Radioterapia posoperatoria para el cáncer de pulmón de células no pequeñas
Resumen
Antecedentes
La función de la radioterapia posoperatoria (RTPO) en el tratamiento de los pacientes con cáncer de pulmón de células no pequeñas (CPCNP) completamente resecado no estuvo clara. Se realizó una revisión sistemática y un metanálisis de los datos de pacientes individuales para evaluar la evidencia disponible a partir de los ensayos controlados aleatorizados (ECA). Estos resultados se publicaron por primera vez en Lung Cancer en 2013.
Objetivos
Evaluar los efectos de la RTPO sobre la supervivencia y la recidiva en pacientes con CPCNP completamente resecado. Investigar si subgrupos de pacientes predeterminados se benefician más o menos de la RTPO.
Métodos de búsqueda
Las búsquedas en MEDLINE y CANCERLIT (1965 hasta el 8 de julio de 2016) se complementaron con información de registros de ensayos, búsquedas manuales en las actas de congresos relevantes y la discusión con autores de ensayos y organizaciones.
Criterios de selección
Se incluyeron los ensayos de cirugía versus cirugía más radioterapia, siempre que los pacientes con CPCNP se asignaran al azar mediante un método que impidiera el conocimiento previo de la asignación al tratamiento.
Obtención y análisis de los datos
Se realizó un metanálisis cuantitativo con información actualizada de los pacientes individuales de todos los ensayos aleatorizados. Se solicitaron los datos de todos los participantes a los responsables de los ensayos. Se obtuvieron los datos de pacientes individuales (DPI) actualizados sobre la supervivencia y la fecha del último seguimiento, así como detalles sobre la asignación al tratamiento, la fecha de la asignación al azar, la edad, el sexo, el tipo histológico de las células, el estadio y el estado funcional. Para evitar posibles sesgos, se solicitó información de todos los pacientes asignados al azar, incluidos los que se habían excluido de los análisis originales realizados por los investigadores. Todos los análisis se realizaron por intención de tratar para la variable principal de evaluación supervivencia.
Resultados principales
Se identificaron 14 ensayos que evaluaron cirugía versus cirugía más radioterapia. Se dispuso de los datos de pacientes individuales de 11 de estos ensayos y los análisis de esta revisión se basan en 2343 participantes (1511 muertes). Los resultados muestran un efecto adverso significativo de la RTPO en la supervivencia, con un cociente de riesgos instantáneos de 1,18, o un aumento relativo del 18% en el riesgo de muerte. Este resultado equivale a un perjuicio absoluto del 5% a los dos años (intervalo de confianza [IC] del 95%: 2% a 9%), que reduce la supervivencia general del 58% al 53%. Los análisis de subgrupos no mostraron diferencias en los efectos de la RTPO por alguna covariable de los subgrupos de participantes.
No se realizó un análisis de los efectos de la RTPO sobre la calidad de vida ni los eventos adversos. Los investigadores no obtuvieron de forma habitual información sobre la calidad de vida durante estos ensayos y fue poco probable que cualquier efecto beneficioso de la RTPO compensara la desventaja observada en la supervivencia. Se consideró que el riesgo de sesgo en los ensayos incluidos fue bajo.
Conclusiones de los autores
Los resultados de 11 ensayos y 2343 participantes muestran que la RTPO es perjudicial para los pacientes con cáncer de pulmón de células no pequeñas completamente resecado y no se debe utilizar en el tratamiento habitual de esos pacientes. Los resultados de los ECA en curso aclararán los efectos de la radioterapia moderna en los pacientes con tumores N2.
PICOs
Resumen en términos sencillos
Radioterapia posoperatoria para el cáncer de pulmón de células no pequeñas
Pregunta de la revisión
¿Los pacientes con cáncer de pulmón de células no pequeñas viven más tiempo si se le administra radioterapia después de la cirugía?
Antecedentes
El cáncer de pulmón de células no pequeñas es el tipo más frecuente de cáncer de pulmón. Si el tumor está en estadio temprano, no es demasiado grande y no se ha diseminado a otras partes del cuerpo, habitualmente los médicos operan para extraerlo. A veces, después de la cirugía se proporciona radioterapia (tratamiento con rayos X), con el objetivo de matar cualquier célula cancerosa restante.
En 1998 se realizó una revisión sistemática y un metanálisis de los datos de pacientes individuales que examinó los ensayos de este tratamiento: la radioterapia posoperatoria (RTPO). Esta revisión reunió información de todos los pacientes que participaron en ensayos similares. Estos ensayos compararon lo que le sucedió a los pacientes con cáncer de pulmón de células no pequeñas a los que se les administró radioterapia después de la cirugía y a los que se sometieron a una cirugía sin radioterapia. Los resultados se publicaron por primera vez en The Lancet en 1998.
Desde que se completó esta revisión, se han realizado muchos ensayos. Para asegurar que la evidencia disponible esté lo más actualizada posible, se realizó una nueva revisión sistemática y un metanálisis de los datos de pacientes individuales que incluyó todos los ensayos, antiguos y nuevos. En cuanto a la revisión de 1998, esta revisión se propuso determinar si la administración de radioterapia después de la cirugía (1) ayuda a los pacientes a vivir más tiempo, (2) impide que el cáncer regrese (recidiva) y (3) impide que el cáncer se disemine a otras partes del cuerpo (metástasis).
Estos resultados actualizados se publicaron por primera vez en Lung Cancer en 2013.
Características de los estudios
Se buscaron ensayos relevantes hasta el 8 de julio de 2016. Estos estudios reunieron los datos de ensayos disponibles de todo el mundo, con 11 ensayos y 2343 pacientes. Los ensayos se realizaron entre 1966 y 1998.
Resultados clave
Los resultados mostraron que menos pacientes que recibieron RTPO vivieron durante dos años después de la cirugía (53 de cada 100 pacientes) con comparación con los que no recibieron RTPO después de la cirugía (58 de cada 100 pacientes). Los investigadores no informaron de una diferencia en los efectos de la RTPO por tipos de pacientes incluidos en los ensayos.
Los investigadores no obtuvieron de forma habitual información sobre la calidad de vida durante los ensayos, y fue poco probable que cualquier efecto beneficioso de la RTPO compensara la desventaja observada en la supervivencia.
La radioterapia administrada después de la extirpación satisfactoria del tumor mediante cirugía no es beneficiosa para los pacientes con cáncer de pulmón de células no pequeñas y no se debe utilizar como tratamiento habitual; sin embargo, se realizan nuevas investigaciones sobre nuevos tipos de radioterapia para los pacientes con mayor riesgo de recidiva.
Calidad de la evidencia
Esta revisión sistemática y los metanálisis utilizaron los datos de pacientes individuales, que se consideran el valor de referencia (gold standard) de este tipo de revisión. De ser posible se incluyeron todos los ensayos elegibles, sin importar el idioma en el que se publicaron ni si se publicaron o no. Este metanálisis incluyó el 88% de todos los participantes en los ensayos elegibles.
Los estudios estuvieron bien diseñados y realizados y abordaron la pregunta de revisión con efectos consistentes entre los ensayos. El impacto de cualquier dato no incluido en los análisis de esta revisión es pequeño.
Authors' conclusions
Background
Description of the condition
Worldwide, carcinoma of the lung is the main cause of cancer death. More than 1.5 million new cases are diagnosed each year (Jemal 2011), about 85% of which involve non‐small cell lung cancer (American Cancer Society 2007). Surgery is the treatment of choice for early non‐small cell lung cancer (NSCLC) (NSCLCCG 1995), but only about 20% of tumours are suitable for potentially curative surgery (Datta 2003). Even for patients with apparently completely resected disease, survival is only around 40% at five years. In an effort to improve local‐regional control of the disease and to increase survival, investigators have explored adjuvant postoperative radiotherapy (PORT) as a therapeutic option.
Description of the intervention
This review concentrated on randomised controlled trials (RCTs) that tested surgery alone compared with surgery followed by radiotherapy. Radiotherapy in these trials was given by cobalt therapy, by cobalt therapy and linear accelerators or by linear accelerators only.
How the intervention might work
Radiotherapy may be given after surgery with the aim of killing any remaining cancer cells.
Why it is important to do this review
Despite the conduct of several RCTs (most in the 1980s and 1990s) that recruited a total of more than 2000 patients, the role of PORT in the treatment of patients with NSCLC has remained unclear. Individually, trials showed inconclusive and conflicting results. However, because of their size (74 to 539 participants), individual trials did not have sufficient statistical power to detect the moderate survival differences that might be expected with PORT. We therefore initiated an individual participant data meta‐analysis to assess this question. This approach to meta‐analysis and systematic review involves the central collection, validation and analysis of original trial data. It does not rely on data extracted from publications. At the outset, the project management group contacted the investigators responsible for each trial and established the PORT Meta‐analysis Trialists Group, under whose auspices the meta‐analysis was conducted and published. This review was first published in The Lancet in 1998 (PORT 1998). In 2005, the meta‐analysis was updated (PORT 2005) with data from one new trial (Italy 2002). In 2009, the meta‐analysis was updated again to include data from another new trial (Korea 2007). However, since this review was last updated, new methods developed to assess treatment by patient covariates that are methodologically more appropriate and less prone to bias have prompted this latest update of the Cochrane review (Fisher 2011). Furthermore, changes over time to the tumour‐node‐metastasis (TNM) staging system have been taken into account in this update, and although the data did not permit use of the seventh TNM edition, they did allow us to convert tumour stage from the fourth (Mountain 1987) to the fifth/sixth (Mountain 1997) edition.
Objectives
To evaluate effects of PORT on survival and recurrence in patients with completely resected NSCLC. To investigate whether predefined patient subgroups benefit more or less from PORT.
Methods
Criteria for considering studies for this review
Types of studies
To be included, both published and unpublished completed trials had to be properly randomised using established methods (not quasi‐randomised). Trials could not have been confounded by additional therapeutic differences between the two arms and must have commenced randomisation on or after 1 January 1965. Trials should have aimed to randomise participants with completely resected non‐small cell lung cancer between radiotherapy and no immediate further treatment. Trials should not have used orthovoltage radiotherapy.
Types of participants
Eligible trials included individuals with histologically confirmed NSCLC who had undergone a potentially curative resection. We included in the meta‐analyses individual participant data from all randomised participants and, when possible, obtained data for individuals who had been excluded from the original trial analyses. We excluded from the meta‐analyses, participants with small cell lung cancer, who were included in early trials that randomised all types of lung cancer.
Types of interventions
-
Surgery versus surgery + postoperative radiotherapy (PORT).
Types of outcome measures
-
Survival.
-
Recurrence‐free survival.
-
Local recurrence‐free survival.
-
Distant recurrence‐free survival.
Primary outcomes
The primary outcome of overall survival was defined as the time from randomisation until death by any cause. Living participants were censored on the date of last follow‐up.
Secondary outcomes
Recurrence‐free survival was defined as the time from randomisation until first recurrence, or death by any cause. Participants alive without disease were censored on the date of last follow‐up. To avoid bias from under‐reporting of subsequent events, time to local‐regional recurrence was defined as the time from randomisation until first local‐regional recurrence, with participants experiencing earlier distant recurrences censored at the time of distant recurrence. Similarly, for time to distant recurrence, participants experiencing earlier local‐regional recurrences were censored on that date. Participants who died without recurrence were censored on the date of death. Data on quality of life were not routinely collected in these trials; therefore we could not analyse the data in this review.
Search methods for identification of studies
To limit publication bias, we included published and unpublished trials with no restrictions based on language. We carried out searches of MEDLINE (Appendix 1) and CANCERLIT from 1965 (using The Cochrane Collaboration's optimal strategy (Lefebvre 2001; Lefebvre 2008). We supplemented searches of trial registers by conducting handsearches of conference proceedings and reference lists of trial publications and review articles. We asked our collaborators if they knew of additional trials. We carried out the most recent searches in July 2016.
In 1997, we identified trials by electronic searches of MEDLINE and CANCERLIT, using a modified version of the optimal search strategy of The Cochrane Collaboration (Dickersin 1995), supplemented by handsearches of Proceedings of the American Society of Clinical Oncology (ASCO) (1990 to 2016) and the World Conference on Lung Cancer (1990 to 2015) and bibliographies of books, reviews and specialist journals. We also searched trial registers (Cochrane Central Register of Controlled Trials (CENTRAL), the National Cancer Institute Physicians Data Query Clinical Protocols and United Kingdom Co‐ordinating Committee for Cancer Research)and asked all trialists who took part in the meta‐analysis to help to identify additional trials. We regularly updated searches to identify new trials and to assess the status of any ongoing trials. Search updates in October 2002 and August 2003 identified one new eligible trial (Italy 2002), and the search update carried out in 2009 identified two new eligible trials: a Polish trial (Dymek 2003) and a Korean trial published as an abstract (Korea 2007). We last carried out searches in July 2016 and identified no new eligible trials.
Electronic searches
We modified The Cochrane Collaboration's optimum search strategy for retrieving RCTs from MEDLINE (Appendix 1) to specifically retrieve RCTs of radiotherapy for NSCLC, and we used this search strategy to search MEDLINE and CANCERLIT (1965 to 2016).
In addition, we searched the following electronic bibliographic databases.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (1995 to 8 July 2016) (Appendix 2).
-
Proceedings of annual meetings of the American Society for Clinical Oncology (ASCO) (1995 to 2016).
We used the following trial registers to supplement searches of electronic databases with trials that were not (yet) published or were still recruiting patients.
-
United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Trials Register.
-
ClinicalTrials.gov.
-
Physicians Data Query Protocols (open and closed).
-
Current Controlled Trials ‘metaRegister’ of controlled trials.
Searching other resources
Handsearches
We carried out the following handsearches to identify trials that may have been reported only as abstracts, or that might have been missed in the searches described above.
-
Proceedings of the American Society for Clinical Oncology (ASCO), 1990 to 1994.
-
Proceedings of the International Association for the Study of Lung Cancer (IASLC) World Lung Cancer Conference 1990 to 2015.
Searches of reference lists
We searched the bibliographies of all identified trials and review articles.
Correspondence
We asked all participating trialists to review and supplement a provisional list of trials.
Data collection and analysis
Selection of studies
Two members of the Project Management Group (SB, LR) checked all titles and abstracts identified by electronic searching and handsearching of conference proceedings, and obtained full publications for those thought to be potentially relevant. We sought individual participant data (IPD) from trial authors, including updated follow‐up, when available.
Data extraction and management
We sought IPD for all eligible trials, as well as updated information on survival, recurrence and date of last follow‐up, and details of treatment allocated, date of randomisation, age, sex, histological cell type, stage and performance status.
We used standard checks to identify missing data. We verified data, for example, by checking the order of the dates of randomisation, and assessed data validity and consistency. To assess randomisation integrity, we checked patterns of treatment allocation and balance of baseline characteristics by treatment arm. We checked follow‐up of surviving participants to ensure that it was balanced by treatment arm and up‐to‐date. We resolved queries, and each trial investigator or statistician verified the final database.
As stage was recorded using different classification systems, for the purposes of this meta‐analysis we translated all stage data to a common staging system, which has been updated since the original analysis to reflect the TNM sixth edition classification (Table 1; Table 2).
| T stage | N stage | M stage | Meta‐analysis stage | AJCC stage |
| 0, 1, 2, X, iS | 0 | 0 | I | I |
| 0, 1, 2, X, iS | 1 | 0 | II | II |
| Any | 2, 3 | 0 | III | III non‐metastatic |
| 3, 4 | Any | 0 | III | III non‐metastatic |
| Any | Any | 1 | IV | Any metastatic |
AJCC = American Joint Committee on Cancer.
| T stage | N stage | M stage | Meta‐analysis stage |
| 1, 2 | 0 | 0 | I |
| 1, 2 | 1 | 0 | II |
| 3 | 0 | 0 | II |
| 1, 2 | 2 | 0 | III |
| 3 | 1, 2 | 0 | III |
| Any | Any | 1 | IV |
Assessment of risk of bias in included studies
We assessed included studies using the risk of bias tool of The Cochrane Collaboration, as outlined in Table 8.5c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), two authors (SB, LR) checked these studies. We considered adequate sequence generation and allocation concealment to be most important; therefore a judgement of low risk was desirable for these domains for all trials. Blinding was not appropriate owing to the nature of the treatments, and any issues surrounding reporting of incomplete outcome data, selective outcome reporting or attrition bias were overcome by collection of IPD.
Measures of treatment effect
Unless otherwise stated, we prespecified all analyses in the protocols and carried out an intention‐to‐treat analysis. For each outcome, we used the log‐rank expected number of events and variance to calculate individual trial hazard ratios (HRs), which we pooled across trials using the fixed‐effect model (Yusuf 1985). We presented overall survival using simple (non‐stratified) Kaplan‐Meier curves (Kaplan 1958) and computed median follow‐up for all participants by using the reverse Kaplan‐Meier method (Schemper 1996).
We analysed 'raw' IPD using in‐house software (SCHARP), then entered the log‐rank summary statistics of these analyses (O‐E and variance) into RevMan (RevMan 2014). We presented results as absolute differences at five years, calculated with the HR and baseline event rate on the surgery alone arm; we assumed proportional hazards (Parmar 1995). We similarly calculated confidence intervals for absolute differences from the baseline event rate and the HR at 95% confidence interval boundary values.
To explore any impact of trial characteristics on effects of adjuvant chemotherapy on overall survival, we calculated pooled HRs for each prespecified trial group and used Chi2 tests for interaction to investigate differences in treatment effect across trial groups.
Dealing with missing data
We outlined all desired variables in a protocol (available on request) and requested missing variables from those who carried out the trials.
Assessment of heterogeneity
We used Chi2 tests and the I2 statistic (Higgins 2002) to test for differences in treatment effect across groups of trials or groups of participants.
Assessment of reporting biases
As we collected IPD, we encountered no reporting biases.
Data synthesis
When we could get data, we included all eligible trials in the analyses. We carried out these analyses in SCHARP (in‐house software), Stata (Stata 2013) and RevMan (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
To investigate differences in treatment effect across participant subgroups, we undertook Cox regressions, including the relevant treatment by subgroup interaction term within each trial. We pooled these interaction coefficients (HRs) across trials (Fisher 2011) and investigated whether differences in treatment effect could be identified that varied with participant age, sex, histological cell type, tumour stage or performance status.
Sensitivity analysis
We outlined in the protocol that HRs for overall survival would be calculated, excluding any trials that were clear outliers.
Results
Description of studies
We identified 14 eligible trials (one unpublished) and included 11 trials in the review (see Characteristics of included studies). We could not include three trials: Data for two trials were unavailable (Dymek 2003 (150 participants); LCSG 841 (five participants)), and it was unclear whether one study of 155 participants, which was reported as a randomised controlled trial (RCT), was indeed randomised. We were unable to obtain appropriate data for this trial (Austria 1996) (see Characteristics of excluded studies).
Therefore, this update is based on the results of 11 RCTs (Belgium 1966; CAMS 1981; EORTC 08861; GETCB 04CB86; GETCB 05CB88; Italy 2002; Korea 2007; LCSG 773; Lille 1985; MRC LU11; Slovenia 1988) and 2343 individuals. Across these trials, PORT doses ranged from 30 Gy to 60 Gy, given in between 10 and 30 fractions, and considerable diversity was evident in other aspects of radiotherapy planning. All trials included participants with completely resected tumours for which the disease stage was no greater than IIIA. Most trials provided updated follow‐up giving a median of 4.4 years for surviving participants (2.3 to 11.4 years for individual trials). Baseline participant characteristics show that most participants were male with stage II/III squamous cell carcinoma (although histology was unknown for a relatively large number of participants) with good performance status (Table 3).
| Characteristic | Postoperative RT | Surgery only | Total |
| AGE (data from 11 trials) | |||
| < 54 years | 294 | 327 | 621 |
| 55 to 59 years | 267 | 261 | 528 |
| 60 to 64 years | 290 | 276 | 566 |
| > 65 years | 312 | 315 | 627 |
| Unknown | 0 | 1 | 1 |
| SEX (data from 11 trials) | |||
| Male | 988 | 992 | 1980 |
| Female | 175 | 187 | 362 |
| Not recorded | 0 | 1 | 1 |
| HISTOLOGY (data from 9 trials) | |||
| Adenocarcinoma | 195 | 218 | 413 |
| Squamous | 522 | 545 | 1067 |
| Other | 66 | 54 | 120 |
| Unknown | 380 | 363 | 743 |
| META‐ANALYSIS STAGE (data from 11 trials) | |||
| I | 328 | 338 | 666 |
| II | 353 | 366 | 719 |
| III | 463 | 455 | 918 |
| IV | 1 | 0 | 1 |
| Unknown | 18 | 21 | 39 |
| WHO PERFORMANCE STATUS (data from 4 trials; not used) | |||
| Good (0, 1) | 195 | 196 | 391 |
| Poor (2, 3, 4) | 77 | 83 | 160 |
| Unknown | 22 | 21 | 43 |
Risk of bias in included studies
We included only trials with adequate methods of randomisation. We excluded trials that used quasi‐random methods, such as birth date. We thoroughly checked all raw data received on individual participants to ensure both the accuracy of the meta‐analysis database and the quality of randomisation and follow‐up. We resolved all queries and verified final database entries through discussion with the responsible trial investigator or statistician. No RCTs were blinded owing to the nature of the intervention, but the primary outcome is not likely to be influenced by lack of blinding. For two trials, allocation concealment was unclear ‐ one trial was unpublished (EORTC 08861), and one was published only as an abstract (Korea 2007) ‐ but checks on IPD and correspondence with those who supplied the data reassured us that the data had been adequate. We received IPD for all outcomes of interest; therefore we considered reporting bias to be low for all RCTs. We considered all included trials to be at low risk of bias (see Figure 1 and Figure 2).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
Results were based on information from 11 RCTs (2343 participants), representing 88% of individuals from all eligible randomised trials. We collected data for 140 out of 142 participants who had been excluded from the original published trial analyses and were reinstated in this meta‐analysis. For one trial, which randomised all histological types of lung cancer (Belgium 1966), we excluded the 20 participants with small cell tumours from the meta‐analysis. Survival and recurrence data were available for all trials. All trials provided information on age, sex and stage, and nine trials provided data on histology (Belgium 1966; CAMS 1981; EORTC 08861; Italy 2002; Korea 2007; LCSG 773; Lille 1985; MRC LU11; Slovenia 1988). Performance status data were available for only four trials (EORTC 08861; Italy 2002; MRC LU11; Slovenia 1988) and were insufficient for assessment of treatment by covariate interactions. All but two trials provided cause of death data (coded as NSCLC, treatment‐related or other) (Italy 2002; LCSG 773), although the trialists themselves questioned the reliability of this information for many trials.
Survival
Survival data were available for all trials and included information from 2343 participants and 1511 deaths (777 PORT, 734 surgery alone). Although the confidence intervals (CIs) for individual trial results were wide, combined results showed a significant adverse effect of PORT on survival (P = 0.001), with a hazard ratio (HR) of 1.18 (95% CI 1.07 to 1.31) (Analysis 1.1), or an 18% relative increase in risk of death. This was equivalent to an absolute detriment of 5% at two years (95% CI 2% to 9%), reducing overall survival from 58% to 53%. Survival curves (Figure 3) appeared to diverge at around four months and remained apart for the five years to which they could be drawn with reasonable reliability. There was some evidence of increased statistical heterogeneity between trials in the current update (I2 = 40%, P = 0.08), compared with the original 1998 meta‐analysis. However, the random‐effects result is similar (HR 1.17, 95% CI 1.02 to 1.34, P = 0.02), and heterogeneity appears largely driven by the Italian trial (Italy 2002). A sensitivity analysis excluding this trial reduces heterogeneity (I2 = 31%, P = 0.16) and gives similar fixed‐effect (HR 1.20, 95% CI 1.08 to 1.33, P = 0.0005) and random‐effects results (HR 1.20, 95% CI 1.06 to 1.37, P = 0.005).
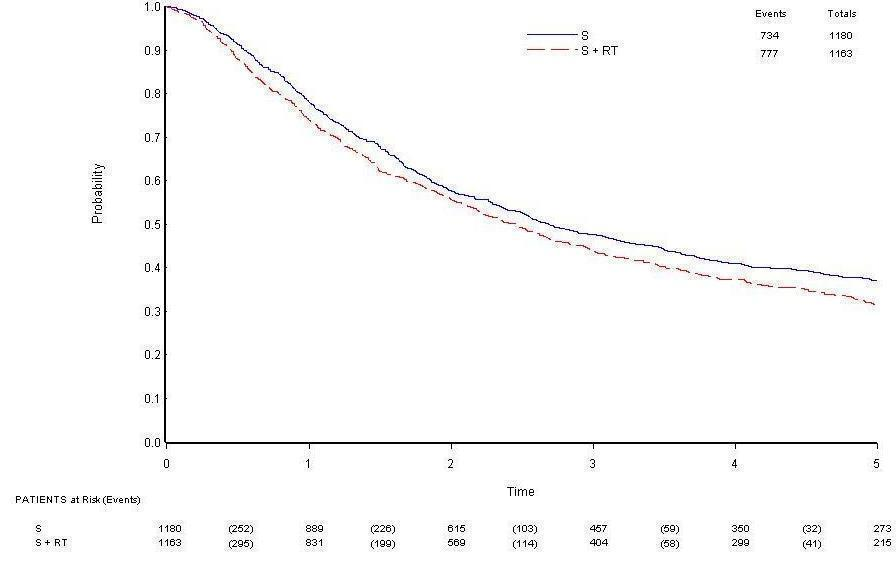
Overall survival.
Cause of death information coded as NSCLC, treatment related or other was available for nine trials. Of 595 coded deaths on PORT, 82% were attributed to NSCLC, 4% to treatment‐related causes and 14% to other causes. For the 565 coded deaths on surgery alone, these figures are 89%, 2% and 9%, respectively.
Local recurrence‐free survival
Data on local‐regional recurrence were available from all trials. Analysis of local‐regional recurrence‐free survival, based on 1556 events (498 local‐regional recurrences (200 on PORT, 298 on surgery alone) and 1058 deaths (593 on PORT, 465 on surgery alone)), gave a HR of 1.12 (95% CI 1.01 to 1.24), significantly in favour of surgery alone (P = 0.03) (Analysis 1.2). There was evidence of statistical heterogeneity between trials (I2 = 47%, P = 0.04), which was not apparent in the 1998 analysis (I2 = 29%, P = 0.19), and for this outcome, the random‐effects result is less convincing (HR 1.10, 95% CI 0.95 to 1.27, P = 0.19) than the fixed‐effect result. However, exclusion of the Italian trial (Italy 2002) again reduces heterogeneity to non‐significant levels (I2 = 22%, P = 0.23), as well as giving similar fixed‐effect (HR 1.15, 95% CI 1.04 to 1.27, P = 0.008) and random‐effects estimates (HR 1.15, 95% CI 1.02 to 1.29, P = 0.02). Results may suggest an increase in local‐regional recurrence on the PORT arm, but the number of local‐regional recurrences alone shows less local‐regional recurrence on the PORT arm and more events when deaths without local‐regional recurrence are included.
Distant recurrence‐free survival
All trials provided data on distant recurrence. Analysis of distant recurrence‐free survival based on 1570 events (892 distant recurrences (438 on PORT, 454 on surgery alone) and 678 deaths (361 on PORT, 317 on surgery alone)) gave an HR of 1.13 (95% CI 1.02 to 1.24) in favour of surgery alone (P = 0.02) (Analysis 1.3). There was no evidence of gross statistical heterogeneity between trials (I2 = 31%, P = 0.15).
Overall recurrence‐free survival
A total of 1597 events were observed, 810 on PORT and 787 on surgery alone. Of these, 445 first events were deaths, 260 participants had local‐regional recurrences and 654 had distant recurrences (238 participants had both local‐regional and distant recurrences, of which 110 were recorded on the same date). The overall HR of 1.10 (95% CI 0.99 to 1.21) potentially suggests an adverse effect of PORT (P = 0.07) (Analysis 1.4). This 10% relative increase in risk of recurrence or death was equivalent to an absolute detriment of 3% at two years (95% CI 0% to 7%), reducing the recurrence‐free survival rate from 48% to 45%. As with local‐regional recurrence‐free survival, there was some evidence of increased statistical heterogeneity between trials (I2 = 44%, P = 0.06) that was not present in the 1998 analysis (I2 = 26%, P = 0.21), and a random‐effects analysis produces a less convincing result (HR 1.09, 95% CI 0.95 to 1.25, P = 0.23). However, a sensitivity analysis excluding the Italian trial (Italy 2002) not only reduces heterogeneity (I2 = 20%, P = 0.26) but also gives similar fixed‐effect (HR 1.13, 95% CI 1.02 to 1.24, P = 0.02) and random‐effects (HR 1.13, 95% CI 1.00 to 1.26, P = 0.04) results.
Analyses by trial characteristics
We planned analysis for overall survival by trial characteristic based on the planned energy beam delivery method (cobalt only, cobalt and linac, linac only) and radiotherapy dose (< 45 Gy, ≥ 45 Gy). We found no difference in effects of treatment on overall survival depending on delivery method (P = 0.18) (Analysis 1.5). We did find a difference by dose of radiotherapy (P = 0.02) (Analysis 1.6), but 80% of data is in the >=45 Gy group, and the result in the < 45 Gy group subgroup alone is not significant.
Analyses by participant covariates
Based on data from all trials, for survival there was no evidence to suggest that PORT was differentially effective by age (interaction P = 0.67), sex (P = 0.49) or histology (P = 0.38). For analysis by stage, we could not include three trials because all participants were in a single stage category (Italy 2002, Lille 1985 stage I only; Slovenia 1988 stage III only). Data from the remaining eight trials provide no evidence to suggest that PORT was differentially effective by stage within individual trials (Figure 4), but the meta‐analysis of these interactions suggests that PORT may be most detrimental in earlier‐stage patients, although the result was not significant (HR = 0.87, 95% CI 0.72 to 1.04, P = 0.12) (Figure 5). Similar results were observed whether or not trials included all three stages or only stage II and III participants (Figure 6; Figure 7). Exploratory analyses of how the effect of PORT on local‐regional, distant and overall recurrence‐free survival varies by stage gave similar results. For analysis by nodal status, we could not include four trials because all participants were in a single subgroup category with N0 (Belgium 1966; Italy 2002; Lille 1985) or N2/3 (Slovenia 1988) disease. Data from the remaining seven trials provided no evidence to suggest that PORT was differentially effective by nodal status within individual trials (Figure 8), nor in a meta‐analysis of these interactions (HR = 0.92, 95% CI 0.76 to 1.11, P = 0.39) (Figure 3).

PORT effect on overall survival by trial according to stage.
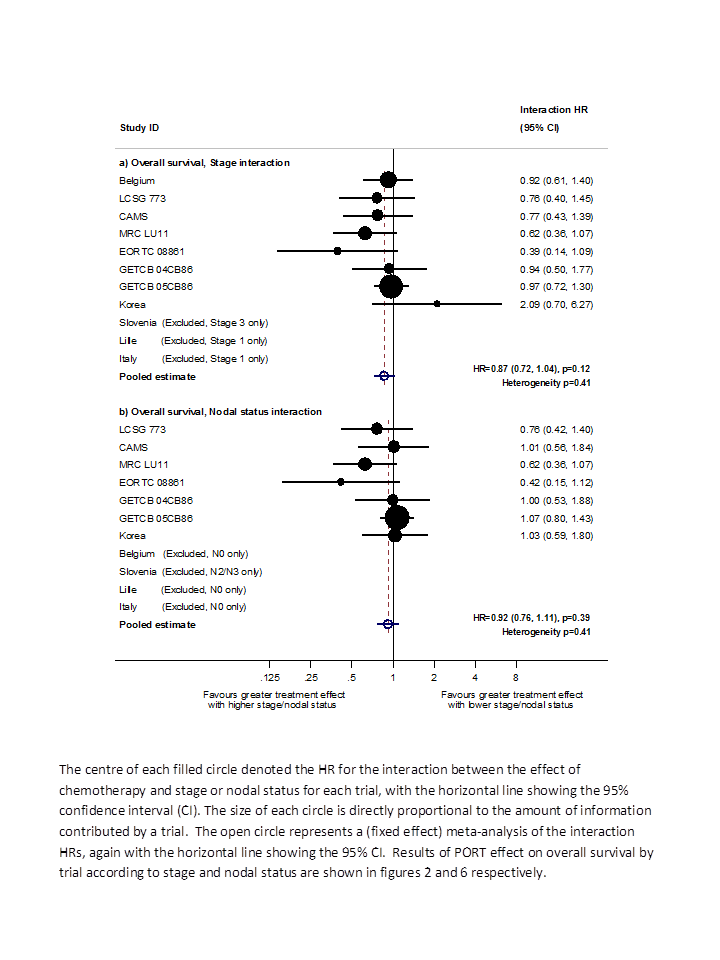
Hazard ratio (HR) for the interaction between the effect of PORT on survival and (a) stage or (b) nodal status.
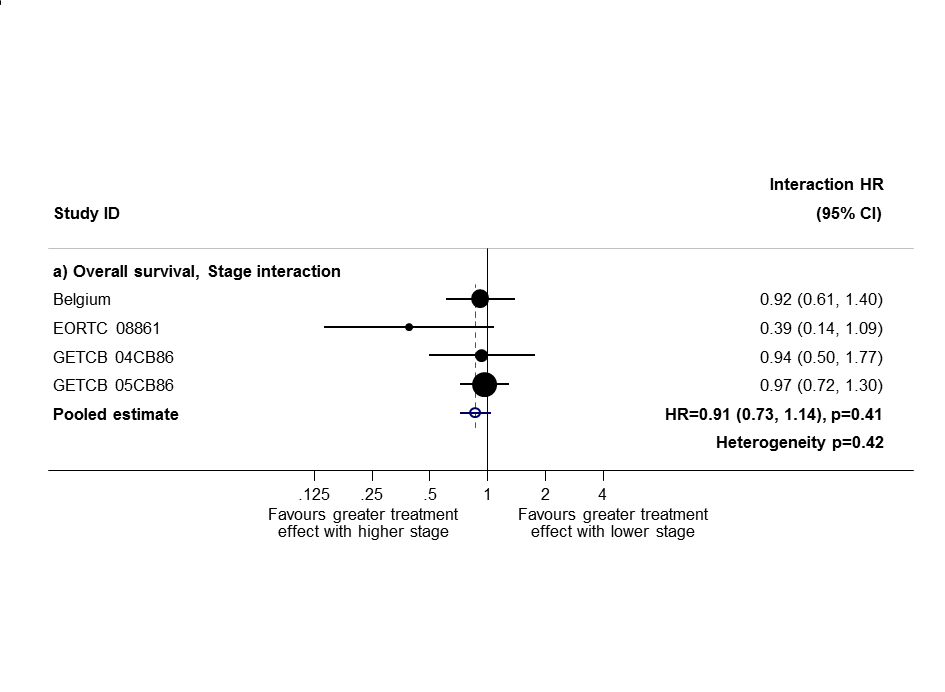
Sensitivity analysis 1: only trials with all stage subgroups included.

Sensitivity analysis (2): only trials with stage II and III subgroups represented.
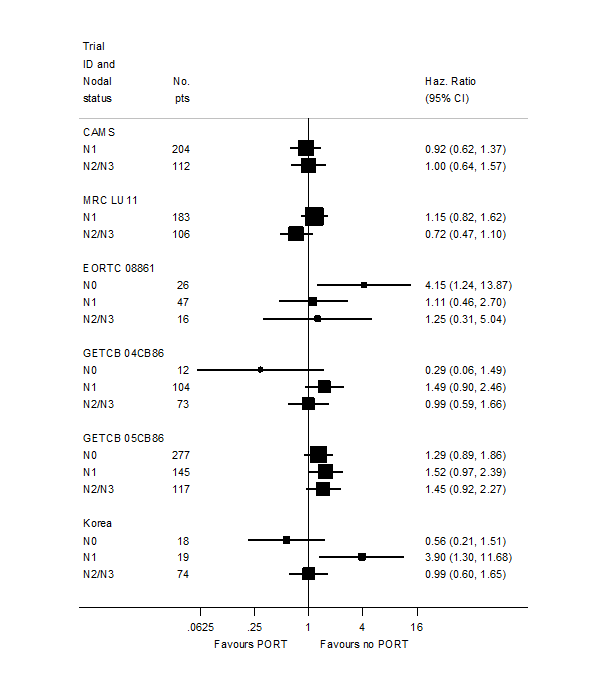
PORT effect on overall survival by trial according to nodal status.
Results for stage and nodal status are different from previously published results (PORT 1998; PORT 2005) largely for two reasons. Here, we have used a method (Fisher 2011) to examine whether the effect of PORT varied by participant covariates that was different from the method used in the original review in 1998 (PORT 1998), and in the previous updates in 2005 and 2009 (PORT 2005). This new method is more appropriate and is less prone to bias. We could not calculate several covariate interactions for trials contributing participants in only a single covariate category; therefore we did not include these studies; this approach, although correct, can provide less power than the methods used previously. Trials not included here also happen to have quite extreme results and have had undue influence on previous analyses.
In this update, we wanted to take account of changes to the TNM staging system; although the data do not allow us to use the seventh edition, they do allow us to convert stage from the fourth to the sixth edition; the major impact of this is that patients previously classified as T3N0M0 (stage IIIA) have been reclassified as stage IIB. This change affected 98 participants and Table 4 shows results for stage and nodal status according to the combination of changes made.
| Trend or interaction 1998 | Trend or interaction 2005 | Trend or interaction 2010 'old' methods | Trend or interaction 2010 'new' methods and TNM changes | |
| Age | P = 0.34 | P = 0.44 | P = 0.32 | P = 0.20 |
| Sex | P = 0.94 | P = 0.92 | P = 0.84 | P = 0.49 |
| Histology | P = 0.75 | P = 0.61 | P = 0.42 | P = 0.38 |
| Stage | P = 0.0003 | P = 0.003 | P = 0.003 | P = 0.12 |
| Nodal status | P = 0.016 | P = 0.02 | P = 0.03 | P = 0.39 |
Discussion
At the outset of this project, despite enrolment of more than 2000 participants in randomised trials, it remained unclear whether postoperative radiotherapy (PORT) was effective for the treatment of patients with non‐small cell lung cancer (NSCLC). The original 1998 meta‐analysis found a significant adverse effect of PORT on survival (P = 0.001) with a hazard ratio (HR) of 1.21 (95% confidence interval (CI) 1.08 to 1.34), or a 21% relative increase in risk of death. We undertook this systematic review and individual participant data meta‐analysis to produce a comprehensive, reliable and up‐to‐date summary of the average effect of PORT in patients with NSCLC, to provide reliable guidance for clinical practice and future research. Therefore, when a new trial of 111 participants was published (Korea 2007), we included this study in an update of the analyses.
Overall, for the primary endpoint of survival, there was clear evidence of a detrimental effect of PORT for patients with completely resected NSCLC. The 18% relative increase in risk of death associated with PORT, equivalent to an overall reduction in survival from 58% to 53% at five years, represents a considerable hazard for these patients. In contrast to the original meta‐analysis and previous updates, this update, using new and more appropriate methods, did not provide evidence that the relative effect of PORT was smaller or larger for patients of any category defined by age, sex or histology. For analysis by stage, PORT tended to be most detrimental in patients with earlier‐stage disease, but this result was not significant once 98 patients had been reclassified according to the updated TNM system. Likewise, analysis by nodal status shows a much less convincing relationship between the effect of PORT and nodal status. Also, in the case of both stage and nodal status analyses, trials with participants in only a single stage or nodal status category (Lille 1985; Italy 2002; Slovenia 1988) had a major impact on the original analyses, and so their appropriate exclusion from these analyses is significant. However, this means that despite inclusion of more trials overall, less data were included in this compared with the original analyses so power is more limited.
All analyses of local‐regional (P = 0.02), distant (P = 0.02) and overall (P = 0.08) recurrence‐free survival (i.e. time to recurrence or death) have suggested an overall adverse effect of PORT. However, the observed detriment was less for these endpoints than for overall survival. For local‐regional recurrence‐free survival, results were driven largely by survival (as deaths account for the majority of events). This suggests that antitumour activity may be attributable to radiotherapy, and that increased risk of death from PORT may be attributable to other mechanisms. Analysis of the local‐regional recurrence‐free interval (i.e. the time to local‐regional recurrence with death and distant recurrence censored) was not presented because such analysis would be difficult to interpret and would be potentially seriously flawed. This difficulty arose because increased risk of death with PORT may mean that patients treated with PORT die before their tumour has had time to recur locally. Thus, such measurement was likely to be an overestimation of local‐regional control.
Inclusion of the most recent trial (Korea 2007) has brought the total number of participants to 2343 across 11 randomised controlled trials (RCTs). As would be expected, the addition of this modestly sized trial has not substantially changed the overall effect of PORT on survival. As evidence from new trials has accumulated, there has been some increase in heterogeneity, particularly in relation to the Italian trial (Italy 2002), which, it should be noted, included only participants with stage I disease.
However, a significant detriment of PORT for survival persists, with similar estimates, irrespective of whether a fixed‐effect or a random‐effects model is used. Results for local‐regional and overall recurrence‐free survival are less convincing. Furthermore, although trials have been conducted over a period of 40 years, with changes in diagnosis and assessment of recurrence and radiotherapy, no clear evidence indicates that the effect of PORT has improved over the decades.
In particular, much discussion over the past few years has focused on modern radiotherapy techniques such as those used in some of the trials included here; the suggestion is that modern radiotherapy (delivered by linear accelerator) may be less detrimental than older methods (delivered by cobalt machines). Recent literature‐based meta‐analyses (Billiet 2014) could not confirm this, providing a reported risk ratio (RR) for overall survival of 0.85 (95% confidence interval (CI) 0.59 to 1.22, P = 0.38) for trials that used only linear accelerators. Indeed when we ran this same analysis using our individual participant data, for those trials that used only linear accelerators (albeit on a different selection of trials), we observed a hazard ratio (HR) for overall survival of 1.02 (95% CI 0.80 to 1.31, P = 0.85; Analysis 1.5). Another recent literature‐based meta‐analysis (Patel 2014) has suggested benefit of PORT for overall survival when radiotherapy has been given only with linear accelerators (HR = 0.77, 95% CI 0.62 to 0.92, P = 0.02); however, this review used a combination of three RCTs (some of which included chemotherapy) and eight retrospective studies. Results of an ongoing trial may clarify this matter (Lung ART‐IGR 2006/1202).
Although this meta‐analysis did not directly address quality of life (none of the trials collected data on patient‐reported quality of life measures), it was unlikely that any benefits of PORT would offset the observed survival disadvantage. Indeed the additional time spent undergoing treatment and the side effects of radiation could reasonably be expected to impair at least short‐term quality of life.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

PORT effect on overall survival by trial according to stage.

Hazard ratio (HR) for the interaction between the effect of PORT on survival and (a) stage or (b) nodal status.

Sensitivity analysis 1: only trials with all stage subgroups included.

Sensitivity analysis (2): only trials with stage II and III subgroups represented.

PORT effect on overall survival by trial according to nodal status.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 1 Survival.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 2 Local recurrence‐free survival.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 3 Distant recurrence‐free survival.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 4 Recurrence‐free survival.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 5 RT delivery method.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 6 RT dose.
| T stage | N stage | M stage | Meta‐analysis stage | AJCC stage |
| 0, 1, 2, X, iS | 0 | 0 | I | I |
| 0, 1, 2, X, iS | 1 | 0 | II | II |
| Any | 2, 3 | 0 | III | III non‐metastatic |
| 3, 4 | Any | 0 | III | III non‐metastatic |
| Any | Any | 1 | IV | Any metastatic |
| AJCC = American Joint Committee on Cancer. | ||||
| T stage | N stage | M stage | Meta‐analysis stage |
| 1, 2 | 0 | 0 | I |
| 1, 2 | 1 | 0 | II |
| 3 | 0 | 0 | II |
| 1, 2 | 2 | 0 | III |
| 3 | 1, 2 | 0 | III |
| Any | Any | 1 | IV |
| Characteristic | Postoperative RT | Surgery only | Total |
| AGE (data from 11 trials) | |||
| < 54 years | 294 | 327 | 621 |
| 55 to 59 years | 267 | 261 | 528 |
| 60 to 64 years | 290 | 276 | 566 |
| > 65 years | 312 | 315 | 627 |
| Unknown | 0 | 1 | 1 |
| SEX (data from 11 trials) | |||
| Male | 988 | 992 | 1980 |
| Female | 175 | 187 | 362 |
| Not recorded | 0 | 1 | 1 |
| HISTOLOGY (data from 9 trials) | |||
| Adenocarcinoma | 195 | 218 | 413 |
| Squamous | 522 | 545 | 1067 |
| Other | 66 | 54 | 120 |
| Unknown | 380 | 363 | 743 |
| META‐ANALYSIS STAGE (data from 11 trials) | |||
| I | 328 | 338 | 666 |
| II | 353 | 366 | 719 |
| III | 463 | 455 | 918 |
| IV | 1 | 0 | 1 |
| Unknown | 18 | 21 | 39 |
| WHO PERFORMANCE STATUS (data from 4 trials; not used) | |||
| Good (0, 1) | 195 | 196 | 391 |
| Poor (2, 3, 4) | 77 | 83 | 160 |
| Unknown | 22 | 21 | 43 |
| Trend or interaction 1998 | Trend or interaction 2005 | Trend or interaction 2010 'old' methods | Trend or interaction 2010 'new' methods and TNM changes | |
| Age | P = 0.34 | P = 0.44 | P = 0.32 | P = 0.20 |
| Sex | P = 0.94 | P = 0.92 | P = 0.84 | P = 0.49 |
| Histology | P = 0.75 | P = 0.61 | P = 0.42 | P = 0.38 |
| Stage | P = 0.0003 | P = 0.003 | P = 0.003 | P = 0.12 |
| Nodal status | P = 0.016 | P = 0.02 | P = 0.03 | P = 0.39 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 2 Local recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.12 [1.01, 1.23] |
| 3 Distant recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.13 [1.02, 1.24] |
| 4 Recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.10 [0.99, 1.21] |
| 5 RT delivery method Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 5.1 Cobalt‐60 only | 1 | 202 | Hazard Ratio (95% CI) | 1.48 [1.09, 2.02] |
| 5.2 Cobalt‐60 and linac | 6 | 1746 | Hazard Ratio (95% CI) | 1.18 [1.05, 1.33] |
| 5.3 Linac only | 4 | 395 | Hazard Ratio (95% CI) | 1.02 [0.80, 1.31] |
| 6 RT dose Show forest plot | 11 | 2343 | Peto Odds Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 6.1 < 45 Gy | 2 | 382 | Peto Odds Ratio (95% CI) | 0.93 [0.75, 1.17] |
| 6.2 ≥ 45 Gy | 9 | 1961 | Peto Odds Ratio (95% CI) | 1.25 [1.12, 1.40] |

