Antibióticos para la neumonía adquirida en la comunidad en pacientes ambulatorios adultos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Date and duration of study: not specified. Follow‐up: 6 to 8 weeks. Patients were included from 57 general practitioners in the UK. Double‐blind, double‐dummy technique, intention‐to‐treat results provided | |
| Participants | Patients with CAP older than 18 years. CAP diagnosis confirmed by 3 of the following features: pyrexia, dyspnoea, tachypnoea, rales, localised reduced breath sounds and cough. Diagnosis of CAP was later confirmed radiographically. Total: n = 208. Evaluable for efficacy: n = 108 (exclusion usually due to failure to confirm initial diagnosis on CXR), n = 64 (clarithromycin), n = 44 (erythromycin). Exclusion criteria clear | |
| Interventions | Clarithromycin 250 mg twice daily for 14 days or erythromycin 500 mg 4 times daily for 14 days, each given at least 1 hour before or 2 hours after meals, mean treatment duration: 13 days (clarithromycin), or 10 days (erythromycin). Compliance assessment: tablet count | |
| Outcomes | Primary outcome: clinical response at 2 weeks (test‐of‐cure visit): 98% (clarithromycin), 91% (erythromycin). Treatment‐related adverse events: 16% (clarithromycin) versus 33% (erythromycin), P value = 0.004, mainly gastrointestinal side effects | |
| Notes | 3 of 5 authors from Abbott Laboratories, source of funding not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not specified |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | 3 of 5 authors from Abbott Laboratories, source of funding not specified |
| Methods | Date and duration of study: January 1989 to June 1990. Follow‐up: 4 to 6 weeks. Multicentre study (15 centres of the Canada‐Sweden Clarithromycin‐Pneumonia Study Group, 11 in Canada, 4 in Sweden). Double‐blind, double‐dummy technique, no intention‐to‐treat results provided | |
| Participants | Ambulatory patients older than 12 years with CAP. N = 268 all patients, after exclusions 173 "evaluable patients": n = 92 (clarithromycin), n = 81 (erythromycin). Patients with mild or moderate infection. Drop‐outs: 35% (due to less than minimum therapy, premature discontinuation, unavailable for follow‐up, misdiagnosis, inadequate data collection, concomitant medication, underlying condition). Exclusion criteria clear | |
| Interventions | Clarithromycin: 250 mg every 12 hours, or erythromycin stearate: 500 mg every 6 hours. Mean treatment duration not specified (minimum duration: 7 days, intended duration: 7 to 14 days). Compliance assessment: tablet count | |
| Outcomes | Primary outcome: clinical success (cure and improvement) 97% clarithromycin, 96% erythromycin. Treatment‐related adverse events: 31% clarithromycin, 59% erythromycin (P value < 0.001) | |
| Notes | Research supported by Abbott Laboratories, Chicago | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation was blinded to both the investigators and the patients." |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | Research supported by Abbott Laboratories, Chicago |
| Methods | Date and duration of study: April 2003 to April 2004. Double‐blind, double‐dummy, non‐inferiority trial, patients were recruited from 56 centres worldwide (Canada, Chile, India, Lithuania, Mexico, Peru, Russia, United States) | |
| Participants | 427 outpatients, 18 years or older, 423 patients received the study medication. Patients were eligible for enrolment if they had a clinical diagnosis of mild to moderate CAP (signs and symptoms) and radiographic evidence of new pulmonary infiltrate. Patients also had to have a Fine Mortality risk class of I, II or III (< 90 points). Exclusion criteria are clearly listed | |
| Interventions | Single, 2 g dose of azithromycin microspheres versus levofloxacin 500 mg once daily for 7 days | |
| Outcomes | Primary endpoint: clinical response in the clinical per protocol population, on the basis of signs and symptoms of CAP, assessed at test‐of‐cure visit (TOC; day 13 to 21): azithromycin 89.7%, levofloxacin 93.7%, non‐significant difference. Treatment‐related adverse effects: azithromycin 19.9%, levofloxacin 12.3%, P value = 0.0063, mainly gastrointestinal side effects | |
| Notes | Study funded by Pfizer Inc., 3 of 5 authors are employees of Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not specified |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | Study funded by Pfizer Inc., 3 of 5 authors are employees of Pfizer |
| Methods | Date and duration of study: not specified. Double‐blind, double‐dummy, phase III trial, conducted in 58 outpatient centres worldwide (United States, Canada, Argentina, Russia, India, Estonia, Lithuania) | |
| Participants | 501 outpatients, 16 years or older. Patients were eligible for enrolment if they had a clinical diagnosis of mild to moderate CAP (signs and symptoms) and radiographic evidence of new pulmonary infiltrate. Patients also had to have a modified Fine risk score of I or II (< 70 points) | |
| Interventions | Single 2 g dose of azithromycin microspheres administered as an oral suspension versus extended‐release clarithromycin administered orally as 2 x 500 mg capsules once daily for 7 days | |
| Outcomes | Primary endpoint: clinical response in the clinical per protocol population, on the basis of signs and symptoms of CAP, assessed at test‐of‐cure visit (TOC; day 14 to 21): azithromycin 91.8% versus clarithromycin 94.7% (non‐significant difference). Treatment‐related adverse effects: azithromycin 26.3% versus clarithromycin 24.6% (non‐significant difference), mainly gastrointestinal side effects | |
| Notes | Study funded by Pfizer Inc., 2 of 4 authors are employees of Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised according to computer‐generated pseudo random code using the method of permutated blocks, balanced within investigational site" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization numbers were assigned by a central Web/telephone computer‐based telerandomization system" |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | Study sponsored by Pfizer |
| Methods | 2 phase 3 prospective, double‐blinded RCTs, parallel‐group, multicentre, multinational studies | |
| Participants | Male or female participants > 18 years from outpatient clinics | |
| Interventions | Oral cethromycin versus oral clarithromycin | |
| Outcomes | Primary: clinical and radiological response. Secondary: bacteriological response, adverse effects | |
| Notes | This trial included results from 2 separate RCTs. However, results were pooled and the interventions were identical. The results of the 2 studies are reported as 1 in the article. Study setting was South Africa, USA, Canada, South America, Europe, Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done by interactive voice response system (IVRS) |
| Allocation concealment (selection bias) | Low risk | IVRS assigned numerically coded blister cards to each participant containing either cethromycin or clarithromycin or placebo |
| Blinding (performance bias and detection bias) | Low risk | Participants and assessors were blinded to treatment and assessments. Study medications and placebos were over‐encapsulated and appeared identical |
| Incomplete outcome data (attrition bias) | Low risk | All inclusions and exclusions were explicitly stated |
| Selective reporting (reporting bias) | Low risk | All enrolled participants were accounted for. Reasons for participant exclusion from the final analysis were provided |
| Other bias | High risk | All authors were members of Advanced Life Sciences Inc, a biopharmaceutical company (USA) |
| Methods | Date and duration of study: December 2000 to June 2001. Double‐blind, double dummy, randomised phase III trial, conducted in 117 centres in Japan | |
| Participants | 270 patients (mix of in‐ and outpatients), aged 16 to 80 years. 123 outpatients were included. Exclusion criteria are clearly stated and patient selection flow chart is provided | |
| Interventions | Telithromycin 600 mg (= 2 x 300 mg) once daily after breakfast versus levofloxacin 100 mg 3 times daily for 7 days | |
| Outcomes | Results are reported separately for in‐ and outpatients. Primary endpoint: clinical response in the clinical per protocol population, on the basis of signs and symptoms of CAP, assessed at end of treatment (EOT; 7 days after treatment initiation) telithromycin 95.7% versus clarithromycin 96.3% (non‐significant difference). Treatment‐related adverse effects: telithromycin 33.6% versus levofloxacin 33.9% (non‐significant difference), mostly gastrointestinal side effects | |
| Notes | Study report is in Japanese, but extensive figures and tables are provided in English, so that most of the necessary information could be extracted. Missing information was kindly provided by the main author, Prof. Shigeru Kohno, MD, PhD, of Nagasaki University, Japan. Funding provided by Astellas (Fujisawa) Pharmaceutical Co. Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Text in Japanese; tables and figures in English |
| Allocation concealment (selection bias) | Unclear risk | Text in Japanese; tables and figures in English |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) | Low risk | Detailed flow chart of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Unclear risk | Text in Japanese; tables and figures in English |
| Other bias | Unclear risk | Study sponsored by Fujisawa |
| Methods | Date and duration of study: May 1998 to September 1999. Double‐blind, double‐dummy, parallel‐group clinical trial conducted at 54 centres in 4 countries (United States, Canada, Argentina and Chile) | |
| Participants | 493 outpatients aged 18 and over. Patients were eligible for enrolment if they had a clinical diagnosis of acute CAP (signs and symptoms) and chest radiographic findings supporting the clinical diagnosis of bacterial pneumonia | |
| Interventions | Telithromycin 800 mg (= 2 x 400 mg capsules) once daily in the morning versus clarithromycin 500 mg (= 2 x 250 mg capsules) twice daily for 10 days | |
| Outcomes | Primary endpoint: clinical response in the clinically assessable per protocol population, on the basis of signs and symptoms of CAP, assessed at test‐of‐cure visit (TOC; day 17 to 24): telithromycin 88.3% versus clarithromycin 88.5% (non‐significant difference). Treatment‐related adverse effects: telithromycin 38.5% versus clarithromycin 27.9%, mostly gastrointestinal side effects | |
| Notes | Funded by an unrestricted educational grant from Aventis Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients... were randomised according to a schedule generated by the sponsor for each centre. The schedule linked sequential treatment assignment number to treatment codes allocated at random." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization schedules were held by the sponsor." |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy technique. Quote: "Treatment blinding was ensured by encapsulation of both active study medication and placebo within identical opaque capsules." |
| Incomplete outcome data (attrition bias) | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | Funded by an unrestricted educational grant from Aventis Pharmaceuticals |
| Methods | Randomised, double‐blind, multicentre, phase 2 study | |
| Participants | Males and females > 18 years | |
| Interventions | Oral solithromycin versus oral levofloxacin | |
| Outcomes | Primary: clinical response. Secondary: bacteriological response, adverse effects | |
| Notes | Study done in USA and Canada. Did not explicitly state the setting in which patients were recruited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stratified by age and pneumonia severity index (PORT score), but method of randomisation not explicitly stated |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not clearly stated |
| Incomplete outcome data (attrition bias) | Unclear risk | CONSORT diagram of patient inclusion and reasons for exclusion is not provided in the paper |
| Selective reporting (reporting bias) | Unclear risk | Since CONSORT diagram missing, we are unclear regarding the reasons patients were excluded from the outcome analysis |
| Other bias | High risk | Study was funded by Cempra Inc., a pharmaceutical company. All investigators are member of Cempra Inc. USA, or InClin Inc. USA. InClin is a consulting firm for biotechnology and pharmaceutical companies |
| Methods | Randomised study | |
| Participants | Males and females 18 to 55 years of age | |
| Interventions | Oral clarithromycin/oral azithromycin or oral levofloxacin/oral high‐dose amoxicillin | |
| Outcomes | Primary: clinical response. Secondary: adverse effects | |
| Notes | Study conducted in Rural Health Training Centre in India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No stated method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not explicitly stated |
| Incomplete outcome data (attrition bias) | Unclear risk | No CONSORT diagram presented, therefore reasons for exclusion of patients at the entry level are unclear |
| Selective reporting (reporting bias) | Low risk | The study reported on every patient that was included in the trial (n = 31) |
| Other bias | Low risk | No explicit mention of biopharmaceutical industry involvement |
| Methods | Randomised, double‐blinded study (outpatients), single‐blinded (inpatients) | |
| Participants | Adult males and females > 18 years | |
| Interventions | Oral clarithromycin versus oral amoxicillin for outpatients study arm | |
| Outcomes | Primary: clinical response. Secondary: adverse effects | |
| Notes | Article in Spanish; translation done by Dr. RA Rodriguez, Dr. Gonzalo Alvarez and Dr. Jacqueline Sandoz (Department of Medicine, University of Ottawa) Hospitalised patients are included in the study but separate results for inpatients and outpatients are provided. Study conducted in Uruguay | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by PORT score was used |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated |
| Blinding (performance bias and detection bias) | Unclear risk | No explicit mention of patient and assessor blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No CONSORT diagram presented, therefore reasons for exclusion of patients at the entry level are unclear |
| Selective reporting (reporting bias) | Low risk | The study reported on every patient that was included in the trial |
| Other bias | Low risk | No financial conflicts |
| Methods | Randomised, double‐blind, multicentre, multinational study, with 3 treatment arms | |
| Participants | Adult males and females > 18 years | |
| Interventions | Oral nemonoxacin 750 mg versus levofloxacin 500 mg daily for 7 days | |
| Outcomes | Primary: clinical response. Secondary: bacteriological response | |
| Notes | Study was partially supported by grant from Government of Taiwan. Study conducted in Taiwan and South Africa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explicit mention of randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | No explicit mention of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No explicit mention of patient and assessor blinding |
| Incomplete outcome data (attrition bias) | High risk | CONSORT diagram presented and numbers of included and excluded patients stated. However, reasons for exclusions are not clearly stated |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting; all outcomes are reported in the included study population |
| Other bias | High risk | Several authors of the study are employees of TaiGen Biotechnology Co. Ltd. |
| Methods | Randomised, double‐blind, multicentre, multinational study, with 3 treatment arms | |
| Participants | Adult males and females > 18 years | |
| Interventions | Oral nemonoxacin 500 mg versus levofloxacin 500 mg daily for 7 days | |
| Outcomes | Primary: clinical response. Secondary: bacteriological response | |
| Notes | Study was partially supported by grant from Government of Taiwan. Study conducted in Taiwan and South Africa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explicit mention of randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | No explicit mention of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No explicit mention of patient and assessor blinding |
| Incomplete outcome data (attrition bias) | High risk | CONSORT diagram presented and numbers of included and excluded patients stated. However, reasons for exclusions are not clearly stated |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting; all outcomes are reported in the included study population |
| Other bias | High risk | Several authors of the study are employees of TaiGen Biotechnology Co. Ltd. |
CAP: community‐acquired pneumonia
CXR: chest x‐ray
EOT: end of treatment
IVRS: interactive voice response system
n: number
RCT: randomised controlled trial
TOC: test‐of‐cure
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Hospitalised patients only included | |
| Conference abstract only | |
| Meta‐analysis of RCTs on ertapenem and ceftriaxone: inpatients only | |
| Mix of in‐ and outpatients (unspecified proportions) | |
| Review of a series of unblinded trials | |
| Mix of diagnoses (acute bronchitis and pneumonia) | |
| Mix of diagnoses (bronchitis and pneumonia) | |
| No chest X‐ray for every patient with suspected pneumonia | |
| Patients with chronic bronchitis were included, not CAP | |
| Children with a mean age of 11; comparison of same antibiotic with different doses; indication for treatment consists of mix of diagnoses besides CAP; some diagnoses not confirmed by chest X‐ray | |
| Focus on pneumococcal CAP; positive sputum cultures used as a selection criterion | |
| Study not randomised, expert opinion only | |
| Study did not address antibiotic treatment for CAP and URTIs | |
| Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors | |
| Study not randomised, expert opinion only | |
| Only bacteriologically evaluable patients were included | |
| Hospitalised patients only included | |
| Hospitalised patients included; study uses trovafloxacin ‐ not recommended for treatment of CAP | |
| Hospitalised patients included; study uses trovafloxacin ‐ not recommended for treatment of CAP | |
| Mix of diagnoses (bronchitis and pneumonia) | |
| Pooled analysis of 2 phase 3 studies on tigecycline versus levofloxacin iv ‐ inpatients only | |
| Open‐label study | |
| Mix of diagnoses (acute bronchitis, acute superinfection of chronic bronchitis, pneumonia) and results reported separately | |
| Open‐label study | |
| Focus on bacterial pneumonia (acute onset, purulent sputum, Gram stain and chest X‐ray consistent with bacterial pneumonia were required for inclusion into the study) | |
| Review article, not a RCT | |
| Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors | |
| Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors | |
| Focus on bacterial pneumonia | |
| Report on safety and efficacy of ceftaroline fosamil; not a RCT; hospitalised patients included | |
| Hospitalised patients included | |
| Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors | |
| Focus on bacterial pneumonia. Serologic evidence of M. pneumoniae or C. pneumoniae was an exclusion criterion; serologic testing was systematically carried out pre‐treatment as well as twice after treatment | |
| Hospitalised patients only included | |
| Too small (n < 30) | |
| Not a RCT | |
| Study on pharmacokinetics and pharmacodynamics of sitafloxacin, not a RCT | |
| Culture confirmation of bacterial CAP required | |
| Too small (n < 30) | |
| Mix of in and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors | |
| Open‐label study | |
| Patients were recruited as outpatients. However, 30% were hospitalised in the course of the study, data for in‐ and outpatients are not reported separately, and hospitalisation was not considered treatment failure | |
| Mix of diagnoses (infective exacerbation of chronic obstructive pulmonary disease, purulent bronchitis and pneumonia) and results not reported separately | |
| Mix of diagnoses (infective exacerbation of chronic obstructive pulmonary disease, purulent bronchitis and pneumonia) and results not reported separately; only 3 of 144 patients had CAP | |
| Only bacteriologically evaluable patients were included | |
| Mix of in and outpatients, data not reported separately | |
| Focus on bacterial pneumonia (purulent sputum and leucocytosis were required for inclusion into study, patients without sputum pathogens were excluded from efficacy analysis, etc.) | |
| Hospitalised patients only included | |
| Mix of diagnoses (infection of soft tissue, infection of the upper respiratory tract, otitis, skin infection, conjunctivitis, pneumonia) and results not reported separately | |
| Comparison of same antibiotic with different doses (abstract only) | |
| Mix of diagnoses (acute bronchitis, acute infectious exacerbations of chronic bronchitis or pneumonia) but results reported separately | |
| Hospitalised patients only included | |
| Focus on pneumococcal pneumonia | |
| Dirithromycin withdrawn from market | |
| Patients with CAP excluded, only patients with lower respiratory tract infection were included | |
| Only inpatients (personal communication H. Lode), contrary to study report (patients reportedly treated as either in‐ or outpatients) | |
| Combines patients from 4 other RCTs, including only those patients with S. pneumoniae pneumonia confirmed by blood culture, i.e. highly select subgroup, generalisability questionable. The data from the 4 studies can be obtained from the original reports (one of which is Örtqvist 1996, already excluded from this review because open‐label) | |
| Gatifloxacin withdrawn from market | |
| Gatifloxacin withdrawn from market | |
| Not comparing treatment looking at antibiotic reduction, not comparing antibiotic use | |
| Comparison levofloxacin versus ceftriaxone/clarithromycin iv ‐ inpatients only | |
| Single‐blind study, no chest X‐ray required | |
| A study about tissue pharmacokinetics and pharmacodynamics, not a RCT | |
| A critical and synthetic review of the literature, not a RCT | |
| Grepafloxacin withdrawn from market | |
| Only bacteriologically evaluable patients were included | |
| A pharmacokinetic study on healthy volunteers, not a RCT | |
| Fleroxacin withdrawn from market | |
| Hospitalised patients only included, not clear if diagnosis confirmed by chest X‐ray | |
| Mix of diagnoses (acute bacterial exacerbation of chronic bronchitis or asthmatic bronchitis and bacterial pneumonia) and data not reported separately | |
| A review of interventions to improve outcomes in CAP patients, not a RCT | |
| Grepafloxacin withdrawn from worldwide market in October 1999 due to risk of severe arrhythmias | |
| Open‐label study | |
| Descriptive study, not a RCT | |
| Focus on pneumococcal pneumonia | |
| Open‐label study | |
| Hospitalised patients only | |
| Excluded because trovafloxacin no longer available for outpatients due to severe hepatotoxicity. Study recruitment was terminated because FDA restricted use of trovafloxacin to hospitalised patients with severe life‐ or limb‐threatening infections (1999) | |
| Open‐label study | |
| Sparfloxacin withdrawn from market due to increased risk of severe phototoxicity and rash, as well as rhabdomyolysis | |
| Cohort of RCTs, not a RCT | |
| Comparison of different dosage regimens of the same drug | |
| Mix of in‐ and outpatients, separate data for outpatients not available Note: article in Japanese. However, tables and figures in English; additional information obtained through personal communication with one of the authors (Kohno S) | |
| Open‐label study | |
| Mix of diagnoses (bronchitis, pneumonia) | |
| Hospitalised patients included | |
| Hospitalised patients only | |
| Focus on pneumococcal pneumonia | |
| A systematic review and meta‐analysis of RCTs for CAP. We searched the back references of this paper to check for any studies suitable for our review. Not a RCT | |
| Cost‐effectiveness analysis, not a RCT | |
| Comparison of same antibiotic; mix of diagnoses not just CAP | |
| Excluded because trovafloxacin no longer indicated for outpatients, later removed from market due to severe hepatotoxicity. Study recruitment was terminated because FDA restricted use of trovafloxacin to hospitalised patients with severe life‐ or limb‐threatening infections (1999) | |
| Open‐label study | |
| Hospitalised patients | |
| Healthy participants | |
| Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors | |
| Too small (n < 30) | |
| Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors | |
| Trovafloxacin withdrawn from market due to severe hepatotoxicity | |
| Focus on bacterial pneumonia | |
| Focus on bacterial pneumonia; purulent sputum sample required; patients with serologic evidence of Mycoplasma pneumoniae or Chlamydophila pneumoniae were excluded | |
| A review of CAP treatment | |
| Not a RCT | |
| Not a RCT | |
| Hospitalised patients only | |
| Meta‐analysis of RCTs, not a RCT | |
| Systematic review and meta‐analysis, not a RCT | |
| Open‐label, unblinded study comparing oral administration twice daily versus intramuscular administration once daily. Included only high‐risk patients; unclear whether treated as in‐ or outpatients | |
| Patients had CAP but were treated exclusively as inpatients |
CAP: community‐acquired pneumonia
FDA: Food and Drug Administration
iv: intravenous
RCT: randomised controlled trial
URTIs: upper respiratory tract infections
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.32, 2.26] |
| Analysis 1.1  Comparison 1 Solithromycin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.28, 6.98] |
| Analysis 1.2 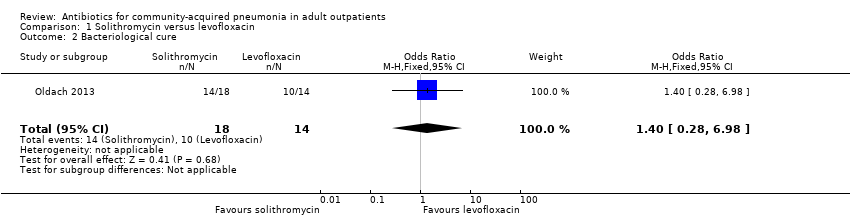 Comparison 1 Solithromycin versus levofloxacin, Outcome 2 Bacteriological cure. | ||||
| 3 Adverse events Show forest plot | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.40] |
| Analysis 1.3  Comparison 1 Solithromycin versus levofloxacin, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 176 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.55, 2.53] |
| Analysis 2.1  Comparison 2 Nemonoxacin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 1 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.19, 3.44] |
| Analysis 2.2  Comparison 2 Nemonoxacin versus levofloxacin, Outcome 2 Bacteriological cure. | ||||
| 3 Adverse events Show forest plot | 1 | 176 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.73, 2.39] |
| Analysis 2.3  Comparison 2 Nemonoxacin versus levofloxacin, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.38, 1.54] |
| Analysis 3.1  Comparison 3 Nemonoxacin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.13, 1.78] |
| Analysis 3.2 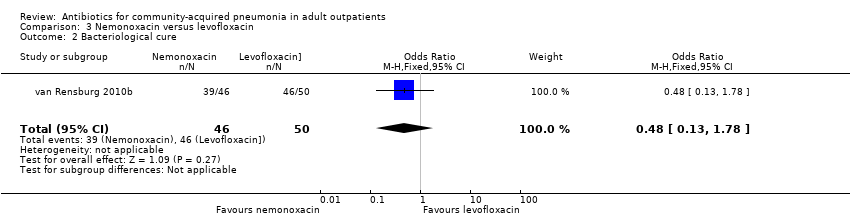 Comparison 3 Nemonoxacin versus levofloxacin, Outcome 2 Bacteriological cure. | ||||
| 3 Adverse events Show forest plot | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.47, 1.54] |
| Analysis 3.3  Comparison 3 Nemonoxacin versus levofloxacin, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.1 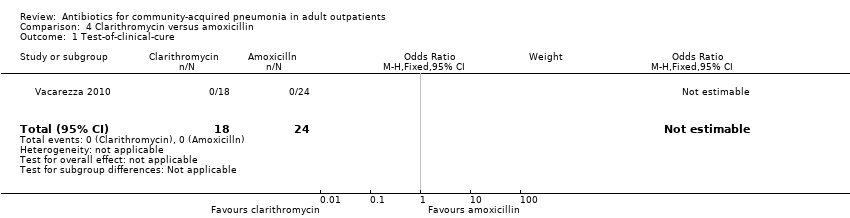 Comparison 4 Clarithromycin versus amoxicillin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||
| 1 Test‐of‐clinical‐cure Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||
| Analysis 5.1
Comparison 5 Clarithromycin versus azithromycin versus levofloxacin versus amoxicillin, Outcome 1 Test‐of‐clinical‐cure. | ||||||||||||||||||||||||||||
| 2 Adverse events Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||
| Analysis 5.2
Comparison 5 Clarithromycin versus azithromycin versus levofloxacin versus amoxicillin, Outcome 2 Adverse events. | ||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 1025 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.22] |
| Analysis 6.1  Comparison 6 Cethromycin versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 1 | 363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.58] |
| Analysis 6.2 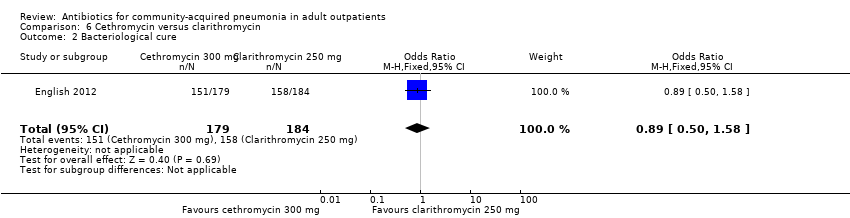 Comparison 6 Cethromycin versus clarithromycin, Outcome 2 Bacteriological cure. | ||||
| 3 Adverse events Show forest plot | 1 | 1096 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.32, 2.15] |
| Analysis 6.3  Comparison 6 Cethromycin versus clarithromycin, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.66, 7.80] |
| Analysis 7.1  Comparison 7 Clarithromycin versus erythromycin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 2 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.57] |
| Analysis 7.2 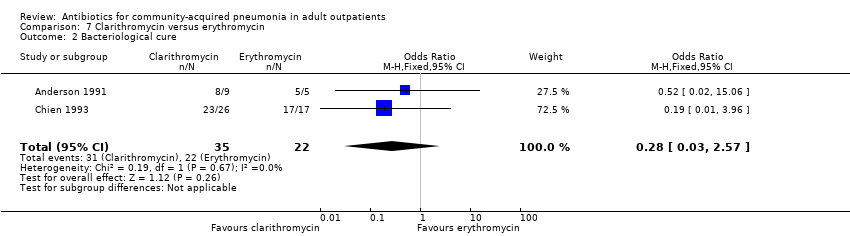 Comparison 7 Clarithromycin versus erythromycin, Outcome 2 Bacteriological cure. | ||||
| 3 Radiological cure Show forest plot | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.33, 2.49] |
| Analysis 7.3  Comparison 7 Clarithromycin versus erythromycin, Outcome 3 Radiological cure. | ||||
| 4 Adverse events Show forest plot | 2 | 476 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.20, 0.46] |
| Analysis 7.4 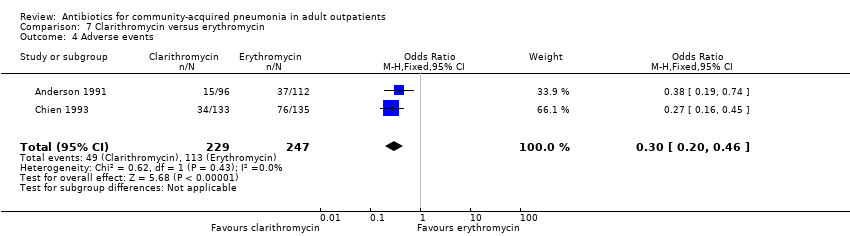 Comparison 7 Clarithromycin versus erythromycin, Outcome 4 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.26] |
| Analysis 8.1 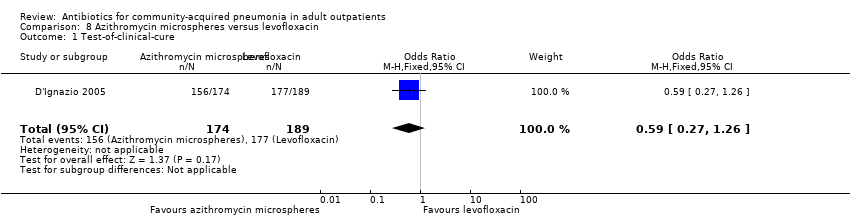 Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.02] |
| Analysis 8.2 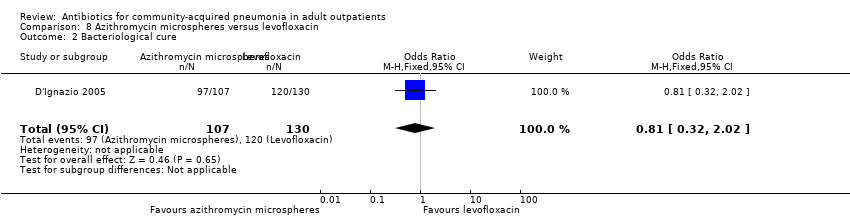 Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 2 Bacteriological cure. | ||||
| 3 Adverse events Show forest plot | 1 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.04, 3.03] |
| Analysis 8.3 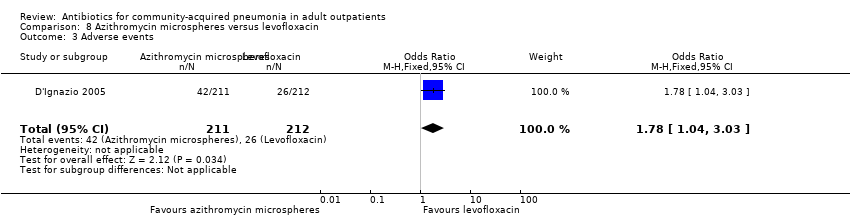 Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 411 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.31, 1.55] |
| Analysis 9.1 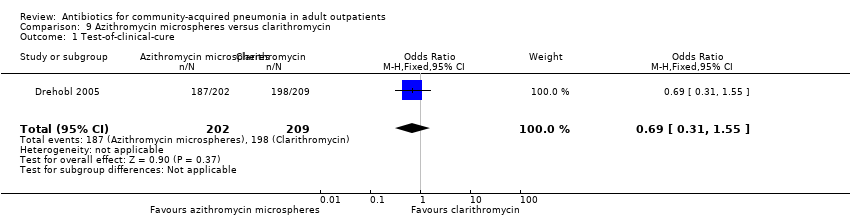 Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 1 | 303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.52, 2.61] |
| Analysis 9.2  Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 2 Bacteriological cure. | ||||
| 3 Adverse events Show forest plot | 1 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
| Analysis 9.3  Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 318 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.49, 1.95] |
| Analysis 10.1 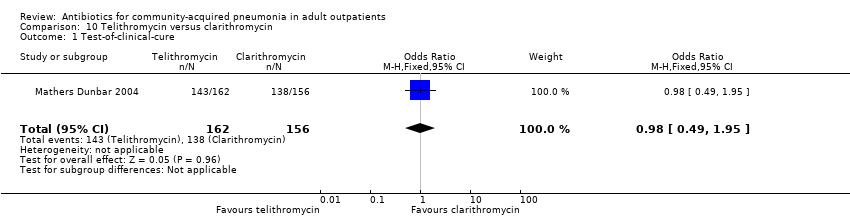 Comparison 10 Telithromycin versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.29] |
| Analysis 10.2  Comparison 10 Telithromycin versus clarithromycin, Outcome 2 Bacteriological cure. | ||||
| 3 Adverse events Show forest plot | 1 | 443 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.08, 2.40] |
| Analysis 10.3 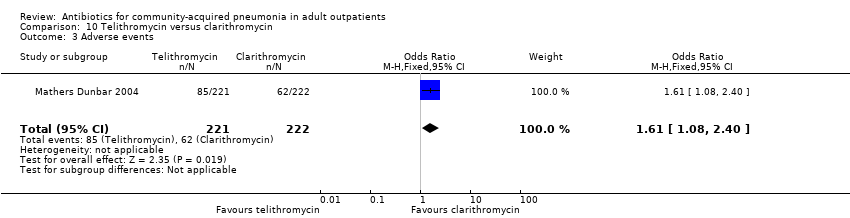 Comparison 10 Telithromycin versus clarithromycin, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.14, 5.25] |
| Analysis 11.1 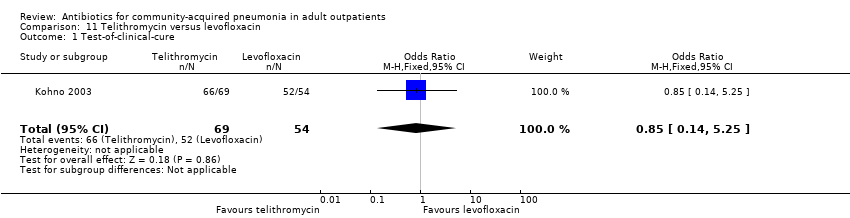 Comparison 11 Telithromycin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure. | ||||
| 2 Bacteriological cure Show forest plot | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.60] |
| Analysis 11.2  Comparison 11 Telithromycin versus levofloxacin, Outcome 2 Bacteriological cure. | ||||
| 3 Clinical efficacy against H. influenzae Show forest plot | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.38, 55.51] |
| Analysis 11.3 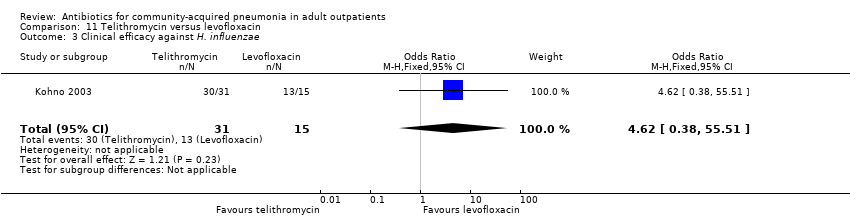 Comparison 11 Telithromycin versus levofloxacin, Outcome 3 Clinical efficacy against H. influenzae. | ||||
| 4 Adverse events Show forest plot | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.58, 1.68] |
| Analysis 11.4  Comparison 11 Telithromycin versus levofloxacin, Outcome 4 Adverse events. | ||||

Figure1: Antibiotic comparisons in new studies included in this review. The red arrow indicates antibiotic comparisons studied in Udupa 2011.
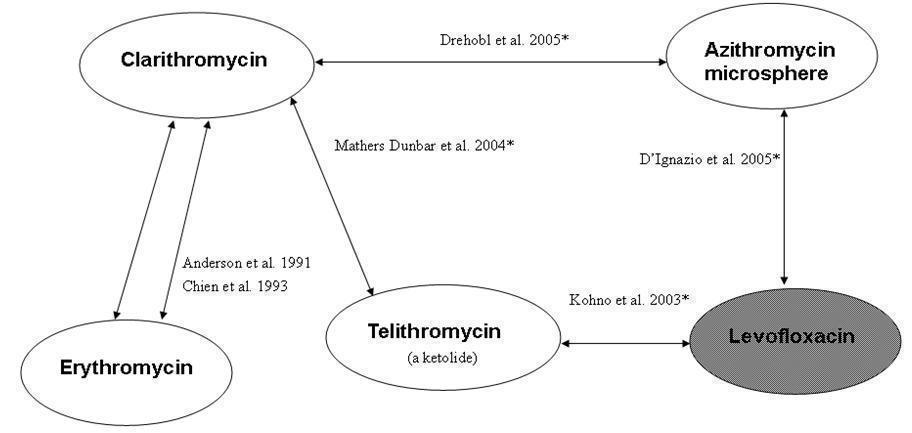
Figure 2. Overview of included studies and antibiotic pairs studied in 2009 review. *Indicates studies new to this review; shaded ovals indicate quinolones (gyrase inhibitors), white ovals indicate macrolides
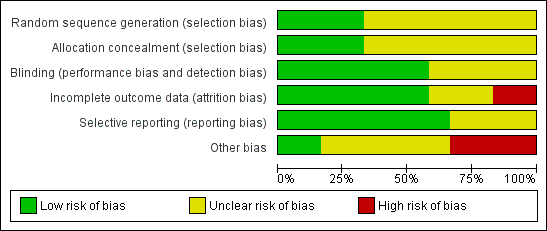
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Solithromycin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.

Comparison 1 Solithromycin versus levofloxacin, Outcome 2 Bacteriological cure.
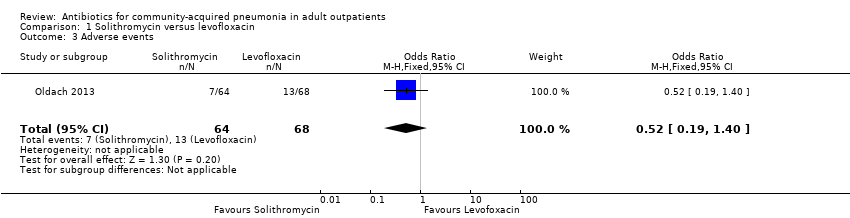
Comparison 1 Solithromycin versus levofloxacin, Outcome 3 Adverse events.

Comparison 2 Nemonoxacin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.
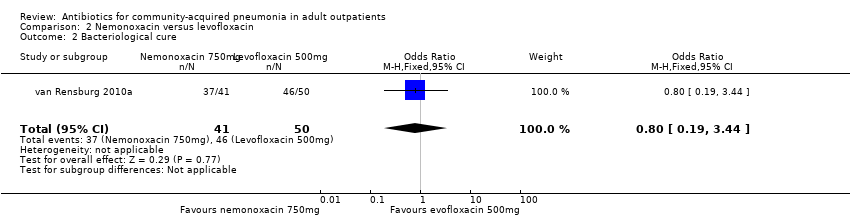
Comparison 2 Nemonoxacin versus levofloxacin, Outcome 2 Bacteriological cure.
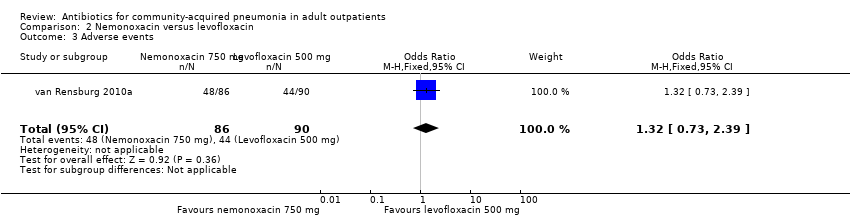
Comparison 2 Nemonoxacin versus levofloxacin, Outcome 3 Adverse events.

Comparison 3 Nemonoxacin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.

Comparison 3 Nemonoxacin versus levofloxacin, Outcome 2 Bacteriological cure.

Comparison 3 Nemonoxacin versus levofloxacin, Outcome 3 Adverse events.

Comparison 4 Clarithromycin versus amoxicillin, Outcome 1 Test‐of‐clinical‐cure.
| Study | Antibiotics | Events | Total |
| Udupa 2011 | Clarithromycin | 8 | |
| Udupa 2011 | Azithromycin | 7 | |
| Udupa 2011 | Levofloxacin | 7 | |
| Udupa 2011 | High‐dose amoxicillin | 9 | |
Comparison 5 Clarithromycin versus azithromycin versus levofloxacin versus amoxicillin, Outcome 1 Test‐of‐clinical‐cure.
| Study | Antibiotic | Events | Total |
| Udupa 2011 | Clarithromycin | 4 | 8 |
| Udupa 2011 | Azithromycin | 5 | 7 |
| Udupa 2011 | Levofloxacin | 5 | 7 |
| Udupa 2011 | Amoxicillin | 7 | 9 |
Comparison 5 Clarithromycin versus azithromycin versus levofloxacin versus amoxicillin, Outcome 2 Adverse events.
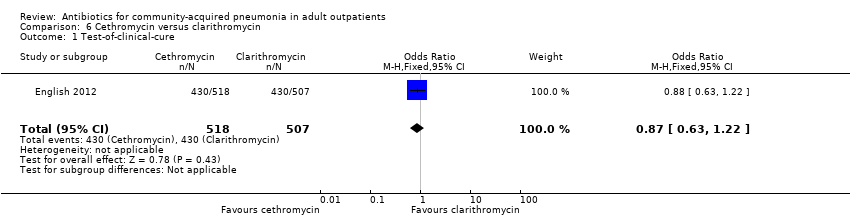
Comparison 6 Cethromycin versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure.
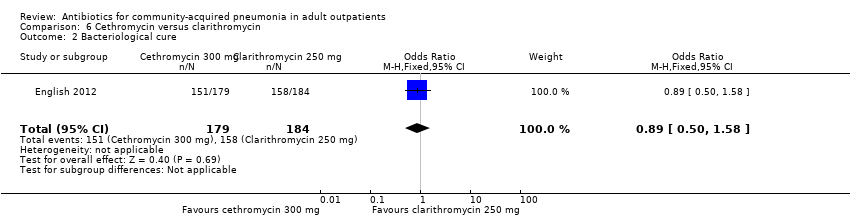
Comparison 6 Cethromycin versus clarithromycin, Outcome 2 Bacteriological cure.

Comparison 6 Cethromycin versus clarithromycin, Outcome 3 Adverse events.

Comparison 7 Clarithromycin versus erythromycin, Outcome 1 Test‐of‐clinical‐cure.

Comparison 7 Clarithromycin versus erythromycin, Outcome 2 Bacteriological cure.

Comparison 7 Clarithromycin versus erythromycin, Outcome 3 Radiological cure.

Comparison 7 Clarithromycin versus erythromycin, Outcome 4 Adverse events.

Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.
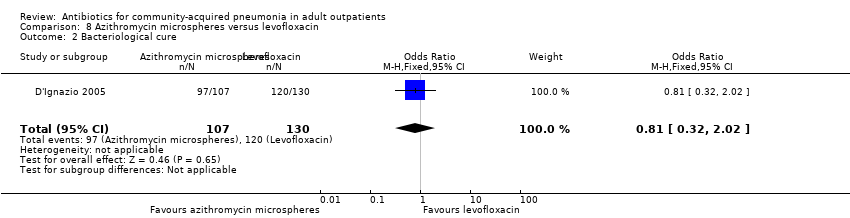
Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 2 Bacteriological cure.

Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 3 Adverse events.

Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure.

Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 2 Bacteriological cure.
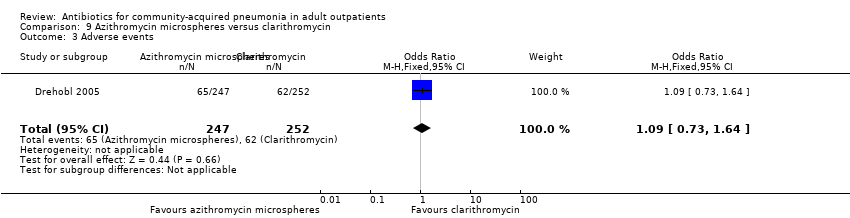
Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 3 Adverse events.

Comparison 10 Telithromycin versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure.

Comparison 10 Telithromycin versus clarithromycin, Outcome 2 Bacteriological cure.

Comparison 10 Telithromycin versus clarithromycin, Outcome 3 Adverse events.

Comparison 11 Telithromycin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.

Comparison 11 Telithromycin versus levofloxacin, Outcome 2 Bacteriological cure.
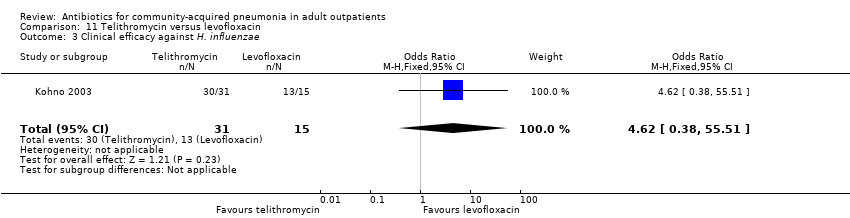
Comparison 11 Telithromycin versus levofloxacin, Outcome 3 Clinical efficacy against H. influenzae.

Comparison 11 Telithromycin versus levofloxacin, Outcome 4 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.32, 2.26] |
| 2 Bacteriological cure Show forest plot | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.28, 6.98] |
| 3 Adverse events Show forest plot | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 176 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.55, 2.53] |
| 2 Bacteriological cure Show forest plot | 1 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.19, 3.44] |
| 3 Adverse events Show forest plot | 1 | 176 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.73, 2.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.38, 1.54] |
| 2 Bacteriological cure Show forest plot | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.13, 1.78] |
| 3 Adverse events Show forest plot | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.47, 1.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | Other data | No numeric data | ||
| 2 Adverse events Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 1025 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.22] |
| 2 Bacteriological cure Show forest plot | 1 | 363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.58] |
| 3 Adverse events Show forest plot | 1 | 1096 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.32, 2.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.66, 7.80] |
| 2 Bacteriological cure Show forest plot | 2 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.57] |
| 3 Radiological cure Show forest plot | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.33, 2.49] |
| 4 Adverse events Show forest plot | 2 | 476 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.20, 0.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.26] |
| 2 Bacteriological cure Show forest plot | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.02] |
| 3 Adverse events Show forest plot | 1 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.04, 3.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 411 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.31, 1.55] |
| 2 Bacteriological cure Show forest plot | 1 | 303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.52, 2.61] |
| 3 Adverse events Show forest plot | 1 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 318 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.49, 1.95] |
| 2 Bacteriological cure Show forest plot | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.29] |
| 3 Adverse events Show forest plot | 1 | 443 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.08, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Test‐of‐clinical‐cure Show forest plot | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.14, 5.25] |
| 2 Bacteriological cure Show forest plot | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.60] |
| 3 Clinical efficacy against H. influenzae Show forest plot | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.38, 55.51] |
| 4 Adverse events Show forest plot | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.58, 1.68] |

