Introdução tardia de dieta enteral progressiva para prevenção de enterocolite necrosante em recém‐nascidos de muito baixo peso
Resumo
Introdução
A introdução de dieta enteral para recém‐nascidos muito prematuros (menores de 32 semanas de idade gestacional) ou de muito baixo peso (abaixo de 1.500 g) é frequentemente adiada por vários dias depois do nascimento, devido à preocupação de que a introdução precoce pode não ser tolerada e aumentar o risco de enterocolite necrosante. Entretanto, adiar a alimentação enteral pode diminuir a adaptação funcional do trato gastrointestinal do bebê e prolongar sua necessidade de nutrição parenteral, com os esperados riscos infecciosos e metabólicos.
Objetivos
Avaliar o efeito da introdução tardia da dieta enteral progressiva na incidência de enterocolite necrosante, mortalidade e outras morbidades em recém‐nascidos muito prematuros ou de muito baixo peso.
Métodos de busca
Pesquisamos as seguintes bases de dados: Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Edição 8), MEDLINE (1966 a setembro de 2014), EMBASE (1980 a setembro de 2014), CINAHL (1982 a setembro de 2014), atas de conferências e revisões já existentes.
Critério de seleção
Incluímos ensaios clínicos randomizados ou quasi‐randomizados que avaliaram o efeito de se adiar a introdução de dieta enteral progressiva (mais de quatro dias após o nascimento) versus introduzir precocemente a dieta sobre a incidência de enterocolite necrosante, mortalidade e outras morbidades em recém‐nascidos muito prematuros ou de muito baixo peso.
Coleta dos dados e análises
Dois autores avaliaram independentemente a elegibilidade e o risco de viés dos estudos, e realizaram a extração dos dados. Analisamos os efeitos do tratamento nos estudos individuais. Apresentamos o risco relativo (RR) e a diferença de risco para dados dicotômicos e a diferença de média para dados contínuos, com os respectivos intervalos de confiança de 95% (95% CI). Para as metanálises, usamos um modelo de modelo e efeito fixo e fizemos análises de sensibilidade para explorar as possíveis causas de heterogeneidade.
Principais resultados
Identificamos nove ensaios clínicos randomizados com 1.106 recém‐nascidos participantes. Poucos participantes eram extremamente prematuros (menos de 28 semanas de idade gestacional) ou tinham extremo baixo peso (menos de 1.000 g). Os estudos definiram introdução tardia de dieta enteral progressiva como uma espera de mais que quatro a sete dias e a introdução precoce como quatro dias ou menos depois nascimento. Nas metanálises, não houve diferenças estatisticamente significativas no risco de enterocolite necrosante (RR 0.93, 95% CI 0.64 a 1.34; 8 estudos; 1.092 recém‐nascidos) ou na mortalidade por qualquer causa (RR 1.18, 95% CI 0.75 a 1.88; 7 estudos; 967 recém‐nascidos). Quatro estudos incluíram apenas recém‐nascidos com crescimento restrito com circulação fetal anormal evidente no Doppler antenatal. As análises de subgrupo desses estudos não encontraram diferenças estatisticamente significantes no risco de enterocolite necrosante ou de mortalidade por qualquer causa. Os recém‐nascidos no grupo de introdução tardia de dieta enteral demoraram mais para alcançar alimentação enteral plena (diferença mediana de dois a quatro dias).
Conclusão dos autores
As evidências disponíveis a partir dos ensaios clínicos randomizados sugeriram que o atraso na introdução de dieta enteral progressiva para além de quatro dias após o nascimento não reduziu o risco de desenvolver enterocolite necrosante em recém‐nascidos muito prematuros ou de muito baixo peso, incluindo recém‐nascidos com crescimento restrito. A demora na introdução de dieta enteral progressiva resultou em atraso de poucos dias em alcançar a alimentação enteral plena, mas a importância clínica desse efeito não foi clara. A aplicabilidade desses achados para prematuros extremos ou de extremo baixo peso é incerta. São necessários novos estudos controlados e randomizados nessa população.
PICO
Resumo para leigos
Não há evidência de que a introdução tardia de dieta enteral progressiva previna a enterocolite necrosante em recém‐nascidos de muito baixo peso.
Introdução
Recém‐nascidos muito prematuros (que nascem antes da 32ª de gravidez) ou de muito baixo peso (menos que 1.500 g) têm risco de desenvolver um problema grave do intestino, chamado enterocolite necrosante, na qual partes do intestino se tornam inflamadas e começam a morrer. Uma maneira possível de prevenir essa condição é atrasar a introdução de leite até vários dias (ou mais) após o nascimento.
Características dos estudos
Pesquisamos bancos de dados científicos em busca de ensaios clínicos que compararam a introdução tardia (mais que quatro dias após o nascimento) versus a introdução precoce de dieta enteral progressiva (onde o leite materno ou fórmula láctea é administrada diretamente por sonda dentro do estômago). Esses estudos deveriam comparar o efeito desses dois tipos de alimentação sobre a incidência de enterocolite necrosante, o risco de morte e o estado de saúde em geral em recém‐nascidos de muito baixo peso. Todos os estudos publicados até setembro de 2014 foram levantados.
Resultados principais
Encontramos nove estudos, com 1.106 recém‐nascidos, que avaliaram o efeito da introdução precoce versus tardia de leite para recém‐nascidos prematuros ou de muito baixo peso. Os resultados desses estudos indicam que não existe nenhuma evidência de que a introdução tardia da alimentação enteral reduza o risco de enterocolite necrosante.
Qualidade da evidência
Os estudos incluídos foram geralmente de qualidade metodológica razoável mas, assim como em outros estudos de intervenções alimentares em recém‐nascidos, não foi possível cegar os cuidadores e avaliadores clínicos para o tratamento oferecido.
Authors' conclusions
Background
Description of the condition
Necrotising enterocolitis (NEC) is an important cause of morbidity, mortality and neuro‐disability in very preterm (less than 32 weeks' gestation) or very low birth weight (VLBW: less than 1500 g) infants. Extremely low birth weight (ELBW: less than 1000 g) and extremely preterm (less than 28 weeks' gestation) infants are at greatest risk (Bisquera 2002; Holman 2006; Rees 2007; Berrington 2012). Intrauterine growth restriction may be an additional specific risk factor, especially if associated with circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in antenatal Doppler studies of the fetal aorta or umbilical artery (Bernstein 2000; Garite 2004; Dorling 2005; Kamoji 2008).
Description of the intervention
Most very preterm or VLBW infants who develop NEC have received enteral milk feeds. Evidence exists that feeding with artificial formula rather than human milk increases the risk of developing NEC (Quigley 2014). The timing of the introduction and the rate of progression of enteral feed volumes may also be modifiable risk factors for the development of NEC (Brown 1978; Uauy 1991; Henderson 2009). Data from observational studies suggest that using feeding regimens that include delaying the introduction of progressive enteral feeds until beyond about four to seven days after birth reduces the risk of NEC (Patole 2005; Hay 2008).
Why it is important to do this review
In current clinical practice, the introduction of progressive enteral feeds for very preterm or VLBW infants is often preceded by a period of enteral fasting or 'minimal enteral nutrition' (Boyle 2004; Patole 2004; Hay 2008; Klingenberg 2012). However, there may also be potential disadvantages associated with delaying the introduction of progressive enteral feeds. Because gastrointestinal hormone secretion and motility are stimulated by enteral milk, delayed enteral feeding could diminish the functional adaptation of the gastrointestinal tract (Berseth 1990; Burrin 2002). Prolonging the duration of use of parenteral nutrition may be associated with infectious and metabolic complications that increase mortality and morbidity, prolong hospital stay, and adversely affect growth and development (Flidel‐Rimon 2004; Stoll 2004). It has been argued that the risk of NEC should not be considered in isolation of these other potential clinical outcomes when determining feeding policies and practice for very preterm or VLBW infants (Flidel‐Rimon 2006; Hay 2008; Hartel 2009).
This review focused on the comparison of delayed versus earlier introduction of progressive enteral feeding; that is, advancing the volume of milk feeds beyond minimal enteral nutrition levels. We addressed the effect of minimal enteral nutrition, the early introduction of small volume enteral feeds (up to 24 mL/kg/day) without advancing the feed volumes for at least five days versus enteral fasting in another Cochrane review (Morgan 2013a).
Objectives
To determine the effect of delayed introduction of progressive enteral feeds on the incidence of NEC, mortality and other morbidities in very preterm or VLBW infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials or cluster‐randomised trials.
Types of participants
VLBW (less than 1500 g) or very preterm (less than 32 weeks' gestation) newborn infants.
Types of interventions
Delayed introduction (four or more days after birth) of progressive enteral feeds versus earlier introduction of enteral feeds. We defined progressive enteral feeding as the intention to advance feed volumes in excess of minimal enteral nutrition levels (24 mL/kg/day) within five days of commencement or by one week after birth.
Infants in each group should have received the same type of milk (breast milk or formula), the same route and mode of feeding (intragastric or transpyloric, bolus gavage or continuous) and the same rate of feed volume advancement in both groups.
Types of outcome measures
Primary outcomes
1. NEC confirmed by at least two of the following features:
a. abdominal radiograph showing pneumatosis intestinalis or gas in the portal venous system or free air in the abdomen;
b. abdominal distension with abdominal radiograph with gaseous distension or frothy appearance of bowel lumen (or both);
c. blood in stool;
d. lethargy, hypotonia or apnoea (or combination of these).
Or by a diagnosis confirmed at surgery or autopsy (Walsh 1986).
2. All‐cause mortality during the neonatal period and prior to hospital discharge.
Secondary outcomes
3. Growth:
a. time to regain birth weight and subsequent rates of weight gain, linear growth, head growth or skinfold thickness growth up to six months (corrected for preterm birth);
b. long‐term growth: weight, height or head circumference (with or without proportion of infants who remain below the 10th percentile for the index population's distribution) assessed at intervals from six months of age.
4. Neurodevelopment:
a. death or severe neurodevelopmental disability defined as any one or combination of the following: non‐ambulant cerebral palsy, developmental delay (developmental quotient less than 70), auditory and visual impairment. Each component was analysed individually as well as part of the composite outcome;
b. neurodevelopmental scores in survivors aged 12 months or greater measured using validated assessment tools;
c. cognitive and educational outcomes in survivors aged more than five years old.
5. Time to establish full enteral feeding (independently of parenteral nutrition).
6. Time to establish oral feeding (independently of parenteral nutrition or enteral tube feeding, or both).
7. Feed intolerance (defined as a requirement to cease enteral feeds).
8. Incidence of invasive infection as determined by culture of bacteria or fungus from blood, cerebrospinal fluid, urine or from a normally sterile body space.
9. Duration of hospital stay (days).
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Review Group (neonatal.cochrane.org/).
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Issue 8), MEDLINE (1966 to September 2014), EMBASE (1980 to September 2014) and CINAHL (1982 to September 2014) using a combination of the following text words and MeSH terms: [Infant, Newborn OR Infant, Premature OR Infant, Low Birth Weight OR Infant, Very Low Birth Weight/ OR infan* OR neonat* OR preterm OR prem*] AND "Infant‐Nutrition"/ all subheadings OR Infant Formula OR milk OR formula OR trophic feeding OR minimal enteral nutrition OR gut priming]. We limited the search outputs with the relevant search filters for clinical trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We applied no language restrictions.
We searched ClinicalTrials.gov (clinicaltrials.gov) and Current Controlled Trials (www.controlled‐trials.com/) for completed or ongoing trials.
Searching other resources
We examined the references in all studies identified as potentially relevant.
We searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993 to 2014), the European Society for Pediatric Research (1995 to 2014), the UK Royal College of Paediatrics and Child Health (2000 to 2014) and the Perinatal Society of Australia and New Zealand (2000 to 2013). Trials reported only as abstracts were eligible if sufficient information was available from the report, or from contact with the authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group (neonatal.cochrane.org/).
Selection of studies
Two review authors screened the title and abstract of all studies identified by the above search strategy. We assessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed any disagreements until we achieved consensus.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Two review authors extracted the data separately. We discussed any disagreements until we reached consensus. We contacted the investigators for further information if data from the trial reports were insufficient.
Assessment of risk of bias in included studies
We used the criteria and standard methods of the Cochrane Neonatal Review Group to assess the methodological quality of any included trials (neonatal.cochrane.org/). We requested additional information from the trial authors to clarify methodology and results as necessary. We evaluated and reported the following issues in the 'Risk of bias' tables:
-
Sequence generation: we categorised the method used to generate the allocation sequence as:
-
low risk: any random process (e.g. random number table, computer random number generator);
-
high risk: any non‐random process (e.g. odd or even date of birth, participant case‐record number);
-
unclear risk.
-
-
Allocation concealment: we categorised the method used to conceal the allocation sequence as:
-
low risk (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes);
-
high risk (e.g. open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk.
-
-
Blinding: we assessed blinding of participants, clinicians and carers, and outcome assessors separately for different outcomes and categorised the methods as:
-
low risk;
-
high risk;
-
unclear risk.
-
-
Incomplete outcome data: we described the completeness of data including attrition and exclusions from the analysis for each outcome and any reasons for attrition or exclusion where reported. We assessed whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised completeness as:
-
low risk: less than 20% missing data;
-
high risk: 20% or greater missing data;
-
unclear risk.
-
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CI). We planned to determine the number needed to treat for an additional beneficial outcome (NNTB) or additional harmful outcome (NNTH) for any statistically significant differences in the RD.
Unit of analysis issues
The unit on analysis was the participating infant in individually randomised trials and the neonatal unit (or sub‐unit) for cluster‐randomised trials.
Assessment of heterogeneity
If we included more than one trial in a meta‐analysis, we examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I2 statistic for each analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be due to heterogeneity rather than sampling error. If we detected substantial (I2 greater than 50%) heterogeneity, we explored the possible causes (e.g. differences in study design, participants, interventions or completeness of outcome assessments) in sensitivity analyses.
Data synthesis
We used the fixed‐effect model in Review Manager 5 for meta‐analysis (RevMan 2011).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses:
-
trials in which most infants were exclusively formula‐fed;
-
trials in which most infants were at least partially fed with human milk (maternal or donor);
-
trials in which most participants were of ELBW (less than 1000 g) or extremely preterm (less than 28 weeks' gestation);
-
trials in which participants were infants with intrauterine growth restriction, or infants with absent or reversed end‐diastolic flow velocities detected on antenatal Doppler studies of the fetal aorta or umbilical artery.
Results
Description of studies
We identified 13 reports for screening.
Included studies
Nine trials fulfilled the review eligibility criteria: Ostertag 1986; Khayata 1987; Davey 1994; Karagianni 2010; Pérez 2011; Abdelmaaboud 2012; Leaf 2012; Armanian 2013; Arnon 2013 (see Characteristics of included studies table).
Population
A total of 1106 infants participated in the included trials.
The three smallest trials were undertaken in neonatal care centres in North America during the 1980s and early 1990s.
-
Ostertag 1986: 38 VLBW infants assessed to be at high risk of developing NEC.
-
Khayata 1987: 12 VLBW infants.
-
Davey 1994: 62 clinically stable preterm infants of birth weight less than 2000 g who had a low umbilical artery catheter in situ. Since most participants were of birth weight less than 1500 g or gestational age less than 32 weeks, we made a consensus decision to include the trial.
The six more recent trials were performed in the 2000s to 2010s.
-
Karagianni 2010: single‐centre study in Greece, 84 infants less than 35 weeks' gestation with a birth weight less than 10th percentile and evidence of abnormal fetal blood flow patterns on Doppler ultrasound of the umbilical artery.
-
Pérez 2011: single‐centre study in Columbia, 239 very preterm or VLBW infants.
-
Leaf 2012: 54‐centre trial in the UK and Ireland, 404 infants (a) less than 35 weeks' gestation, (b) birth weight less than 10th percentile and (c) evidence of abnormal fetal blood flow patterns on Doppler ultrasound studies. Since most participants were of birth weight less than 1500 g, we made a consensus decision to include the trial.
-
Abdelmaaboud 2012: single‐centre study in Qatar, 125 preterm infants with intrauterine growth restriction and abnormal Doppler flow patterns on ultrasound of the umbilical artery. Since most participants were of birth weight less than 1500 g, we made a consensus decision to include the trial.
-
Armanian 2013: single‐centre study in Iran, 82 VLBW infants.
-
Arnon 2013: single‐centre study in Israel, 60 small for gestation age preterm infants. Since most participants were of birth weight less than 1500 g, we made a consensus decision to include the trial.
Interventions/comparisons
-
Eight trials defined 'delayed' introduction of enteral feeds as later than day four to seven after birth (Ostertag 1986; Davey 1994; Karagianni 2010; Pérez 2011; Abdelmaaboud 2012; Leaf 2012; Armanian 2013; Arnon 2013) and one trial defined it as day 10 after birth (Khayata 1987).
-
'Early' feeding varied from day one to four after birth.
In seven trials, infants received breast milk, artificial formula or a combination of the two (Davey 1994; Karagianni 2010; Pérez 2011; Abdelmaaboud 2012; Leaf 2012; Armanian 2013; Arnon 2013). In two trials, infants received only formula‐feed (Ostertag 1986; Khayata 1987). Infants received enteral feeds by gavage at one‐ or two‐hourly intervals in all of the trials except Ostertag 1986, where infants received feeds by continuous intragastric infusion. In Ostertag 1986, infants were initially fed with a sterile water infusion slowly progressing to a 2.5% dextrose solution followed by half‐strength formula. They reached full‐strength formula milk seven days after initiating enteral feeds.
All of the trial protocols, except that of the smallest trial (Khayata 1987), specified criteria and indications for advancing (daily increments of 15 to 30 mL/kg) or interrupting enteral feed (e.g. residual gastric contents not greater than 3 to 5 mL or one‐third to one‐half of the previous feed volume, frequent vomiting, abdominal distention or detection of blood in the stools).
Outcomes
Eight of the trials reported the incidence of NEC (confirmed radiologically, or at surgery or autopsy) (Ostertag 1986; Davey 1994; Karagianni 2010; Pérez 2011; Abdelmaaboud 2012; Leaf 2012; Armanian 2013; Arnon 2013). The other reported outcomes included mortality, time to establish full enteral feeding, growth and duration of hospital stay. Only two trials reported the incidence of invasive infection (Leaf 2012; Arnon 2013).
Excluded studies
We excluded eight studies after full‐text screening (Higgs 1974; Glass 1984; LaGamma 1985; Wilson 1997; Weiler 2006; Said 2008; Sanghvi 2013; Chetry 2014) (see Characteristics of excluded studies table).
Risk of bias in included studies
Quality assessments are described in the Characteristics of included studies table and summarised in Figure 1.
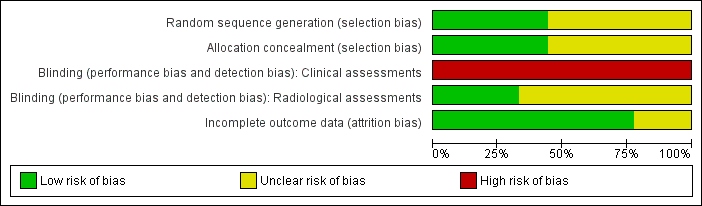
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
The smallest trial (12 infants) was reported in abstract form only and methodological details were not described (Khayata 1987).
The other trials had various methodological weaknesses. In five trials, methods to ensure adequate allocation concealment were not described. None of the trials concealed the feeding strategies from parents, carers or clinical investigators. Complete or near‐complete assessments of the primary outcomes were reported and data were available to undertake intention‐to‐treat analyses as required.
Effects of interventions
Primary outcomes
Necrotising enterocolitis (Outcome 1.1)
Meta‐analysis did not detect a statistically significant effect (typical RR 0.93, 95% CI 0.64 to 1.34; typical RD ‐0.01, 95% CI ‐0.04 to 0.03; 8 trials; 1092 infants). There was no statistical evidence of heterogeneity (Figure 2).
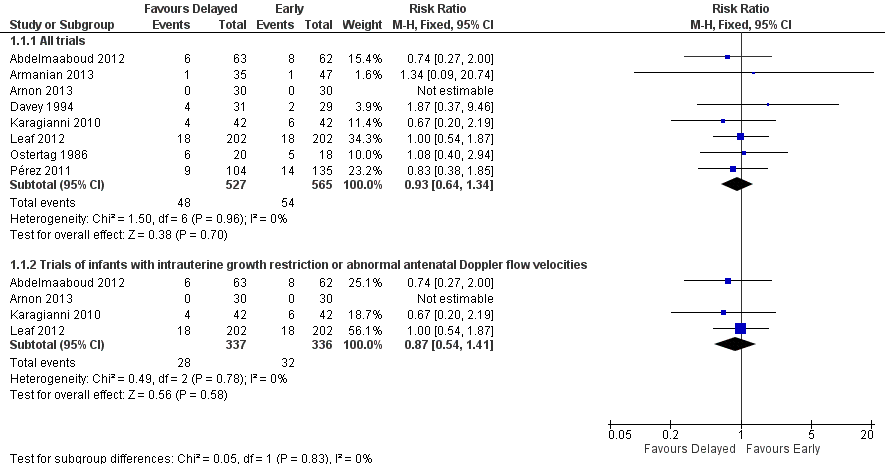
Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.1 Necrotising enterocolitis.
Mortality prior to discharge (Outcome 1.2)
Meta‐analysis did not detect a statistically significant effect (typical RR 1.18, 95% CI 0.75 to 1.88; typical RD 0.01, 95% CI ‐0.02 to 0.04; 7 trials, 967 infants). There was no statistical evidence of heterogeneity (Figure 3).
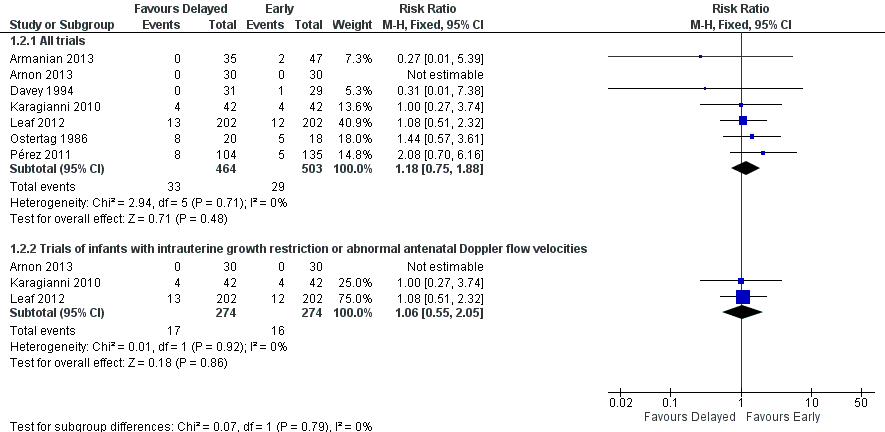
Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.2 Mortality prior to discharge.
Secondary outcomes
Growth
Two trials did not detect a statistically significant difference in the median time to regain birth weight:
-
Davey 1994: 13 days for both groups (range not reported; 62 infants);
-
Abdelmaaboud 2012: 13 days in the delayed group compared with 14 days in the early introduction group (range not reported; 125 infants).
Two trials did not detect statistically significant differences in the rate of weight gain but the reports did not provide data to allow quantitative synthesis (Khayata 1987; Pérez 2011).
None of the other trials reported hospital growth parameters.
None of the trials assessed any long‐term (post‐hospital discharge) growth parameters.
Neurodevelopment
None of the trials assessed neurodevelopmental outcomes.
Time to establish full enteral feeding
The median time to establish full enteral feeding was longer in the delayed introduction group but the reports did not provide data to allow quantitative synthesis:
-
Ostertag 1986: not reported;
-
Khayata 1987: not reported;
-
Davey 1994: three days;
-
Karagianni 2010: three days;
-
Pérez 2011: four days;
-
Abdelmaaboud 2012: two days;
-
Leaf 2012: three days;
-
Armanian 2013: five days;
-
Arnon 2013: three days.
Time to establish full oral feeding
None of the trials assessed time to establish full oral feeding.
Feed intolerance (Outcome 1.3)
Meta‐analysis of data from three trials did not detect a statistically significant difference in feed intolerance (typical RR 0.84, 95% CI 0.62 to 1.15; typical RD ‐0.06, 95% CI ‐0.17 to 0.05; 288 infants; Figure 4) (Karagianni 2010;Abdelmaaboud 2012;Armanian 2013).
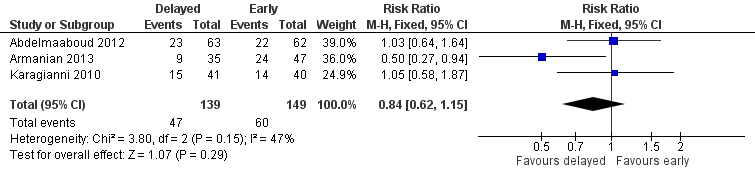
Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.3 Feed intolerance.
One trial did not detect a statistically significant difference but the report did not provide data to allow quantitative synthesis (Davey 1994).
None of the other trials reported the incidence of feed intolerance.
Incidence of invasive infection (Outcome 1.4)
Meta‐analysis of data from two trials did not detect a statistically significant difference (typical RR 1.27, 95% CI 0.95 to 1.70; typical RD 0.07, 95% CI ‐0.01 to 0.15; 457 infants) (Leaf 2012;Arnon 2013).
Duration of hospital stay (Outcome 1.5)
Meta‐analysis from three trials showed a statistically significant longer duration in the delayed feeding group (MD 2.11 days, 95% CI 0.31 to 3.90; 346 infants; Figure 5). The meta‐analysis contained substantial heterogeneity (Chi2 = 7.66; degrees of freedom (df) = 2; P value = 0.02; I2 = 74%).
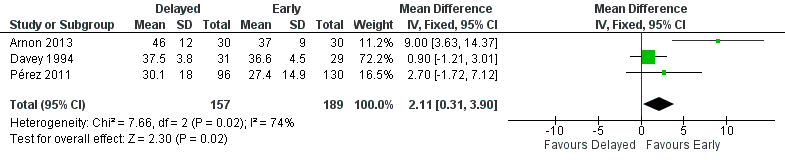
Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.5 Duration of hospital admission (days).
Another two trials did not detect a statistically significant effect but the reports did not provide data to allow quantitative synthesis (Abdelmaaboud 2012; Leaf 2012).
Subgroup analyses
-
Two trials only recruited exclusively formula‐fed infants (Ostertag 1986; Khayata 1987). Only Ostertag 1986 reported the effect on NEC (RR 1.08, 95% CI 0.40 to 2.94; RD 0.02, 95% CI ‐0.27 to 0.31; 38 infants) or mortality (RR 1.44, 95% CI 0.57 to 3.61; RD 0.12, 95% CI ‐0.18 to 0.42; 38 infants).
-
Trials in which most infants were at least partially fed with human milk (maternal or donor): subgroup data not available.
-
ELBW or extremely preterm infants: none of the trials recruited predominantly ELBW or extremely preterm infants.
-
Four trials recruited only infants with intrauterine growth restriction and abnormal flow velocities detected on antenatal Doppler studies (Karagianni 2010; Abdelmaaboud 2012; Leaf 2012; Arnon 2013). Meta‐analysis did not detect any statistically significant differences in the incidence of NEC (typical RR 0.87, 95% CI 0.54 to 1.41; typical RD ‐0.01, 95% CI ‐0.06 to 0.03; 673 infants; Figure 2) or mortality (typical RR 1.06, 95% CI 0.55 to 2.05; typical RD 0.00, 95% CI ‐0.04 to 0.05; 548 infants; Figure 3).
Discussion
Summary of main results
Analyses of data from nine randomised controlled trials with 1106 infants did not provide evidence that delayed introduction of progressive enteral feeds reduced the risk of NEC. The boundaries of the 95% CI for the estimate of effect are consistent with either four fewer or three extra cases of NEC in every 100 infants who had delayed introduction of progressive enteral feeds. Meta‐analysis of data from these trials did not indicate an effect on all‐cause mortality with the 95% CI boundaries being consistent with either two fewer or four more deaths in every 100 infants who had delayed introduction of progressive enteral feeds. Similarly, pre‐specified subgroup meta‐analyses showed no effect of delayed introduction of enteral feeds on NEC or mortality in infants with growth restriction or antenatal evidence of absent end‐diastolic flow velocities.
Infants who had delayed introduction of feeds achieved full enteral feeding several days later than infants who had earlier introduction. Whether this was associated with important clinical adverse consequences, such as a higher rate of nosocomial infection secondary to prolonged use of parenteral nutrition or a longer duration of hospital admission, remains unclear.
Overall completeness and applicability of evidence
These data are relevant to current practice since most of the included trials were conducted in the 2000s with infants receiving 'modern' antenatal care including exposure to antenatal corticosteroids and exogenous surfactant, interventions that reduce the risk of NEC or death in this population (Roberts 2006; Seger 2009; Soll 2009; Soll 2010). Four of these trials specifically recruited infants thought to be at higher risk of developing NEC due to intrauterine growth restriction and abnormal fetal circulatory distribution or flow. This widens the applicability of the findings since this is the population for which most clinical uncertainty and variation in practice with regard to early feeding strategies exists (Boyle 2004). Previously, this population of infants has been specifically excluded from participating in many trials of early enteral feeding practices (Tyson 2007).
Evidence exists that artificial formula feeding increases the risk NEC (Quigley 2014). The risk‐benefit balance of enteral feeding strategies may differ between human milk‐fed and formula‐fed very preterm or VLBW infants. Currently there are insufficient data to comment on whether there is a differential effect of the timing of the introduction of enteral feeds depending on whether infants received human breast milk versus formula.
It is also unclear whether the findings can be applied to infants who receive continuous infusion of intragastric feeds, as most of the infants in the included trials received enteral feeds as interval gastric boluses. Randomised controlled trials have reported conflicting findings about the effect on continuous enteral infusion on feed tolerance in VLBW (and especially ELBW) infants (Premji 2011).
Most of the included trials were undertaken in neonatal care centres in middle‐ or high‐income countries. It is less clear how applicable this evidence is to neonatal care practices in low‐income countries. Conservative strategies, such as delayed introduction of enteral feeds, may confer substantial nutritional disadvantage in settings with less technologically developed healthcare provision where adjunctive parenteral nutrition is not readily and safely available. In some low‐ or middle‐income countries where severe infection (diarrhoea, pneumonia, septicaemia) is a much more important cause of mortality and morbidity, the nutritional and immunological advantages of early feeding, particularly with breast milk, may outweigh any risks associated with enteral feeding for very preterm or VLBW infants (Narayanan 1981; de Silva 2004). We identified two feasibility trials undertaken in India in the late 2000s/early 2010s that compared exclusive enteral feeding (no parenteral fluid) from birth with gradual introduction of enteral feeds over several days in VLBW infants with birth weight greater than 1000 g (Sanghvi 2013; Chetry 2014). While these trials were not eligible for inclusion in this review, neither found evidence of an effect on NEC or other adverse outcomes.
Quality of the evidence
The included trials were generally of reasonable methodological quality but, in common with other trials of feeding interventions in this population, it was not possible to mask carers and clinical assessors to the nature of the intervention. Although the lack of blinding may have resulted in surveillance and ascertainment biases, this is more likely to have caused an underestimation of the incidence of NEC in infants whose enteral feeding was delayed. The assessment of abdominal radiographs was masked in three studies to ensure that the diagnosis of stage II/III NEC (confirmed by the radiological detection of gas in the bowel wall or portal tract) was not prone to bias. However, since the microbial generation of gas in the bowel wall is substrate dependent, infants who received more enteral milk (substrate) may have been more likely to demonstrate this radiological sign than infants with equally severe bowel disease who had less intraluminal substrate. This 'substrate effect' is also more likely to cause under‐ascertainment of NEC in the infants whose enteral feeding was delayed (Tyson 2007).
Potential biases in the review process
The definition of delayed introduction of progressive feeds may vary between different subpopulations of very preterm or VLBW infants who have different empiric risks for developing feed intolerance and NEC. The effects of enteral feeding are likely to be very different for a mechanical ventilator‐dependent or inotrope‐dependent infant of birth weight less than 700 g compared with a clinically stable infant of birth weight greater than 1400 g. For this Cochrane review, we defined delayed introduction as later than four days after birth since some observational studies have found the risk of NEC to be lower when feeds are introduced five to seven days after birth (Patole 2005). For ELBW or extremely preterm infants, it may be more appropriate to define delayed introduction as more than seven days after birth (or even later). Small‐intestinal motility is poorly organised before about 28 weeks' gestation resulting in a higher risk of feed intolerance. In addition, enteral feeds are often delayed in this population because of respiratory or metabolic instability or because of other putative risk factors for NEC, such as the existence of a patent ductus arteriosus, the use of non‐steroidal anti‐inflammatory drugs or the presence of an umbilical arterial catheter (Boyle 2004).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.1 Necrotising enterocolitis.

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.2 Mortality prior to discharge.

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.3 Feed intolerance.

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.5 Duration of hospital admission (days).

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 1 Necrotising enterocolitis.

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 2 Mortality prior to discharge.

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 3 Feed intolerance.

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 4 Incidence of invasive infection.
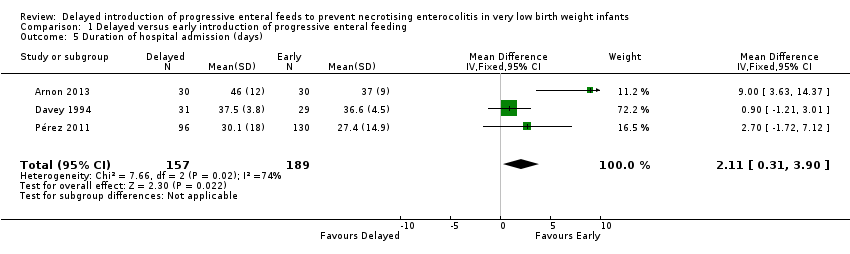
Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 5 Duration of hospital admission (days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Necrotising enterocolitis Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All trials | 8 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.34] |
| 1.2 Trials of infants with intrauterine growth restriction or abnormal antenatal Doppler flow velocities | 4 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.54, 1.41] |
| 2 Mortality prior to discharge Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 All trials | 7 | 967 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.75, 1.88] |
| 2.2 Trials of infants with intrauterine growth restriction or abnormal antenatal Doppler flow velocities | 3 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.55, 2.05] |
| 3 Feed intolerance Show forest plot | 3 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.62, 1.15] |
| 4 Incidence of invasive infection Show forest plot | 2 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.95, 1.70] |
| 5 Duration of hospital admission (days) Show forest plot | 3 | 346 | Mean Difference (IV, Fixed, 95% CI) | 2.11 [0.31, 3.90] |

