXCLUSION: Not described | |
| Interventions | PROTOCOL Duration CONTROL GROUP: Fluticasone propionate 88 μg twice a day TEST GROUP: Zafirlukast 20 mg twice a day DEVICE: Metered dose inhaler Criteria for withdrawal from study: Reported | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | PARTICIPANTS WHO ARE RECEIVING β2‐AGONISTS ALONE RANDOMISED: WITHDRAWALS: AGE (± SD): GENDER (% male): ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described FEV1 (± SD): MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION: Not described | |
| Interventions | PROTOCOL Duration CONTROL GROUP: Fluticasone propionate 88 μg twice a day TEST GROUP: Zafirlukast 20 mg twice a day DEVICE: Metered dose inhaler Criteria for withdrawal from study: Reported | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 1, 2, 4, 6, 8, 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Randomised clinical study | |
| Participants | PARTICIPANTS WITH MILD ASTHMA RANDOMISED: WITHDRAWALS: AGE (± SD): GENDER (% male): ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described FEV1 (± SD): MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Described ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration CONTROL GROUP: Fluticasone propionate 50 μg twice a day TEST GROUP: Zafirlukast 20 mg twice a day DEVICE: Metered dose inhaler Criteria for withdrawal from study: Reported | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 1, 4 weeks PULMONARY FUNCTION TESTS: FEV1 SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported. INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi centre, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | PARTICIPANTS WITH PERSISTENT ASTHMA RANDOMISED: WITHDRAWALS: AGE (± SD): GENDER (% male): ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described % Pred. FEV1 (± SD): MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration CONTROL GROUP: Fluticasone propionate 50 μg twice a day TEST GROUP: Montelukast 5 mg QD DEVICE: Diskus Criteria for withdrawal from study: Reported | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change from baseline in percent of symptom‐free days FUNCTIONAL STATUS: Change from baseline in total albuterol use INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parellel‐group, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE: years (range): M: 9 (6‐14) GENDER (% male): ASTHMA SEVERITY: Mild persistent asthma at step 2 of GINA guidelines % Pred. FEV1 ± range: β2‐agonist use (puffs per day): median (range) ATOPY: Not reported ASTHMA DURATION: (years): Not reported HISTORY OF ALLERGY:(%) ELIGIBILITY CRITERIA:
(2) a positive methacholine or histamine provocative concentration causing a 20% decrease in FEV1 of 8 mg/mL; or (3) a decrease in FEV1 of 15% after an exercise challenge.
EXCLUSION: Not reported | |
| Interventions | PROTOCOL Duration CONTROL GROUP: Fluticasone 100 μg twice a day TEST GROUP: Montelukast 5‐mg oral tablet [10 mg if the patient turned 15 years of age during the study], once at bedtime DEVICE: Metered dose inhaler Criteria for withdrawal from study: Described | |
| Outcomes | ANALYSIS ( ITT ) OUTCOMES reported at 52 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 (L and %) SYMPTOM SCORES: Not reported FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Peripheral blood eosinophils level ADVERSE EVENTS: Described WITHDRAWALS: Described * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Merck and Co. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: electronically generated randomisation using blocking factor of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, reasons for withdrawal by group and adverse effects by group presented, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary, secondary and tertiary outcomes were described |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, clinical trial. Confirmation of methodology obtained (08/01) | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE (± SD): GENDER (% male): ASTHMA SEVERITY: Mild % Pred. FEV1 % ± SD: Mean (± SD) β2‐agonist use (puffs/day): ATOPY or ALLERGIC RHINITIS: ELIGIBILITY CRITERIA:
EXCLUSION CRITERIA:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 10 mg CONTROL GROUP 1: Fluticasone 100 μg twice a day CONTROL GROUP 2: Budesonide 200 μg twice a day DEVICE: Aerosol inhaler for Fluticasone and Dry powder inhaler for Budesonide CO‐INTERVENTION: None allowed other than rescue B2‐agonist and systemic corticosteroids | |
| Outcomes | ANALYSIS ( ITT) OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Change from baseline in serum eosinophils ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Abstract & Poster (1999) Funded by Merck Letter, meth sent to Hughes December 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by opaque consecutive numbered envelopes containing assignment |
| Blinding (performance bias and detection bias) | High risk | Open label |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, withdrawal and adverse effects, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcome was described |
| Other bias | Low risk | No apparent other bias |
| Methods | as above | |
| Participants | as above | |
| Interventions | as above | |
| Outcomes | as above | |
| ICS dose in HFA beclomethasone ‐ equivalent | as above | |
| Notes | as above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by opaque consecutive numbered envelopes containing assignment |
| Blinding (performance bias and detection bias) | High risk | Open label |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, withdrawal and adverse effects, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcome was described |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 782 WITHDRAWALS: n (%) AGE: years (range): M = 33.5 (15‐71) GENDER: n (% male) M = 162 (47.8%) ASTHMA SEVERITY: Not mentioned ASTHMA DURATION: Not mentioned % PRED. FEV1% ±SD: M = 67.4 ± 11.1 MEAN (± SD) β2‐AGONIST USE (puffs/day): DOSE OF inhaled corticosteroids AT STUDY ENTRY AND DURING RUN‐IN: Not reported ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION
| |
| Interventions | PROTOCOL DURATION TEST GROUP: Montelukast: 10 mg once daily CONTROL GROUP 1: BDP: 200 μg twice a day CONTROL GROUP 2: Placebo DEVICE: MDI + spacer CRITERIA FOR WITHDRAWAL FROM STUDY: Not described CO‐INTERVENTION: Short‐acting anti‐histamines | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 6 weeks PULMINARY FUNCTION TESTS SYMPTOM SCORES: % of days with asthma control FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported *primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Merck Confirmation of methodology and data extraction not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: blinded allocation schedule produced by the study sponsor |
| Allocation concealment (selection bias) | Low risk | A block of allocation numbers that were assigned sequentially to consecutively randomised patients |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, withdrawal rate per group was mentioned |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were specified |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: Not mentioned AGE (years): Not mentioned GENDER (% female): Not mentioned ASTHMA SEVERITY: Not described ASTHMA DURATION: Not mentioned % PRED. FEV1% ± SD: Not mentioned MEAN ± SD β2‐AGONIST USE (puffs/day): Not mentioned DOSE OF inhaled corticosteroids AT STUDY ENTRY AND DURING RUN‐IN: Not reported ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION± Not mentioned | |
| Interventions | PROTOCOL DURATION TEST GROUP: Montelukast (dose un‐specified: probably 10 mg per day) CONTROL GROUP 1: Fluticasone (dose un‐specified) DEVICE: Not described CRITERIA FOR WITHDRAWAL FROM STUDY: Not described | |
| Outcomes | ANALYSIS (ITT not specified) OUTCOMES ‐reported at 8 weeks PULMINARY FUNCTION TESTS
SYMPTOM SCORES: Change in symptoms FUNCTIONAL STATUS: Change from baseline in use of β2‐agonist INFLAMMATORY MEDULATOR: *Change in sputum Eo (%) ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported *primary outcomes | |
| ICS dose in HFA beclomethasone ‐ equivalent | Not reported | |
| Notes | Abstract 2002 Funding not mentioned Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, multicentric, placebo controlled, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED WITHDRAWALS: N (%) AGE: years ± SD GENDER: N (% male) ASTHMA SEVERITY: Persistent symptomatic asthma % Pred. FEV1 % (± SD) β2‐agonist use (puffs per day) Mean (± SD): ATOPY: ELIGIBILITY CRITERIA:
| |
| Interventions | PROTOCOL DURATION TEST GROUP: Montelukast (10 mg qd) CONTROL GROUP: Fluticasone (250 μg per day) DEVICE: Not described CRITERIA FOR WITHDRAWAL FROM STUDY: Not described | |
| Outcomes | ANALYSIS: (ITT not specified) OUTCOMES Reported at 8 weeks PULMINARY FUNCTION TESTS
SYMPTOM SCORES: Change in symptoms (5‐35 score) FUNCTIONAL STATUS: Pre and post use of β2‐agonist INFLAMMATORY MEDULATOR: *Change in sputum Eosinophils (%) ADVERSE EVENTS: Reported WITHDRAWALS: Reported *primary outcomes | |
| ICS dose in HFA beclomethasone ‐ equivalent | 250 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Inc; | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | High risk | Data on symptom severity, methacholine tests, PEF, skin prick test were mentioned in methodology but not described in results |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Cross‐over, active controlled, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 58 WITHDRAWALS: n(%): 4 (6.90) AGE: years (range): N = 38.5 (16–70) GENDER (% male): 60.35 ASTHMA SEVERITY: Mild to moderate asthma ASTHMA DURATION: Not described % Pred. FEV1: mean±SD: N = 76.1 ± 11.9 β2‐AGONIST USE: puffs per day (range): 3.0 (1.0–5.5) ATOPY: 93% ELIGIBILITY CRITERIA:
EXCLUSION CIRTERIA:
| |
| Interventions | PROTOCOL DURATION TEST GROUP: Montelukast (over‐encapsulated montelukast 10 mg tablet q.d.) CONTROL GROUP: Fluticasone propionate (Accuhaler/Diskus 250 mg twice a day) DEVICE: (Accuhaler/Diskus) CRITERIA FOR WITHDRAWAL FROM STUDY: Described | |
| Outcomes | ANALYSIS: (ITT) OUTCOMES Reported at 6 weeks PULMINARY FUNCTION TESTS:
SYMPTOM SCORES: *Change in symptoms (1‐6 score) FUNCTIONAL STATUS:
INFLAMMATORY MEDULATOR: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported *primary outcomes | |
| ICS dose in HFA beclomethasone ‐ equivalent | 500 μg | |
| Notes | Full‐text publication Funding: Not reported; | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, intention‐to‐treat analysis,reasons for withdrawal by group and adverse effects by group described |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, active and placebo controlled, clinical trial. | |
| Participants | ASTHMA PATIENTS RANDOMISED: WITHDRAWALS: Not reported AGE: years ± SD GENDER: % male ASTHMA SEVERITY: Not mentioned % Pred. FEV1 % (± SD) β2‐agonist use (puffs/day) Mean (± SD): Not reported ATOPY: Not reported Exhaled NO, parts per billion (± SD) M: 12.5 (4.8) SPUTUM EOSINOPHILS, % (± SD) SPUTUM ECP, ng/mL (± SD) ELIGIBILITY CRITERIA:
| |
| Interventions | PROTOCOL DURATION TEST GROUP: Montelukast capsule (10 mg at night) CONTROL GROUP: Fluticasone propionate (200 μg twice a day) DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL FROM STUDY: Not mentioned | |
| Outcomes | ANALYSIS: (ITT not mentioned) OUTCOMES Reported at 12 weeks PULMINARY FUNCTION TESTS:
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS AND INFLAMMATORY MEDULATOR:
ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined but no bias is observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Cross‐over, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 40 WITHDRAWALS: Not reported AGE mean years (range): 37 (18‐60) GENDER: 24 male (60%) ASTHMA SEVERITY: Moderate allergic bronchial asthma FEV1 % pred (Mean ±SD): 74.2 (10.6) MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported ATOPY: 100% of patients ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL DURATION TEST GROUP: Fluticasone 100 μg twice a day CONTROL GROUP: Montelukast 10 mg at night‐time DEVICE: Diskus CRITERIA FOR WITHDRAWAL FROM STUDY: Not described CO‐INTERVENTION: Not permitted | |
| Outcomes | ANALYSIS (not by ITT) OUTCOMES PULMINARY FUNCTION TESTS:
SYMPTOM SCORES: Symptom score daytime and night‐time (range 0‐4) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported WITHDRAWALS: Not reported PRIMARY OUTCOMES: Not reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by GlaxoSmithKline, d20354 Hamburg, Germany Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, active controlled, randomised, clinical trial. | |
| Participants | ASTHMA PATIENTS RANDOMISED: WITHDRAWALS: Not reported AGE: years±SD: Not mentioned GENDER: % male ASTHMA SEVERITY: Not mentioned % Pred. FEV1 % (± SD): Not reported β2‐agonist use (puffs/day) Mean (± SD): Not reported ATOPY: Not reported ELIGIBILITY CRITERIA Not mentioned EXCLUSION CRITERIA:
| |
| Interventions | PROTOCOL DURATION TEST GROUP: Beclomethasone 250 μg daily CONTROL GROUP: Montelukast 10 mg at night‐time DEVICE: Not reported CRITERIA FOR WITHDRAWAL FROM STUDY: Not described CO‐INTERVENTION: Not permitted | |
| Outcomes | ANALYSIS (ITT Not reported) OUTCOMES PULMINARY FUNCTION TESTS: Peak expiratory flow rate SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported PRIMARY OUTCOMES: Not reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 125 μg | |
| Notes | Full‐text publication Funded : Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open label study |
| Incomplete outcome data (attrition bias) | High risk | No proper statistical analysis was performed; severity of patient was not determined using any guideline, |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not defined but no bias is observed |
| Other bias | High risk | No details on ethical committee approval mentioned; no details on informed consent was mentioned |
| Methods | DESIGN: Parallel‐group, clinical trial. Confirmation of methodology: Obtained | |
| Participants | ASYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE (years, range): GENDER (%male): ASTHMA SEVERITY: Mild stable FEV1 (Mean ± SD): Mean (± SD) β2‐agonist use (puffs/day): Not reported ATOPY: ASTHMA DURATION: 10 years ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 6 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: MDI CO‐INTERVENTION: None | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptom score (6‐point scale) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data extraction received from Gerry Hogan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated in block of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented including withdrawal with reasons by group and adverse effects by group, analysis was done by intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary out come was presented |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parellel‐group, randomised, clinical trial. | |
| Participants | ASTHMA PATIENTS RANDOMISED: WITHDRAWALS: Not reported AGE: years (SD): GENDER (%male): ASTHMA SEVERITY: Asthma patients with FEV1 between 40% and 85% of predicted normal FEV1 % of predicated: Mean: ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 16 weeks TEST GROUP: Montelukast 10 mg qd CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Flovent Diskus CO‐INTERVENTION: None | |
| Outcomes | ANALYSIS (ITT Not reported) OUTCOMES reported AM PEF: at every week up to 16 weeks FEV1 (L): at every 4 week up to 16 weeks Other parameters: baseline and at the end of 16 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptom score (0‐5 point scale) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented; analysis by intention‐to‐treat; reasons for withdrawal and adverse effects described |
| Selective reporting (reporting bias) | Low risk | Primary and secondary end outcomes were described |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parellel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH ASTHMA LIKE SYMPTOMS RANDOMISED: WITHDRAWALS: Reported AGE: mean years ± SD: GENDER (% male): ASTHMA SEVERITY: Children with asthma‐like symptoms (wheeze, cough and/or shortness of breath) of sufficient severity to justify the use of prophylactic asthma treatment ATOPY: ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 12 weeks TEST GROUP: Montelukast 4 mg chewable tablet qd CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Metered dose inhaler via a plastic spacer device (Aerochambers) CO‐INTERVENTION: None | |
| Outcomes | ANALYSIS by intention‐to‐treat manner OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: *Symptom score (wheeze, cough, shortness of breath) as recorded by caregivers in a symptom diary card FUNCTIONAL STATUS: Rescue medication free days, INFLAMMATORY MEDIATORS: Blood eosinophils ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding: from Merck Sharp and Dohme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented; intention‐to‐treat analysis; reasons for withdrawal and adverse effects were described by group; |
| Selective reporting (reporting bias) | Low risk | Primary and secondary end outcomes were defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parellel‐group, randomised, clinical trial. | |
| Participants | MILD PERSISTENT ASTHMA PATIENTS RANDOMISED: WITHDRAWALS: AGE: years±SD GENDER (%male): ASTHMA SEVERITY: Mild persistent asthma was defined as asthma symptoms occurring more than two times in a week but < 1 episode per day and exacerbation may affect activity, night‐time symptoms more than two times a month and peak expiratory flow rate (PEFR) more than or equal to 80% of the predicted FEV1 % of predicted: Mean(range) ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA
exacerbation may affect activity, night‐time symptoms more than two times a month
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 12 weeks TEST GROUP: Montelukast 10 mg qd CONTROL GROUP: Budesonide 400 μg per day DEVICE: Metered dose inhaler CO‐INTERVENTION: None | |
| Outcomes | ANALYSIS (ITT Not reported) OUTCOMES reported: at every 4 weeks up to 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Symptom score FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Packets of drugs for each patient were labelled |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy, identical MDIs or tables of drugs were used |
| Incomplete outcome data (attrition bias) | High risk | Total of 13.3% (4) and 6.25% (2) patients withdrew from montelukast and budesonide group, respectively and the reasons for withdrawals were reported by group. |
| Selective reporting (reporting bias) | Low risk | Analysis was not done with intention‐to‐treat analysis; the data on secondary outcomes was insufficiently reported to be aggregated; no data on visit to emergency or local practitioner. |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 481 WITHDRAWALS: AGE: GENDER (% male): % Pred. FEV1 (mean ± SD): Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY/ALLERGIC RHINITIS: ASTHMA DURATION (Years, SD): ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 6 weeks TEST GROUP 1: Zafirlukast (Accolate) 20 mg twice a day p.o. TEST GROUP 2: Zafirlukast (Accolate) 80 mg twice a day p.o. CONTROL GROUP: Inhaled beclomethasone dipropionate 200‐250 μg twice a day DEVICE: Not described CO‐INTERVENTION: Not specified | |
| Outcomes | ANALYSIS (ITT not specified) OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Change from baseline daytime symptom scores (max = 21) FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Change in eosinophil counts ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 225 μg | |
| Notes | Abstract (1997) Funded by Zeneca Confirmation of methodology and data extraction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation with block of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, withdrawals and adverse effects were reported by groups, |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE: GENDER (% male): % Pred. FEV1 (mean ±SD): Mean (± SD) β2‐agonist use (puffs/day): ATOPY/ALLERGIC RHINITIS: ELIGIBILITY CRITERIA:
| |
| Interventions | PROTOCOL Duration Intervention Period: 16 weeks TEST GROUP 1: Montelukast 10 mg per day + Placebo (after blind beclomethasone removal) TEST GROUP 2: Montelukast 10 mg per day + inhaled beclomethasone 200 μg twice a day (not used in this review) CONTROL GROUP 1: Placebo + inhaled beclomethasone 200 μg twice a day CONTROL GROUP 2: Placebo + Placebo (not used in this review) DEVICE: Aero‐Chamber spacer device CO‐INTERVENTION: Not specified | |
| Outcomes | ANALYSIS ( Intention‐to‐treat) OUTCOMES reported at 6 and 16 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Change from baseline daytime asthma symptoms scores (6‐point scale) FUNCTIONAL STATUS:
INFLAMMATORY MARKERS: Change from baseline peripheral blood eosinophil counts ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication (1999) Funded by Merck Confirmation of methodology and data received from Reiss 01 Sept 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation with block of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | High risk | All data presented, 20.9% and 11.0% withdrawal in montelukast and beclomethasone respectively, no intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN (ONLY DATA OF NONSMOKER PATIENTS ARE PRESENTED IN THIS REVIEW) | |
| Participants | CORTICOSTEROID‐NAIVE ASTHMATIC PATIENT RANDOMISED: N = 44 WITHDRAWALS: 1 (4.6%) AGE: mean years: 28.98 ± 5.92 GENDER (% male): Not reported % Pred. FEV1 (mean ± SD): 80.16 ± 9.16 Mean (±SD) β2‐agonist use (puffs/day): Not reported ATOPY/ALLERGIC RHINITIS: Not reported DURATION OF ASTHMA: More than 10 years ELIGIBILITY CRITERIA:
| |
| Interventions | PROTOCOL Duration Intervention Period: 16 weeks TEST GROUP 1: Montelukast 10 mg qd TEST GROUP 2: Inhaled beclomethasone 160 μg twice daily DEVICE: Inhaled (HFA)–beclomethasone dipropionate or QVAR CO‐INTERVENTION: Not specified | |
| Outcomes | ANALYSIS (ITT‐ Not reported) OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: AQOL average score INFLAMMATORY MARKERS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full Text publication Funded: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomisation was performed online from the data coordinating centre |
| Allocation concealment (selection bias) | Low risk | Matching placebos were used; Treatment medication for each subject was packaged with unique number and distributed to the clinical centres |
| Blinding (performance bias and detection bias) | Low risk | Triple blind |
| Incomplete outcome data (attrition bias) | Unclear risk | All data presented; analysis was not done using intention‐to‐treat analysis; data for drop out patients was not addressed |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Cross‐over, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: Not reported group wise AGE: 34.11 ± 11.85 GENDER (% male): N: 48.0% % PRED FEV1 (mean, SD): Not reported Mean (± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 10 mg per day at bedtime p.o. CONTROL GROUP 1: Beclomethasone 200 μg twice a day CONTROL GROUP 2: Placebo (not used in this review) DEVICE: Not reported | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT ANALYSIS OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Daytime asthma symptoms score FUNCTIONAL STATUS: Total daily β2‐agonist use INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication Funded: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind |
| Incomplete outcome data (attrition bias) | Unclear risk | All data presented, intention‐to‐treat analysis, clinical and laboratory adverse experiences were reported however withdrawal were not reported by group |
| Selective reporting (reporting bias) | Low risk | Primary, secondary and tertiary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 895 WITHDRAWALS: AGE: GENDER (% male): ‐%PRED FEV1 (mean, SD): Mean (± SD) β2‐agonist use (puffs/day): ATOPY: ASTHMA DURATION years(range): ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 10 mg per day at bedtime p.o. CONTROL GROUP 1: Beclomethasone 200 μg twice a day CONTROL GROUP 2: Placebo (not used in this review) DEVICE: Spacer for Beclomethasone CO‐INTERVENTION: Theophylline (10%) | |
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 6 and 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change from baseline daytime symptom scores FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Change from baseline peripheral blood eosinophil counts ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication (1999) Funded by Merck Confirmation of methodology and data extraction obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation with block of 7 |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, reasons for withdrawal and adverse effects were mentioned by group |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS: AGE: 6‐11 years GENDER (% male): BASELINE SEVERITY: Moderate Mean % Predicted FEV1: ATOPY/ALLERGEN TRIGGERS: ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 5 mg per day at bedtime p.o. CONTROL GROUP: Inhaled BPD 100 μg tid DEVICE: MDI, spacer optional CO‐INTERVENTION: Not specified | |
| Outcomes | ANALYSIS by Intention‐to‐treat OUTCOMES reported at 12, 24 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 (L) SYMPTOM SCORES: Not reported FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: *LTC4 in nasal wash ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 150 μg | |
| Notes | Full‐text publication of primary and extension study (1999) Funded by Merck Frosst confirmation of methodology and data extraction obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | High risk | Open label, the criteria for election of participants from last study not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | All data presented |
| Selective reporting (reporting bias) | Unclear risk | Peak expiratory flow rate was mentioned in method but data was not presented in results, primary and secondary outcomes not specified |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 522 WITHDRAWALS; N (%) AGE: mean (range) GENDER (% male): BASELINE SEVERITY: Moderate % Predicted FEV1 (Mean ± SE): ATOPY/ALLERGEN TRIGGERS: Not reported ASTHMA DURATION (years) ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention: 24 weeks TEST GROUP: Montelukast 10 mg per day at bedtime p.o. CONTROL GROUP: Inhaled FP 100 μg twice a day DEVICE: MDI with spacer CO‐INTERVENTION: Rx permitted: antihistamines, nasal decongestants, intranasal medications (including corticosteroids) for the treatment of rhinitis | |
| Outcomes | ANALYSIS by intention‐to‐treat OUTCOMES reported at 24 weeks or endpoint PULMONARY FUNCTION TESTS
SYMPTOM SCORES
FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2002) Funded by GlaxoSmithKline Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated in block of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented including withdrawal with reasons by group and adverse effects by group, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | PARTICIPANTS WITH PERSISTENT ASTHMA RANDOMISED: WITHDRAWALS: AGE (± SD): GENDER (% male): ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described % Pred. FEV1 (±SD): MON: 75.7 (± 7.0) MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION:: Subjects with chronic obstructive pulmonary disease. | |
| Interventions | PROTOCOL Duration CONTROL GROUP: Fluticasone propionate 250 twice a day TEST GROUP: Montelukast 10 mg QD DEVICE: Not reported Criteria for withdrawal from study: Reported | |
| Outcomes | ANALYSIS: Not reported OUTCOMES reported at 24 weeks PULMONARY FUNCTION TESTS: Change from baseline in morning PEFR SYMPTOM SCORES: Change from baseline in mean day time symptom score FUNCTIONAL STATUS: *Percentage of asthma‐control days over the 6‐month treatment period, INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 500 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation:not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐groups, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS; N (%): AGE: Mean ( range) GENDER (% male): BASELINE SEVERITY: Not mentioned % Pred. FEV1 (Mean ± SD): MEAN (± SD) β2‐AGONIST USE (puffs/day): ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention: 4 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Not reported CO‐INTERVENTION: None permitted | |
| Outcomes | PER PROTOCOL ANALYSIS (not ITT) OUTCOMES reported at 4 and endpoint PULMONARY FUNCTION TESTS
SYMPTOM SCORES
FUNCTIONAL STATUS: % Change from baseline mean daily B2‐agonist use (puffs/day) INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2001) Funded by: Not reported Confirmation of methodology and data extraction obtained/not requested/not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Double blind |
| Incomplete outcome data (attrition bias) | Unclear risk | All data presented, adverse events by group were mentioned, 3% and 8% patients were withdrawn in fluticasone and zafirlukast, not a intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐groups, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS; N (%): AGE: Mean ( years, SD): GENDER: n (% male): 39 (20.4%) BASELINE SEVERITY: Not mentioned % Pred. FEV1 (Mean ± SD): Not reported β2‐AGONIST USE (puffs per day): MEAN (± SD): Not reported ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 4 ‐ 5 mg per day CONTROL GROUP: ICS DEVICE: Not reported CO‐INTERVENTION: None permitted | |
| Outcomes | PER PROTOCOL ANALYSIS (not ITT) OUTCOMES reported at 12 week endpoint PULMONARY FUNCTION TESTS: Not reported SYMPTOM SCORES:
FUNCTIONAL STATUS: Not reported INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | Not reported | |
| Notes | Unpublised data (2008) Funded by: Merck | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Incomplete outcome data (attrition bias) | Low risk | High withdrawal rate but balanced in both groups |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Cross over, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: WITHDRAWALS; N (%) AGE: Mean±SD GENDER (% male): BASELINE SEVERITY: Not mentioned % Pred. FEV1 (Mean ± SD): Not mentioned β2‐AGONIST USE (puffs per day): MEAN (± SD): Not mentioned ATOPY: Not mentioned ASTHMA DURATION: Not mentioned ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Not reported CO‐INTERVENTION: None permitted | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT ANALYSIS OUTCOMES reported at 8 week endpoint PULMONARY FUNCTION TESTS: *FEV‐1 % predicted SYMPTOM SCORES: Proportion of symptom‐free day FUNCTIONAL STATUS: % Change from baseline mean daily B2‐agonist use (puffs/day) INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2007) Funded by: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomisation table was prepared by pharmacist |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Dobule blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented; intention‐to‐treat analysis was done; reason for drop outs by groups were mentioned |
| Selective reporting (reporting bias) | High risk | Primary outcome was presented; however the day time asthma symptoms and nocturnal awakenings (secondary outcomes) were not presented in details |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | ASTHMATIC PATIENTS WITH AT LEAST 6‐MONTH HISTORY OF CHRONIC ASTHMA AS PER ATS GUIDELINES RANDOMISED: WITHDRAWALS; N(%) AGE: Mean years (range): GENDER (% male): BASELINE SEVERITY: % Pred. FEV1 (Mean ± SD) MEAN ± SD) β2‐AGONIST USE (puffs/day) ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast sodium chewable tablet 5 mg once daily CONTROL GROUP: Fluticasone propionate 50 μg twice daily DEVICE: DISKUS multi‐dose powder inhaler CO‐INTERVENTION: None permitted | |
| Outcomes | ANALYSIS by intention‐to‐treat OUTCOMES reported at 12 weeks or endpoint PULMONARY FUNCTION TESTS
SYMPTOM SCORES
FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full‐text publication Funded: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes are reported including withdrawal rate and reasons for withdrawals were well described by group, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcomes was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | ATOPIC MILD ASTHMA PATIENTS RANDOMISED: WITHDRAWALS; % AGE: mean (range) years M = 24.5 (19–49) GENDER (% male): 55.56% % Pred. FEV1 Mean ± SE ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast sodium capsule (10 mg nocte) CONTROL GROUP: Fluticasone propionate (100 μg twice a day) by Diskus inhaler DEVICE: DISKUS multi‐dose inhaler CO‐INTERVENTION: None permitted | |
| Outcomes | ANALYSIS by intention‐to‐treat. Not reported OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MARKERS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by GlaxoSmithKline R&D, UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | There was no withdrawal rate; all patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the manuscript are reported, including adverse events |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | MILD ASTHMATIC CHILDREN ALLERGIC TO HOUSE DUST MITE RANDOMISED: WITHDRAWALS; N(%): 3 (12.5%) AGE: range years: 6‐13 years GENDER (% male): 41.67% % Pred. FEV1 (Median%) ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast (5 mg administered once a day in the evening), CONTROL GROUP: Budesonide (100 µg twice daily) DEVICE: Turbuhaler CO‐INTERVENTION: None permitted | |
| Outcomes | ANALYSIS by intention‐to‐treat: Not reported PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MARKERS: Sputum eosinophil cell count (%) ADVERSE EVENTS: Not reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding source: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: used randomisation table prepared by a person not included in the study |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope were used to allocate drug however the concealment method is not described |
| Blinding (performance bias and detection bias) | Low risk | Double blind; centralized randomisation table and placebo preparation |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis was not done by intention‐to‐treat; Data from 8.3% and 16.6% of patients were not evaluated due to lack of compliance to treatment. |
| Selective reporting (reporting bias) | Low risk | Primary outcome was not defined but not bias was observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | ASTHMATIC PATIENTS RANDOMISED: WITHDRAWALS; N (%) AGE: Mean ± SD GENDER (% male): % of predicted value: mean ± SD At randomisation ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast (Singulair, GlaxoSmithKline), 5 mg once daily for children ages 6 to 14 years and 10 mg once daily for persons 15 years or older CONTROL GROUP: Inhaled fluticasone propionate (100 μg twice daily) DEVICE: Flovent Diskus CO‐INTERVENTION: None permitted | |
| Outcomes | ANALYSIS by intention‐to‐treat OUTCOMES reported at 16 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES
FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding source: Unrestricted grant from GlaxoSmithKline, a grant from the American Lung Association. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomised using permuted‐block design stratified by clinic and age of patient. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Placebo and matching montelukast tablets used for the study. |
| Incomplete outcome data (attrition bias) | Low risk | All data reported in detail, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary data were well defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 48 WITHDRAWALS; AGE: mean (± SD) years GENDER: N (% male): BASELINE SEVERITY: Mild persistent % Predicted FEV1 (Mean ± SD) ATOPY/ALLERGEN TRIGGERS: Not reported ASTHMA DURATION (years): 1 year ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Zafirlukast 20 mg twice a day TEST GROUP 2: Zafirlukast 20 mg twice a day + Budesonide 400 μg twice a day CONTROL GROUP: Budesonide 400 μg twice a day CONTROL GROUP 2: Placebo DEVICE: Not reported CO‐INTERVENTION: Not reported | |
| Outcomes | ANALYSIS PER PROTOCOL (not by ITT) OUTCOMES PULMONARY FUNCTION TEST
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not mentioned WITHDRAWALS: Not reported Primary outcome: Not specified. | |
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication (2001) Funded by University Confirmation of methodology and data extraction: obtained from Graziano Riccioni, Oct 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Data on asthma diary, number of puffs used, compliance of treatment were mentioned in methodology but not addressed in the results |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Obtained (Oct 2003) | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 40 WITHDRAWALS: Reported AGE: years ± SD GENDER (% male): ASTHMA SEVERITY: Mild and persistent ASTHMA DURATION: Not mentioned % Pred. FEV1: Mean (± SD) β2‐agonist use (puffs per day): Not described ATOPY: PARTICIPANTS WITH ALLERGIC RHINITIS: ELIGIBILITY CRITERIA:
EXCLUSION CRITERIA:
| |
| Interventions | PROTOCOL Duration TEST GROUP: 10 mg Montelukast per day CONTROL GROUP: 400 μg Budesonide twice a day DEVICE: Turbuhaler CRITERIA FOR WITHDRAWAL: Mentioned CO‐INTERVENTION: Not permitted | |
| Outcomes | ANALYSIS PER PROTOCOL OUTCOMES: Reported at 16 weeks PULMONARY FUNCTION TEST:
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not mentioned INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported (overall, not in details) WITHDRAWALS: Reported Primary outcome: not specified | |
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication Funded by the University "G. D'Annunzio" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind (patient and assessor) |
| Incomplete outcome data (attrition bias) | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined but no bias was observed |
| Other bias | Low risk | Not found |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. CONFIRMATION OF METHODOLOGY: obtained (Oct 2003) | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 45 WITHDRAWALS: AGE: years ± SD GENDER: N (% male): ASTHMA SEVERITY: Mild ASTHMA DURATION: 1 year % Pred. FEV1: mean (range): Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY: 100% (Definition not mentioned) ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention: 16 weeks TEST GROUP 1: 10 mg Montelukast once daily TEST GROUP 2: 10 mg Montelukast once daily + 400 μg Budesonide twice a day CONTROL GROUP: 400 μg Budesonide twice a day DEVICE: Turbo haler CRITERIA FOR WITHDRAWAL: Not described CO‐INTERVENTION: None permitted | |
| Outcomes | ANALYSIS PER PROTOCOL: not by ITT OUTCOMES: Reported at 16 weeks PULMONARY FUNCTION TEST:
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not mentioned WITHDRAWALS: Reported Primary outcome: Not specified | |
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication Funded by University Confirmation of methodology and data extraction: obtained by Graziono Riccioni, Oct 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Data on compliance of treatment and adverse effects were mentioned in methodology but not addressed in the results |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | ATOPIC NON‐SMOKING MILD‐PERSISTENT BRONCHIAL ASTHMATICS RANDOMISED: N = 45 WITHDRAWALS: Not mentioned AGE: years ± SD: GENDER (% male): ASTHMA SEVERITY: Mild ASTHMA DURATION: 1 year % Pred. FEV1: mean ± SD: ATOPY: 100% (Dermatophagoides Pteronyssinus) ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP 1: 10 mg Montelukast once daily CONTROL GROUP: 400 μg Budesonide twice a day DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL: Not described CO‐INTERVENTION: None permitted | |
| Outcomes | ANALYSIS : Not mentioned OUTCOMES PULMONARY FUNCTION TEST:
SYMPTOM SCORES: Not reported INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not mentioned WITHDRAWALS: Not reported Primary outcome: Not specified | |
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication Funded: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | There is no report as to whether some patients withdrew from the study after randomisation |
| Selective reporting (reporting bias) | High risk | The primary outcome was not specified. The data on several outcomes was insufficiently reported to be aggregated namely, number of puffs used each day; daily asthma diary; adverse events, FVC, compliance of treatment were not reported |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: unclear if stratified randomisation on baseline FEV1 value FEV1 < 70% WITHDRAWALS: Not reported AGE: mean (± SD) years: Not reported GENDER (% male): Not reported BASELINE SEVERITY: Not reported % Predicted FEV1: (FEV1 < 70%) ATOPY/ALLERGEN TRIGGERS: Not reported ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION: Not reported | |
| Interventions | PROTOCOL Duration TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL: Not mentioned CO‐INTERVENTION: Not mentioned | |
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Change in symptom‐free days (%) FUNCTIONAL STATUS: Change from baseline in rescue β2‐agonist use (puffs/day) INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Abstract Funding not mentioned, probably by GSK Confirmation of methodology and data extraction: Not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind ‐double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, clinical trial. | |
| Participants | CHILDREN WITH MILD TO MODERATE PERSISTENT ASTHMA RANDOMISED: N = 285 M = 95 WITHDRAWALS: AGE: mean ± SD years GENDER: % male ASTHMA SEVERITY: Mild to moderate % Pred. FEV1 (mean ± SD): FP:97.8 ± 12.2 ATOPY or ALLERGIC RHINITIS: ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration Intervention Period: 486 weeks TEST GROUP: Montelukast 10 mg qd p.o. CONTROL GROUP 1: Fluticasone propionate 100 μcg morning and 100 μcg evening DEVICE: Flovent Diskus; GlaxoSmithKline CO‐INTERVENTION: None permitted | |
| Outcomes | ANALYSIS by Intention‐to‐treat OUTCOMES reported at 48 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Asthma control questionnaire FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: ENO, % ADVERSE EVENTS: Reported WITHDRAWALS: Reported *Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomisation was stratified by centre |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind; double‐dummy with identical placebo |
| Incomplete outcome data (attrition bias) | Unclear risk | Approximately 12% of withdrawal, balanced between groups but the reasons for withdrawal were not specified by group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the manuscript are reported, including adverse events |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 91 WITHDRAWALS: AGE: years ± SD: (without withdrawals): GENDER (% male): ASTHMA SEVERITY: Moderate % Pred. FEV1 % (± SD): Mean (± SD) β2‐agonist use (puffs/day): Not described ALLERGIC RHINITIS: ATOPY (to dust mites): DURATION OF ASTHMA: ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 5 mg per day if <=14 years (10 mg per day, otherwise) CONTROL GROUP 1: Inhaled Triamcinolone acetonide 100ug/day qid CONTROL GROUP 2: Placebo (not used in this review) CONTROL GROUP 3: Formoterol 12 μg twice a day (not used in this review) DEVICE: MDI (actuation inhaler) CO‐INTERVENTION: No other Rx allowed other than rescue b2‐agonists or rescue oral corticosteroids | |
| Outcomes | ANALYSIS per protocol OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 ‐Change from baseline in PC 20 SYMPTOM SCORES: Total score (3‐point for daytime symptoms + 3‐point for night‐time symptoms + 3 points for use of rescue β2‐agonists) FUNCTIONAL STATUS: Patients with exacerbations requiring systemic corticosteroids INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome not specified | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication (2002) Self‐funded by authors Confirmation of methodology and data obtained from I. Stelmach June 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, reasons for withdrawal were mentioned |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N =154 WITHDRAWALS (N = 14) AGE: years ± SD: GENDER (% male): ASTHMA SEVERITY: Moderate asthma % Pred. FEV1 % ±SD: ‐ after withdrawals Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): ALLERGIC RHINITIS: DURATION OF ASTHMA (years): ASTHMA SYMPTOMS: ELIGIBILITY CRITERIA:
EXCLUSION:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 5 mg per day if <= 14 years (10 mg per day, otherwise) CONTROL GROUP 1: Inhaled Triamcinolone acetonide 200ug twice a day CONTROL GROUP 2: Placebo (not used in this review) CONTROL GROUP 3: Formoterol 12 μg twice a day (not used in this review) CONTROL GROUP 4: Nedocromil 0.002 g/inhalation 2 inhalations qid (not used in this review) DEVICE: MDI (actuation inhaler) CO‐INTERVENTION: No other Rx allowed other than rescue β2‐agonist or rescue systemic corticosteroids. | |
| Outcomes | ANALYSIS per protocol OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 ‐Change from baseline in PC 20 SYMPTOM SCORES: Total score (3‐point for daytime symptoms + 3‐point for night‐time symptoms + 3 points for use of rescue β2‐agonists) FUNCTIONAL STATUS INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported overall (not by group) Primary outcome: Not specified | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication (2002) Self‐Funded Confirmation of methodology and data obtained from I. Stelmach June 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, overall reasons for withdrawal were mentioned |
| Selective reporting (reporting bias) | Low risk | Primary outcome was not defined but no bias was observed |
| Other bias | Low risk | Not found |
| Methods | DESIGN: Three armed, parallel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH HISTORY OF ASTHMA AND SENSITIVE TO HOUSE DUST MITES (DERMATOPHAGOIDES PTERONYSSINUS AND/OR DERMATOPHAGOIDES FARINE) RANDOMISED: N =250 WITHDRAWALS: N = 4 AGE: years ± SD: GENDER (% male): ASTHMA SEVERITY: Mild to moderate asthma % Pred. FEV1 % ±SD ‐ after withdrawals: ATOPY (described as chronic atopic): DURATION OF ASTHMA (years): ASTHMA SYMPTOMS: ELIGIBILITY CRITERIA: Age= 6‐18 years
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 5 mg tablets in children aged 6–14 yr or 10 mg tablets in children over 14 yr of age CONTROL GROUP 1: Inhaled Triamcinolone acetonide two puffs twice a day (400 μg/day), DEVICE: Azmacort, Aventis, USA CO‐INTERVENTION: No other Rx allowed other than rescue b2‐agonists or rescue oral corticosteroids | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Total asthma score INFLAMMATORY MEDIATORS: Eosinophil blood count ADVERSE EVENTS: Not reported WITHDRAWALS: Reported overall (not by group) Primary outcome not specified | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication Funded: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open labelled |
| Incomplete outcome data (attrition bias) | Low risk | The withdrawal rate was low (2%) and balanced between groups and the reasons for withdrawal were specified by group. |
| Selective reporting (reporting bias) | High risk | The primary outcome was not specified and the results of the Paediatric asthma quality of life questionnaire and adverse effects were not presented. The data was not reported as analysed by intention‐to‐treat analysis. |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH NEWLY DIAGNOSED ASTHMA AND SENSITIVE TO HOUSE‐DUST MITES (DERMATOPHAGOIDES PTERONYSSINUS AND/OR DERMATOPHAGOIDES FARINE) RANDOMISED: N = 51 High dose BD (800 mg/day): 18 WITHDRAWALS: N = 2 AGE: years ± SD Medium dose BD (400 mg/day): 11.6 ± 1.4 High dose BD (800 mg/day): 11.6 ± 1.8 GENDER: % male: M: 56.3 Medium dose BD (400 mg/day): 60 High dose BD (800 mg/day): 55.6 ASTHMA SEVERITY: Newly diagnosed asthma and sensitive to house‐dust mites (Dermatophagoides pteronyssinus or/and Dermatophagoides farinae) % Pred. FEV1 % ±SD ‐ after withdrawals: M: 83.4 ± 0.4 Medium dose BD (400 mg/day): 84.3 ± 0.5 High dose BD (800 mg/day): 83.4 ± 0.3 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years): Not described ASTHMA SYMPTOMS: Not described ELIGIBILITY CRITERIA:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast sodium 5 mg tablets in children aged 6–14 yr or 10 mg tablets in children over 14 yr of age CONTROL GROUP 1 (Medium dose): Inhaled Budesonide 400 mg/day CONTROL GROUP 2 (High dose): Inhaled Budesonide 800 mg/day DEVICE: Dry powder capsule (Miflonide, Novartis, Switzerland) CO‐INTERVENTION:: No other Rx allowed other than rescue b2‐agonists | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 6 months PULMONARY FUNCTION TESTS: FEV1% of predicted SYMPTOM SCORES: Total asthma score INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported by group *Primary outcomes | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication Funded: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Doubl blind, double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | The withdrawal rate was low, balanced between groups with reasons for withdrawal reported by group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the manuscript are reported, including adverse events |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH NEWLY DIAGNOSED ASTHMA AND SENSITIVE TO HOUSE‐DUST MITES (DERMATOPHAGOIDES PTERONYSSINUS AND/OR DERMATOPHAGOIDES FARINE) RANDOMISED: N = 51 BD: 29 M + BD: 29 Formoterol + BD: 29 Placebo: 29 WITHDRAWALS: N = 2 M: 0 BD: 0 M + BD: 0 Formoterol + BD: 0 Placebo: 2 AGE: years ± SD M: 10.4 ± 2.9 BD: 12 ± 3.1 M + BD: 11 ± 3.2 Formoterol + BD: 8.79 ± 2.4 Placebo: 11.4 ± 3.3 GENDER: % male M: 62.1 BD: 69 M + BD: 69 Formoterol + BD: 58.6 Placeboe: 70.4 ASTHMA SEVERITY: Newly diagnosed asthma and sensitive to house‐dust mites (Dermatophagoides pteronyssinus or/and Dermatophagoides farinae) % Pred. FEV1 % ± SD: M: 95.5 ± 2.1 BD: 94.4 ± 1.8 M + BD: 94.8 ± 2.1 Formoterol + BD: 93.1 ± 2.3 Placebo: 94.8 ± 1.8 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years ± SD): M: 3.85 ± 0.61 BD: 3.97 ± 0.52 M + BD: 3.79 ± 0.58 Formoterol + BD: 3.93 ± 0.63 Placebo: 3.88 ± 0.47 ASTHMA SYMPTOMS: Not described ELIGIBILITY CRITERIA:
EXCLUSION CRITERIAS:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast sodium 5 mg tablets in children aged 6–14 yr or 10 mg tablets in children over 14 yr of age CONTROL GROUP 1: Inhaled Budesonide 200 mg/day CONTROL GROUP 2: Montelukast 5 or 10 mg tablets and Inhaled Budesonide200 mg/day CONTROL GROUP 3: Formoterol mg/day and Inhaled Budesonide200 mg/day CONTROL GROUP 4: Placebo DEVICE: Turbuhaler | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Not described INFLAMMATORY MEDIATORS: Not described ADVERSE EVENTS: Not reported WITHDRAWALS: Reported by group | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication Funded: Grants from the Medical University of Lodz, Poland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind; double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, balanced numbers in all groups, reason for withdrawal by group reported, |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcome was not specified but no bias was observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH SYMPTOMATIC ASTHMA AND SENSITIVE TO HOUSE‐DUST MITES (DERMATOPHAGOIDES PTERONYSSINUS AND/OR DERMATOPHAGOIDES FARINE) RANDOMISED: N =100 BD: 20 M + BD: 20 Formoterol + BD: 20 Placebo: 20 WITHDRAWALS: N = 9 BD: 0 M + BD: 3 Formoterol + BD: 2 Placebo: 1 AGE: years (range) M: 11.8 (7‐17) BD: 11.9 (6‐16) M + BD: 12.2 (7‐18) Formoterol + BD: 11.3 (6‐18) Placebo: 12.1 (7‐15) GENDER (% male): Not reported ASTHMA SEVERITY: Not reported % Pred. FEV1 % ±SD: M: 93.5 ± 11.4 BD:92.2 ± 13.5 M + BD: 90.2 ± 10.2 Formoterol + BD: 91.1 ± 11.2 Placebo: 92.4 12.7 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years): Not reported ASTHMA SYMPTOMS: Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast sodium 5 mg tablets in children aged 6–14 yr or 10 mg tablets in children over 14 yr of age CONTROL GROUP 1: Inhaled Budesonide 200 mg/day CONTROL GROUP 2: Montelukast 5 or 10 mg tablets and Inhaled Budesonide200 mg/day CONTROL GROUP 3: Formoterol mg/day and Inhaled Budesonide200 mg/day CONTROL GROUP 4: Placebo DEVICE: Turbuhaler | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: AUC0‐20min of FEV1 Maximum % fall in FEV1 SYMPTOM SCORES: Not described INFLAMMATORY MEDIATORS: Not described ADVERSE EVENTS: Not reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication Funded: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double blind; double‐dummy |
| Incomplete outcome data (attrition bias) | Unclear risk | The overall withdrawal rate was 10% (N = 9) but it was not presented by group. This might be of importance as the only reason for withdrawal was poor efficacy. |
| Selective reporting (reporting bias) | Low risk | Primary outcome was not pre‐specified however no bias was observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Cross‐over, randomised, clinical trial. | |
| Participants | PATIENTS WITH MILD TO MODERATE PERSISTENT ASTHMA RANDOMISED: N = 144 WITHDRAWALS: no (%) N = 17, (11.81%) M: 11 (7.64%) FP: 6 (4.17%) AGE: years ± SD: Not reported GENDER (% male): Not reported ASTHMA SEVERITY: Not reported % Pred. FEV1 % ± SD: Not reported Mean (± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Not reported ALLERGIC RHINITIS: Not reported DURATION OF ASTHMA (years): Not reported ASTHMA SYMPTOMS:Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast sodium 5‐mg chewable tablet for those 6 to 14 years of age and 10‐mg tablet for those 15 to 18 years of age CONTROL GROUP 1: Flovent Diskus, 100 μg per inhalation administered as one inhalation twice a day DEVICE: Diskus | |
| Outcomes | ANALYSIS NOT BY INTENTION‐TO‐TREAT OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS:
FUNCTIONAL TESTS:
SYMPTOM SCORES: Not reported INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported by groups WITHDRAWALS: Reported *Primary outcomes | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication Funded: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: by minimization method for several factors |
| Allocation concealment (selection bias) | Unclear risk | Nnot reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | No intention‐to‐treat analysis, 8.4% and 15.1% of withdrawal in ICS and montelukast groups respectively |
| Selective reporting (reporting bias) | Low risk | Primary outcome was specified |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | PATIENTS WITH MILD PERSISTENT ASTHMA RANDOMISED: N = 395 BD: 197 WITHDRAWALS: no (%) N = 115, (29.1%) M: 52 (26.40%) BD: 63 (31.98%) AGE: years ± SD M: 4.7 ± 1.9 BD: 4.6 ± 2.0 GENDER (% male): M: 118 ± 59.9 BD: 122 ± 61.9 ASTHMA SEVERITY: Not reported % Pred. FEV1 % ± SD: M: 91.67 ± 18.07 BD:89.88 ± 17.75 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years): Not reported ASTHMA SYMPTOMS: Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast sodium 4 or 5 mg tablets CONTROL GROUP 1: Budesonide inhalation suspension 0.5 mg/day DEVICE: Not described | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT OUTCOMES reported at 52 weeks PULMONARY FUNCTION TESTS:
FUNCTIONAL TESTS:
SYMPTOM SCORES
INFLAMMATORY MEDIATORS: Not described ADVERSE EVENTS: Reported by groups WITHDRAWALS: Reported *Primary outcomes | |
| ICS dose in HFA beclomethasone ‐ equivalent | 250 μg | |
| Notes | Full Text publication Funded: AstraZeneca LP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open labelled and study subjects could be discontinued at any time at the discretion of the investigators. |
| Incomplete outcome data (attrition bias) | Unclear risk | the withdrawal data is presented by group, however the large withdrawal rate (29%), similar in both group, while acceptable for the main outcome (time to event) raise doubt about the validity of the secondary outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | NEWLY DIAGNOSED MILD INTERMITTENT ASTHMA PATIENTS RANDOMISED: N = 85 BD: 43 WITHDRAWALS: no (%) N = 11 (12.94%) PRAN: 6 (14.29%) BD: 5 (11.63%) AGE: years ± SD PRAN: 36 ± 3 BD: 38 ± 4 GENDER (% male) PRAN: 27.7 BD: 26.32 ASTHMA SEVERITY: Not reported % Pred. FEV1 % ± SD: PRAN: 84.6 ± 2.0 BD: 85.0 ± 2.1 Mean (±SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years): PRAN: 1.6 ± 1.1 BD: 2.0 ± 1.2 ASTHMA SYMPTOMS SCORE: BD: 5.7 ± 0.5 INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Pranlukast 225 mg twice a day CONTROL GROUP: Inhaled BDP 100 μg twice a day DEVICE: Metered‐dose inhaler using a spacing chamber | |
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS:
FUNCTIONAL TESTS: Changes in the use of supplemental 2‐agonist SYMPTOM SCORES: Asthma symptom score INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication Funded: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomised using balanced block of four |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, drop‐outs are reported by group and were balanced between the two groups |
| Selective reporting (reporting bias) | Low risk | No apparent other bias |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. ‐Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 30 WITHDRAWALS: Not reported AGE (± SD) years: GENDER (% male): ASTHMA SEVERITY: Mild persistent or mild intermittent % Pred. FEV1 (mean ± SD) Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY: ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION CRITERIA: Not described. | |
| Interventions | PROTOCOL Duration TEST GROUP: Pranlukast 450 mg/day CONTROL GROUP: Inhaled Beclomethasone 400 μg/day DEVICE: Not reported CO‐INTERVENTION: Theophylline | |
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: Change in morning and evening PEFR SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported Primary outcome: Not specified | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2001) Funded by the Ministry of Education, Science, Sports and Culture in Japan Confirmation of data and methodology requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | No details on blinding provided |
| Incomplete outcome data (attrition bias) | Unclear risk | All data presented, no intention‐to‐treat analysis and withdrawal rate is not reported |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 74 BDP: 25 WITHDRAWALS: Reported AGE (years ± SD): THEO: 33.5 ± 5 GENDER (% male): M: 20% THEO: 25% ASTHMA SEVERITY: Mild persistent % Pred. FEV1 (mean ± SD): THEO: 86.6 ± 5.5 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY: Not reported ASTHMA DURATION (years): Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Pranlukast 450 mg/day CONTROL GROUP: Inhaled Beclomethasone 400 μg/day twice a day DEVICE: Not reported CO‐INTERVENTION: Theophylline | |
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS: Mean number of rescue inhalations, puffs/day INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported exacerbation by groups WITHDRAWALS: Not reported *Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2003) Funded by: Not reported Confirmation of data and methodology: Not done | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: simple random sampling method according to random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open label |
| Incomplete outcome data (attrition bias) | High risk | No intention‐to‐treat analysis and no reported withdrawal rate |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the manuscript are reported, including adverse events by group |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 56 FP: 29 WITHDRAWALS: Reported AGE: years ± SD: GENDER (% male): 64.15% ASTHMA SEVERITY: Moderate persistent asthma % Pred. FEV1 (mean ± SD): Not reported Mean (±SD) β2‐agonist use (puffs/day): Not described ATOPY: Not reported ASTHMA DURATION (years): N: 5.36 ELIGIBILITY CRITERIAS:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 5 mg at bed time CONTROL GROUP: Fluticasone propionate (100 μg twice daily) DEVICE: Pressurized metered‐dose inhaler CO‐INTERVENTION: Short‐acting β2 agonists | |
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: FEV1 SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2009) Funded by: Not reported Confirmation of data and methodology: Not done | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Non‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, withdrawal rate per group presented |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 380 WITHDRAWALS; N (%): AGE: Mean ( years ± SD): GENDER (% male): BASELINE SEVERITY: Mild and moderate % Pred. FEV1 ± SD: MEAN (± SD) β2‐AGONIST USE (puffs/day): ATOPY: Not reported ASTHMA DURATION: >=4 months ELIGIBILITY CRITERIA
EXCLUSION: Not reported | |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 10 mg od CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Not reported CO‐INTERVENTION: Not reported | |
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Asthma symptoms FUNCTIONAL STATUS:
INFLAMMATORY MARKERS: Blood eosinophil count ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full paper (2005) Funded by Merck & Co. Inc Confirmation of methodology and data extraction: Not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind,double dummies: "appropriate matching placebo' |
| Incomplete outcome data (attrition bias) | Low risk | Modified intention‐to‐treat with low and similar withdrawal rate (6% LTRA versus 9% FP); |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome (rescue‐free days) reported; poorly reported clinical and laboratory adverse health events |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Cross over, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 144 WITHDRAWALS; N (%): AGE: Mean years ± SD: GENDER (% male): Not reported BASELINE SEVERITY: Mild and moderate persistent asthma % Pred. FEV1 (Mean ± SD): Not reported MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 5‐mg chewable tablet for those 6 to 14 years of age or as a 10‐mg for 15 to 18 years of age CONTROL GROUP: Fluticasone propionate 100 mg per inhalation administered as twice a day DEVICE: Flovent Diskus CO‐INTERVENTION: Not reported | |
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS
INFLAMMATORY MARKERS: eNO ADVERSE EVENTS: Not reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full paper (2006) Funded by: Not reported Confirmation of methodology and data extraction: Not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: minimization method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind using double dummies: " placebo for the alternative drug" |
| Incomplete outcome data (attrition bias) | High risk | No intention‐to‐treat analysis; withdrawal rate 12.5% and most withdrew because of treatment failure (i.e. requiring treatment with systemic corticosteroids with an imbalance between treatment period (2 FP and 10 in Montelukast) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | DESIGN: Parallel‐group, single centre, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 102 WITHDRAWALS; N AGE: N: 4.94 GENDER (% male): N: 58.82% BASELINE SEVERITY: Episodic asthmatics % Pred. FEV1 (Mean ± SD): N: 98.8% MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION: Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
| |
| Interventions | PROTOCOL Duration TEST GROUP: Montelukast 4‐mg chewable tablet for those 4 to 6 years of age or as a 5‐mg for older than 6 years CONTROL GROUP: Inhaled fluticasone 100 mg twice daily DEVICE: By spacer CO‐INTERVENTION: Inhaled salbutamol | |
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Asthma symptoms FUNCTIONAL STATUS: Number of exacerbations INFLAMMATORY MARKERS:
ADVERSE EVENTS: Reported WITHDRAWALS: Reported | |
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full paper (2010) Funded by: Disclosed for all funds for authors Confirmation of methodology and data extraction: Not obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open label |
| Incomplete outcome data (attrition bias) | Low risk | All data presented, |
| Selective reporting (reporting bias) | Low risk | Primary outcome and secondary outcomes reported, withdrawal by group reported |
| Other bias | Low risk | No apparent other bias |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Intervention was given < 4 weeks | |
| Participants were not people with asthma | |
| Participants were not people with asthma Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial (meta‐analysis) | |
| Participants received additional non‐permitted co‐interventions (cetirizine with montelukast and intranasal beclomethasone with inhaled beclomethasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroids | |
| Participants received additional non‐permitted co‐interventions (fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplicate of published paper in Eur Respir J 2003;21:123‐128. | |
| Participants received additional non‐permitted co‐interventions (budesonide with zafirlukast and oral loratadine/pseudoephedrine with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (combination of budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplicate of published paper in Journal of Allergy & Clinical Immunology 2007;119(4):916‐923. | |
| Duplicate of published paper in Journal of Allergy & Clinical Immunology 2007;119(4):916‐923. | |
| Control intervention was not inhaled corticosteroids. | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not placebo | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone and salmeterole with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone and salmeterole with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone and salmeterole with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid | |
| Intervention administered < 4 weeks | |
| Participants were not people with asthma | |
| Participants were not people with asthma | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Outcome measures did not reflect asthma control | |
| Duplicate of published paper in Annals of Allergy Asthma & Immunology 2003;91(3):309‐313. | |
| Duplicate of published paper in Annals of Allergy Asthma & Immunology 2003;91(3):309‐313. | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not placebo | |
| Participants received additional non‐permitted co‐interventions (zafirlukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐intervention other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Acute asthma setting | |
| Control intervention was not inhaled corticosteroid Acute asthma setting | |
| Not a randomised controlled trial Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid | |
| Participants were non‐asthmatics (atopic dermatitis) | |
| Participants received additional non‐permitted co‐interventions (formoterol with budesonide and montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroids | |
| Intervention was not anti‐leukotriene | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (fexofenadine with montelukast and fexofenadine with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Duplicate of published paper in Journal of Allergy & Clinical Immunology 2007;119(1):64‐72. | |
| Participants received additional non‐permitted co‐interventions (cromoglycate, formoterol with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid; Intervention was given < 4 weeks | |
| Intervention was given < 4 weeks | |
| Intervention was given < 4 weeks; Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplicate of published paper in Journal of Respiratory & Critical Care Medicine 2003;167(9):1232‐1238. | |
| Ongoing trial | |
| Control intervention was not inhaled corticosteroid; | |
| Participants were not people with asthma | |
| Not a randomised controlled trial; All patients not received inhaled corticosteroid as controlled intervention | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid | |
| Intervention administered for < 4 weeks | |
| Intervention administered <4 weeks | |
| Control intervention was not placebo | |
| Control intervention was not placebo | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (inhaled steroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with montelukast and salmeterol with beclomethasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroidervention administered for < 4 weeks | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Outcome measures did not reflect asthma control | |
| Participants received additional non‐permitted co‐interventions (fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Not a randomised controlled trial Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial. Intervention was not anti‐leukotriene. | |
| Outcome measures did not reflect asthma control | |
| Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks | |
| Not a randomised controlled trial. Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid (but placebo). A small number of placebo‐treated patients also received co‐intervention with inhaled steroids (N = 42), but co‐intervention with inhaled steroids was not randomly assigned. | |
| Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Co‐intervention with inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone and fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial. Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Outcome measures did not reflect asthma control | |
| Control intervention was not placebo | |
| Control intervention was not placebo | |
| Control intervention was not placebo. | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with beclomethasone and salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene. Participants were not people with asthma. | |
| Participants received additional non‐permitted co‐interventions (fluticasone+salmeterol with montelukast and salmeterol+levocetirizine with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplicate of published paper in Journal of Allergy & Clinical Immunology 2006;118(1):78‐83. | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (formoterole with budesonide) other than short‐acting beta2‐agonists and/or short course of oral ccorticosteroid | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants were not people with asthma. | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (zafirlukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplicate of published paper in Chang Gung Medical Journal. 2003;26(8):554‐560 | |
| Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Duplicate of published paper in J Fam Pract 2001;50:595‐602. | |
| Participants received additional non‐permitted co‐interventions (montelukast with other antihistaminc drugs) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Control intervention was not placebo | |
| Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled coticosteroid and long acting beta2 agoinit with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid. | |
| Not a randomised controlled trial (meta‐analysis of RCTs) | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Control intervention were not placebo | |
| Duplication of paper in The Journal of Asthma 2008;45( 8):681‐687 | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication of paper in Pulmonary Pharmacology and Therapeutics 2008;21(5):798‐804 | |
| Use of higher than licensed dose of leukotriene receptor antagonists (Pranlukast 600 mcg/day vs. 450 mcg/day) | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroids. | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid Intervention administered for < 4 weeks | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Duplication of paper in Chest 2004;125(4):1372‐1377 | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroids. | |
| Control intervention was not inhaled corticosteroid. | |
| Duplication of paper in American Journal of Respiratory and Critical Care Medicine 2002. 165 (Suppl 8): B4 | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone and fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention administered for < 4 weeks | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not placebo | |
| Control intervention was not placebo (it was sodium cromoglycate) | |
| Intervention was not anti‐leukotriene | |
| Ongoing trial | |
| Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (ICS with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (formoterol/fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with budesonide) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Intervention was given < 4 weeks | |
| Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. | |
| Participants received additional non‐permitted co‐interventions (fluticasone with montelukast and salmeterol with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial; Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Intervention administered for < 4 weeks | |
| Intervention administered for < 4 weeks | |
| Duplicate of published paper in J Allergy Clin Immunol 2000;105:1123‐1129; Outcomes did not reflect control of chronic asthma | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not placebo | |
| Intervention was not anti‐leukotriene. Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Duplicate of published paper in New Engl J of Med 2007;356(20):2027‐2039 | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (montelukast with other anti‐asthma therapies) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Acute asthma setting. | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (beclomethazone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Participantsreceived additional non‐permitted co‐interventions (salmeterol with ICS and ICS with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication of paper in Chest 2005;128(4):1910‐1920 | |
| Participants received additional non‐permitted co‐interventions (fluticasone+salmeterol with montelukast) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Test group is not anti‐leukotrienes. Control group is not ICS. | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Terminated and results are not available | |
| Terminated and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Control group received non‐permitted drugs (Montelukast and long‐acting beta 2 agonist) | |
| Ongoing and results are not available | |
| Test group received non‐permitted drug (Mometasone) | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Ongoing and results are not available | |
| Control group is not ICS | |
| Ongoing and results are not available | |
| Ongoing and results are not available. Test group is not anti‐leukotrienes. | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Control intervention were not placebo | |
| Participants received additional non‐permitted co‐interventions (fluticasone+salmeterol with montelukast) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (fluticasone+salmeterol with montelukast) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Participants were not people with asthma. | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention were not placebo | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Control intervention was not placebo | |
| Control intervention was not placebo | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants were not people with asthma | |
| Duplicate of published abstract in European Respiratory Journal 2002;20(Suppl 38):388s | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not placebo | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone+salmeterold) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (pranlukast with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (pranlukast with inhaled corticosteroids+long acting beta2 agonists) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene; Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone+salmeterold) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Duplicate of published abstract in Journal of Pediatrics 2005;147(2):213‐220 | |
| Outcomes measures did not reflect asthma control | |
| Intervention administered for < 4 weeks | |
| Intervention administered for < 4 weeks | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide+formoterol with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention administered for < 4 weeks | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Intervention administered for < 4 weeks | |
| Participants were not people with asthma | |
| Participants received additional non‐permitted co‐interventions (zafirlukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast and formoterol with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication of paper published in Annals of Allergy Asthma & Immunology 2003;91(1):49‐54 | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (fluticasone with montelukast and salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication of paper published in Pediatrics 2005;116(2):360‐369 | |
| Participants received additional non‐permitted co‐interventions (budesonide+salmeterol with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention were not placebo | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication; Participants received additional non‐permitted co‐interventions (eg. montelukast with budesonide) | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants were not people with asthma (allergic rhinitis) | |
| Participants were not people with asthma | |
| Participants were not people with asthma | |
| Intervention was not anti‐leukotriene; | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Duplication of full paper in Journal of Allergy & Clinical Immunology 2007;119(4):916‐923 | |
| Participants were not people with asthma | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. | |
| Control intervention was not inhaled corticosteroid. Duplication of full paper in Clin Exp Allergy 2001;31:1‐10. | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. | |
| Control intervention was not inhaled corticosteroid | |
| Duplication of paper published in Journal of Asthma 2009;46(5):465‐469 | |
| Duplication of International Journal of Immunopathology and Pharmacology 2001:14(2):87‐92 | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not placebo | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Outcomes did not reflect control of chronic or acute asthma | |
| Participants received additional non‐permitted co‐interventions (fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (inhaled corticosteroids with montelukast and salmeterol with inhaled corticosteroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide with zafirlukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid | |
| Duplication | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. | |
| Duplication | |
| Control intervention was not inhaled corticosteroid. | |
| Treatments were administered for <4 weeks | |
| Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication of full paper in Annals of Allergy Asthma & Immunology 2010;104(6):511‐517 | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial | |
| Non‐permitted drugs | |
| Not a randomised controlled trial | |
| Intervention was not anti‐leukotriene | |
| Intervention was not anti‐leukotriene | |
| Not a randomised controlled trial. Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (budesonide+salmeterol with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was given < 4 weeks | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial‐retrospective analysis | |
| Not a randomised controlled trial | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial. Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (ICS with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (antiasthmatic drugs with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not placebo | |
| Control intervention was not placebo | |
| Participants received additional non‐permitted co‐interventions (inhaled and oral corticosteroids) other than short‐acting beta2‐agonists | |
| Not a randomised controlled trial; Participants received additional non‐permitted co‐interventions (antihistaminic drugs) other than short‐acting beta2‐agonists | |
| Control intervention was not inhaled corticosteroid | |
| Not a randomised controlled trial. Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. | |
| Participants received additional non‐permitted co‐interventions (beclomethasone with pranlukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication of work presented in Am J Respir Crit Care Med 2009;179:A2416 | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not inhaled corticosteroid. Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid. | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene; Control intervention was not inhaled corticosteroid | |
| Control intervention was not placebo | |
| Intervention was not anti‐leukotriene | |
| Participants were not asthmatics (COPD) | |
| Participants received additional non‐permitted co‐interventions (fluticasone with montelukast and salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. | |
| Intervention was not anti‐leukotriene | |
| Intervention administered for < 4 weeks | |
| Intervention administered for < 4 weeks | |
| Intervention administered for < 4 weeks | |
| Control intervention was not inhaled corticosteroid | |
| Duplication | |
| Control intervention was not inhaled corticosteroid | |
| Participants received additional non‐permitted co‐interventions (other antiasthmatic drugs with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Duplication of full paper in Curr Med Res Opin 2001;17(2):96‐104 | |
| Intervention was not anti‐leukotriene | |
| Participants received additional non‐permitted co‐interventions (inhaled steroids) other than short‐acting beta2‐agonists | |
| Control intervention was not inhaled corticosteroid | |
| Intervention administered for < 4 weeks. | |
| Ongoing clinical trial. | |
| Intervention was not anti‐leukotriene | |
| Not a randomised controlled trial | |
| Outcomes did not reflect control of chronic or acute asthma | |
| Control intervention was not inhaled corticosteroids | |
| Control intervention was not inhaled corticosteroid. | |
| Intervention was not anti‐leukotriene | |
| Control intervention was not inhaled corticosteroid | |
| Control participants were not people with asthma. | |
| Duplication of data published in American Journal of Respiratory & Critical Care Medicine 1998;157:A411 | |
| Intervention was not anti‐leukotriene | |
| Intervention administered for < 4 weeks | |
| Intervention administered for < 4 weeks | |
| Duplication of three studies | |
| Control intervention was not placebo | |
| Control intervention was not inhaled corticosteroid; | |
| Intervention administered for < 4 weeks | |
| Participants were not prople with asthma | |
| Intervention administered for < 4 weeks | |
| Outcome measures did not reflect asthma control | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Participants received additional non‐permitted co‐interventions (ICS with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Control intervention was not inhaled corticosteroid | |
| Control intervention was not placebo | |
| Participants received additional non‐permitted co‐intervention (ICS with montelukast) | |
| Control intervention was not inhaled corticosteroid. | |
| Control intervention was not placebo | |
| Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid | |
| Participants received additional non‐permitted co‐interventions (inhaled corticosteroids) other than short‐acting beta2‐agonists | |
| Intervention administered for < 4 weeks | |
| Intervention administered for < 4 weeks | |
| Not a randomised controlled trial | |
| Duplication of full paper published in American Journal of Medicine 2005;118(6):649‐657 | |
| Not a randomised controlled trial (product monograph) | |
| Control intervention was not inhaled corticosteroid | |
| Intervention was not anti‐leukotriene |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with at least one exacerbation requiring systemic steroids Show forest plot | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| Analysis 1.1  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 1 Patients with at least one exacerbation requiring systemic steroids. | ||||
| 1.1 Paediatrics | 6 | 1662 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.99, 1.86] |
| 1.2 Adults | 15 | 4415 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.12, 2.31] |
| 2 Patients with at least one exacerbation requiring hospital admission Show forest plot | 12 | 2715 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.02, 10.94] |
| Analysis 1.2  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 2 Patients with at least one exacerbation requiring hospital admission. | ||||
| 2.1 Paediatrics | 4 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 73.98] |
| 2.2 Adults | 8 | 2157 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.94, 12.17] |
| 3 Change from baseline FEV1 (L) at 4 ‐ 8 weeks Show forest plot | 12 | 3020 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| Analysis 1.3  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 3 Change from baseline FEV1 (L) at 4 ‐ 8 weeks. | ||||
| 3.1 Paediatrics | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.69, 0.13] |
| 3.2 Adults | 11 | 2964 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| 4 Change from baseline FEV1( L) 12 ‐ 16 weeks Show forest plot | 8 | 1778 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| Analysis 1.4  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 4 Change from baseline FEV1( L) 12 ‐ 16 weeks. | ||||
| 4.1 Paediatrics | 2 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 4.2 Adults | 6 | 1599 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.24, ‐0.05] |
| 5 Change from baseline FEV1 (L) at 24 ‐ 26 weeks Show forest plot | 3 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.22, ‐0.04] |
| Analysis 1.5  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 5 Change from baseline FEV1 (L) at 24 ‐ 26 weeks. | ||||
| 5.1 Paediatrics | 1 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.14, 0.12] |
| 5.2 Adults | 2 | 1055 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.23, ‐0.11] |
| 6 Change from baseline FEV1 (L) at 36 ‐ 52 weeks Show forest plot | 2 | 1040 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| Analysis 1.6  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 6 Change from baseline FEV1 (L) at 36 ‐ 52 weeks. | ||||
| 6.1 Paediatrics | 2 | 1040 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| 7 FEV1 irrespective of time of treatment Show forest plot | 23 | 7016 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.14, ‐0.08] |
| Analysis 1.7  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 7 FEV1 irrespective of time of treatment. | ||||
| 7.1 Paediatrics | 4 | 1398 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| 7.2 Adults | 19 | 5618 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.16, ‐0.09] |
| 8 Responders (defined as change from baseline in FEV1 >= 7.5% Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 8 Responders (defined as change from baseline in FEV1 >= 7.5%. | ||||
| 9 Change from baseline FEV1 (%) at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 9 Change from baseline FEV1 (%) at 4 ‐ 8 weeks. | ||||
| 9.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change from baseline FEV1 (%) 12 ‐ 16 weeks Show forest plot | 2 | 603 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐9.81, ‐1.59] |
| Analysis 1.10  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 10 Change from baseline FEV1 (%) 12 ‐ 16 weeks. | ||||
| 10.1 Adults | 2 | 603 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐9.81, ‐1.59] |
| 11 Change from baseline FEV1 (%) at 24 ‐ 26 weeks Show forest plot | 2 | 838 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐10.85, ‐5.55] |
| Analysis 1.11  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 11 Change from baseline FEV1 (%) at 24 ‐ 26 weeks. | ||||
| 11.1 Adults | 2 | 838 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐10.85, ‐5.55] |
| 12 Change from baseline FEV1 % of predicted at 4 ‐ 8 weeks Show forest plot | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐6.56, 1.40] |
| Analysis 1.12  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 12 Change from baseline FEV1 % of predicted at 4 ‐ 8 weeks. | ||||
| 12.1 Paediatrics | 1 | 183 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐4.82, 3.74] |
| 12.2 Adults | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐4.6 [‐8.86, ‐0.34] |
| 13 Change from baseline FEV1 % of predicated at 12 ‐ 16 weeks Show forest plot | 3 | 948 | Mean Difference (IV, Fixed, 95% CI) | ‐3.76 [‐5.01, ‐2.50] |
| Analysis 1.13  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 13 Change from baseline FEV1 % of predicated at 12 ‐ 16 weeks. | ||||
| 13.1 Paediatrics | 1 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐6.02 [‐9.45, ‐2.59] |
| 13.2 Adults | 2 | 613 | Mean Difference (IV, Fixed, 95% CI) | ‐3.41 [‐4.76, ‐2.06] |
| 14 Change from baseline FEV1 % of predicated at 36 ‐ 52 weeks Show forest plot | 3 | 1229 | Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐7.14, 0.12] |
| Analysis 1.14  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 14 Change from baseline FEV1 % of predicated at 36 ‐ 52 weeks. | ||||
| 14.1 Paediatrics | 3 | 1229 | Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐7.14, 0.12] |
| 15 Change from baseline AM PEFR (L/min) at 4 ‐ 8 weeks Show forest plot | 8 | 1926 | Mean Difference (IV, Random, 95% CI) | ‐15.12 [‐20.80, ‐9.44] |
| Analysis 1.15  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 15 Change from baseline AM PEFR (L/min) at 4 ‐ 8 weeks. | ||||
| 15.1 Paediatrics | 1 | 332 | Mean Difference (IV, Random, 95% CI) | ‐7.76 [‐13.43, ‐2.09] |
| 15.2 Adults | 7 | 1594 | Mean Difference (IV, Random, 95% CI) | ‐17.63 [‐22.56, ‐12.69] |
| 16 Change from baseline AM PEFR (L/min) at 12 ‐ 16 weeks Show forest plot | 9 | 2601 | Mean Difference (IV, Random, 95% CI) | ‐19.07 [‐25.86, ‐12.27] |
| Analysis 1.16  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 16 Change from baseline AM PEFR (L/min) at 12 ‐ 16 weeks. | ||||
| 16.1 Paediatrics | 1 | 335 | Mean Difference (IV, Random, 95% CI) | ‐16.9 [‐28.54, ‐5.26] |
| 16.2 Adults | 8 | 2266 | Mean Difference (IV, Random, 95% CI) | ‐19.57 [‐27.27, ‐11.87] |
| 17 Change from baseline AM PEFR (L/min) at 24 ‐ 26 weeks Show forest plot | 3 | 1718 | Mean Difference (IV, Random, 95% CI) | ‐21.62 [‐40.19, ‐3.05] |
| Analysis 1.17  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 17 Change from baseline AM PEFR (L/min) at 24 ‐ 26 weeks. | ||||
| 17.1 Adults | 3 | 1718 | Mean Difference (IV, Random, 95% CI) | ‐21.62 [‐40.19, ‐3.05] |
| 18 Change from baseline AM PEFR (L/min) at 36 ‐ 52 weeks Show forest plot | 3 | 1028 | Mean Difference (IV, Random, 95% CI) | ‐5.06 [‐10.58, 0.45] |
| Analysis 1.18  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 18 Change from baseline AM PEFR (L/min) at 36 ‐ 52 weeks. | ||||
| 18.1 Adults | 3 | 1028 | Mean Difference (IV, Random, 95% CI) | ‐5.06 [‐10.58, 0.45] |
| 19 Change from baseline daytime symptom scores at 4 ‐ 8 weeks Show forest plot | 6 | 1925 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.08, 0.32] |
| Analysis 1.19  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 19 Change from baseline daytime symptom scores at 4 ‐ 8 weeks. | ||||
| 19.1 Paediatrics | 1 | 393 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.14, 0.26] |
| 19.2 Adults | 5 | 1532 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.11, 0.36] |
| 20 Change from baseline daytime symptom scores at 12 ‐ 16 weeks Show forest plot | 9 | 2650 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.18, 0.33] |
| Analysis 1.20  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 20 Change from baseline daytime symptom scores at 12 ‐ 16 weeks. | ||||
| 20.1 Paediatrics | 2 | 388 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.08, 0.48] |
| 20.2 Adults | 7 | 2262 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.16, 0.33] |
| 21 Change from baseline daytime symptom scores at 24 ‐ 26 weeks Show forest plot | 3 | 1719 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.02, 0.42] |
| Analysis 1.21  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 21 Change from baseline daytime symptom scores at 24 ‐ 26 weeks. | ||||
| 21.1 Adults | 3 | 1719 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.02, 0.42] |
| 22 Change from baseline daytime symptom scores at 36 ‐ 52 weeks Show forest plot | 2 | 582 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.02, 0.34] |
| Analysis 1.22  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 22 Change from baseline daytime symptom scores at 36 ‐ 52 weeks. | ||||
| 22.1 Paediatrics | 2 | 582 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.02, 0.34] |
| 23 Change from baseline night‐time awakenings at 4 ‐ 8 week Show forest plot | 3 | 798 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| Analysis 1.23  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 23 Change from baseline night‐time awakenings at 4 ‐ 8 week. | ||||
| 23.1 Adults | 3 | 798 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| 24 Change from baseline night‐time awakenings at 12 ‐ 16 weeks Show forest plot | 9 | 2916 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.11, 0.26] |
| Analysis 1.24  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 24 Change from baseline night‐time awakenings at 12 ‐ 16 weeks. | ||||
| 24.1 Adults | 9 | 2916 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.11, 0.26] |
| 25 Change from baseline night‐time awakenings at 24 ‐ 26 weeks Show forest plot | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| Analysis 1.25  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 25 Change from baseline night‐time awakenings at 24 ‐ 26 weeks. | ||||
| 25.1 Adults | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 26 Change from baseline mean daily use of β2‐agonists at 4 ‐ 8 weeks Show forest plot | 10 | 3264 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.07, 0.34] |
| Analysis 1.26  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 26 Change from baseline mean daily use of β2‐agonists at 4 ‐ 8 weeks. | ||||
| 26.1 Paediatrics | 1 | 393 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.17] |
| 26.2 Adults | 9 | 2871 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.10, 0.38] |
| 27 Change from baseline mean daily use of β2‐agonists at 12 ‐ 16 weeks Show forest plot | 11 | 3367 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.17, 0.30] |
| Analysis 1.27  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 27 Change from baseline mean daily use of β2‐agonists at 12 ‐ 16 weeks. | ||||
| 27.1 Paediatrics | 1 | 335 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.20, 0.23] |
| 27.2 Adults | 10 | 3032 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.19, 0.33] |
| 28 Change from baseline mean daily use of β2‐agonists at 24 ‐ 26 weeks Show forest plot | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.19, 0.43] |
| Analysis 1.28  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 28 Change from baseline mean daily use of β2‐agonists at 24 ‐ 26 weeks. | ||||
| 28.1 Adults | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.19, 0.43] |
| 29 Change from baseline mean daily use of β2‐agonists at 36 ‐ 52 weeks Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.29  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 29 Change from baseline mean daily use of β2‐agonists at 36 ‐ 52 weeks. | ||||
| 29.1 Paediatrics | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 30 Change in rescue‐free days (%) at 4 ‐ 8 weeks Show forest plot | 5 | 1315 | Mean Difference (IV, Random, 95% CI) | ‐6.83 [‐17.73, 4.07] |
| Analysis 1.30  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 30 Change in rescue‐free days (%) at 4 ‐ 8 weeks. | ||||
| 30.1 Paediatrics | 1 | 393 | Mean Difference (IV, Random, 95% CI) | 2.72 [‐3.11, 8.55] |
| 30.2 Adults | 4 | 922 | Mean Difference (IV, Random, 95% CI) | ‐13.25 [‐18.11, ‐8.39] |
| 31 Change in rescue‐free days (%) at 12 ‐ 16 weeks Show forest plot | 7 | 2304 | Mean Difference (IV, Random, 95% CI) | ‐9.64 [‐13.71, ‐5.56] |
| Analysis 1.31  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 31 Change in rescue‐free days (%) at 12 ‐ 16 weeks. | ||||
| 31.1 Paediatrics | 1 | 335 | Mean Difference (IV, Random, 95% CI) | ‐10.10 [‐18.97, ‐1.23] |
| 31.2 Adults | 6 | 1969 | Mean Difference (IV, Random, 95% CI) | ‐9.64 [‐14.39, ‐4.89] |
| 32 Change in rescue‐free days (%) at 24 ‐ 26 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.32  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 32 Change in rescue‐free days (%) at 24 ‐ 26 weeks. | ||||
| 32.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 Change in rescue‐free days (%) at 36 ‐ 52 weeks Show forest plot | 2 | 1350 | Mean Difference (IV, Fixed, 95% CI) | ‐2.59 [‐4.97, ‐0.21] |
| Analysis 1.33  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 33 Change in rescue‐free days (%) at 36 ‐ 52 weeks. | ||||
| 33.1 Paediatrics | 2 | 1350 | Mean Difference (IV, Fixed, 95% CI) | ‐2.59 [‐4.97, ‐0.21] |
| 33.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Change in proportion of symptom‐free days (%) at 4 ‐ 8 weeks Show forest plot | 3 | 1154 | Mean Difference (IV, Fixed, 95% CI) | ‐10.46 [‐14.56, ‐6.36] |
| Analysis 1.34  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 34 Change in proportion of symptom‐free days (%) at 4 ‐ 8 weeks. | ||||
| 34.1 Adults | 3 | 1154 | Mean Difference (IV, Fixed, 95% CI) | ‐10.46 [‐14.56, ‐6.36] |
| 35 Change in proportion of symptom‐free days (%) at 12 ‐ 16 weeks Show forest plot | 8 | 2423 | Mean Difference (IV, Fixed, 95% CI) | ‐8.89 [‐11.92, ‐5.87] |
| Analysis 1.35  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 35 Change in proportion of symptom‐free days (%) at 12 ‐ 16 weeks. | ||||
| 35.1 Paediatrics | 1 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐6.40 [‐15.82, 3.02] |
| 35.2 Adults | 7 | 2088 | Mean Difference (IV, Fixed, 95% CI) | ‐9.18 [‐12.38, ‐5.98] |
| 36 Change in proportion of symptom‐free days (%) at 24 ‐ 26 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.36  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 36 Change in proportion of symptom‐free days (%) at 24 ‐ 26 weeks. | ||||
| 36.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 Change in proportion of symptom‐free days (%) at 36 ‐ 52 weeks Show forest plot | 3 | 1190 | Mean Difference (IV, Fixed, 95% CI) | ‐5.49 [‐9.06, ‐1.91] |
| Analysis 1.37  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 37 Change in proportion of symptom‐free days (%) at 36 ‐ 52 weeks. | ||||
| 37.1 Paediatrics | 2 | 575 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐9.73, ‐0.08] |
| 37.2 Adults | 1 | 615 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐11.53, ‐0.87] |
| 38 Change from baseline quality of life (QOL) at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.38  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 38 Change from baseline quality of life (QOL) at 4 ‐ 8 weeks. | ||||
| 38.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 39 Change from baseline quality of life (QOL) at 12 ‐ 16 weeks Show forest plot | 2 | 1065 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.34, ‐0.09] |
| Analysis 1.39  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 39 Change from baseline quality of life (QOL) at 12 ‐ 16 weeks. | ||||
| 39.1 Adults | 2 | 1065 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.34, ‐0.09] |
| 40 Change from baseline quality of life (QOL) at 24 ‐ 26 weeks Show forest plot | 2 | 1028 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.54, ‐0.21] |
| Analysis 1.40  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 40 Change from baseline quality of life (QOL) at 24 ‐ 26 weeks. | ||||
| 40.1 Adults | 2 | 1028 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.54, ‐0.21] |
| 41 Change from baseline quality of life (QOL) at 36 ‐ 52 weeks Show forest plot | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.33, 0.07] |
| Analysis 1.41  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 41 Change from baseline quality of life (QOL) at 36 ‐ 52 weeks. | ||||
| 41.1 Paediatrics | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.33, 0.07] |
| 41.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 42 Days with use of β2‐agonists at 36 ‐ 52 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.42  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 42 Days with use of β2‐agonists at 36 ‐ 52 weeks. | ||||
| 42.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 43 Change from baseline blood eosinophils at 4 ‐ 8 weeks Show forest plot | 4 | 1294 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| Analysis 1.43  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 43 Change from baseline blood eosinophils at 4 ‐ 8 weeks. | ||||
| 43.1 Adults | 4 | 1294 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 44 Change from baseline blood eosinophils at 12 ‐ 16 weeks Show forest plot | 2 | 1013 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| Analysis 1.44  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 44 Change from baseline blood eosinophils at 12 ‐ 16 weeks. | ||||
| 44.1 Adults | 2 | 1013 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 45 % Change in sputum eosinophils at 4 ‐ 8 weeks Show forest plot | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐2.06, 3.47] |
| Analysis 1.45  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 45 % Change in sputum eosinophils at 4 ‐ 8 weeks. | ||||
| 45.1 Adults | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐2.06, 3.47] |
| 46 % Change in sputum eosinophils at 36 ‐ 52 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.46  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 46 % Change in sputum eosinophils at 36 ‐ 52 weeks. | ||||
| 46.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 47 LTC4 concentration (ng/mL) in nasal wash at 24 ‐ 26 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.47  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 47 LTC4 concentration (ng/mL) in nasal wash at 24 ‐ 26 weeks. | ||||
| 47.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 48 % Asthma control days during intervention period at 4 ‐ 8 weeks Show forest plot | 2 | 1293 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐10.86, ‐0.59] |
| Analysis 1.48  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 48 % Asthma control days during intervention period at 4 ‐ 8 weeks. | ||||
| 48.1 Adults | 2 | 1293 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐10.86, ‐0.59] |
| 49 % Asthma control days during intervention period at 24 ‐ 26 weeks Show forest plot | 2 | 1185 | Mean Difference (IV, Random, 95% CI) | ‐8.19 [‐19.46, 3.07] |
| Analysis 1.49  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 49 % Asthma control days during intervention period at 24 ‐ 26 weeks. | ||||
| 49.1 Adults | 2 | 1185 | Mean Difference (IV, Random, 95% CI) | ‐8.19 [‐19.46, 3.07] |
| 50 Change in PC20 at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.50  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 50 Change in PC20 at 4 ‐ 8 weeks. | ||||
| 50.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 51 % rescue ‐ free days at 24 ‐ 26 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.51  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 51 % rescue ‐ free days at 24 ‐ 26 weeks. | ||||
| 51.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 52 Days off work or school at 24 ‐ 26 weeks Show forest plot | 2 | 606 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.01, 0.26] |
| Analysis 1.52  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 52 Days off work or school at 24 ‐ 26 weeks. | ||||
| 52.1 Paediatrics | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.31, 0.83] |
| 52.2 Adults | 1 | 482 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.00, 0.26] |
| 53 Change in height (cm) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.53  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 53 Change in height (cm). | ||||
| 53.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 54 Patient's satisfaction at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.54  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 54 Patient's satisfaction at 4 ‐ 8 weeks. | ||||
| 54.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 55 Physician's satisfaction at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.55  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 55 Physician's satisfaction at 4 ‐ 8 weeks. | ||||
| 55.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 56 Overall Withdrawals Show forest plot | 42 | 10939 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.38] |
| Analysis 1.56  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 56 Overall Withdrawals. | ||||
| 56.1 Paediatrics | 18 | 3397 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.88, 1.21] |
| 56.2 Adults | 24 | 7542 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.11, 1.54] |
| 57 Withdrawal due to poor asthma control/exacerbations Show forest plot | 26 | 7669 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [2.01, 3.27] |
| Analysis 1.57  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 57 Withdrawal due to poor asthma control/exacerbations. | ||||
| 57.1 Paediatrics | 7 | 1219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.20, 3.94] |
| 57.2 Adults | 19 | 6450 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [2.02, 3.45] |
| 58 Withdrawals due to adverse effects Show forest plot | 25 | 8518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.95, 1.63] |
| Analysis 1.58  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 58 Withdrawals due to adverse effects. | ||||
| 58.1 Paediatrics | 8 | 2330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.70, 2.33] |
| 58.2 Adults | 17 | 6188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.91, 1.67] |
| 59 Overall Adverse effects Show forest plot | 22 | 7818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| Analysis 1.59  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 59 Overall Adverse effects. | ||||
| 59.1 Paediatrics | 3 | 1460 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 59.2 Adults | 19 | 6358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.05] |
| 60 Elevated liver enzymes Show forest plot | 7 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.58, 2.19] |
| Analysis 1.60  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 60 Elevated liver enzymes. | ||||
| 60.1 Paediatrics | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.03, 7.68] |
| 60.2 Adults | 6 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.36] |
| 61 Upper respiratory tract infections Show forest plot | 8 | 2729 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| Analysis 1.61  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 61 Upper respiratory tract infections. | ||||
| 61.1 Paediatrics | 5 | 1514 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.36] |
| 61.2 Adults | 3 | 1215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.50] |
| 62 Headache Show forest plot | 24 | 8872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| Analysis 1.62  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 62 Headache. | ||||
| 62.1 Paediatrics | 6 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.37] |
| 62.2 Adults | 18 | 6283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.10] |
| 63 Nausea Show forest plot | 17 | 5563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| Analysis 1.63  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 63 Nausea. | ||||
| 63.1 Paediatrics | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.28, 2.31] |
| 63.2 Adults | 15 | 5098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.09] |
| 64 Oral candidiasis Show forest plot | 3 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.19] |
| Analysis 1.64  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 64 Oral candidiasis. | ||||
| 64.1 Adults | 3 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.19] |
| 65 Hoarseness Show forest plot | 2 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| Analysis 1.65  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 65 Hoarseness. | ||||
| 65.1 Adults | 2 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| 66 Death Show forest plot | 13 | 5489 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.32, 29.26] |
| Analysis 1.66  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 66 Death. | ||||
| 66.1 Paediatrics | 2 | 1114 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.46] |
| 66.2 Adults | 11 | 4375 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.13, 75.82] |
| 67 Primary outcome ‐ stratified by anti‐leukotrienes Show forest plot | 21 | 6077 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.20, 2.16] |
| Analysis 1.67  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 67 Primary outcome ‐ stratified by anti‐leukotrienes. | ||||
| 67.1 Monelukast | 15 | 4352 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [1.14, 2.12] |
| 67.2 Zafirlukast | 6 | 1725 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [0.88, 4.20] |
| 68 Primary outcome ‐ stratified by duration of intervention Show forest plot | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| Analysis 1.68  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 68 Primary outcome ‐ stratified by duration of intervention. | ||||
| 68.1 4‐8 weeks | 9 | 2346 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.78, 3.87] |
| 68.2 12‐16 weeks | 7 | 1541 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.43, 2.96] |
| 68.3 24‐26 weeks | 2 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.55, 2.45] |
| 68.4 36‐52 weeks | 3 | 1533 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.87, 1.91] |
| 69 Main outcome ‐stratified by severity of airway obstruction Show forest plot | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| Analysis 1.69  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 69 Main outcome ‐stratified by severity of airway obstruction. | ||||
| 69.1 Mean FEV1 60‐80% of predicted | 11 | 3922 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.41, 2.91] |
| 69.2 Mean FEV1 ≥80% of predicted | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.97, 1.61] |
| 70 Primary outcome ‐ stratified by methodological quality Show forest plot | 21 | 6061 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| Analysis 1.70  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 70 Primary outcome ‐ stratified by methodological quality. | ||||
| 70.1 High quality | 11 | 4366 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.29, 2.03] |
| 70.2 Poor quality | 10 | 1695 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.74, 2.43] |
| 71 Primary outcome‐ stratified by funding source Show forest plot | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| Analysis 1.71  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 71 Primary outcome‐ stratified by funding source. | ||||
| 71.1 Funded by producers of ICS | 9 | 2638 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.05, 2.80] |
| 71.2 Funded by producers of AL | 5 | 2797 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.99, 2.35] |
| 71.3 No industry funding or not reported | 7 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.90, 1.66] |
| 72 Primary outcome ‐ stratified by HFC‐BDP equivalent Show forest plot | 21 | 6077 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.20, 2.16] |
| Analysis 1.72  Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 72 Primary outcome ‐ stratified by HFC‐BDP equivalent. | ||||
| 72.1 100‐150 μg HFA‐BDP equivalent | 3 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.26, 2.08] |
| 72.2 200‐250 μg HFA‐BDP equivalent | 15 | 5767 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [1.29, 2.38] |
| 72.3 400‐500 μg HFA‐BDP equivalent | 3 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.11, 2.78] |
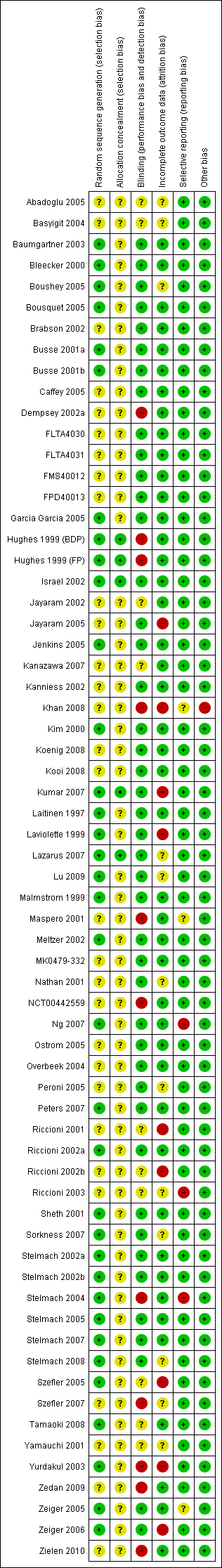
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), outcome: 1.1 Patients with at least one exacerbation requiring systemic steroids.
In the control group (on ICS) 7 people out of 100 had at least one exacerbation requiring systemic steroids over 4 to 52 weeks, compared to 10 (95% CI 8 to 13) out of 100 for the active treatment group given LRTA.

Funnel plot of comparison: 1 Anti‐leukotriene (AL) versus. Inhaled glucocorticoids (in HFC‐BDP equivalent), outcome: Patients with at least 1 exacerbation requiring systemic corticosteroids.
In the control group (on ICS) 2 people out of 100 had withdrawal due to poor control over 4 to 52 weeks, compared to 6 (95% CI 4 to 7) out of 100 for the active treatment group (given LRTA).

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 1 Patients with at least one exacerbation requiring systemic steroids.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 2 Patients with at least one exacerbation requiring hospital admission.
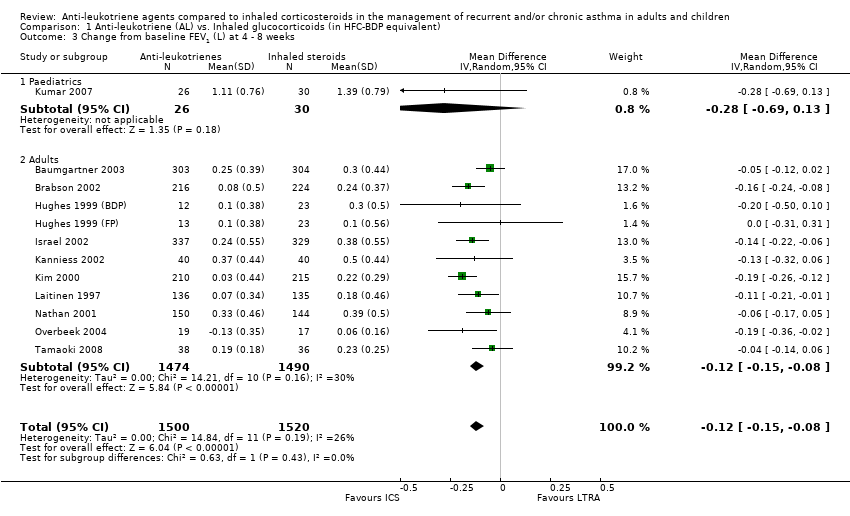
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 3 Change from baseline FEV1 (L) at 4 ‐ 8 weeks.
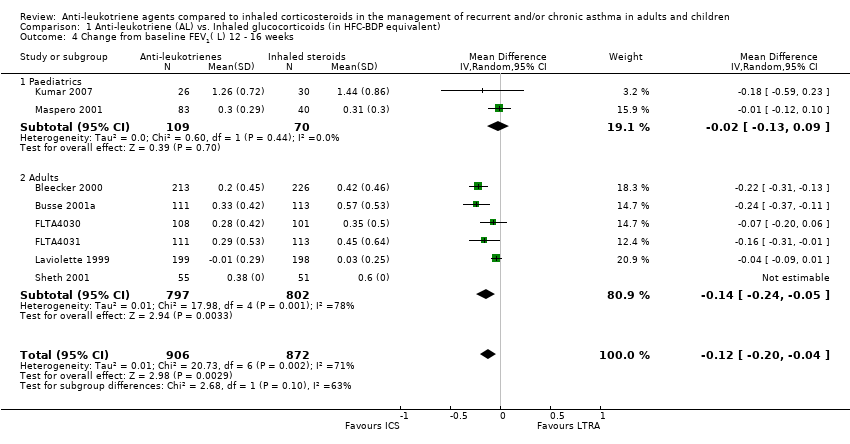
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 4 Change from baseline FEV1( L) 12 ‐ 16 weeks.
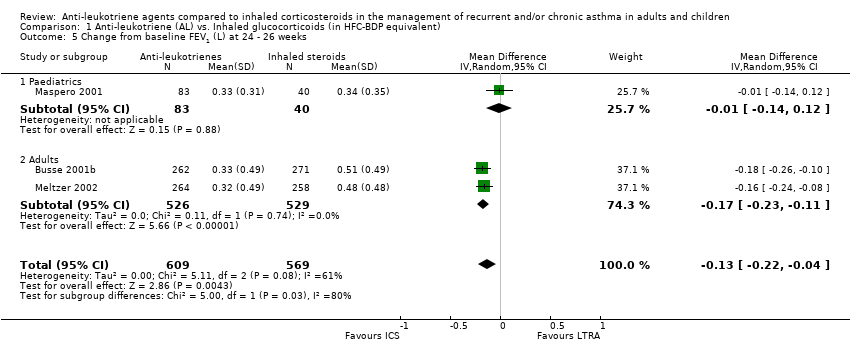
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 5 Change from baseline FEV1 (L) at 24 ‐ 26 weeks.
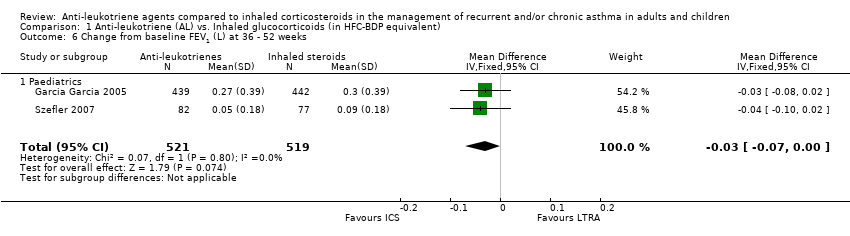
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 6 Change from baseline FEV1 (L) at 36 ‐ 52 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 7 FEV1 irrespective of time of treatment.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 8 Responders (defined as change from baseline in FEV1 >= 7.5%.
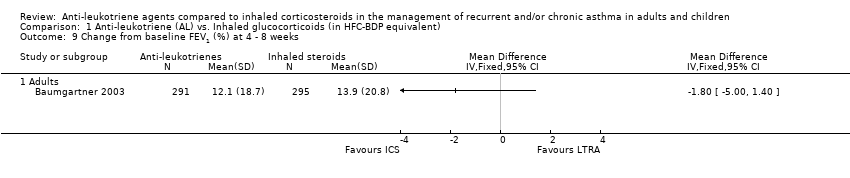
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 9 Change from baseline FEV1 (%) at 4 ‐ 8 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 10 Change from baseline FEV1 (%) 12 ‐ 16 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 11 Change from baseline FEV1 (%) at 24 ‐ 26 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 12 Change from baseline FEV1 % of predicted at 4 ‐ 8 weeks.
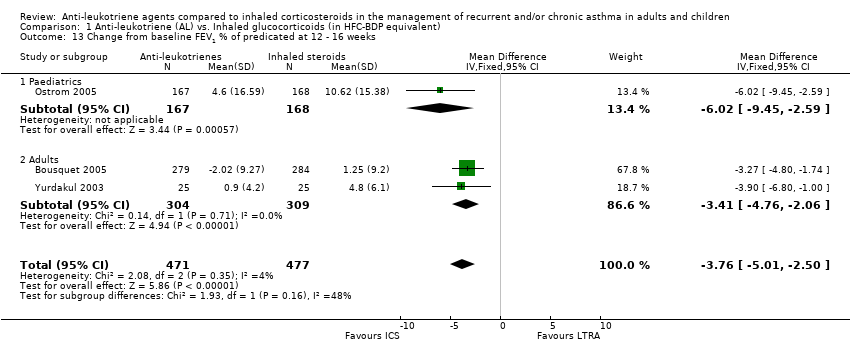
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 13 Change from baseline FEV1 % of predicated at 12 ‐ 16 weeks.
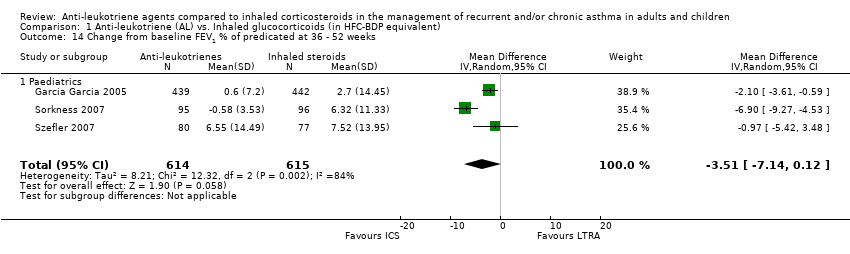
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 14 Change from baseline FEV1 % of predicated at 36 ‐ 52 weeks.
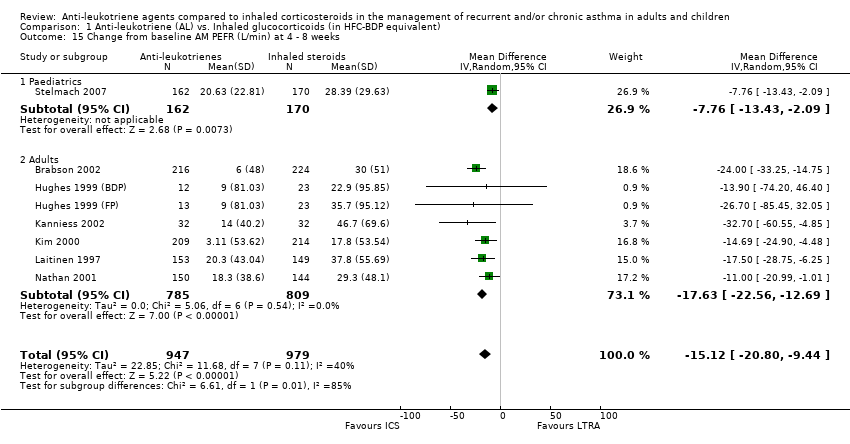
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 15 Change from baseline AM PEFR (L/min) at 4 ‐ 8 weeks.
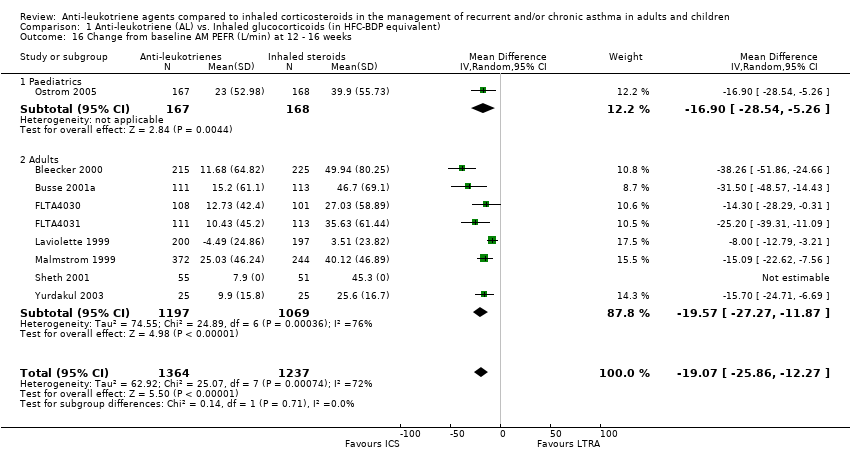
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 16 Change from baseline AM PEFR (L/min) at 12 ‐ 16 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 17 Change from baseline AM PEFR (L/min) at 24 ‐ 26 weeks.
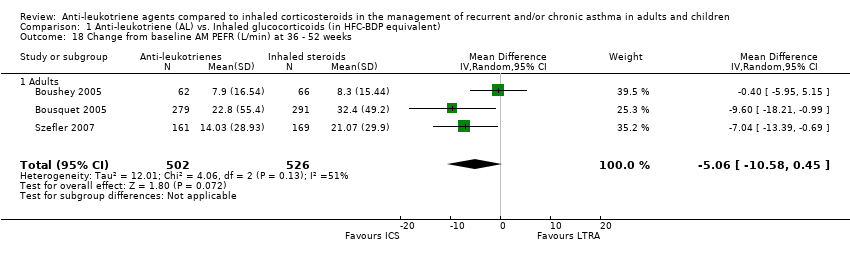
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 18 Change from baseline AM PEFR (L/min) at 36 ‐ 52 weeks.
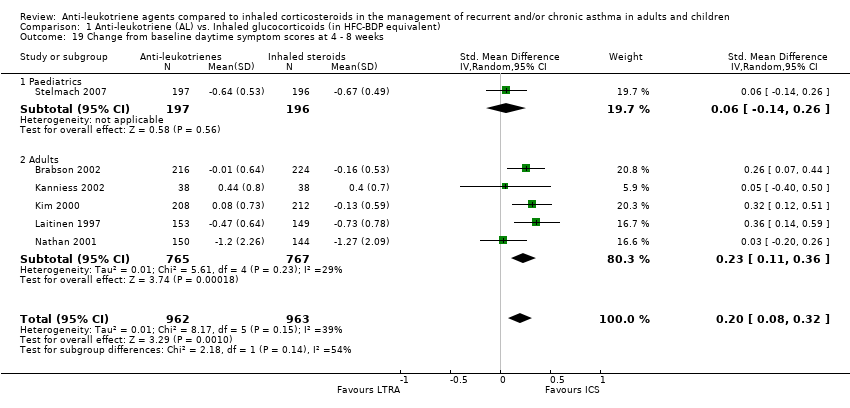
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 19 Change from baseline daytime symptom scores at 4 ‐ 8 weeks.
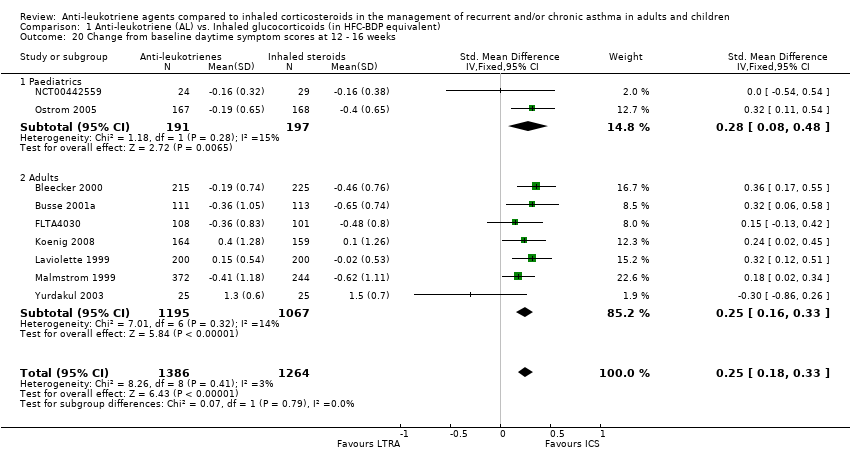
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 20 Change from baseline daytime symptom scores at 12 ‐ 16 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 21 Change from baseline daytime symptom scores at 24 ‐ 26 weeks.
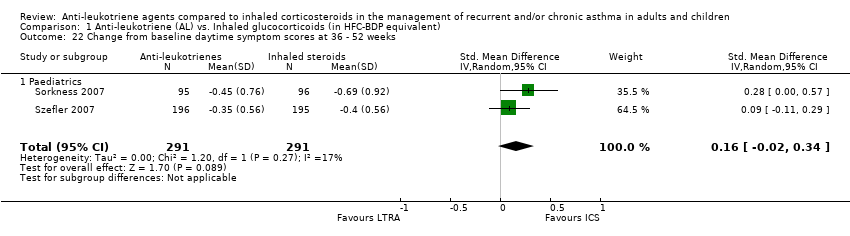
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 22 Change from baseline daytime symptom scores at 36 ‐ 52 weeks.
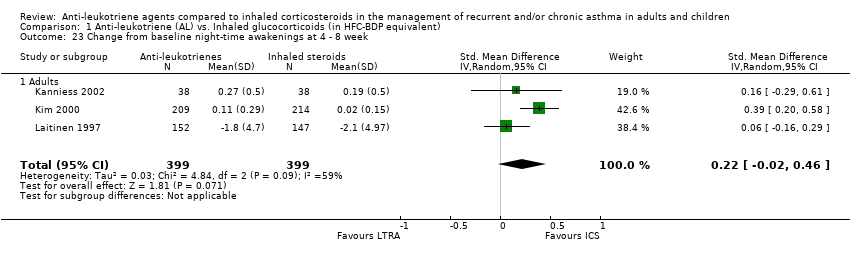
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 23 Change from baseline night‐time awakenings at 4 ‐ 8 week.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 24 Change from baseline night‐time awakenings at 12 ‐ 16 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 25 Change from baseline night‐time awakenings at 24 ‐ 26 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 26 Change from baseline mean daily use of β2‐agonists at 4 ‐ 8 weeks.
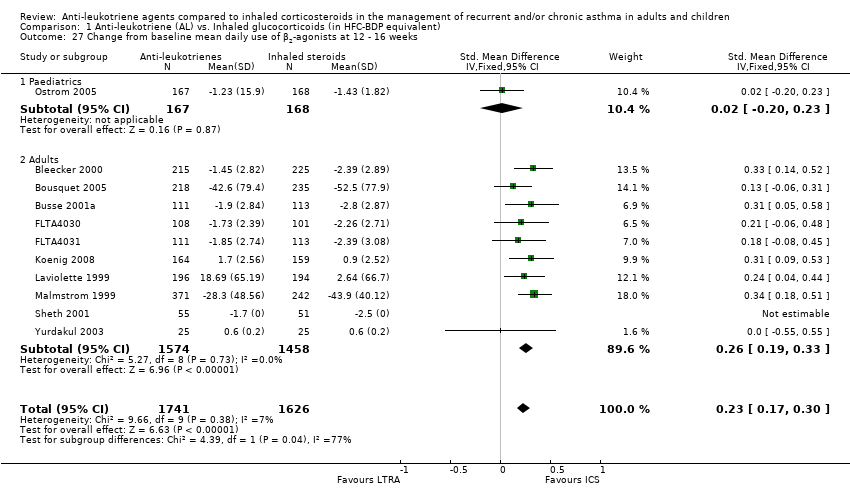
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 27 Change from baseline mean daily use of β2‐agonists at 12 ‐ 16 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 28 Change from baseline mean daily use of β2‐agonists at 24 ‐ 26 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 29 Change from baseline mean daily use of β2‐agonists at 36 ‐ 52 weeks.
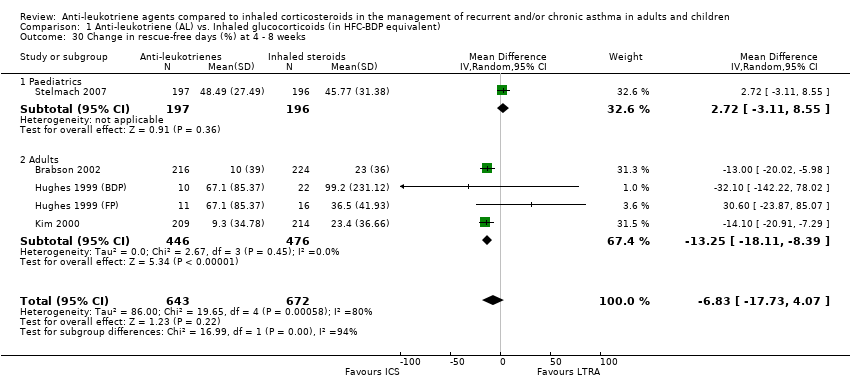
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 30 Change in rescue‐free days (%) at 4 ‐ 8 weeks.
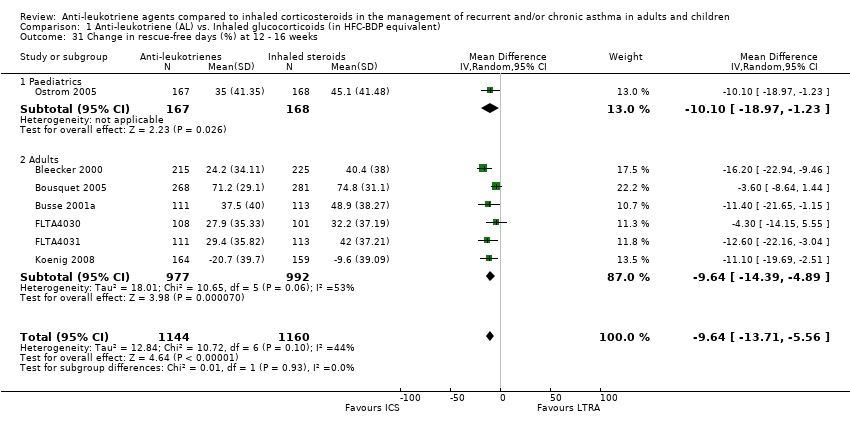
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 31 Change in rescue‐free days (%) at 12 ‐ 16 weeks.
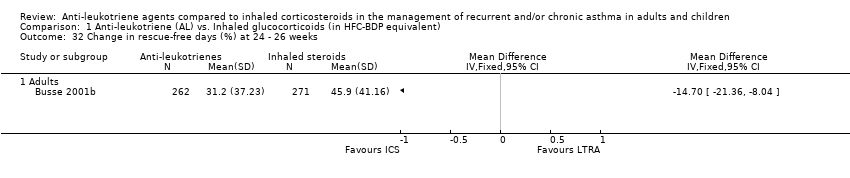
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 32 Change in rescue‐free days (%) at 24 ‐ 26 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 33 Change in rescue‐free days (%) at 36 ‐ 52 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 34 Change in proportion of symptom‐free days (%) at 4 ‐ 8 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 35 Change in proportion of symptom‐free days (%) at 12 ‐ 16 weeks.
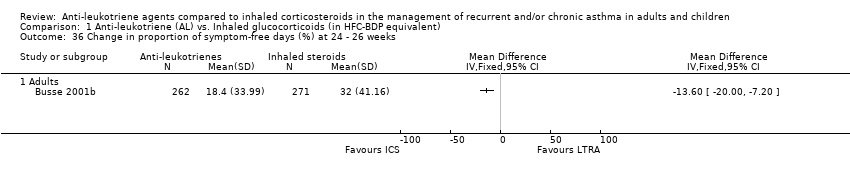
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 36 Change in proportion of symptom‐free days (%) at 24 ‐ 26 weeks.
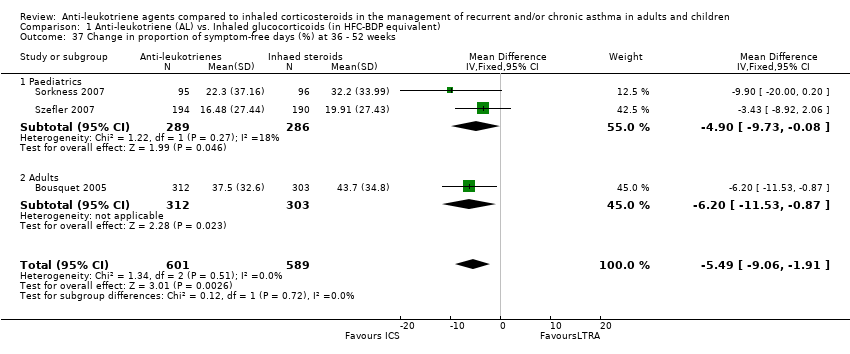
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 37 Change in proportion of symptom‐free days (%) at 36 ‐ 52 weeks.
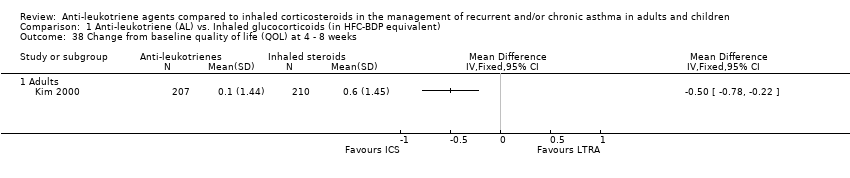
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 38 Change from baseline quality of life (QOL) at 4 ‐ 8 weeks.
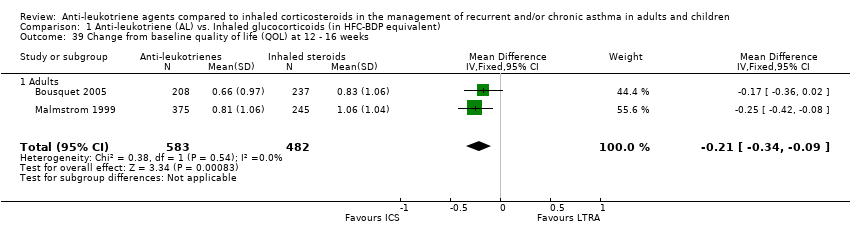
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 39 Change from baseline quality of life (QOL) at 12 ‐ 16 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 40 Change from baseline quality of life (QOL) at 24 ‐ 26 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 41 Change from baseline quality of life (QOL) at 36 ‐ 52 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 42 Days with use of β2‐agonists at 36 ‐ 52 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 43 Change from baseline blood eosinophils at 4 ‐ 8 weeks.
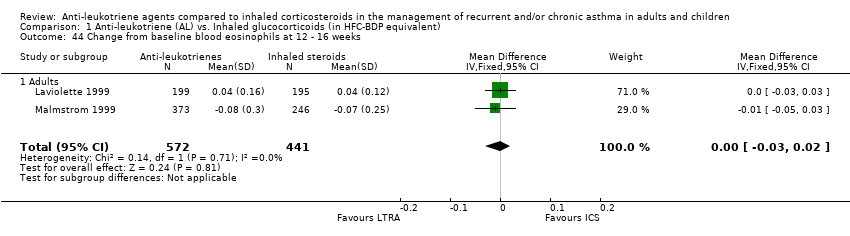
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 44 Change from baseline blood eosinophils at 12 ‐ 16 weeks.
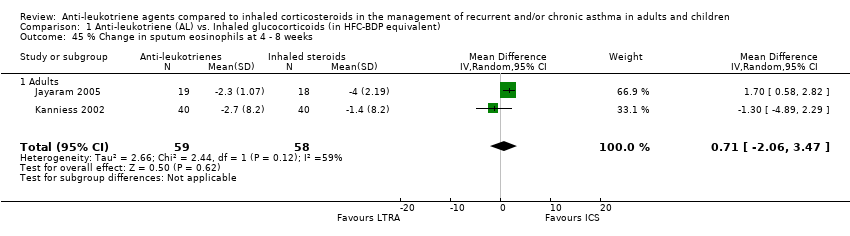
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 45 % Change in sputum eosinophils at 4 ‐ 8 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 46 % Change in sputum eosinophils at 36 ‐ 52 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 47 LTC4 concentration (ng/mL) in nasal wash at 24 ‐ 26 weeks.
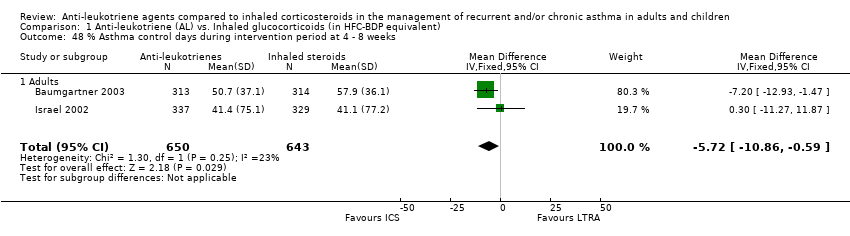
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 48 % Asthma control days during intervention period at 4 ‐ 8 weeks.
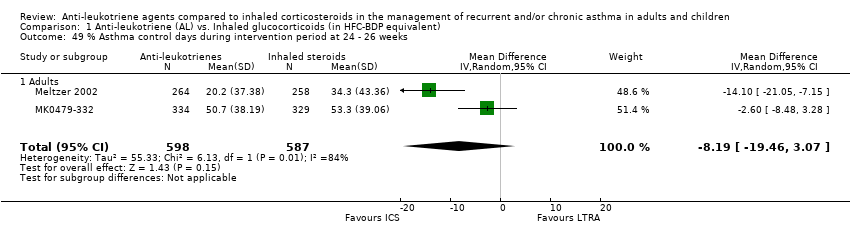
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 49 % Asthma control days during intervention period at 24 ‐ 26 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 50 Change in PC20 at 4 ‐ 8 weeks.
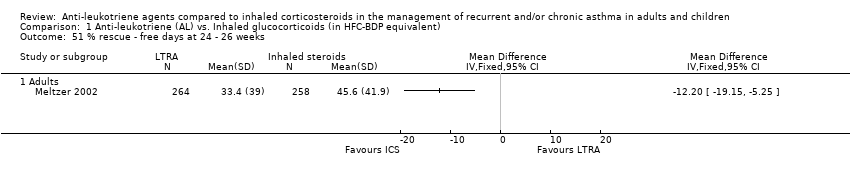
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 51 % rescue ‐ free days at 24 ‐ 26 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 52 Days off work or school at 24 ‐ 26 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 53 Change in height (cm).

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 54 Patient's satisfaction at 4 ‐ 8 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 55 Physician's satisfaction at 4 ‐ 8 weeks.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 56 Overall Withdrawals.
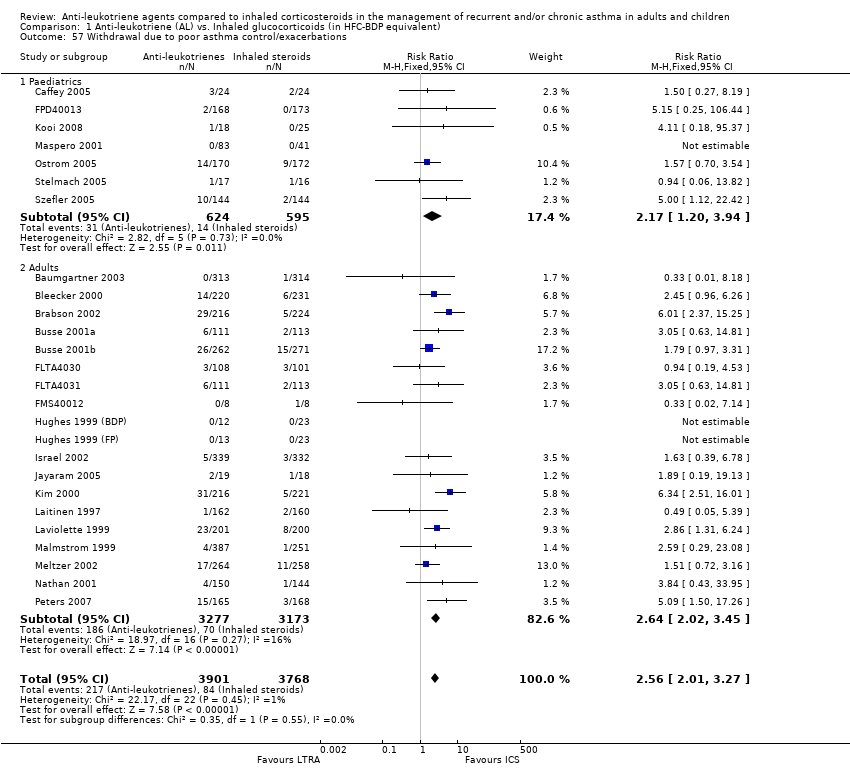
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 57 Withdrawal due to poor asthma control/exacerbations.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 58 Withdrawals due to adverse effects.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 59 Overall Adverse effects.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 60 Elevated liver enzymes.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 61 Upper respiratory tract infections.
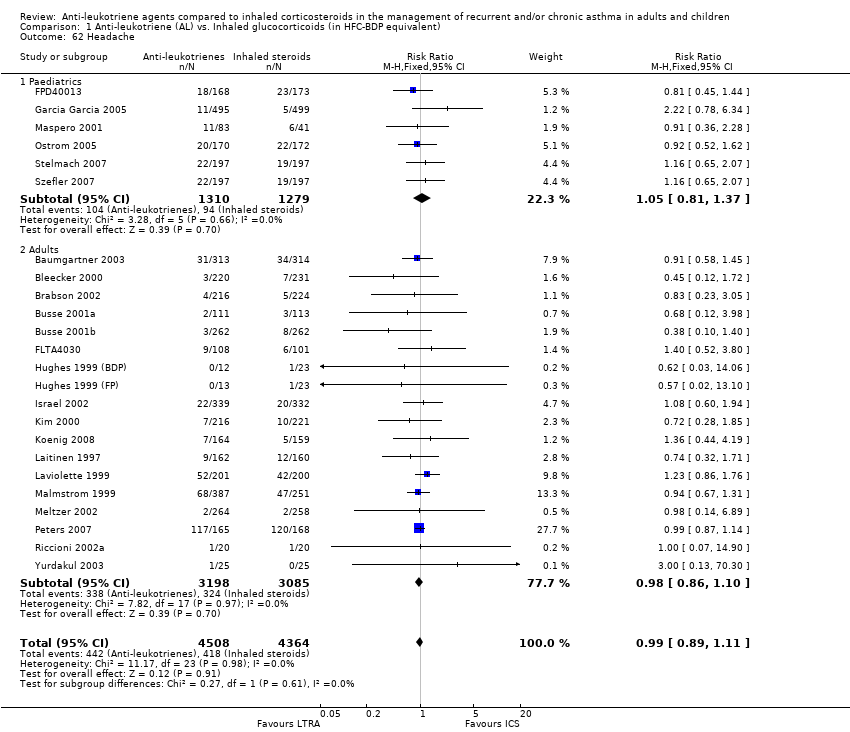
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 62 Headache.
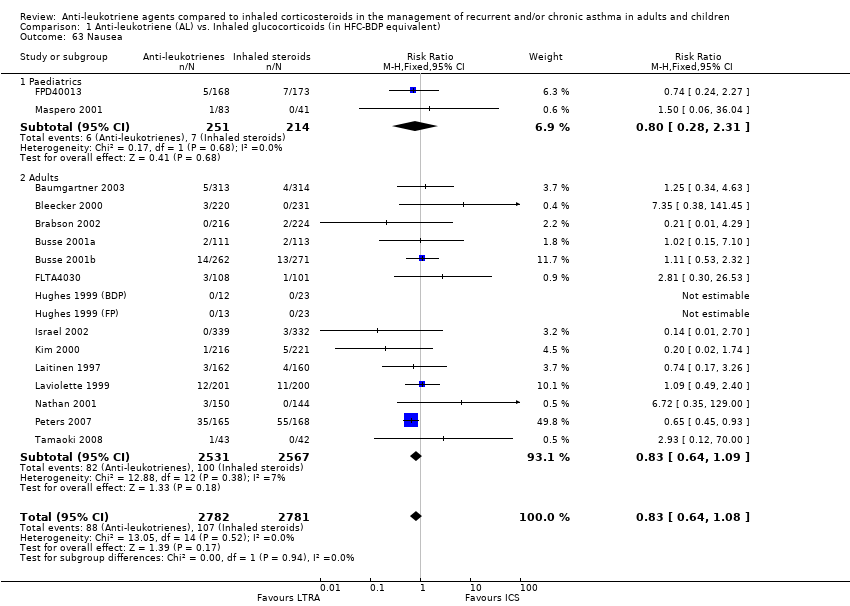
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 63 Nausea.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 64 Oral candidiasis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 65 Hoarseness.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 66 Death.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 67 Primary outcome ‐ stratified by anti‐leukotrienes.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 68 Primary outcome ‐ stratified by duration of intervention.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 69 Main outcome ‐stratified by severity of airway obstruction.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 70 Primary outcome ‐ stratified by methodological quality.
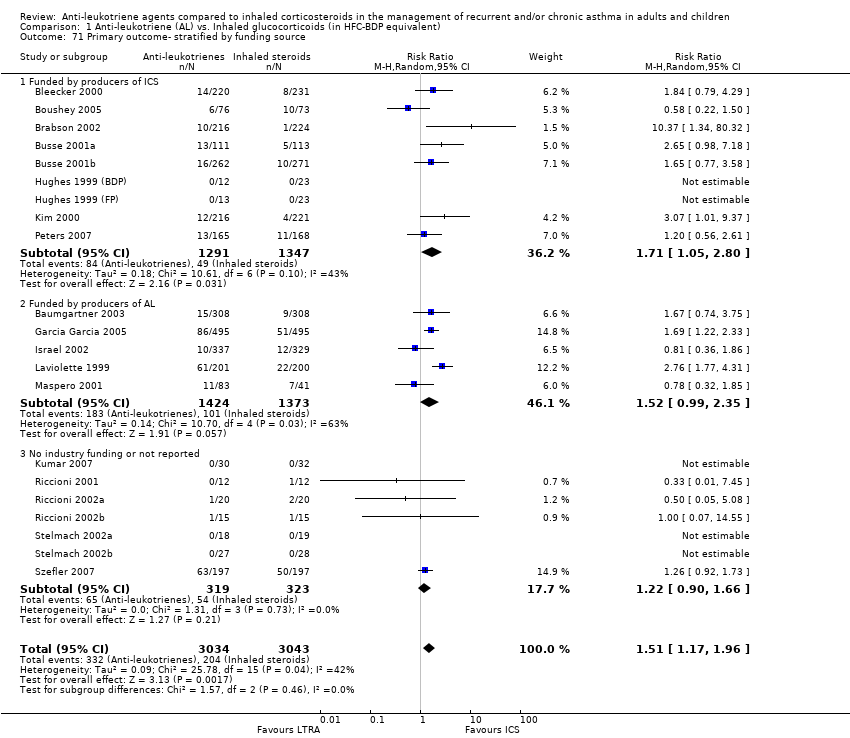
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 71 Primary outcome‐ stratified by funding source.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 72 Primary outcome ‐ stratified by HFC‐BDP equivalent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients with at least one exacerbation requiring systemic steroids Show forest plot | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 1.1 Paediatrics | 6 | 1662 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.99, 1.86] |
| 1.2 Adults | 15 | 4415 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.12, 2.31] |
| 2 Patients with at least one exacerbation requiring hospital admission Show forest plot | 12 | 2715 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.02, 10.94] |
| 2.1 Paediatrics | 4 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 73.98] |
| 2.2 Adults | 8 | 2157 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.94, 12.17] |
| 3 Change from baseline FEV1 (L) at 4 ‐ 8 weeks Show forest plot | 12 | 3020 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| 3.1 Paediatrics | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.69, 0.13] |
| 3.2 Adults | 11 | 2964 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| 4 Change from baseline FEV1( L) 12 ‐ 16 weeks Show forest plot | 8 | 1778 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 4.1 Paediatrics | 2 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 4.2 Adults | 6 | 1599 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.24, ‐0.05] |
| 5 Change from baseline FEV1 (L) at 24 ‐ 26 weeks Show forest plot | 3 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.22, ‐0.04] |
| 5.1 Paediatrics | 1 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.14, 0.12] |
| 5.2 Adults | 2 | 1055 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.23, ‐0.11] |
| 6 Change from baseline FEV1 (L) at 36 ‐ 52 weeks Show forest plot | 2 | 1040 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| 6.1 Paediatrics | 2 | 1040 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| 7 FEV1 irrespective of time of treatment Show forest plot | 23 | 7016 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.14, ‐0.08] |
| 7.1 Paediatrics | 4 | 1398 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| 7.2 Adults | 19 | 5618 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.16, ‐0.09] |
| 8 Responders (defined as change from baseline in FEV1 >= 7.5% Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 9 Change from baseline FEV1 (%) at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change from baseline FEV1 (%) 12 ‐ 16 weeks Show forest plot | 2 | 603 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐9.81, ‐1.59] |
| 10.1 Adults | 2 | 603 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐9.81, ‐1.59] |
| 11 Change from baseline FEV1 (%) at 24 ‐ 26 weeks Show forest plot | 2 | 838 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐10.85, ‐5.55] |
| 11.1 Adults | 2 | 838 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐10.85, ‐5.55] |
| 12 Change from baseline FEV1 % of predicted at 4 ‐ 8 weeks Show forest plot | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐6.56, 1.40] |
| 12.1 Paediatrics | 1 | 183 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐4.82, 3.74] |
| 12.2 Adults | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐4.6 [‐8.86, ‐0.34] |
| 13 Change from baseline FEV1 % of predicated at 12 ‐ 16 weeks Show forest plot | 3 | 948 | Mean Difference (IV, Fixed, 95% CI) | ‐3.76 [‐5.01, ‐2.50] |
| 13.1 Paediatrics | 1 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐6.02 [‐9.45, ‐2.59] |
| 13.2 Adults | 2 | 613 | Mean Difference (IV, Fixed, 95% CI) | ‐3.41 [‐4.76, ‐2.06] |
| 14 Change from baseline FEV1 % of predicated at 36 ‐ 52 weeks Show forest plot | 3 | 1229 | Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐7.14, 0.12] |
| 14.1 Paediatrics | 3 | 1229 | Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐7.14, 0.12] |
| 15 Change from baseline AM PEFR (L/min) at 4 ‐ 8 weeks Show forest plot | 8 | 1926 | Mean Difference (IV, Random, 95% CI) | ‐15.12 [‐20.80, ‐9.44] |
| 15.1 Paediatrics | 1 | 332 | Mean Difference (IV, Random, 95% CI) | ‐7.76 [‐13.43, ‐2.09] |
| 15.2 Adults | 7 | 1594 | Mean Difference (IV, Random, 95% CI) | ‐17.63 [‐22.56, ‐12.69] |
| 16 Change from baseline AM PEFR (L/min) at 12 ‐ 16 weeks Show forest plot | 9 | 2601 | Mean Difference (IV, Random, 95% CI) | ‐19.07 [‐25.86, ‐12.27] |
| 16.1 Paediatrics | 1 | 335 | Mean Difference (IV, Random, 95% CI) | ‐16.9 [‐28.54, ‐5.26] |
| 16.2 Adults | 8 | 2266 | Mean Difference (IV, Random, 95% CI) | ‐19.57 [‐27.27, ‐11.87] |
| 17 Change from baseline AM PEFR (L/min) at 24 ‐ 26 weeks Show forest plot | 3 | 1718 | Mean Difference (IV, Random, 95% CI) | ‐21.62 [‐40.19, ‐3.05] |
| 17.1 Adults | 3 | 1718 | Mean Difference (IV, Random, 95% CI) | ‐21.62 [‐40.19, ‐3.05] |
| 18 Change from baseline AM PEFR (L/min) at 36 ‐ 52 weeks Show forest plot | 3 | 1028 | Mean Difference (IV, Random, 95% CI) | ‐5.06 [‐10.58, 0.45] |
| 18.1 Adults | 3 | 1028 | Mean Difference (IV, Random, 95% CI) | ‐5.06 [‐10.58, 0.45] |
| 19 Change from baseline daytime symptom scores at 4 ‐ 8 weeks Show forest plot | 6 | 1925 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.08, 0.32] |
| 19.1 Paediatrics | 1 | 393 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.14, 0.26] |
| 19.2 Adults | 5 | 1532 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.11, 0.36] |
| 20 Change from baseline daytime symptom scores at 12 ‐ 16 weeks Show forest plot | 9 | 2650 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.18, 0.33] |
| 20.1 Paediatrics | 2 | 388 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.08, 0.48] |
| 20.2 Adults | 7 | 2262 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.16, 0.33] |
| 21 Change from baseline daytime symptom scores at 24 ‐ 26 weeks Show forest plot | 3 | 1719 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.02, 0.42] |
| 21.1 Adults | 3 | 1719 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.02, 0.42] |
| 22 Change from baseline daytime symptom scores at 36 ‐ 52 weeks Show forest plot | 2 | 582 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.02, 0.34] |
| 22.1 Paediatrics | 2 | 582 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.02, 0.34] |
| 23 Change from baseline night‐time awakenings at 4 ‐ 8 week Show forest plot | 3 | 798 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| 23.1 Adults | 3 | 798 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| 24 Change from baseline night‐time awakenings at 12 ‐ 16 weeks Show forest plot | 9 | 2916 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.11, 0.26] |
| 24.1 Adults | 9 | 2916 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.11, 0.26] |
| 25 Change from baseline night‐time awakenings at 24 ‐ 26 weeks Show forest plot | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 25.1 Adults | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 26 Change from baseline mean daily use of β2‐agonists at 4 ‐ 8 weeks Show forest plot | 10 | 3264 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.07, 0.34] |
| 26.1 Paediatrics | 1 | 393 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.17] |
| 26.2 Adults | 9 | 2871 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.10, 0.38] |
| 27 Change from baseline mean daily use of β2‐agonists at 12 ‐ 16 weeks Show forest plot | 11 | 3367 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.17, 0.30] |
| 27.1 Paediatrics | 1 | 335 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.20, 0.23] |
| 27.2 Adults | 10 | 3032 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.19, 0.33] |
| 28 Change from baseline mean daily use of β2‐agonists at 24 ‐ 26 weeks Show forest plot | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.19, 0.43] |
| 28.1 Adults | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.19, 0.43] |
| 29 Change from baseline mean daily use of β2‐agonists at 36 ‐ 52 weeks Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 29.1 Paediatrics | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 30 Change in rescue‐free days (%) at 4 ‐ 8 weeks Show forest plot | 5 | 1315 | Mean Difference (IV, Random, 95% CI) | ‐6.83 [‐17.73, 4.07] |
| 30.1 Paediatrics | 1 | 393 | Mean Difference (IV, Random, 95% CI) | 2.72 [‐3.11, 8.55] |
| 30.2 Adults | 4 | 922 | Mean Difference (IV, Random, 95% CI) | ‐13.25 [‐18.11, ‐8.39] |
| 31 Change in rescue‐free days (%) at 12 ‐ 16 weeks Show forest plot | 7 | 2304 | Mean Difference (IV, Random, 95% CI) | ‐9.64 [‐13.71, ‐5.56] |
| 31.1 Paediatrics | 1 | 335 | Mean Difference (IV, Random, 95% CI) | ‐10.10 [‐18.97, ‐1.23] |
| 31.2 Adults | 6 | 1969 | Mean Difference (IV, Random, 95% CI) | ‐9.64 [‐14.39, ‐4.89] |
| 32 Change in rescue‐free days (%) at 24 ‐ 26 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 32.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 Change in rescue‐free days (%) at 36 ‐ 52 weeks Show forest plot | 2 | 1350 | Mean Difference (IV, Fixed, 95% CI) | ‐2.59 [‐4.97, ‐0.21] |
| 33.1 Paediatrics | 2 | 1350 | Mean Difference (IV, Fixed, 95% CI) | ‐2.59 [‐4.97, ‐0.21] |
| 33.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Change in proportion of symptom‐free days (%) at 4 ‐ 8 weeks Show forest plot | 3 | 1154 | Mean Difference (IV, Fixed, 95% CI) | ‐10.46 [‐14.56, ‐6.36] |
| 34.1 Adults | 3 | 1154 | Mean Difference (IV, Fixed, 95% CI) | ‐10.46 [‐14.56, ‐6.36] |
| 35 Change in proportion of symptom‐free days (%) at 12 ‐ 16 weeks Show forest plot | 8 | 2423 | Mean Difference (IV, Fixed, 95% CI) | ‐8.89 [‐11.92, ‐5.87] |
| 35.1 Paediatrics | 1 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐6.40 [‐15.82, 3.02] |
| 35.2 Adults | 7 | 2088 | Mean Difference (IV, Fixed, 95% CI) | ‐9.18 [‐12.38, ‐5.98] |
| 36 Change in proportion of symptom‐free days (%) at 24 ‐ 26 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 36.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 Change in proportion of symptom‐free days (%) at 36 ‐ 52 weeks Show forest plot | 3 | 1190 | Mean Difference (IV, Fixed, 95% CI) | ‐5.49 [‐9.06, ‐1.91] |
| 37.1 Paediatrics | 2 | 575 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐9.73, ‐0.08] |
| 37.2 Adults | 1 | 615 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐11.53, ‐0.87] |
| 38 Change from baseline quality of life (QOL) at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 38.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 39 Change from baseline quality of life (QOL) at 12 ‐ 16 weeks Show forest plot | 2 | 1065 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.34, ‐0.09] |
| 39.1 Adults | 2 | 1065 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.34, ‐0.09] |
| 40 Change from baseline quality of life (QOL) at 24 ‐ 26 weeks Show forest plot | 2 | 1028 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.54, ‐0.21] |
| 40.1 Adults | 2 | 1028 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.54, ‐0.21] |
| 41 Change from baseline quality of life (QOL) at 36 ‐ 52 weeks Show forest plot | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.33, 0.07] |
| 41.1 Paediatrics | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.33, 0.07] |
| 41.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 42 Days with use of β2‐agonists at 36 ‐ 52 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 42.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 43 Change from baseline blood eosinophils at 4 ‐ 8 weeks Show forest plot | 4 | 1294 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 43.1 Adults | 4 | 1294 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 44 Change from baseline blood eosinophils at 12 ‐ 16 weeks Show forest plot | 2 | 1013 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 44.1 Adults | 2 | 1013 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 45 % Change in sputum eosinophils at 4 ‐ 8 weeks Show forest plot | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐2.06, 3.47] |
| 45.1 Adults | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐2.06, 3.47] |
| 46 % Change in sputum eosinophils at 36 ‐ 52 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 46.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 47 LTC4 concentration (ng/mL) in nasal wash at 24 ‐ 26 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 47.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 48 % Asthma control days during intervention period at 4 ‐ 8 weeks Show forest plot | 2 | 1293 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐10.86, ‐0.59] |
| 48.1 Adults | 2 | 1293 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐10.86, ‐0.59] |
| 49 % Asthma control days during intervention period at 24 ‐ 26 weeks Show forest plot | 2 | 1185 | Mean Difference (IV, Random, 95% CI) | ‐8.19 [‐19.46, 3.07] |
| 49.1 Adults | 2 | 1185 | Mean Difference (IV, Random, 95% CI) | ‐8.19 [‐19.46, 3.07] |
| 50 Change in PC20 at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 50.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 51 % rescue ‐ free days at 24 ‐ 26 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 51.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 52 Days off work or school at 24 ‐ 26 weeks Show forest plot | 2 | 606 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.01, 0.26] |
| 52.1 Paediatrics | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.31, 0.83] |
| 52.2 Adults | 1 | 482 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.00, 0.26] |
| 53 Change in height (cm) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 53.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 54 Patient's satisfaction at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 54.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 55 Physician's satisfaction at 4 ‐ 8 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 55.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 56 Overall Withdrawals Show forest plot | 42 | 10939 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.38] |
| 56.1 Paediatrics | 18 | 3397 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.88, 1.21] |
| 56.2 Adults | 24 | 7542 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.11, 1.54] |
| 57 Withdrawal due to poor asthma control/exacerbations Show forest plot | 26 | 7669 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [2.01, 3.27] |
| 57.1 Paediatrics | 7 | 1219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.20, 3.94] |
| 57.2 Adults | 19 | 6450 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [2.02, 3.45] |
| 58 Withdrawals due to adverse effects Show forest plot | 25 | 8518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.95, 1.63] |
| 58.1 Paediatrics | 8 | 2330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.70, 2.33] |
| 58.2 Adults | 17 | 6188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.91, 1.67] |
| 59 Overall Adverse effects Show forest plot | 22 | 7818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 59.1 Paediatrics | 3 | 1460 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 59.2 Adults | 19 | 6358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.05] |
| 60 Elevated liver enzymes Show forest plot | 7 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.58, 2.19] |
| 60.1 Paediatrics | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.03, 7.68] |
| 60.2 Adults | 6 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.36] |
| 61 Upper respiratory tract infections Show forest plot | 8 | 2729 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| 61.1 Paediatrics | 5 | 1514 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.36] |
| 61.2 Adults | 3 | 1215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.50] |
| 62 Headache Show forest plot | 24 | 8872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 62.1 Paediatrics | 6 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.37] |
| 62.2 Adults | 18 | 6283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.10] |
| 63 Nausea Show forest plot | 17 | 5563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| 63.1 Paediatrics | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.28, 2.31] |
| 63.2 Adults | 15 | 5098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.09] |
| 64 Oral candidiasis Show forest plot | 3 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.19] |
| 64.1 Adults | 3 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.19] |
| 65 Hoarseness Show forest plot | 2 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| 65.1 Adults | 2 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| 66 Death Show forest plot | 13 | 5489 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.32, 29.26] |
| 66.1 Paediatrics | 2 | 1114 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.46] |
| 66.2 Adults | 11 | 4375 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.13, 75.82] |
| 67 Primary outcome ‐ stratified by anti‐leukotrienes Show forest plot | 21 | 6077 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.20, 2.16] |
| 67.1 Monelukast | 15 | 4352 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [1.14, 2.12] |
| 67.2 Zafirlukast | 6 | 1725 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [0.88, 4.20] |
| 68 Primary outcome ‐ stratified by duration of intervention Show forest plot | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 68.1 4‐8 weeks | 9 | 2346 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.78, 3.87] |
| 68.2 12‐16 weeks | 7 | 1541 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.43, 2.96] |
| 68.3 24‐26 weeks | 2 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.55, 2.45] |
| 68.4 36‐52 weeks | 3 | 1533 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.87, 1.91] |
| 69 Main outcome ‐stratified by severity of airway obstruction Show forest plot | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 69.1 Mean FEV1 60‐80% of predicted | 11 | 3922 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.41, 2.91] |
| 69.2 Mean FEV1 ≥80% of predicted | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.97, 1.61] |
| 70 Primary outcome ‐ stratified by methodological quality Show forest plot | 21 | 6061 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 70.1 High quality | 11 | 4366 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.29, 2.03] |
| 70.2 Poor quality | 10 | 1695 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.74, 2.43] |
| 71 Primary outcome‐ stratified by funding source Show forest plot | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 71.1 Funded by producers of ICS | 9 | 2638 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.05, 2.80] |
| 71.2 Funded by producers of AL | 5 | 2797 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.99, 2.35] |
| 71.3 No industry funding or not reported | 7 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.90, 1.66] |
| 72 Primary outcome ‐ stratified by HFC‐BDP equivalent Show forest plot | 21 | 6077 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.20, 2.16] |
| 72.1 100‐150 μg HFA‐BDP equivalent | 3 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.26, 2.08] |
| 72.2 200‐250 μg HFA‐BDP equivalent | 15 | 5767 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [1.29, 2.38] |
| 72.3 400‐500 μg HFA‐BDP equivalent | 3 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.11, 2.78] |

