Antagonistas opiáceos con sedación mínima para la abstinencia de opiáceos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002021.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 29 mayo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Alcohol y drogas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Linda Gowing assessed each potentially relevant study according to identified inclusion and exclusion criteria, extracted key information and compiled a first draft of the review. Robert Ali and Jason White confirmed and commented on review content.
Sources of support
Internal sources
-
Drug and Alcohol Services South Australia, Australia.
External sources
-
Commonwealth Department of Health and Aging, Australia.
Declarations of interest
Linda Gowing: none known.
Robert Ali: none known.
Jason White: none known.
Acknowledgements
Dr Zhao Chengzheng and Dr Liang Jianhui translated and assisted with assessment of a study considered for this review (He 1999) that was published in Chinese.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 May 29 | Opioid antagonists with minimal sedation for opioid withdrawal | Review | Linda Gowing, Robert Ali, Jason M White | |
| 2009 Oct 07 | Opioid antagonists with minimal sedation for opioid withdrawal | Review | Linda Gowing, Robert Ali, Jason M White | |
| 2009 Jul 08 | Opioid antagonists with minimal sedation for opioid withdrawal | Review | Linda Gowing, Robert Ali, Jason M White | |
| 2002 Apr 22 | Opioid antagonists with minimal sedation for opioid withdrawal | Review | Linda Gowing, Robert Ali, Jason M White | |
Notes
The first version of this review was entitled 'Opioid antagonists and adrenergic agonists for the management of opioid withdrawal'.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Adrenergic alpha‐Agonists [*therapeutic use];
- Clonidine [analogs & derivatives, therapeutic use];
- Naloxone [therapeutic use];
- Naltrexone [therapeutic use];
- Narcotic Antagonists [*therapeutic use];
- Non‐Randomized Controlled Trials as Topic;
- Opioid‐Related Disorders [*rehabilitation];
- Prospective Studies;
- Randomized Controlled Trials as Topic;
- Severity of Illness Index;
- Substance Withdrawal Syndrome [*drug therapy];
Medical Subject Headings Check Words
Humans;
PICO

Flow diagram of literature search

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
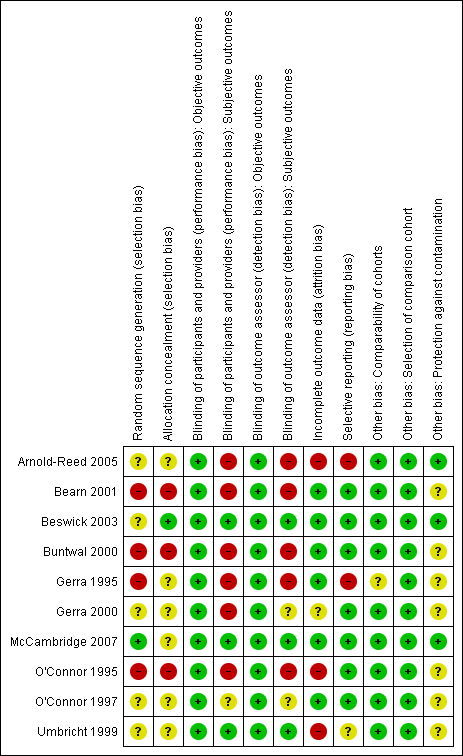
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 1 Peak withdrawal severity.
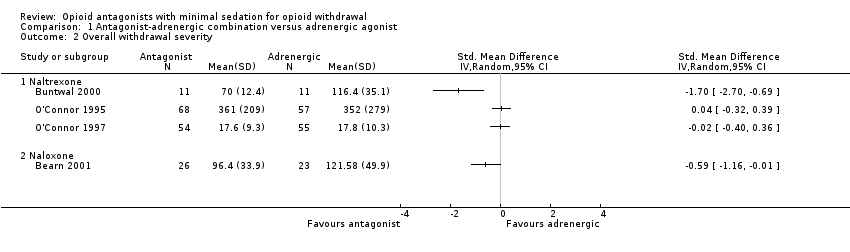
Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 2 Overall withdrawal severity.

Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 3 Completion rate.
| Antagonist‐adrenergic combination compared to adrenergic agonist for opioid withdrawal | |||
| Patient or population: opioid dependent adults | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Peak withdrawal severity ‐ Naltrexone | In 1 study peak withdrawal severity was similar for the 2 types of intervention. In the other study peak withdrawal was more severe in the group receiving antagonist‐adrenergic combination. | 184 | ⊕⊝⊝⊝ |
| Peak withdrawal severity ‐ Naloxone | This comparison reported by only 1 study, which found more severe withdrawal with naloxone‐clonidine combination. | 91 | ⊕⊝⊝⊝ |
| Overall withdrawal severity ‐ Naltrexone | No difference in overall withdrawal severity for 2 studies; in 1 study overall severity significantly less for antagonist‐adrenergic combination. | 256 | ⊕⊝⊝⊝ |
| Overall withdrawal severity ‐ Naloxone | This comparison reported by only 1 study, which found less severe overall withdrawal with naloxone‐lofexidine combination. | 49 | ⊕⊝⊝⊝ |
| Completion rate ‐ Naltrexone | Completion rate with adrenergic agonist only ranged from 42% to 94%. Completion rate with antagonist‐adrenergic agonist combination ranged from 73% to 95% | 330 | ⊕⊝⊝⊝ |
| Completion rate ‐ Naloxone | Completion rate with adrenergic agonist only ranged from 28% to 94%. Completion rate with antagonist‐adrenergic agonist combination ranged from 73% to 98% | 463 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| aOne study at high risk of bias. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peak withdrawal severity Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Naltrexone | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Naloxone | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall withdrawal severity Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Naltrexone | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Naloxone | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Completion rate Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Naltrexone | 4 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Naloxone | 6 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

