Radioterapia posoperatoria para el cáncer de pulmón de células no pequeñas
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002142.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 octubre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer de pulmón
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The writing group for the current version of this review consisted of S Burdett, LHM Rydzewska, JF Tierney, MKB Parmar, D Fisher, R Arriagada, JP Pignon and C Le Pechoux, on behalf of the PORT Meta‐analysis Trialists Group.
Sources of support
Internal sources
-
Medical Research Council, UK.
External sources
-
NHS R&D programme project grant NCP/U03, UK.
Declarations of interest
None known.
Acknowledgements
The following investigators, groups and project management team* form the original PORT Meta‐analysis Group and participated in this meta‐analysis.
R Arriagada, Institut Gustave‐Roussy, Villejuif, France; AH Brichet, JJ Lafitte, Hôpital Calmette, CHRU, Lille, France; S Burdett*, D Fisher, DJ Girling, MKB Parmar*, LHM Rydzewska*, RJ Stephens, LA Stewart*, JF Tierney, MRC Clinical Trials Unit at UCL, UK; B Dautzenberg, Groupe d'Etude et de Traitement des Cancers Bronchiques (GETCB); M Debevec, V Kovac, Institute of Oncology, Zaloska 2 , Ljublijana, Slovenia; A Gregor, European Organization for Research and Treatment of Cancer, Brussels, Belgium; JH Park, Korea Cancer Center, Seoul, Korea; S Piantadosi, Lung Cancer Study Group, USA; JP Pignon, Institut Gustave‐Roussy, Villejuif, France; P Rocmans, Dept of Thoracic Surgery, Hôpitaux St Pierre et Erasme, Brussels, Belgium; R Souhami*, University College London, Medical School, UK; V Torri, Istituto di Ricerche Farmacologiche "Mario Negri", Milan, Italy; P Van Houtte, Dept of Radiotherapy, Institut Jules Bordet, Brussels, Belgium; L Trodella, Department of Radiation Oncology, Università Cattolica del S. Cuore, Rome; M Wang, Dept of Radiation Oncology and Surgery, Chinese Academy of Medical Sciences, Beijing, China.
The authors thank the European Organization for Research and Treatment of Cancer for permission to the the data from EORTC 08861 for this research. The contents of this publication and methods used are solely the responsibility of the authors and do not necessarily represent the official views of the EORTC.
Co‐ordination of the meta‐analysis and the collaborators' meeting was funded by the UK National Health Service Research and Development Cancer Programme (project grant NCP/U03). We would like to thank all those patients who took part in the trials and contributed to this research. The meta‐analysis would not have been possible without their help or without the collaborating institutions, which kindly supplied their trial data.
We are particularly grateful to Desmond Curran, Stanley Dische and Michele Saunders for helpful comments on the 1998 manuscript.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Oct 11 | Postoperative radiotherapy for non‐small cell lung cancer | Review | Sarah Burdett, Larysa Rydzewska, Jayne Tierney, David Fisher, Mahesh KB Parmar, Rodrigo Arriagada, Jean Pierre Pignon, Cecile Le Pechoux, on behalf of the PORT Meta‐analysis Trialists Group | |
| 2016 Sep 29 | Postoperative radiotherapy for non‐small cell lung cancer | Review | Sarah Burdett, Larysa Rydzewska, Jayne Tierney, David Fisher, Mahesh KB Parmar, Rodrigo Arriagada, Jean Pierre Pignon, Cecile Le Pechoux, on behalf of the PORT Meta‐analysis Trialists Group | |
| 2005 Apr 20 | Postoperative radiotherapy for non‐small cell lung cancer | Review | PORT Meta‐analysis Trialists Group | |
| 2003 Jan 20 | Postoperative radiotherapy for non‐small cell lung cancer | Review | G roup PORT Meta‐analysis Trialists, Lesley A Stewart, Sarah S Burdett | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
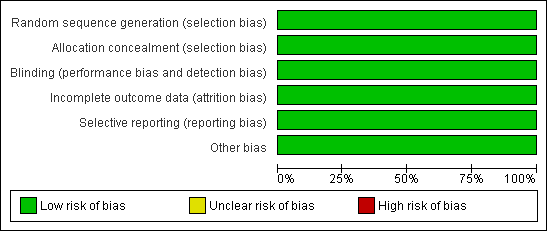
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Overall survival.
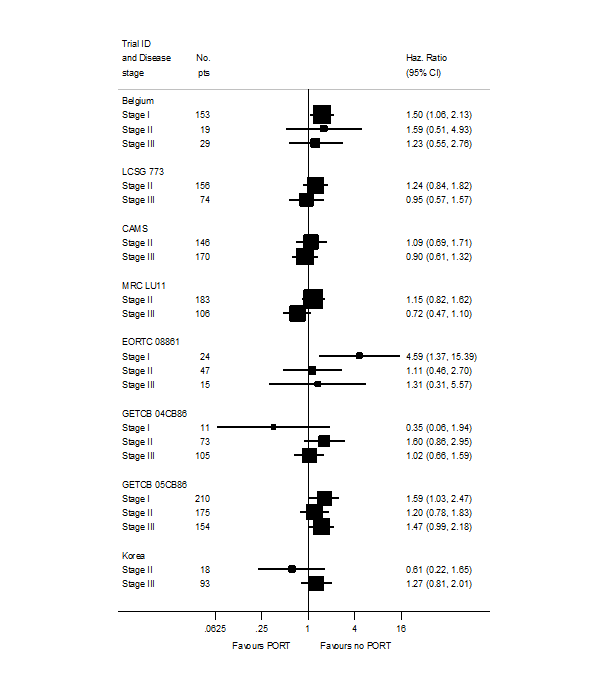
PORT effect on overall survival by trial according to stage.
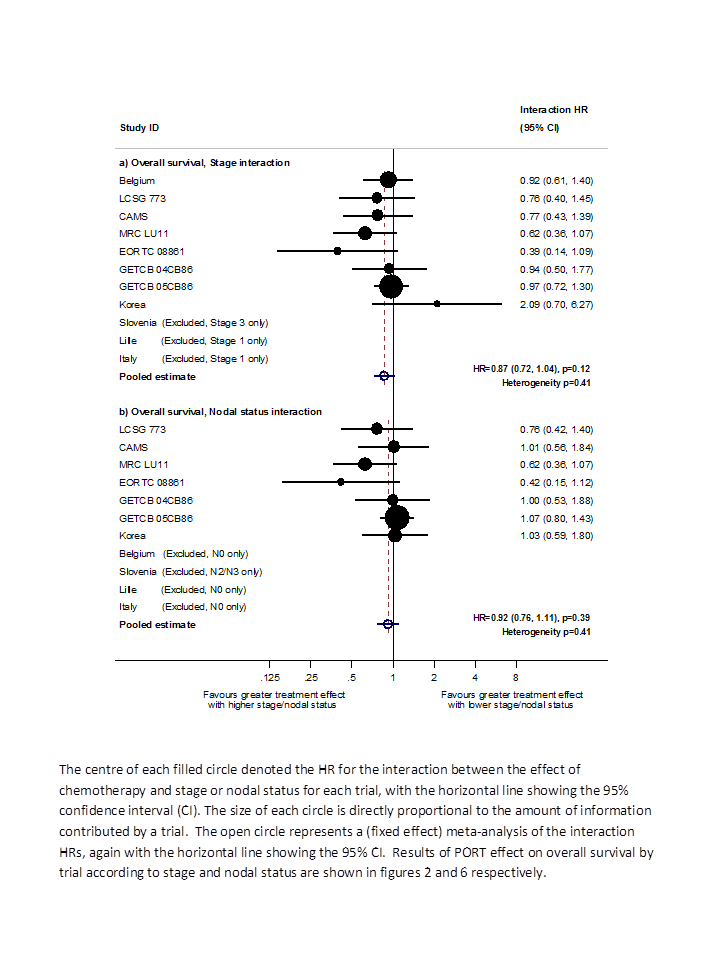
Hazard ratio (HR) for the interaction between the effect of PORT on survival and (a) stage or (b) nodal status.
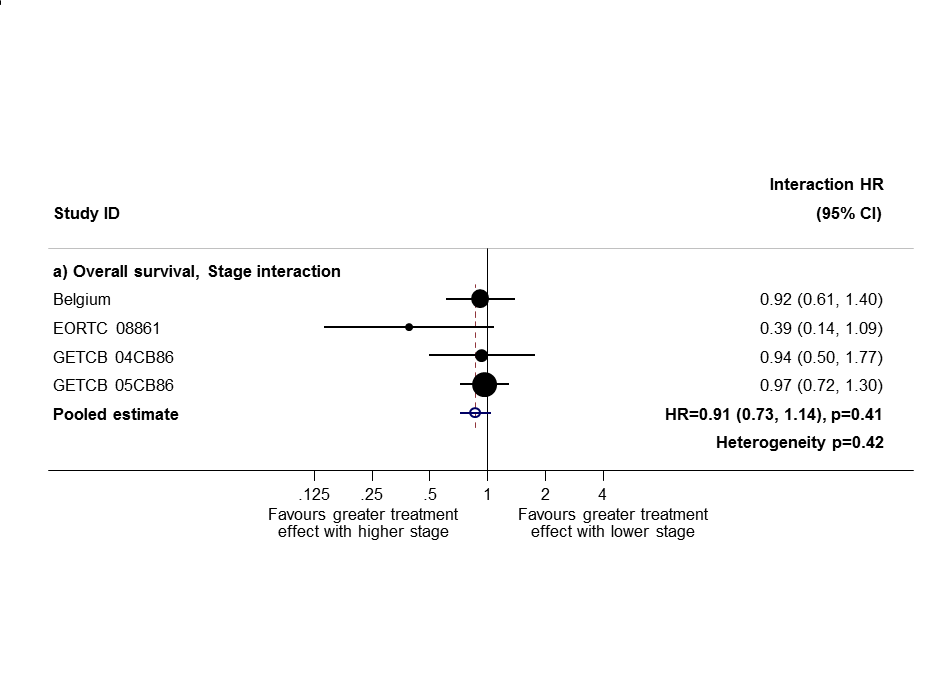
Sensitivity analysis 1: only trials with all stage subgroups included.
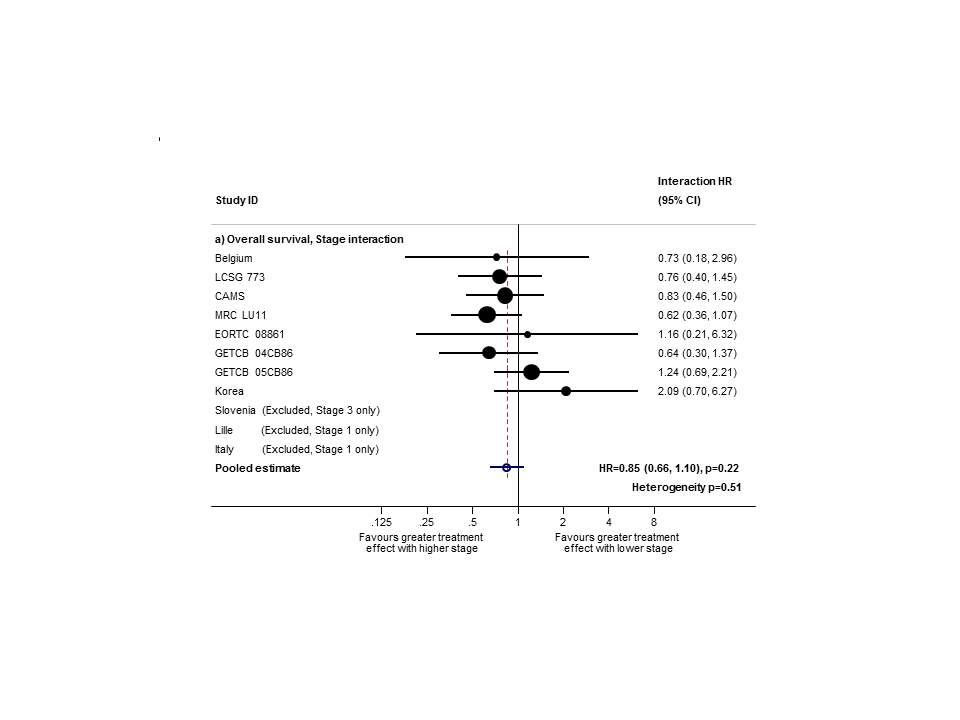
Sensitivity analysis (2): only trials with stage II and III subgroups represented.

PORT effect on overall survival by trial according to nodal status.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 1 Survival.
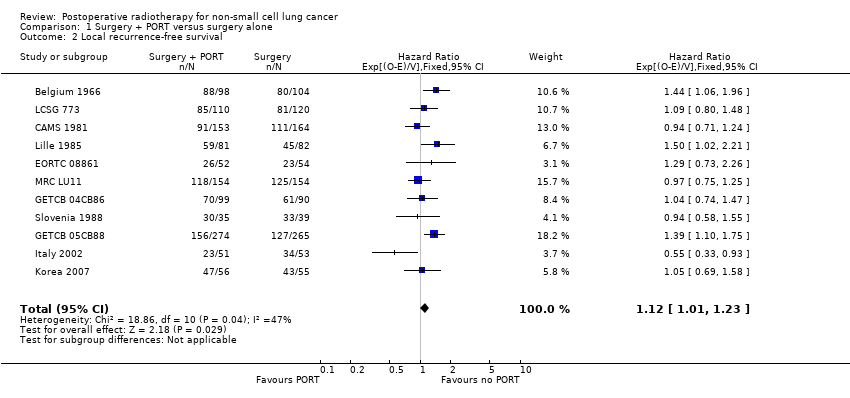
Comparison 1 Surgery + PORT versus surgery alone, Outcome 2 Local recurrence‐free survival.
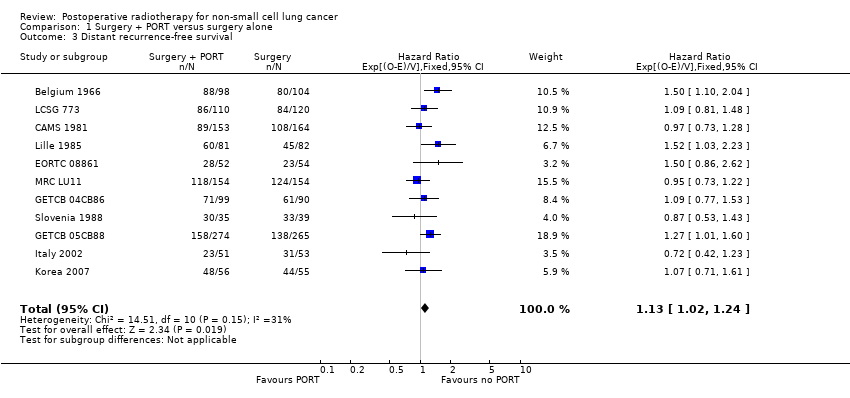
Comparison 1 Surgery + PORT versus surgery alone, Outcome 3 Distant recurrence‐free survival.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 4 Recurrence‐free survival.
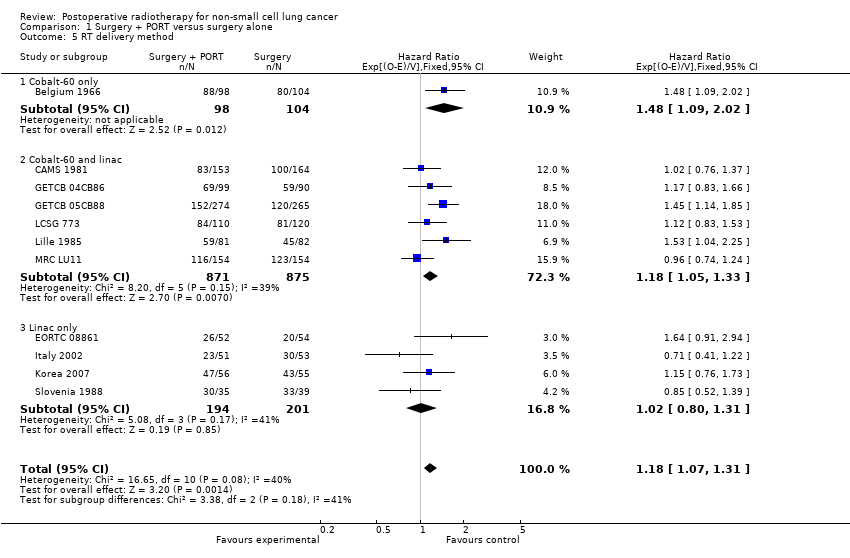
Comparison 1 Surgery + PORT versus surgery alone, Outcome 5 RT delivery method.
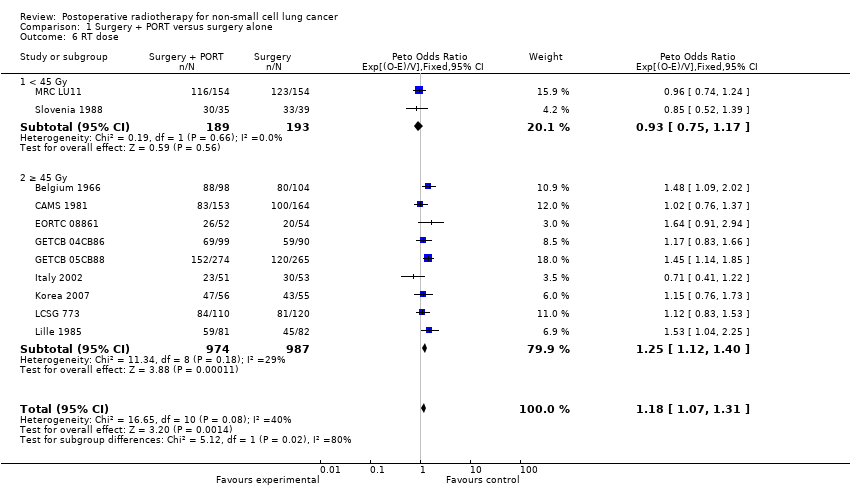
Comparison 1 Surgery + PORT versus surgery alone, Outcome 6 RT dose.
| T stage | N stage | M stage | Meta‐analysis stage | AJCC stage |
| 0, 1, 2, X, iS | 0 | 0 | I | I |
| 0, 1, 2, X, iS | 1 | 0 | II | II |
| Any | 2, 3 | 0 | III | III non‐metastatic |
| 3, 4 | Any | 0 | III | III non‐metastatic |
| Any | Any | 1 | IV | Any metastatic |
| AJCC = American Joint Committee on Cancer. | ||||
| T stage | N stage | M stage | Meta‐analysis stage |
| 1, 2 | 0 | 0 | I |
| 1, 2 | 1 | 0 | II |
| 3 | 0 | 0 | II |
| 1, 2 | 2 | 0 | III |
| 3 | 1, 2 | 0 | III |
| Any | Any | 1 | IV |
| Characteristic | Postoperative RT | Surgery only | Total |
| AGE (data from 11 trials) | |||
| < 54 years | 294 | 327 | 621 |
| 55 to 59 years | 267 | 261 | 528 |
| 60 to 64 years | 290 | 276 | 566 |
| > 65 years | 312 | 315 | 627 |
| Unknown | 0 | 1 | 1 |
| SEX (data from 11 trials) | |||
| Male | 988 | 992 | 1980 |
| Female | 175 | 187 | 362 |
| Not recorded | 0 | 1 | 1 |
| HISTOLOGY (data from 9 trials) | |||
| Adenocarcinoma | 195 | 218 | 413 |
| Squamous | 522 | 545 | 1067 |
| Other | 66 | 54 | 120 |
| Unknown | 380 | 363 | 743 |
| META‐ANALYSIS STAGE (data from 11 trials) | |||
| I | 328 | 338 | 666 |
| II | 353 | 366 | 719 |
| III | 463 | 455 | 918 |
| IV | 1 | 0 | 1 |
| Unknown | 18 | 21 | 39 |
| WHO PERFORMANCE STATUS (data from 4 trials; not used) | |||
| Good (0, 1) | 195 | 196 | 391 |
| Poor (2, 3, 4) | 77 | 83 | 160 |
| Unknown | 22 | 21 | 43 |
| Trend or interaction 1998 | Trend or interaction 2005 | Trend or interaction 2010 'old' methods | Trend or interaction 2010 'new' methods and TNM changes | |
| Age | P = 0.34 | P = 0.44 | P = 0.32 | P = 0.20 |
| Sex | P = 0.94 | P = 0.92 | P = 0.84 | P = 0.49 |
| Histology | P = 0.75 | P = 0.61 | P = 0.42 | P = 0.38 |
| Stage | P = 0.0003 | P = 0.003 | P = 0.003 | P = 0.12 |
| Nodal status | P = 0.016 | P = 0.02 | P = 0.03 | P = 0.39 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 2 Local recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.12 [1.01, 1.23] |
| 3 Distant recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.13 [1.02, 1.24] |
| 4 Recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.10 [0.99, 1.21] |
| 5 RT delivery method Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 5.1 Cobalt‐60 only | 1 | 202 | Hazard Ratio (95% CI) | 1.48 [1.09, 2.02] |
| 5.2 Cobalt‐60 and linac | 6 | 1746 | Hazard Ratio (95% CI) | 1.18 [1.05, 1.33] |
| 5.3 Linac only | 4 | 395 | Hazard Ratio (95% CI) | 1.02 [0.80, 1.31] |
| 6 RT dose Show forest plot | 11 | 2343 | Peto Odds Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 6.1 < 45 Gy | 2 | 382 | Peto Odds Ratio (95% CI) | 0.93 [0.75, 1.17] |
| 6.2 ≥ 45 Gy | 9 | 1961 | Peto Odds Ratio (95% CI) | 1.25 [1.12, 1.40] |

