Kortikosteroid sedutan berbanding sistemik untuk mencegah displasia bronkopulmonary pada bayi pramatang berat badan yang sangat rendah yang menerima ventilasi
Abstract
Background
Bronchopulmonary dysplasia (BPD) remains an important cause of mortality and morbidity in preterm infants and inflammation plays a significant role in its pathogenesis. The use of inhaled corticosteroids may modulate the inflammatory process without concomitant high systemic steroid concentrations and less risk of adverse effects. This is an update of a review published in 2012 (Shah 2012). We recently updated the related review on "Inhaled versus systemic corticosteroids for treating bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates".
Objectives
To determine the effect of inhaled versus systemic corticosteroids started within the first 7 days of life on preventing death or BPD in ventilated very low birth weight infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 1), MEDLINE via PubMed (1966 to 23 February 2017), Embase (1980 to 23 February 2017), and CINAHL (1982 to 23 February 2017). We searched clinical trials registers, conference proceedings and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐randomised trials.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing inhaled versus systemic corticosteroid therapy (irrespective of dose and duration) starting in the first seven days of life in very low birth weight preterm infants receiving assisted ventilation.
Data collection and analysis
Clinical outcomes data were extracted and analysed using Review Manager. When appropriate, meta‐analysis was performed using typical relative risk (RR), typical risk difference (RD) and weighted mean difference (WMD). Meta‐analyses were performed using typical relative risk, typical risk difference (RD), and weighted mean difference with their 95% confidence intervals (CI). If RD was statistically significant, the number needed to benefit or the number needed to harm was calculated. We assessed the quality of evidence was evaluated using GRADE principles.
Main results
We included two trials that involved 294 infants. No new studies were included for the 2017 update. The incidence of death or BPD at 36 weeks' postmenstrual age was not statistically significantly different between infants who received inhaled or systemic steroids (RR 1.09, 95% CI 0.88 to 1.35; RD 0.05, 95% CI ‐0.07 to 0.16; 1 trial, N = 278). The incidence of BPD at 36 weeks' postmenstrual age among survivors was not statistically significant between groups (RR 1.34, 95% CI 0.94 to 1.90; RD 0.11, 95% CI ‐0.02 to 0.24; 1 trial, N = 206). There was no statistically significant difference in the outcomes of BPD at 28 days, death at 28 days or 36 weeks' postmenstrual age and the combined outcome of death or BPD by 28 days between groups (2 trials, N = 294). The duration of mechanical ventilation was significantly longer in the inhaled steroid group compared with the systemic steroid group (typical MD 4 days, 95% CI 0.2 to 8; 2 trials, N = 294; I² = 0%) as was the duration of supplemental oxygen (typical MD 11 days, 95% CI 2 to 20; 2 trials, N = 294; I² = 33%).
The incidence of hyperglycaemia was significantly lower with inhaled steroids (RR 0.52, 95% CI 0.39 to 0.71; RD ‐0.25, 95% CI ‐0.37 to ‐0.14; 1 trial, N = 278; NNTB 4, 95% CI 3 to 7 to avoid 1 infant experiencing hyperglycaemia). The rate of patent ductus arteriosus increased in the group receiving inhaled steroids (RR 1.64, 95% CI 1.23 to 2.17; RD 0.21, 95% CI 0.10 to 0.33; 1 trial, N = 278; NNTH 5, 95% CI 3 to 10). In a subset of surviving infants in the United Kingdom and Ireland there were no significant differences in developmental outcomes at 7 years of age. However, there was a reduced risk of having ever been diagnosed as asthmatic by 7 years of age in the inhaled steroid group compared with the systemic steroid group (N = 48) (RR 0.42, 95% CI 0.19 to 0.94; RD ‐0.31, 95% CI ‐0.58 to ‐0.05; NNTB 3, 95% CI 2 to 20).
According to GRADE the quality of the evidence was moderate to low. Evidence was downgraded on the basis of design (risk of bias), consistency (heterogeneity) and precision of the estimates.
Both studies received grant support and the industry provided aero chambers and metered dose inhalers of budesonide and placebo for the larger study. No conflict of interest was identified.
Authors' conclusions
We found no evidence that early inhaled steroids confer important advantages over systemic steroids in the management of ventilator‐dependent preterm infants. Based on this review inhaled steroids cannot be recommended over systemic steroids as a part of standard practice for ventilated preterm infants. Because they might have fewer adverse effects than systemic steroids, further randomised controlled trials of inhaled steroids are needed that address risk/benefit ratio of different delivery techniques, dosing schedules and long‐term effects, with particular attention to neurodevelopmental outcome.
PICO
Ringkasan bahasa mudah
Kortikosteroid sedutan berbanding sistemik untuk mencegah penyakit kronik paru‐paru pada bayi pramatang berat badan yang sangat rendah yang menerima ventilasi
Soalan ulasan
Objektif utama adalah untuk membandingkan keberkesanan kortikosteroid sedutan berbanding sistemik bermula dalam minggu pertama kehidupan dalam mencegah kematian atau displasia bronkopulmonari (ditakrifkan sebagai keperluan oksigen tambahan pada umur 36 minggu post menstruasi) pada bayi yang menerima ventilasi mekanikal invasif dengan berat lahir ≤ 1500 g atau usia kehamilan ≤ 32 minggu.
Latar belakang
Bayi pramatang yang memerlukan sokongan pernafasan sering menghidapi displasia bronkopulmonari. Keradangan paru‐paru mungkin sebahagian daripada puncanya. Ubat kortikosteroid apabila diberikan secara oral atau melalui vena mengurangkan keradangan ini, tetapi penggunaan kortikosteroid dikaitkan dengan kesan sampingan yang serius. Penggunaan kortikosteroid telah dikaitkan dengan cerebral palsy (masalah motor) dan kelewatan perkembangan. Ada kemungkinan bahawa menyedut steroid, supaya ubat itu terus sampai ke paru‐paru, dapat mengurangkan kesan buruk.
Ciri‐ciri kajian
Ulasan tersebut melihat pada kajian yang membandingkan bayi pramatang yang menerima steroid melalui sedutan kepada mereka yang menerima steroid secara sistematik (melalui vena atau secara oral) ketika mereka menerima ventilasi mekanikal. Penyelidik termasuk dua kajian yang melibatkan 294 bayi. Satu kajian termasuk 278 bayi dan kajian lain termasuk 16 bayi. Tiada kajian baru dimasukkan untuk kemas kini pada 2017.
Satu kajian menerima sokongan geran dan industri menyediakan Aerochambers dan inhaler dos meteran budesonide dan plasebo untuk kajian yang besar. Tiada percanggahan dengan kepentingan yang telah dikenal pasti.
Keputusan utama
Tiada bukti bahawa menyedut steroid berbanding dengan steroid sistemik menghalang hasil utama kematian atau displasia bronkopulmonari. Jumlah hari bayi memerlukan sokongan ventilasi mekanikal atau oksigen tambahan meningkat pada bayi yang mendapat steroid yang disedut berbanding bayi yang menerima steroid sistemik. Hasil ini dilaporkan dalam kedua‐dua kajian. Kadar paten duktus arteriosus (kegagalan untuk duktus arteriosus, peredaran arteri pada hayat janin, menutup selepas kelahiran) dinaikkan dalam kumpulan yang menerima sedutan steroid. Terdapat insiden gula darah tinggi yang lebih rendah dalam kumpulan steroid yang disedut berbanding dengan kumpulan steroid sistemik. Hasil sekunder ini hanya dilaporkan dalam satu kajian sahaja (kajian yang lebih besar). Dalam sub‐sampel 52 kanak‐kanak pada usia 7 tahun tidak ada perbezaan dalam hasil jangka panjang susulan antara kumpulan steroid yang disedut dan sistemik. dalam sampel 48 bayi yang lebih kecil hasil daripada 'pernah diberi diagnosis asma pada usia tujuh tahun' adalah jauh lebih rendah dalam kumpulan steroid yang disedut berbanding dengan kumpulan steroid sistemik.
Kualiti bukti
Berdasarkan GRADE, kualiti bukti adalah sederhana hingga rendah.
Authors' conclusions
Summary of findings
| Inhaled steroids compared with systemic corticosteroids for preventing bronchopulmonary dysplasia among all randomised infants | ||||||
| Patient or population: Preterm neonates with respiratory distress Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age among all randomised | 526 per 1000 | 573 per 1000 | RR 1.09 (95% CI 0.88 to 1.35) | 278 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. |
| Death or BPD at 28 days | 736 per 1000 | 780 per 1000 | RR 1.05 (95% CI 0.93 to 1.20) | 294 (2) | ⊕⊕⊝⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Heterogeneity/Consistency: There was high heterogeneity for this analysis I² = 78%. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| BPD at 36 weeks' postmenstrual age | 237 per 1000 | 343 per 1000 | RR 1.45 (95% CI 0.99 to 2.11) | 278 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. |
| BPD at 28 days | 507 per 1000 | 613 per 1000 | RR 1.21 (95% CI 0.98 to 1.48) | 294 (2) | ⊕⊕⊝⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic corticosteroids for preventing bronchopulmonary dysplasia among all randomised | ||||||
| Patient or population: Preterm neonates with respiratory distress Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Duration of mechanical ventilation (days) | The mean duration of mechanical ventilation ranged across the systemic steroid groups from 15.2 to 17.9 days | The mean duration of mechanical ventilation ranged across the inhaled steroid groups from 20 to 21 days | 3.89 days (0.24 to 7.55) | 294 (2) | ⊕⊕⊕⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| Duration of supplemental oxygen (days) | The mean duration of supplemental oxygen ranged across control groups from 22.7 to 49.3 days | The mean duration of supplemental oxygen in the intervention groups ranged from 38.2 to 53.0 days | 11.10 days (1.97 to 20.22) | 294 (2) | ⊕⊕⊕⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| Hyperglycaemia | 533 per 1000 | 280 per 1000 | RR 0.52, (95% CI 0.39 to 0.71) | 278 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| Patent ductus arteriosus | 333 per 1000 | 546 per 1000 | RR 1.64, (95% CI 1.23 to 2.17) | 278 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic corticosteroids for preventing bronchopulmonary dysplasia among survivors | ||||||
| Patient or population: Preterm neonates with respiratory distress Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| BPD at 36 weeks' postmenstrual age | 333 per 1000 | 446 per 1000 | RR 1.34 (95% CI 0.94 to 1.90) | 206 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| BPD at 28 days | 658 per 1000 | 754 per 1000 | RR 1.14 (95% CI 0.96 to 1.34) | 233 | ⊕⊕⊝⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| Ever diagnosed as asthmatic by 7 years of age | 546 per 1000 | 231 per 1000 | RR 0.42 (95% CI 0.19 to 0.94) | 48 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/Consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Despite the availability of antenatal corticosteroids (Roberts 2017), surfactant replacement therapy (Bahadue 2012; Soll 2002) and other advances in neonatal intensive care, chronic lung disease (bronchopulmonary dysplasia, BPD) remains a substantial cause of mortality and morbidity in preterm infants (Horbar 1993; Lee 2000; Schwartz 1994). The incidence of BPD has an inverse relationship with both birth weight and gestational age (Lee 2000; Sinkin 1990) and has increased partly due to improved survival of extremely low birth weight infants (Shaw 1993). Among survivors, BPD results in prolonged hospitalisation, an increased risk for rehospitalization and adverse neurodevelopmental outcome.
There is increasing evidence from cellular and biochemical research that inflammation plays an important role in the pathogenesis of BPD (Groneck 1994; Gupta 2000; Kotecha 1996; Pierce 1995; Speer 1993; Watterberg 1994; Watterberg 1996; Watts 1992). In many infants, the inflammatory reaction is evident shortly after birth suggesting that the process may have been triggered in utero (Watterberg 1996). Postnatally, a number of factors may also initiate or aggravate this inflammatory process. These include baro or volume‐trauma induced by mechanical ventilation, oxygen toxicity, infections and presence of patent ductus arteriosus (PDA). Interventions aimed at reducing or modulating the inflammatory process may reduce the incidence or severity of BPD.
Description of the intervention
Due to their strong anti‐inflammatory properties, systemic corticosteroids are being used clinically to reduce or limit the inflammatory process associated with development of BPD. The rationale for early administration of postnatal corticosteroids is that these drugs may prevent or minimise the inflammatory changes associated with mechanical ventilation and decrease the need for steroids later to treat BPD. Several systematic reviews on the use of postnatal systemic corticosteroids (early (< 96 hours) and moderately early (7 to 14 days)) have demonstrated a reduction in BPD at 28 days and 36 weeks' postmenstrual age (Arias‐Camison 1999; Bhuta 1998; Doyle 2014a; Doyle 2014b; Halliday 1999; Shah 2001). Marked heterogeneity of the doses and duration of dexamethasone therapy among trials has been noted.
There is growing concern that the beneficial effects on the pulmonary system may be negated by increased risk of short‐ and long‐term adverse effects with corticosteroid therapy (Garland 1999; Ng 1993; Soll 1999; Stark 2001; Yeh 1997; Yeh 1998). Short‐term serious complications with early systemic corticosteroid therapy include gastrointestinal haemorrhage and perforation, hyperglycaemia requiring insulin therapy and hypertension (Garland 1999; Soll 1999; Stark 2001). The potential effects on brain growth and neurodevelopment are the most alarming deficits. Two follow‐up studies of early systemic corticosteroid administration have shown a two‐ to four‐fold increase in neuromotor impairments in surviving dexamethasone‐treated infants compared with controls at two years corrected age (Shinwell 2000; Yeh 1998). Meta‐analysis has shown increased risk of cerebral palsy in infants treated early with dexamethasone (Doyle 2014b).
In statements released by the European Association of Perinatal Medicine (Halliday 2001b), the American Academy of Pediatrics (Watterberg 2012) and the Canadian Pediatric Society (Jefferies 2012) routine use of systemic dexamethasone for the prevention or treatment of BPD is not recommended. Outside the context of randomised controlled trials, the use of corticosteroids should be limited to exceptional clinical circumstances. These recommendations were based on concerns regarding short and long‐term complications, especially cerebral palsy.
Theoretically, the use of inhaled corticosteroids may allow for beneficial effects on the pulmonary system without concomitant high systemic concentrations and less risk of adverse effects. Results of a large multi centre study of early use of inhaled steroids concluded that among extremely preterm infants, the incidence of BPD was lower among those who received early inhaled budesonide than among those who received placebo, but the advantage may have been gained at the expense of increased mortality (Bassler 2015). The results of this study have been included in a Cochrane Review (Shah 2017a) and a meta‐analysis (Shinwell 2016). Shinwell 2016 concluded "Very preterm infants appear to benefit from inhaled corticosteroids with reduced risk for BPD and no effect on death, other morbidities, or adverse events. Data on long‐term respiratory, growth, and developmental outcomes are eagerly awaited". Shah 2017a summarized the results of their Cochrane Review: "There is increasing evidence from the trials reviewed that early administration of inhaled steroids to very low birth weight neonates is effective in reducing the incidence of death or chronic lung disease at 36 weeks' postmenstrual age among either all randomised infants or among survivors. Even though there is statistical significance, the clinical relevance is of question as the upper CI limit for the outcome of death or BPD at 36 weeks' postmenstrual age is infinity. The long‐term follow‐up results of the Bassler 2015 study may affect the conclusions of this review. Further studies are needed to identify the risk/benefit ratio of different delivery techniques and dosing schedules for the administration of these medications. Studies need to address both the short‐ and long‐term benefits and adverse effects of inhaled steroids with particular attention to neurodevelopmental outcome". It is noteworthy that a Cochrane Review by Onland 2017b concluded: "Despite the fact that some studies reported a modulating effect of treatment regimens in favour of higher‐dosage regimens on the incidence of BPD and neurodevelopmental impairment, recommendations on the optimal type of corticosteroid, the optimal dosage, or the optimal timing of initiation for the prevention of BPD in preterm infants cannot be made based on current level of evidence. A well‐designed large RCT is urgently needed to establish the optimal systemic postnatal corticosteroid dosage regimen". Apart from the studies included in this review, we are not aware of any other direct comparisons of early use of inhaled versus systemic corticosteroids.
How the intervention might work
It is thought that inflammation in the lungs may be part of the cause of BPD. As part of a randomised, placebo‐controlled trial of early inhaled beclomethasone therapy, Gupta 2000 measured interleukin‐8 (IL‐8) and interleukin‐1 receptor antagonist (IL‐1ra) concentrations in tracheal aspirates as markers of pulmonary inflammation. Beclomethasone‐treated infants with moderately elevated baseline IL‐8 levels received less subsequent systemic glucocorticoid therapy and had a lower incidence of BPD than non treated infants. Gupta 2000 and co‐authors concluded that early‐inhaled beclomethasone therapy was associated with a reduction in pulmonary inflammation after one week of therapy. Corticosteroid drugs when given orally or intravenously reduces this inflammation in the lungs. However, the use of corticosteroids is associated with serious side effects. Its use has been associated with cerebral palsy and developmental delay. Inhaling steroids, so that the drug directly reaches the lung, has been tried as a way to limit adverse effects.
Why it is important to do this review
Cochrane Reviews have addressed the use of systemic or inhaled corticosteroids in the prevention or treatment of BPD or chronic lung disease. These include reviews of the early use (< 8 days) of systemic postnatal corticosteroids to prevent chronic lung disease (Doyle 2014b) as well as the late use (> 7 days) of systemic postnatal corticosteroids for chronic lung disease (Doyle 2014a).
Other Cochrane Reviews address the use of inhaled corticosteroids in the prevention or treatment of chronic lung disease. Shah 2017a reviewed the effects of early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Shah 2017a reported increasing trial evidence that early administration of inhaled steroids to very low birth weight neonates was effective in reducing the incidence of death or BPD at 36 weeks' postmenstrual age among all randomised infants and survivors. Although there was statistical significance, the clinical relevance was questionable because the upper confidence interval limit for death or BPD at 36 weeks' postmenstrual age was infinity. Onland 2017a reviewed the late use (≥ 7 days) of inhaled corticosteroids to reduce BPD in preterm infants and concluded that: "Based on the results of the currently available evidence, inhalation corticosteroids initiated at ≥ 7 days of life for preterm infants at high risk of developing chronic lung disease cannot be recommended at this point in time".
Cochrane Reviews have compared systemic and inhaled corticosteroids. Shah and colleagues compared the use of inhaled versus systemic corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates (Shah 2012; Shah 2017a) and the use of inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants (Shah 2017b).
The use of corticosteroids for other indications in neonates includes intravenous dexamethasone to facilitate extubation (Davis 2001), treat hypotension (Ibrahim 2011) and meconium aspiration syndrome (Ward 2003).
The aim of this review was to assess the effectiveness of inhaled compared with systemic corticosteroid therapy for ventilated preterm infants in the first week of life to prevent BPD. This is an update of our reviews published in 2003 and 2012 (Shah 2003; Shah 2012).
Objectives
The primary objective was to compare the effectiveness of inhaled versus systemic corticosteroids started within the first 7 days of life in preventing death or BPD (defined as requirement of supplemental oxygen at 36 weeks' postmenstrual age) in ventilated infants with birth weight ≤ 1500 g or gestational age ≤ 32 weeks.
Secondary objectives
To compare the effectiveness of inhaled versus systemic corticosteroids on other indicators of BPD, the incidence of adverse events and long‐term neurodevelopmental outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised clinical trials comparing inhaled versus systemic corticosteroid therapy (regardless of the dose and duration of therapy) starting in the first week of life in very low birth weight preterm infants receiving assisted ventilation.
Types of participants
Preterm infants with birth weight ≤ 1500 g or gestational age ≤ 32 weeks receiving assisted ventilation and postnatal age of less than 7 days.
Types of interventions
Inhaled corticosteroids compared to systemic corticosteroids irrespective of the type, dose and duration of therapy as long as the treatment started before 7 days of age.
Types of outcome measures
Primary outcomes
1. Death or BPD at 36 weeks' postmenstrual age (among all randomised)
Secondary outcomes
1. Other indicators of BPD (among all randomised):
-
BPD at 36 weeks' postmenstrual age (requirement for supplemental oxygen at 36 weeks' postmenstrual age);
-
death at 36 weeks' postmenstrual age;
-
death or BPD 28 days
-
BPD at 28 days of age (requirement for supplemental oxygen at 28 days of age)
-
death at 28 days of age;
-
failure to extubate within 14 days of starting treatment
-
change in pulmonary function tests (lung compliance and resistance);
-
later requirement for systemic corticosteroid therapy;
2. Other indicators of BPD (among survivors):
-
duration of mechanical ventilation (days);
-
duration of requirement for supplemental oxygen (days);
-
duration of hospital stay (days) (post hoc)
3. Adverse events (among all randomised):
-
hyperglycaemia (defined as blood glucose of > 10 mmol/L) during the period of intervention;
-
PDA defined by presence of clinical symptoms or signs or demonstration by echocardiography;
-
gastrointestinal haemorrhage (defined as presence of bloody nasogastric or orogastric aspirate);
-
gastrointestinal perforation (defined by presence of free air in peritoneal cavity on an abdominal x‐ray);
-
infants with free elastase in tracheal aspirate on day 14
-
hypertension (defined as systolic or diastolic blood pressure > 2 standard deviations (SD) above the mean for infant's gestational and postnatal age) during the period of intervention (Zubrow 1995);
-
pneumothorax
-
other air leaks
-
pulmonary haemorrhage
-
necrotizing enterocolitis (Bell's stage II and III) (Bell 1978);
-
retinopathy of prematurity any stage based on international classification (ICROP 1984);
-
retinopathy of prematurity >stage 3 based on international classification (ICROP 1984);
-
sepsis defined by presence of clinical symptoms and signs of infection and a positive culture from normally sterile site (blood, CSF or urine)
-
intraventricular haemorrhage any grade (defined as per Papile 1978);
-
periventricular leukomalacia (defined as cysts in periventricular area on ultrasound or CT scan);
-
hypertrophic cardiomyopathy defined as thickening of interventricular septum or of the left ventricular wall on echocardiography;
-
pneumonia based on clinical and radiologic signs and a positive endotracheal tube aspirate culture;
-
growth (weight, length/height and head circumference) at 36 weeks' postmenstrual age;
-
cataracts (defined by presence of opacities in the lens);
-
hypertrophy of the tongue;
-
nephrocalcinosis (defined by presence of echo densities in the medulla of the kidney on ultrasound) (Saarela 1999);
-
suppression of hypothalamic‐pituitary‐adrenal axis assessed by metyrapone or ACTH stimulation test.
4. Long‐term neurodevelopmental outcome (among survivors):
-
Neurodevelopmental impairment was defined as presence of cerebral palsy or mental retardation (Bayley scales of infant development (BSID), Mental Developmental Index (MDI) < 70) or legal blindness (< 20/200 visual acuity) or deafness (aided or < 60 dB on audiometric testing) assessed at 18 to 24 months.
5. The following outcomes were reported at 7 years of age (among survivors) (post‐hoc analyses based on available data):
-
British Ability Scales, 2nd Edition (provides a global measure of cognitive functioning (the general conceptual ability (GCA) score, with a standardisation mean of 100 and SD of 15);
-
Activities, social, and school competency scales of the Child Behaviour Checklist for children 4 to 18 years of age;
-
Strengths and Difficulties Questionnaire (SDQ) from which overall behavioral, emotional, conduct, hyperactivity, and peer problem scores are derived;
-
Cerebral palsy;
-
Severe disability defined as GCA score < 55, no independent walking, inability to dress or feed oneself, requirement for continuous home oxygen therapy, behavioural disturbance requiring constant supervision, no useful vision, or no useful hearing;
-
Moderate disability was defined as a GCA score of 55 to 69, restricted mobility, admission to an ICU and ventilation within the past year, secondary referral for specialised help with behavior, ability to see gross movement only or hearing loss not corrected with aid;
-
Death or moderate/severe disability;
-
Systolic blood pressure > 95th percentile;
-
Diastolic blood pressure > 95th percentile; and
-
Ever diagnosed as asthmatic by 7 years of age.
Search methods for identification of studies
Electronic searches
See Appendix 1 for the previous search methodologies.
For the 2017 update, we used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 1) in The Cochrane Library; MEDLINE via PubMed (1 January 2011 to 23 February, 2017); Embase (1 January 2011 to 23 February, 2017); and CINAHL (1 January 2011 to 23 February, 2017) using the following search terms: (bronchopulmonary dysplasia OR lung diseases OR chronic lung disease OR BPD OR BPD) AND ((anti‐inflammatory agents OR steroid* OR dexamethasone OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate OR corticosteroid* OR betamethasone OR hydrocortisone) AND (inhalation OR aerosol OR inhale*)), plus database‐specific limiters for RCTs and neonates (see Appendix 2 for the full search strategies for each database). We did not apply language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry).
Searching other resources
We searched the abstracts of the Pediatric Academic Societies Annual Conference electronically at Abstracts 2 view from 2010 to 2016.
Data collection and analysis
We used the methods of the Cochrane Neonatal Review Group for data collection and analysis.
Selection of studies
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria described in the previous section. The review authors independently reviewed the results of the updated search and selected studies for inclusion. We resolved any disagreement by discussion.
Data extraction and management
For each trial, information was sought regarding the method of randomisation, blinding and reporting of all outcomes for all the infants enrolled in the trial. Data from primary investigator were obtained for unpublished trials or when published data were incomplete. Retrieved articles were assessed and data abstracted independently by four review authors (SS, AO, HH, VS). Dr Henry Halliday did not assess the risk of bias for his trial (Halliday 2001a).
For each study, final data were entered into RevMan by one review author and then checked for accuracy by a second review author. We resolved discrepancies through discussion.
We attempted to contact authors of the original reports to provide further details when information regarding any of the above was unclear.
Assessment of risk of bias in included studies
Three review authors (SS, AO, VS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains:
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
Any disagreements were resolved by discussion or by a third assessor (VS). See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using Review Manager software (Review Manager 2014). Dichotomous data were analysed using relative risk (RR), risk difference (RD) and the number needed to benefit (NNTB) or number needed to harm (NNTH). The 95% confidence intervals (CI) were reported on all estimates. If more than one trial was included in an analysis we report on typical RR and typical RD.
We analysed continuous data using weighted mean difference (WMD) (if more than one trial was included in an analysis), or the standardized mean difference to combine trials that measure the same outcome but use different methods.
Unit of analysis issues
For clinical outcomes such as episodes of sepsis, we analysed the data as proportion of neonates having one or more episodes.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by using sensitivity analysis.
All outcomes analyses were on an intention to treat basis i.e. we included all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. If noted, we planned to explore the possible causes of statistical heterogeneity using pre‐specified subgroup analysis (for example, differences in study quality, participants, intervention regimens, or outcome assessments). We used the following criteria for describing the percentages of heterogeneity: < 25% no heterogeneity, > 25% to 49% low heterogeneity, ≥ 50% to 74% moderate heterogeneity and ≥ 75% high heterogeneity.
Assessment of reporting biases
We planned to assess possible publication bias and other biases using symmetry/asymmetry of funnel plots.
For included trials that were performed recently (and prospectively registered), we planned to explore possible selective reporting of study outcomes by comparing the primary and secondary outcomes in the reports with the primary and secondary outcomes proposed at trial registration, using the web sites www.clinicaltrials.gov and www.controlled‐trials.com. If such discrepancies were found, we planned to contact the primary investigators to obtain missing outcome data on outcomes pre‐specified at trial registration.
Data synthesis
Where meta‐analysis was judged to be appropriate, the analysis was done using Review Manager software (Review Manager 2014). We used the Mantel‐Haenszel method for estimates of typical relative risk and risk difference. No continuous outcomes were included in this review. We planned to analyses continuous measures using the inverse variance method, if included. We used the fixed‐effect model for all meta‐analyses.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence. The following (clinically relevant) outcomes were assessed among all randomised infants using GRADE: primary outcome ‐ death or BPD at 36 weeks' postmenstrual age among all randomised. Secondary outcomes: death or BPD at 28 days; BPD at 36 weeks' postmenstrual age; BPD at 28 days; duration of mechanical ventilation; duration of supplemental oxygen; hyperglycaemia; and patent ductus arteriosus. The following secondary outcomes were assessed among survivors; BPD at 36 weeks' postmenstrual age; BPD at 28 days and ever diagnosed as asthmatic at seven years of age.
Three authors (SS, AO, VS) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
-
High: We are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Groups were analysed based on all randomised and survivors only. We reported results of the I² test if more than one study was included in an analysis.
Sensitivity analysis
We planned sensitivity analyses for situations where this might affect the interpretation of significant results (e.g. where there is risk of bias associated with the quality of some of the included trials or missing outcome data). None were thought necessary in this review.
Results
Description of studies
Both studies received grant support and the industry provided aero chambers and metered dose inhalers of budesonide and placebo for the larger study. No conflict of interest was identified.
Results of the search
Five trials comparing inhaled versus systemic corticosteroids for prevention of bronchopulmonary dysplasia (BPD) were identified, of which three were excluded. No new trials were identified for the 2011 update. For this update in 2017, 395 articles were identified through the search after duplicates were removed. One potential study was identified (Mazulov 2013), but after contact with the first author it was excluded because it was not a randomised controlled trial. The study flow diagram is shown in Figure 1.

Study flow diagram: review update
Included studies
We included two trials: Groneck 1999 and Halliday 2001a (both studies published as full text articles; see Characteristics of included studies). Although both studies aimed to include infants thought to be at risk of developing BPD, the inclusion criteria, the intervention type (dose and type of inhaled steroid) and duration of therapy varied between the two studies.
Groneck 1999 was a open comparative trial which enrolled preterm infants < 1200 g while they were mechanically ventilated and had fractional inspired oxygen (FiO₂) requirement > 0.3 on the third day of life. Sixteen infants were enrolled into the study and were alternatively allocated to treatment with inhaled beclomethasone or systemic dexamethasone. Due to poor clinical results (BPD in 6/7 infants), alternate allocation to inhaled steroids was stopped for ethical reasons after inhaled steroid treatment of seven infants. Thus, seven infants were treated with inhaled steroids and nine received systemic steroids. Inhaled beclomethasone was given from day three to day 28 of life administered by an aero chamber into the ventilatory circuit at a dose of 3 x 2 puffs of 250 µg (= 1.5 mg/day). After extubation, inhalation therapy was continued by face mask, and the aero chamber was connected to a ventilation bag. No systemic steroids were given to infants treated with inhaled steroids during the first month of life. Systemic dexamethasone was given at a starting dose of 0.5 mg/kg/day for three days, starting between days 11 to 13; thereafter the dose was gradually tapered over 10 to 28 days, according to clinical status of the infant. Duration of systemic steroids was at the discretion of attending physician. Primary outcome was assessment of lung inflammation and lung permeability. Other outcome measures were days on mechanical ventilation, days on supplemental oxygen and BPD (oxygen dependency and radiological abnormalities on day 28). Pulmonary inflammation and lung permeability were assessed by analysing inflammatory mediators (interleukin ‐8, elastase alpha ‐1 proteinase inhibitor, free elastase, secretory component for IgA and albumin) in tracheal aspirates on day 10 (before starting dexamethasone) and day 14 (three days after starting dexamethasone). The baseline characteristics were similar between groups.
Halliday 2001a enrolled infants born at < 30 weeks' gestation, postnatal age < 72 hours and needing mechanical ventilation and FiO₂ > 0.30. Infants of 30 and 31 weeks could be included if they needed FiO₂ > 0.50. Infants with lethal congenital anomalies, severe intraventricular haemorrhage (grade 3 or 4) and proven systemic infection before entry were excluded from the trial. The trial was designed to evaluate the effectiveness of early (< 72 hours) and delayed (> 15 days) administration of systemic dexamethasone and inhaled budesonide. Infants were randomly allocated to one of four treatment policies in a factorial design: early (< 72 hours) dexamethasone, early budesonide, delayed selective (> 15 days) dexamethasone and delayed selective budesonide. Only the groups allocated to early budesonide or early dexamethasone are included in this review. Budesonide was administered by metered dose inhaler and a spacing chamber in a dose of 400 µg/kg twice daily for 12 days. Dexamethasone was given intravenously (IV) or orally in a tapering course beginning with 0.5 mg/kg/day in two divided doses for three days reducing by half every three days for a total of 12 days of therapy. Halliday 2001a reported that 143 infants were randomised to the early budesonide group and 135 were randomised to the early dexamethasone group. Of 143 infants randomised to early budesonide, 53 received full course, 87 received partial course, and three did not receive budesonide. Of 135 infants randomised to early dexamethasone, 53 received a full course, 76 received a partial course and six infants did not receive dexamethasone. The primary outcome was death or oxygen dependency at 36 weeks. Secondary outcome measures included death or major cerebral abnormality, duration of oxygen treatment, duration of assisted ventilation, duration of hospitalisation, death or oxygen dependency at 28 days and complications of preterm birth. An intention‐to‐treat analysis was performed. Additional data were obtained from the authors for the outcomes of duration of ventilation and duration of supplemental oxygen (expressed as mean and SD). A subset of the infants enrolled in the OSECT study (Halliday 2001a) has been followed to a median age of seven years; 127 (84%) of 152 survivors born in the United Kingdom and Ireland were followed; of these 52 infants belonged to the early dexamethasone and early budesonide groups.
Excluded studies
We excluded four trials. Dimitriou 1997 was excluded because the investigators included non‐ventilator‐dependent infants in the study and the age of commencement of treatment varied from five to 118 days of life. Kovács 1998 was excluded because the participants received systemic dexamethasone initially followed by inhaled steroids while the control group received normal saline systemically and then by nebulization. Parikh 2004 was excluded as all study participants received systemic dexamethasone for seven days and then randomised to receive either inhaled beclomethasone or placebo. Mazulov 2013 was not a randomised controlled trial. See Characteristics of excluded studies.
Risk of bias in included studies
In Groneck 1999 infants were alternately allocated to treatment with inhaled beclomethasone or systemic dexamethasone. Alternate allocation to inhaled steroids was stopped after treatment of seven neonates due to poor clinical results. The study was stopped prematurely. The intervention was not blinded. Outcome data were presented for all 16 babies enrolled in the study. Outcome measures were not blinded.
Halliday 2001a was a multi centre RCT involving 47 centres. The intervention was not blinded in most centres. However, in 11 centres the trial was conducted double‐blind, and in these, placebo metered dose inhalers and intravenous saline were used to mask treatment allocation. Randomisation was performed by telephoning the central randomisation centre. After identifying an eligible infant, the clinician telephoned the randomisation centre to enrol the infant and determine the treatment group. Outcomes have been reported for all infants enrolled in the study. Outcome assessments were not blinded. An intention‐to‐treat analysis was performed. Comparisons were also made for primary outcome variables between the centres observing double blind strategy and other centres. Neurodevelopmental and respiratory follow‐up results at seven years of age for children from the UK and Ireland were reported on 28 infants in the early budesonide group (80% of survivors) and on 24 infants in the early dexamethasone group (75% of survivors).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Groneck 1999 presented no information on how the random sequence was generated and there was no blinding of randomisation (high risk of bias). In Halliday 2001a the random sequence was generated by the trial statistician, independent of the researchers (low risk of bias).
Blinding
In Groneck 1999 there was no blinding of the intervention nor of the outcome measurement (high risk of bias). In Halliday 2001a there was blinding of the intervention and the outcome measurement in 11 centres but not in another 36 centres. All assessors were blinded to the original treatment group allocations (high risk of bias). Long‐term outcomes were assessed at seven years of age and all assessors were blinded at that time to the original treatment group allocations (low risk of bias).
Incomplete outcome data
There was complete follow up in both studies (low risk of bias).
Selective reporting
The study protocols for the Groneck 1999 study was not available to us so we can not judge if there was selective reporting or not (unclear risk). According to the first author (HH) of the Halliday 2001a study there was no selective reporting (low risk of bias)
Other potential sources of bias
We did not identify any other sources of bias in Halliday 2001a (unclear risk of bias). In Groneck 1999 alternate allocation to inhaled steroids was stopped after treatment of seven neonates due to poor clinical results. The study was stopped prematurely (high risk of bias).
Effects of interventions
See: Summary of findings for the main comparison Inhaled steroids compared with systemic corticosteroids for preventing bronchopulmonary dysplasia among all randomised infants; Summary of findings 2 ; Summary of findings 3
Comparison 1: Inhaled versus systemic steroids among all randomised infants
The following (clinically relevant) outcomes among all randomised infants were assessed using GRADE: Primary outcome ‐ Incidence of death or BPD at 36 weeks' postmenstrual age among all randomised. Secondary outcomes: Incidence of death or BPD at 28 days; BPD at 36 weeks' postmenstrual age; BPD at 28 days summary of findings Table for the main comparison; duration of mechanical ventilation; duration of supplemental oxygen; hyperglycaemia; and patent ductus arteriosus summary of findings Table 2
Primary outcomes
Death or BPD by 36 weeks' postmenstrual age
There was no significant difference in death or BPD by 36 weeks' postmenstrual age in the inhaled steroid group compared with the systemic steroid group (RR 1.09, 95% CI 0.88 to 1.35; RD 0.05, 95% CI ‐0.07 to 0.16; Analysis 1.1; Halliday 2001a, N = 278; moderate‐quality evidence). Tests for heterogeneity not applicable.
Secondary outcomes
BPD at 36 weeks' postmenstrual age
There was no significant difference in the outcome of BPD at 36 weeks' in the inhaled steroid group compared with the systemic steroid group (RR 1.45, 95% CI 0.99 to 2.11; RD 0.11, 95% CI ‐0.00 to 0.21; Analysis 1.2; Halliday 2001a, N = 278; P = 0.05; moderate‐quality evidence). Tests for heterogeneity not applicable.
Death at 36 weeks' postmenstrual age
No statistically significant effect on mortality by 36 weeks' postmenstrual age was noted in the inhaled steroid group compared with the systemic steroid group (typical RR 0.83, 95% CI 0.56 to 1.23; typical RD ‐0.05, 95% CI ‐0.15 to 0.05; Analysis 1.3; 2 studies, N = 294). There was no heterogeneity for this outcome for RR (I² = 0%) and low for RD (I² = 36%).
Death or BPD at 28 days
There was no statistically significant difference between the inhaled steroid group compared with the systemic steroid group for the combined outcome of BPD or death at 28 days (typical RR 1.05, 95% CI 0.93 to 1.20; typical RD 0.04, 95% CI ‐0.06 to 0.13; Analysis 1.4; 2 studies, N = 294; low‐quality evidence). There was high heterogeneity for this outcome for RR (I² = 78%) and for RD (I² = 90%).
BPD at 28 days of age
There was no statistically significant difference in the incidence of BPD at 28 days in the inhaled steroid group compared with the systemic steroid group (typical RR 1.21, 95% CI 0.98 to 1.48; typical RD 0.11, 95% CI ‐0.01 to 0.22; Analysis 1.5; 2 studies, N = 294; low‐quality evidence). There was moderate heterogeneity for RR (I² = 72 %) and high for RD (I² = 87).
Death at 28 days
There was no statistically significant difference in the incidence effect of death at 28 days in the inhaled steroid group compared with the systemic steroid group (typical RR 0.80, 95% CI 0.51 to 1.25; RD ‐0.05, 95% CI ‐0.14 to 0.05; Analysis 1.6; 2 studies, N = 294). Test for heterogeneity not applicable for RR; there was no heterogeneity for RD (I² = 0%).
Duration mechanical ventilation (days)
The duration of mechanical ventilation was statistically significantly longer in the inhaled steroid group as compared with the systemic steroid group (typical WMD 3.89 days, 95% CI 0.24 to 7.55; Analysis 1.7; 2 studies, N = 294; moderate‐quality evidence; I² = 0.0%).
Duration of supplemental oxygen (days)
The duration of supplemental oxygen was statistically significantly higher in the inhaled steroid group as compared with the systemic steroid group (typical WMD 11 days, 95% CI 2 to 20; Analysis 1.8; 2 studies, N = 294; moderate‐quality evidence). There was low heterogeneity for this outcome (I² = 33%).
Hyperglycaemia
A statistically significant decrease in the incidence of hyperglycaemia was noted in the inhaled steroid group compared with the systemic steroid group in Halliday 2001a (RR 0.52, 95% CI 0.39 to 0.71; RD ‐0.25, 95% CI ‐0.37 to ‐0.14; Analysis 1.9; N = 278; moderate‐quality evidence). The NNTB was 4.0 (95% CI 3 to 7). Tests for heterogeneity not applicable.
Patent ductus arteriosus
There was a statistically significant increase in the rate of PDA (RR 1.64, 95% CI 1.23 to 2.17; RD 0.21, 95% CI 0.10 to 0.33; Analysis 1.10; N = 278; moderate‐quality evidence) in the group receiving inhaled steroids compared with the systemic steroid group reported by Halliday 2001a. The NNTH was 5 (95% CI 3 to 10). Tests for heterogeneity not applicable.
Gastrointestinal haemorrhage
There was no statistically significant difference in the incidence of gastrointestinal haemorrhage between the inhaled steroid group compared with the systemic steroid group reported by Halliday 2001a (RR 0.40, 95% CI 0.16 to 1.02) but reduced risk for RD (RD ‐0.06, 95%CI ‐0.12 to ‐0.00; Analysis 1.11; N = 278). Because the significance levels were different for RR and RD we elected not to calculate NNTB. Tests for heterogeneity were not applicable.
Gastrointestinal perforation
There was no statistically significant difference in the incidence of gastrointestinal perforation for the inhaled steroid group compared with the systemic steroid group reported by Halliday 2001a (RR 0.16, 95% CI 0.02 to 1.29) but reduced risk for RD (RD ‐0.04, 95% CI ‐0.07 to ‐0.00; Analysis 1.12; N = 278). Because the significance levels were different for RR and RD we elected not to calculate NNTB. Tests for heterogeneity not applicable.
Infants with free elastase (inflammatory mediator) in tracheal aspirate on day 14 (Outcome 1.13):
Groneck 1999 (N = 16) reported this outcome. There was no statistically significant difference in the number of infants with detectable free elastase in tracheobronchial aspirate fluid for the inhaled steroid group compared with the systemic steroid group (RR 8.75, 95% CI 0.52 to 145.86; RD 0.43, 95% CI 0.06 to 0.80; Analysis 1.13) with a higher number in the inhaled steroid group. Tests for heterogeneity not applicable.
There were no statistically significant differences between the inhaled and the systemic corticosteroid groups for the following outcomes in Halliday 2001a (N = 278 infants): pneumothorax (Analysis 1.14), other air leaks (Analysis 1.15), pulmonary haemorrhage (Analysis 1.16), hypertension (Analysis 1.17), necrotizing enterocolitis (Analysis 1.18), retinopathy of prematurity ‐ any stage (Analysis 1.19), retinopathy of prematurity ≥ stage 3 (Analysis 1.20) and sepsis (Analysis 1.21).
Comparison 2: Inhaled versus systemic steroids among survivors
The following secondary outcomes were assessed using GRADE among survivors; BPD at 36 weeks' postmenstrual age; BPD at 28 days and ever diagnosed as asthmatic at 7 years of age (summary of findings Table 3).
Secondary outcomes
BPD at 36 weeks' postmenstrual age
There was no statistically significant difference in the incidence of BPD at 36 weeks' among survivors in the inhaled steroid group compared with the systemic steroid group (RR 1.34, 95% CI 0.94 to 1.90; RD 0.11, 95% CI ‐0.02 to 0.24; Analysis 2.1; Halliday 2001a; N = 206; moderate‐quality evidence). Tests for heterogeneity not applicable.
BPD at 28 days of age
There was no statistically significant difference in the incidence of BPD at 28 days among survivors in the inhaled steroid group compared with the systemic steroid group (typical RR 1.14, 95% CI 0.96 to 1.34; typical RD 0.09, 95% CI ‐0.02 to 0.21; Analysis 2.2; 2 trials, N = 233; low‐quality evidence). There was high heterogeneity for this outcome for RR (I² = 75%) and for RD (I² = 76%).
No relevant data for the following outcomes were available for analyses: failure to extubate within 14 days of starting treatment, change in pulmonary function tests (lung compliance and resistance), later requirement for systemic corticosteroid therapy, intraventricular haemorrhage, periventricular leukomalacia, measurement of pulmonary functions, pneumonia, growth, nephrocalcinosis, cataracts, hypertrophy of tongue, hypertrophic cardiomyopathy and suppression of hypothalamic‐pituitary‐adrenal axis.
Long‐term follow‐up of survivors at 7 years of age
A subset (127/152, 84%) of infants born in the UK and Ireland enrolled in the OSECT study (Halliday 2001a) has been followed to a median age of seven years. Of these children, 52 belonged to the early dexamethasone and early budesonide groups; 28 children belonged to the early budesonide group and 24 children to the early dexamethasone group.
There were no statistically significant differences between the early inhaled and the early systemic corticosteroid groups for the following outcomes in Halliday 2001a which reported on 52 infants: general conceptual ability (GCA) score at seven years (Analysis 2.3), Child Behaviour Checklist at seven years (Analysis 2.4), Strengths and Difficulties Questionnaire at seven years (Analysis 2.5), cerebral palsy at seven years (Analysis 2.6), moderate/severe disability at seven years (Analysis 2.7), death or moderate/severe disability at seven years (Analysis 2.8), systolic blood pressure > 95th percentile at seven years (Analysis 2.9), diastolic blood pressure > 95th percentile at seven years (Analysis 2.10). Test for heterogeneity was not applicable for any of these analyses.
Ever diagnosed as asthmatic by seven years
Halliday 2001a reported on the outcome ever diagnosed as asthmatic by seven years in 48 children. There was a significantly lower risk in the inhaled steroid group compared with the systemic steroid group (RR 0.42, 95% CI 0.19 to 0.94; RD ‐0.31, 95% CI ‐0.58 to ‐0.05; NNTB 3, 95% CI 2 to 20; Analysis 2.11; moderate‐quality evidence). Tests for heterogeneity not applicable.
Discussion
Summary of main results
This review demonstrated that early use of inhaled steroids is not associated with any significant difference in the incidence of death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age or at 28 days of age compared with the early use of systemic steroids. We found no evidence that inhaled steroids decrease the incidence of BPD at 36 weeks' postmenstrual age or at 28 days of age compared to systemic steroids. Inhaled steroid use compared with systemic steroids use was associated with increase in the incidence of PDA, longer duration of mechanical ventilation and longer duration of supplemental oxygen. Inhaled steroid use compared with systemic corticosteroid use was associated with decrease in the incidence hyperglycaemia and in the incidence of children ever diagnosed as asthmatic by age seven years. In a subgroup of 52 infants there were no significant differences in other long‐term outcomes at seven years of age.
We found no evidence that early inhaled steroids confer important advantages over systemic steroids in the management of ventilated preterm infants. Further studies need to be performed before early steroids, either inhaled or systemic, can be recommended as safe for prevention of BPD in preterm infants. Only a small sample of infants have been followed to seven years of age with no differences observed between the inhaled and systemic steroid groups.
Both studies received grant support and the industry provided aero chambers and metered dose inhalers of budesonide and placebo for the larger study. No conflict of interest was identified.
Overall completeness and applicability of evidence
An intriguing observation made by Halliday 2001a was the statistically significant decrease in the incidence of patent ductus arteriosus (PDA) in infants treated with systemic compared with inhaled steroids. Use of antenatal corticosteroids has shown to decrease the PDA incidence (Aghajafari 2001). Early postnatal dexamethasone therapy in preterm infants with respiratory distress syndrome (RDS) has been shown to decrease the incidence of PDA (Doyle 2014b; Yeh 1997). Heyman 1990 proposed that closure of PDA could be achieved by dexamethasone. Glucocorticoids may have an effect on PDA through an interference in prostaglandin synthesis or through a reduction in sensitivity of ductal muscle to prostaglandin E2 (Clyman 1981; Clyman 1987).
In the current review, hyperglycaemia was less common in the inhaled steroid group. There was a decrease in the incidence of gastrointestinal haemorrhage and gastrointestinal perforation in the inhaled steroid group which was of borderline statistical significance. There were no significant differences in incidences of other adverse effects between the groups. Overall, it would appear that inhaled steroids are less likely to have short‐term adverse effects than systemic steroids. However, data from long‐term follow‐up studies are needed before use of inhaled steroids can be said to be preferable to systemic steroids. Early use of inhaled versus systemic steroids cannot presently be recommended for the prevention of BPD in the preterm infant.
Another major concern with studies of inhaled steroid therapy is the uncertainty regarding drug delivery and deposition in the oropharynx and in the peripheral airways. Numerous factors affect drug delivery and deposition including the number of particles in the respirable range, the delivery technique (use of metered dose inhalers with or without a spacer, nebulizers (jet or ultrasonic)) and the presence or absence of an endotracheal tube. Previous reports have shown that the amount of aerosol delivery varies from 0.4% to 14% based on the technique used (Arnon 1992; Grigg 1992; O'Callaghan 1992). Some studies have suggested that the delayed onset of activity (Dimitriou 1997) and similar risk profile of inhaled steroids (Shah 2003) are consistent with their effects being secondary to systemic absorption.
Identification of an effective dose of inhaled steroids and improvements in drug delivery systems guaranteeing selective delivery in the alveoli and smaller airways may improve the clinical efficacy and decrease the side‐effect profile of inhalational steroids.
Quality of the evidence
According to GRADE assessment, the quality of the evidence was moderate to low (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3). Evidence quality was downgraded based on design (risk of bias), consistency across studies (heterogeneity) and precision of the estimates (sample size).
Potential biases in the review process
A review author (HH) is also the author of an included study (Halliday 2001a). Professor Halliday was not involved in the assessment of risk of bias or data abstraction from that study, which was performed by the other three review authors.
Agreements and disagreements with other studies or reviews
Systematic reviews of early postnatal systemic corticosteroids (< 7 days of age) versus placebo or no treatment have shown a significant decrease in the incidence of BPD and the combined outcome of BPD and death at 28 days and 36 weeks' postmenstrual age (Doyle 2014b; Shah 2001). A borderline increased risk of periventricular leukomalacia was noted in the infants who received dexamethasone. In the reviews of systemic postnatal corticosteroid therapy administered after seven days of age a decrease in the combined outcome of BPD at 36 weeks and mortality was shown (Doyle 2014a; Shah 2001). There was no evidence that the duration of hospitalisation or need for supplemental oxygen was decreased (Shah 2001). Early administration of inhaled steroids in the first two weeks of life to ventilated very low birth weight infants showed no evidence of decrease in the incidence of BPD (Shah 2017a). However, there was increasing evidence from the trials reviewed that early administration of inhaled steroids to very low birth weight neonates was effective in reducing the incidence of death or BPD at 36 weeks' postmenstrual age among either all randomised infants or among survivors.

Study flow diagram: review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 1 Death or BPD at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 2 BPD at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 3 Death at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 4 Death or BPD at 28 days.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 5 BPD at 28 days.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 6 Death at 28 days.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 7 Duration of mechanical ventilation (days).

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 8 Duration of supplemental oxygen (days).

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 9 Hyperglycaemia.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 10 Patent ductus arteriosus.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 11 Gastrointestinal haemorrhage.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 12 Gastrointestinal perforation.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 13 Infants with detectable free elastase (inflammatory mediator) in tracheal aspirate fluid on day 14.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 14 Pneumothorax.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 15 Other air leaks.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 16 Pulmonary haemorrhage.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 17 Hypertension.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 18 Necrotising enterocolitis.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 19 Retinopathy of prematurity ‐ any stage.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 20 Retinopathy of prematurity ≥ stage 3.

Comparison 1 Inhaled versus systemic steroids among all randomised, Outcome 21 Sepsis.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 1 BPD at 36 weeks' postmenstrual age.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 2 BPD at 28 days.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 3 General conceptual ability (GCA) score at 7 years.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 4 Child Behaviour Checklist at 7 years.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 5 Strengths and Difficulties Questionnaire at 7 years.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 6 Cerebral palsy at 7 years.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 7 Moderate/severe disability at 7 years.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 8 Death or moderate/severe disability at 7 years.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 9 Systolic blood pressure > 95th percentile at 7 years.

Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 10 Diastolic blood pressure > 95th percentile at 7 years.
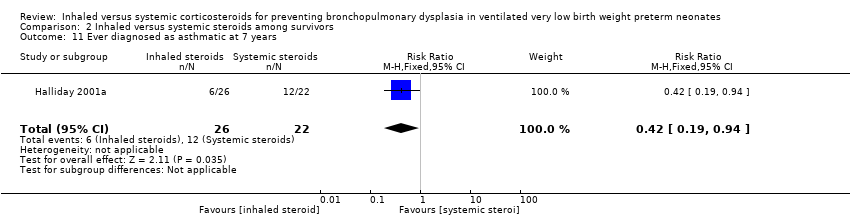
Comparison 2 Inhaled versus systemic steroids among survivors, Outcome 11 Ever diagnosed as asthmatic at 7 years.
| Inhaled steroids compared with systemic corticosteroids for preventing bronchopulmonary dysplasia among all randomised infants | ||||||
| Patient or population: Preterm neonates with respiratory distress Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age among all randomised | 526 per 1000 | 573 per 1000 | RR 1.09 (95% CI 0.88 to 1.35) | 278 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. |
| Death or BPD at 28 days | 736 per 1000 | 780 per 1000 | RR 1.05 (95% CI 0.93 to 1.20) | 294 (2) | ⊕⊕⊝⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Heterogeneity/Consistency: There was high heterogeneity for this analysis I² = 78%. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| BPD at 36 weeks' postmenstrual age | 237 per 1000 | 343 per 1000 | RR 1.45 (95% CI 0.99 to 2.11) | 278 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. |
| BPD at 28 days | 507 per 1000 | 613 per 1000 | RR 1.21 (95% CI 0.98 to 1.48) | 294 (2) | ⊕⊕⊝⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic corticosteroids for preventing bronchopulmonary dysplasia among all randomised | ||||||
| Patient or population: Preterm neonates with respiratory distress Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Duration of mechanical ventilation (days) | The mean duration of mechanical ventilation ranged across the systemic steroid groups from 15.2 to 17.9 days | The mean duration of mechanical ventilation ranged across the inhaled steroid groups from 20 to 21 days | 3.89 days (0.24 to 7.55) | 294 (2) | ⊕⊕⊕⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| Duration of supplemental oxygen (days) | The mean duration of supplemental oxygen ranged across control groups from 22.7 to 49.3 days | The mean duration of supplemental oxygen in the intervention groups ranged from 38.2 to 53.0 days | 11.10 days (1.97 to 20.22) | 294 (2) | ⊕⊕⊕⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| Hyperglycaemia | 533 per 1000 | 280 per 1000 | RR 0.52, (95% CI 0.39 to 0.71) | 278 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| Patent ductus arteriosus | 333 per 1000 | 546 per 1000 | RR 1.64, (95% CI 1.23 to 2.17) | 278 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic corticosteroids for preventing bronchopulmonary dysplasia among survivors | ||||||
| Patient or population: Preterm neonates with respiratory distress Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| BPD at 36 weeks' postmenstrual age | 333 per 1000 | 446 per 1000 | RR 1.34 (95% CI 0.94 to 1.90) | 206 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| BPD at 28 days | 658 per 1000 | 754 per 1000 | RR 1.14 (95% CI 0.96 to 1.34) | 233 | ⊕⊕⊝⊝ | Bias: The risk of bias for these two studies was high. The larger study was not blinded at all sites. Only 53/135 infants randomised to systemic steroids received full course while 53/145 infants randomised to inhaled steroids received full course. The smaller study was not blinded and it was stopped prematurely. We downgraded the Quality of the evidence by one step. Precision: Precison for the point estimate was acceptable. Presence of publication bias: N/A. |
| Ever diagnosed as asthmatic by 7 years of age | 546 per 1000 | 231 per 1000 | RR 0.42 (95% CI 0.19 to 0.94) | 48 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/Consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.88, 1.35] |
| 2 BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.99, 2.11] |
| 3 Death at 36 weeks' postmenstrual age Show forest plot | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.56, 1.23] |
| 4 Death or BPD at 28 days Show forest plot | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.20] |
| 5 BPD at 28 days Show forest plot | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.98, 1.48] |
| 6 Death at 28 days Show forest plot | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.25] |
| 7 Duration of mechanical ventilation (days) Show forest plot | 2 | 294 | Mean Difference (IV, Fixed, 95% CI) | 3.89 [0.24, 7.55] |
| 8 Duration of supplemental oxygen (days) Show forest plot | 2 | 294 | Mean Difference (IV, Fixed, 95% CI) | 11.10 [1.97, 20.22] |
| 9 Hyperglycaemia Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.39, 0.71] |
| 10 Patent ductus arteriosus Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.23, 2.17] |
| 11 Gastrointestinal haemorrhage Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.16, 1.02] |
| 12 Gastrointestinal perforation Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.29] |
| 13 Infants with detectable free elastase (inflammatory mediator) in tracheal aspirate fluid on day 14 Show forest plot | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.75 [0.52, 145.86] |
| 14 Pneumothorax Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.56, 1.39] |
| 15 Other air leaks Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.58, 1.90] |
| 16 Pulmonary haemorrhage Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.48, 2.16] |
| 17 Hypertension Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.44, 1.29] |
| 18 Necrotising enterocolitis Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.60, 3.36] |
| 19 Retinopathy of prematurity ‐ any stage Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.72, 1.71] |
| 20 Retinopathy of prematurity ≥ stage 3 Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.43, 4.06] |
| 21 Sepsis Show forest plot | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.94, 1.90] |
| 2 BPD at 28 days Show forest plot | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.96, 1.34] |
| 3 General conceptual ability (GCA) score at 7 years Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐5.85, 13.45] |
| 4 Child Behaviour Checklist at 7 years Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐7.12, 4.32] |
| 5 Strengths and Difficulties Questionnaire at 7 years Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.12, 4.52] |
| 6 Cerebral palsy at 7 years Show forest plot | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.36, 8.70] |
| 7 Moderate/severe disability at 7 years Show forest plot | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.16, 2.59] |
| 8 Death or moderate/severe disability at 7 years Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.45] |
| 9 Systolic blood pressure > 95th percentile at 7 years Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.06, 13.14] |
| 10 Diastolic blood pressure > 95th percentile at 7 years Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.17, 17.95] |
| 11 Ever diagnosed as asthmatic at 7 years Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.94] |

