敷料处理浅表烧伤及深二度烧伤
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002106.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 28 marzo 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Jason Wasiak: designed and coordinated the review. Extracted data and checked quality of data extraction. Undertook and checked quality assessment. Performed statistical analysis, interpreted data and checked the analysis. Completed first draft of the review and advised on subsequent drafts. Made an intellectual contribution to the review. Approved final review prior to submission. Performed previous work that was the foundation of the current review. Is guarantor of the review. Appraised search, checked data extraction, contributed guidance on data and analyses, and reviewed changes to the text in the most recent update
Heather Cleland: designed the review. Checked quality of data extraction and interpreted data. Completed first draft of the review. Made an intellectual contribution to the review and approved final review prior to submission. Advised on the review. Performed previous work that was the foundation of the current review. Appraised search, checked data extraction, contributed guidance on data and analyses, and reviewed changes to the text in the most recent update.
Fiona Campbell: designed and coordinated the review. Examined all search results. Extracted data and checked quality of data extraction. Wrote to study author/experts/companies and handsearched journals. Undertook and checked quality assessment. Performed statistical analysis, interpreted data and checked the analysis. Completed first draft of the review and advised on subsequent drafts. Made an intellectual contribution to the review. Approved final review prior to submission. Performed previous work that was the foundation of the current review.
Anneliese Spinks: Made an intellectual contribution to the review and approved the overall review process prior to submission. Advised on the review and reviewed changes to the text in the most recent update.
Contributions of editorial base:
Nicky Cullum: edited the review, advised on methodology, interpretation and review content. Undertook extensive redrafting. Approved the final review prior to submission. Commented on and edited the review update.
Sally Bell‐Syer: co‐ordinated the editorial process. Checked data extraction and quality assessment. Performed part of data analysis and interpretation. Advised on methodology, interpretation and content. Undertook extensive redrafting, editing and copy editing of the review and of the review update.
Ruth Foxlee: designed the search strategy and edited the search methods section.
Rachel Richardson: edited and checked the updated review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Royal College of Nursing, UK.
Declarations of interest
None
Acknowledgements
Acknowledgements to Kate Seers for previous work that was the foundation of the current review and to Greg Duncan for his assistance in reading the first draft review. The authors would like to acknowledge the peer referees, Wounds Group Editors (Andrew Jull, Dirk Ubbink, Gill Worthy) and Catriona McDaid, Mary Mondozzi and Janet Yarrow. Acknowledgements to the Cochrane Editorial Unit (Toby Lasserson, John Hilton and Rachel Marshall), Karla‐Soares Weiser, for search appraisal, data extraction and updating the text, and Anne Lethaby for extracting risk of bias data in the most recent review update. This work was funded by the NIHR.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Mar 28 | Dressings for superficial and partial thickness burns | Review | Jason Wasiak, Heather Cleland, Fiona Campbell, Anneliese Spinks | |
| 2008 Oct 08 | Dressings for superficial and partial thickness burns | Review | Jason Wasiak, Heather Cleland, Fiona Campbell | |
| 2007 Jul 18 | Dressings for superficial and partial thickness burns | Protocol | Jason Wasiak, Heather Cleland, Fiona Campbell | |
| 2000 Apr 24 | Dressing and topical agents for burns | Protocol | Fiona Campbell, Kate Seers | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
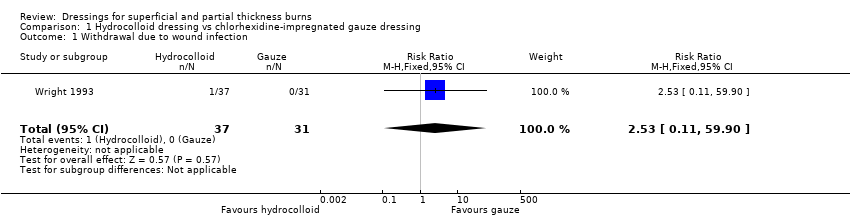
Comparison 1 Hydrocolloid dressing vs chlorhexidine‐impregnated gauze dressing, Outcome 1 Withdrawal due to wound infection.

Comparison 2 Hydrocolloid dressing vs silver sulphadiazine, Outcome 1 Number of dressing changes.
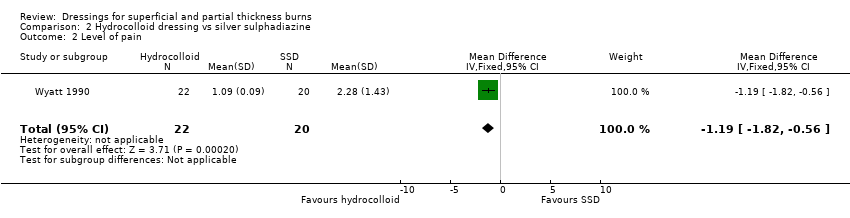
Comparison 2 Hydrocolloid dressing vs silver sulphadiazine, Outcome 2 Level of pain.

Comparison 3 Polyurethane film dressing vs paraffin gauze dressing, Outcome 1 Wound infection.

Comparison 4 Polyurethane film dressing vs chlorhexidine‐impregnated paraffin gauze dressing, Outcome 1 Wound infection.

Comparison 5 Hydrogel dressing vs usual care, Outcome 1 Wound healing: number of people healed at 6 days.
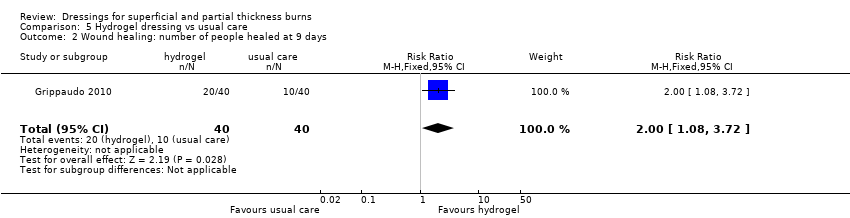
Comparison 5 Hydrogel dressing vs usual care, Outcome 2 Wound healing: number of people healed at 9 days.
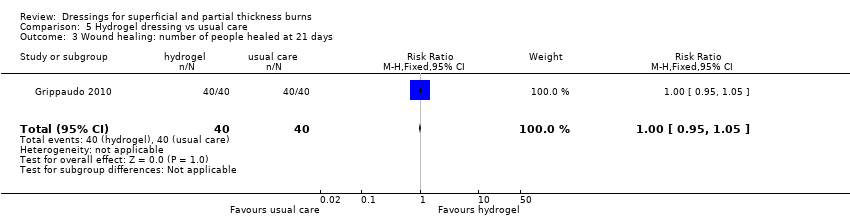
Comparison 5 Hydrogel dressing vs usual care, Outcome 3 Wound healing: number of people healed at 21 days.

Comparison 5 Hydrogel dressing vs usual care, Outcome 4 Wound healing: number of people healed at 12 days.

Comparison 5 Hydrogel dressing vs usual care, Outcome 5 Wound healing: number of people healed at 15 days.

Comparison 5 Hydrogel dressing vs usual care, Outcome 6 Wound healing: number of people healed at 18 days.

Comparison 5 Hydrogel dressing vs usual care, Outcome 7 Assessment of pain at baseline.

Comparison 5 Hydrogel dressing vs usual care, Outcome 8 Pain 30 minutes after treatment.
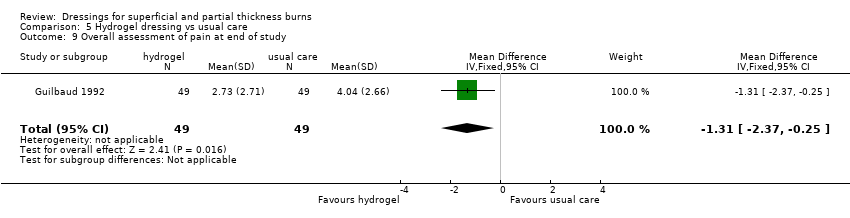
Comparison 5 Hydrogel dressing vs usual care, Outcome 9 Overall assessment of pain at end of study.

Comparison 5 Hydrogel dressing vs usual care, Outcome 10 Infection with Pseudomonas aeruginosa requiring antibiotic therapy.

Comparison 6 Silicon nylon dressing vs silver sulphadiazine, Outcome 1 Number of dressing changes.
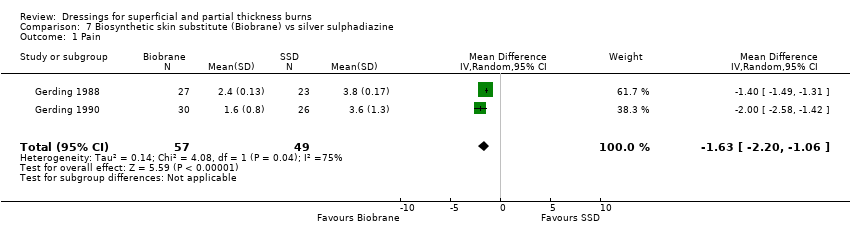
Comparison 7 Biosynthetic skin substitute (Biobrane) vs silver sulphadiazine, Outcome 1 Pain.

Comparison 7 Biosynthetic skin substitute (Biobrane) vs silver sulphadiazine, Outcome 2 Need for surgery.
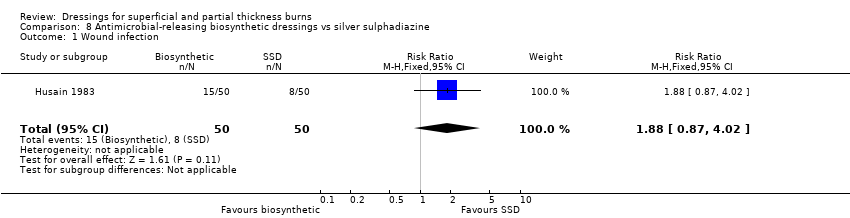
Comparison 8 Antimicrobial‐releasing biosynthetic dressings vs silver sulphadiazine, Outcome 1 Wound infection.

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 1 Wound healing time (days).

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 2 Wound healing: number of people healed at 7 days.
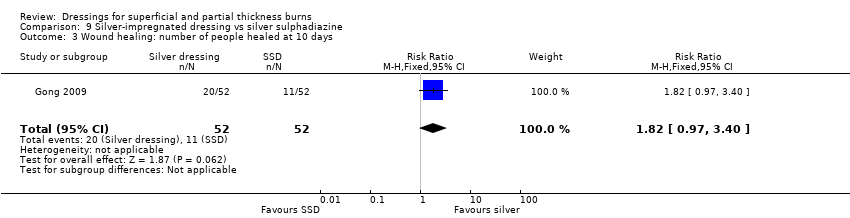
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 3 Wound healing: number of people healed at 10 days.

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 4 Wound healing: number of people healed at 15 days.

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 5 Wound healing: number of people healed at 17 days.

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 6 Wound healing: number of people healed at 21 days.
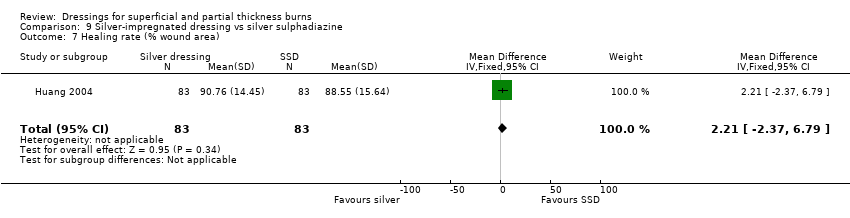
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 7 Healing rate (% wound area).

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 8 Pain.

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 9 Need for surgery.

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 10 Number of infections.

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 11 Number of wound dressings.

Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 12 Nursing time (minutes).

Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 1 Wound healing time (days).

Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 2 Pain at day 1.

Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 3 Pain at day 3.

Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 4 Pain at day 7.
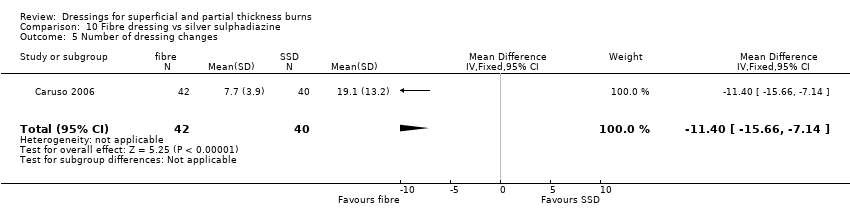
Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 5 Number of dressing changes.
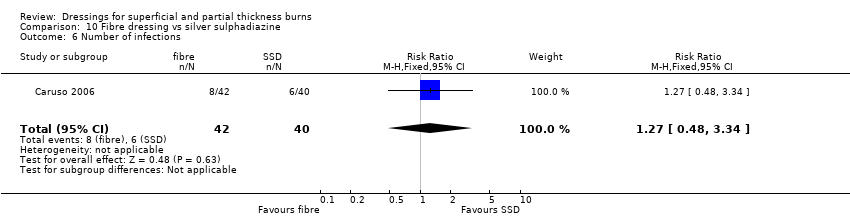
Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 6 Number of infections.
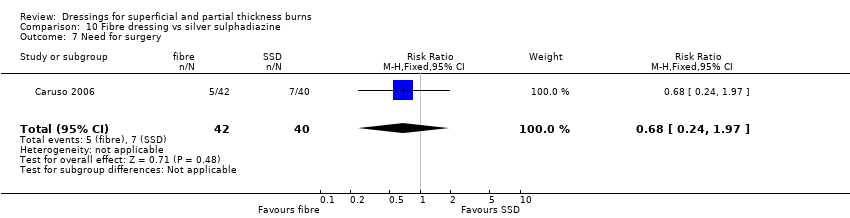
Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 7 Need for surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Withdrawal due to wound infection Show forest plot | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.11, 59.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of dressing changes Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐18.65 [‐22.54, ‐14.76] |
| 2 Level of pain Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐1.82, ‐0.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound infection Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.23, 6.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound infection Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing: number of people healed at 6 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.46, 4.91] |
| 2 Wound healing: number of people healed at 9 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.08, 3.72] |
| 3 Wound healing: number of people healed at 21 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.95, 1.05] |
| 4 Wound healing: number of people healed at 12 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.17, 2.42] |
| 5 Wound healing: number of people healed at 15 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.95, 1.41] |
| 6 Wound healing: number of people healed at 18 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.21] |
| 7 Assessment of pain at baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Pain 30 minutes after treatment Show forest plot | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.64, 0.06] |
| 9 Overall assessment of pain at end of study Show forest plot | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐1.31 [‐2.37, ‐0.25] |
| 10 Infection with Pseudomonas aeruginosa requiring antibiotic therapy Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of dressing changes Show forest plot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐2.64, ‐0.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 2 | 106 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.20, ‐1.06] |
| 2 Need for surgery Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.21, 2.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound infection Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.87, 4.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing time (days) Show forest plot | 2 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐4.22 [‐5.92, ‐2.52] |
| 2 Wound healing: number of people healed at 7 days Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.58, 3.91] |
| 3 Wound healing: number of people healed at 10 days Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.97, 3.40] |
| 4 Wound healing: number of people healed at 15 days Show forest plot | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 5 Wound healing: number of people healed at 17 days Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.98, 1.54] |
| 6 Wound healing: number of people healed at 21 days Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.06, 1.37] |
| 7 Healing rate (% wound area) Show forest plot | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | 2.21 [‐2.37, 6.79] |
| 8 Pain Show forest plot | 3 | 135 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐5.89, 0.21] |
| 9 Need for surgery Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.21, 2.08] |
| 10 Number of infections Show forest plot | 4 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.67] |
| 11 Number of wound dressings Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐11.07 [‐19.58, ‐2.56] |
| 12 Nursing time (minutes) Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐4.82 [‐19.42, 9.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing time (days) Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐5.44, ‐1.96] |
| 2 Pain at day 1 Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.03, ‐0.97] |
| 3 Pain at day 3 Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.1 [‐4.02, ‐2.18] |
| 4 Pain at day 7 Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐3.18, ‐1.62] |
| 5 Number of dressing changes Show forest plot | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐11.40 [‐15.66, ‐7.14] |
| 6 Number of infections Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.48, 3.34] |
| 7 Need for surgery Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.97] |

