Introducción tardía de la alimentación enteral progresiva para la prevención de la enterocolitis necrosante en lactantes de muy bajo peso al nacer
Resumen
Antecedentes
Con frecuencia la alimentación enteral en lactantes muy prematuros o de muy bajo peso al nacer (MBPN) se retrasa varios días desde el nacimiento por preocupaciones de que la introducción temprana de la alimentación no sea tolerada y pueda aumentar el riesgo de enterocolitis necrosante. Sin embargo, existen dudas sobre si retrasar la alimentación enteral podría disminuir la adaptación funcional del sistema digestivo y prolongar la necesidad de nutrición parenteral con sus consiguientes riesgos infecciosos y metabólicos.
Objetivos
Determinar los efectos de la introducción tardía de la alimentación enteral progresiva sobre el riesgo de enterocolitis necrosante, mortalidad y otras morbilidades en lactantes muy prematuros o de MBPN.
Métodos de búsqueda
Un documentalista en consulta con los autores de la revisión desarrolló las estrategias de búsqueda. Se hicieron búsquedas en las siguientes bases de datos en octubre de 2021 sin restricciones de idioma ni fecha: CENTRAL (2021, número 10), MEDLINE vía OVID (1946 a octubre de 2021), EMBASE vía OVID (1974 a octubre de 2021), Maternity and Infant Care vía OVID (1971 a octubre de 2021), CINAHL vía OVID (1982 a octubre de 2021). También se realizaron búsquedas de ensayos elegibles en bases de datos de ensayos clínicos, resúmenes de congresos y en las listas de referencias de artículos identificados.
Criterios de selección
Ensayos controlados aleatorizados que evaluaran los efectos de la introducción tardía (á partir de cuatro días desde el nacimiento) versus más temprana de la alimentación enteral progresiva en la enterocolitis necrosante, la mortalidad y otras morbilidades en lactantes muy prematuros o de MBPN.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron por separado el riesgo de sesgo de los ensayos, extrajeron los datos y resumieron las estimaciones del efecto mediante la razón de riesgos (RR), la diferencia de riesgos (DR) y la diferencia de medias. Se utilizó el método GRADE para evaluar la certeza de la evidencia de los efectos sobre la enterocolitis necrosante, la mortalidad, la intolerancia alimentaria y la infección invasiva.
Resultados principales
Se incluyeron 14 ensayos en los que participaron un total de 1551 lactantes. Las posibles fuentes de sesgo fueron la falta de claridad sobre la metodología para generar las secuencias aleatorias y ocultar la asignación en la mitad de los ensayos, así como la falta de cegamiento de los cuidadores o los investigadores en todos los ensayos. Los ensayos solían definir la introducción tardía de la alimentación enteral progresiva como posterior a cuatro a siete días después del nacimiento y la introducción temprana como menos de cuatro días después del nacimiento. Los lactantes de seis ensayos (que corresponden a cerca de la mitad del total de participantes) tuvieron restricción del crecimiento intrauterino o redistribución circulatoria, observado por ausencia o inversión de velocidades del flujo diastólico final en la aorta fetal o la arteria umbilical.
Los metanálisis mostraron que retrasar la introducción de la alimentación enteral progresiva podría no reducir el riesgo de enterocolitis necrosante (razón de riesgos [RR] 0,81; intervalo de confianza [IC] del 95%: 0,58 a 1,14; diferencia de riesgos [DR] ‐0,02; IC del 95%: ‐0,04 a 0,01; 13 ensayos, 1507 lactantes; evidencia de certeza baja por riesgo de sesgo e imprecisión) ni la mortalidad por todas las causas antes del alta hospitalaria (RR 0,97; IC del 95%: 0,70 a 1,36; DR ‐0,00; IC del 95%: ‐0,03 a 0,03; 12 ensayos, 1399 lactantes; evidencia de certeza baja por riesgo de sesgo e imprecisión). Retrasar la introducción de la alimentación enteral progresiva podría reducir ligeramente el riesgo de intolerancia alimentaria (RR 0,81; IC del 95%: 0,68 a 0,97; DR ‐0,09; IC del 95%: ‐0,17 a ‐0,02; número necesario a tratar para un desenlace beneficioso adicional = 11; IC del 95%: 6 a 50; seis ensayos, 581 lactantes; evidencia de certeza baja por riesgo de sesgo e imprecisión) y probablemente aumenta el riesgo de infección invasiva (RR 1,44; IC del 95%: 1,15 a 1,80; DR 0,10; IC del 95%: 0,04 a 0,15; número necesario a tratar para un desenlace perjudicial = 10; IC del 95%: 7 a 25; siete ensayos, 872 lactantes; evidencia de certeza moderada por riesgo de sesgo).
Conclusiones de los autores
Retrasar la introducción de la alimentación enteral progresiva más allá de los cuatro días después del nacimiento (en comparación con una introducción más temprana) podría no reducir el riesgo de enterocolitis necrosante ni la muerte en lactantes muy prematuros o de MBPN. Retrasar la introducción podría reducir ligeramente la intolerancia alimentaria y probablemente aumenta el riesgo de infección invasiva.
PICO
Resumen en términos sencillos
Introducción tardía de la alimentación enteral progresiva para la prevención de la enterocolitis necrosante en lactantes de muy bajo peso al nacer
Antecedentes
Los recién nacidos muy prematuros (que nacen con más de ocho semanas de antelación) o de muy bajo peso (MBPN; menos de 1500 gramos) están en riesgo de presentar un trastorno intestinal grave llamado enterocolitis necrosante (en el que el intestino se inflama y muere). Se cree que los recién nacidos cuyo crecimiento en el útero está afectado tienen un alto riesgo de sufrir enterocolitis necrosante. Los recién nacidos muy prematuros o de MBPN se alimentan inicialmente con cantidades bajas de leche, que se aumentan gradualmente a lo largo de varios días. Retrasar la introducción y el aumento del volumen de las tomas de leche varios días (o más) después del nacimiento podría ser una forma de reducir el riesgo de esta enfermedad.
Características de los estudios
Se buscaron ensayos clínicos que evaluaran el efecto de la introducción tardía (más de cuatro días después del nacimiento) versus la introducción más temprana de las tomas de leche (en las que la leche materna o artificial se introduce directamente por un tubo en el estómago) en el riesgo de enterocolitis necrosante, muerte y en la salud general en los recién nacidos muy prematuros o de MBPN. La búsqueda está actualizada hasta octubre de 2021.
Resultados clave
Se encontraron 14 ensayos con 1551 recién nacidos participantes. Cerca de la mitad de estos recién nacidos presentaban evidencias de una afectación del crecimiento mientras estaban en el útero. El análisis combinado de estos ensayos muestra que la introducción tardía de la alimentación enteral progresiva podría no reducir el riesgo de enterocolitis necrosante o de muerte. Retrasar la alimentación podría reducir ligeramente el riesgo de intolerancia alimentaria, pero probablemente aumenta el riesgo de que se produzca una infección grave.
Conclusiones y certeza de la evidencia
Esta revisión proporciona evidencia de certeza baja de que retrasar la introducción de la alimentación enteral podría no reducir el riesgo de enterocolitis necrosante ni la muerte en lactantes muy prematuros o de MBPN, incluidos los recién nacidos cuyo crecimiento en el útero estuvo afectado.
Authors' conclusions
Summary of findings
| Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very preterm or very low birth weight infants | |||||
| Patient or population: very preterm (< 32 weeks' gestation) or very low birth weight (< 1500 g) infants | |||||
| Outcomes | Anticipated absolute effects* | Relative effect | No. of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with early introduction | Risk with delayed introduction | ||||
| Necrotising enterocolitis prior to hospital discharge | 85 per 1000 | 69 per 1000 (95% CI 49 to 97) | RR 0.81 (95% CI 0.58 to 1.14] | 1507 (13) | ⊕⊕⊝⊝ |
| Mortality prior to hospital discharge | 84 per 1000 | 81 per 1000 (95% CI 59 to 114) | RR 0.97 (95% CI 0.70 to 1.36) | 1399 (12) | ⊕⊕⊝⊝ |
| Feed intolerance prior to hospital discharge | 461 per 1000 | 374 per 1000 (95% CI 314 to 447) | RR 0.81 (95% CI 0.68 to 0.97) | 581 (6) | ⊕⊕⊝⊝ |
| Invasive infection prior to hospital discharge | 266 per 1000 | 383 per 1000 (95% CI 306 to 479) | RR 1.44 (95% CI 1.15 to 1.80) | 872 (7) | ⊕⊕⊕⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group certainty of evidence
| |||||
| aDowngraded one level for serious study limitations (risk of bias due to lack of masking of clinicians, caregivers, and investigators in trials) bDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial benefit or harm) cDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial benefit or slight/no benefit) | |||||
Background
Description of the condition
Necrotising enterocolitis (NEC), a syndrome of acute intestinal necrosis of unknown aetiology, affects about 5% of very preterm (< 32 weeks' gestation) or very low birth weight (VLBW) (< 1500 g) infants (Horbar 2012; Samuels 2017). Intrauterine growth restriction may be an additional risk factor, especially if associated with circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in Doppler studies of the fetal aorta or umbilical artery (Bernstein 2000; Garite 2004; Kamoji 2008). Infants who develop NEC experience more infections, have lower levels of nutrient intake, grow more slowly, and have longer durations of intensive care and hospital stay than gestation‐comparable infants who do not develop NEC (Battersby 2018; Berrington 2012). The associated mortality rate is more than 20%, and infants who develop NEC, especially if associated with bloodstream infections, have a higher risk of developmental delay and neurodisability compared with their unaffected peers (Hickey 2018; Shah 2012).
Most very preterm or VLBW infants who develop NEC have received enteral milk feeds. Feeding with human milk rather than cow milk formula reduces the risk of NEC (Quigley 2019). Other differences in enteral feeding regimens, such as the timing of introduction of feeds and the size of daily feeds volume increments, may also contribute to inter‐unit variation in the incidence of NEC (Walsh 2019). Observational studies have suggested that delaying the introduction of enteral feeds beyond the first few days after birth, or increasing the volume of feeds by less than about 24 mL/kg body weight each day, is associated with a lower risk of developing NEC in very preterm or VLBW infants (Henderson 2009; Patole 2005).
Description of the intervention
Oral feeding for very preterm or VLBW infants is not usually possible because of neurological immaturity or respiratory distress challenging breathing, sucking, and swallowing coordination (Viswanathan 2019). Consequently, most very preterm or VLBW infants receive milk via a gastric feeding tube. The timing of introduction of milk feeds, and the rate of advancement of feed volumes, is determined and monitored by clinicians and care‐givers. Substantial variation in early enteral feeding practices for very preterm or VLBW infants exists (Hay 2018). In high‐income countries, clinical policy and practice has tended to favour a conservative approach to introducing enteral feeds because of concerns about adverse effects including gastro‐oesophageal reflux and aspiration of stomach contents, and whether early feeding might increase the risk of NEC (de Waard 2018). One commonly recommended and widely used approach is to limit any enteral feeding to 'trophic' levels (minimal enteral nutrition) during the first few days after birth, and to delay introducing progressive enteral feeding (beyond trophic levels) until clinicians are reassured that the trophic feeding volumes are well‐tolerated and absorbed (Klingenberg 2012). In many low‐ and middle‐income countries with fewer resources for neonatal care, practice has tended to be more pragmatic and to favour early introduction and advancement of enteral feeds (often facilitated by 'kangaroo' mother care) for clinically stable very preterm or VLBW infants (Conde‐Agudelo 2016).
How the intervention might work
Delaying the introduction of milk feeds aims to reduce the risk of feed intolerance (inability to absorb and digest milk) and NEC by limiting the physiological and metabolic stresses on the immature gastrointestinal tract during the first few days after birth. Potential disadvantages, however, are associated with this conservative approach to early enteral feeding (Flidel‐Rimon 2004; Leaf 2013). Because gastrointestinal hormone secretion and motility are stimulated by milk feeds, delayed introduction of progressive enteral feeds may delay the functional adaptation of the gastrointestinal tract and disrupt the patterns of microbial colonisation (Burrin 2002; Embleton 2017). Intestinal dysmotility and dysbiosis might exacerbate feed intolerance and delay the establishment of enteral feeding independently of parenteral nutrition (Pammi 2017). Prolonging the duration of parenteral nutrition is associated with infectious and metabolic complications that increase mortality and morbidity, prolong hospital stay, and adversely affect growth and development (Embleton 2013; el Manouni el Hassani 2019). It has been argued that the risk of NEC should not be considered in isolation from these other potential clinical outcomes when evaluating enteral feeding practices for very preterm or VLBW infants (Flidel‐Rimon 2006; Hartel 2009).
Why it is important to do this review
Given the potential for the timing of the introduction of progressive enteral feeding to affect important outcomes for very preterm or VLBW infants, it is important to identify, appraise, and synthesise available evidence from randomised controlled trials to inform practice and research. This review focuses on the comparison of delayed versus earlier introduction of progressive enteral feeding; that is, advancing the volume of milk feeds beyond minimal enteral nutrition levels. Other Cochrane Reviews have assessed the evidence for the effect of prolonged minimal enteral nutrition (restricting feed volumes to trophic levels) versus a period of enteral fasting, and different rates advancement of enteral feed volumes (including full enteral feeding from birth) on the risk of NEC and mortality in very preterm or VLBW infants (Morgan 2013; Oddie 2017; Walsh 2020).
Objectives
To determine the effectiveness of delayed introduction of progressive enteral feeds on reducing the risk of NEC, mortality and other morbidities in very preterm or VLBW infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), quasi‐RCTs or cluster‐RCTs.
Types of participants
VLBW (< 1500 g) or very preterm (< 32 weeks' gestation) newborn infants.
If studies included some infants of > 32 weeks' gestation and > 1500 g birth weight (and subgroup data were not provided), we included data if they had enrolled a majority (> 50%) of very preterm or VLBW infants.
Types of interventions
Delayed introduction (four or more days after birth) of progressive enteral feeds versus earlier introduction of enteral feeds. We defined progressive enteral feeding as the intention to advance feed volumes in excess of minimal enteral nutrition levels (24 mL/kg/day) within five days of commencement or by one week after birth.
Infants in each group should have received the same type of milk (breast milk or formula), the same route and mode of feeding (intragastric or transpyloric, bolus gavage or continuous) and the same rate of feed volume advancement in both groups.
Types of outcome measures
We focused on assessing effects on infant‐ and family‐important outcomes, principally neonatal morbidities that plausibly affect rates of mortality or neurodevelopmental impairment or disability.
Primary outcomes
-
NEC confirmed at surgery or autopsy or using standardised clinical and radiological criteria (VON 2020):
-
at least one of: bilious gastric aspirate or emesis; or abdominal distention; or blood in stool; and
-
at least one of: abdominal radiograph showing pneumatosis intestinalis; or gas in the portal venous system; or free air in the abdomen.
-
-
All‐cause mortality before discharge from hospital
Secondary outcomes
-
Growth
-
Time to regain birth weight and rates of weight gain, linear growth, head growth, or skinfold thickness growth up to six months (corrected for preterm birth)
-
Long‐term growth: weight, height, or head circumference (or proportion of infants who remained below the 10th percentile for the index population's distribution) assessed at intervals from six months of age
-
-
Neurodevelopmental disability defined as one or more of: moderate or severe developmental delay (> two standard deviations (SD) below the mean of standardised infant developmental assessment aged > 18 months), and classifications of disability, including non‐ambulant cerebral palsy and auditory or visual impairment
-
Time to establish full enteral feeding independently of parenteral nutrition
-
Time to establish oral feeding (independently of parenteral nutrition or enteral tube feeding, or both)
-
Feed intolerance (requirement to cease enteral feeds > 4 hours)
-
Invasive infection confirmed by culture of bacteria or fungus from blood, cerebrospinal fluid, or another normally sterile body space
-
Duration of hospital stay (days)
Search methods for identification of studies
An information specialist developed search strategies in consultation with the authors. Search strategies used three conceptual approaches:
-
enteral nutrition terms and neonate;
-
necrotising enterocolitis and prevention and neonate;
-
parenteral nutrition and adverse effects and neonate.
The neonatal terms are a standardised set developed by Cochrane Neonatal.
A methodological filter was used to limit retrieval to RCTs.
Electronic searches
The following databases were searched in October 2021 without language, publication year, publication status, or publication type restrictions:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 10) (Appendix 1)
-
MEDLINE via OVID (1946 to October 2021) (Appendix 2)
-
Embase via OVID (1974 to October 2021) (Appendix 3)
-
Maternity and Infant Care via OVID (1971 to October 2021) (Appendix 4)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to October 2021) (Appendix 5)
We searched the US National Library of Medicine trial registry (ClinicalTrials.gov) for ongoing or recently completed trials (Appendix 6).
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review.
Data collection and analysis
We used the standard methods of Cochrane Neonatal (neonatal.cochrane.org/).
Selection of studies
WM screened titles and abstracts of all records identified by the search and coded records as “order” or “exclude". A second review author (LY or SO) assessed all records coded as “order” and made the final decision about which records should be ordered as full‐text articles. Two review authors read the full texts and used a checklist to assess each article's eligibility for inclusion on the basis of prespecified inclusion and exclusion criteria.
Data extraction and management
WM and LY extracted data independently using a data collection form to aid extraction of information on design, methods, participants, interventions, outcomes, and treatment effects from each included study. We discussed disagreements until we reached consensus. If data from trial reports were insufficient, we contacted trialists to ask for further information.
Assessment of risk of bias in included studies
Two review authors (WM and SO) independently assessed risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011), for the following domains.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 7 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). When we deemed it appropriate to combine two or more study arms, we obtained treatment effects from combined data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We determined the number needed to treat for an additional beneficial outcome (NNTB) or harmful outcome (NNTH) for a statistically significant difference in RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. For cluster‐randomised trials (had we identified any for inclusion), we planned to undertake analyses at the level of the individual while accounting for clustering in the data by using methods recommended in the Cochrane Handbook (Higgins 2020).
Dealing with missing data
We requested additional data from trial investigators when data on important outcomes were missing or were reported unclearly. When data remained missing, we examined the impact on effect size estimates by performing sensitivity analyses.
Assessment of heterogeneity
We examined treatment effects in individual trials and heterogeneity between trial results by inspecting forest plots if more than one trial was included in a meta‐analysis. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate or high (I² > 50%) levels of heterogeneity, we explored possible causes by performing subgroup analyses.
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary outcomes and secondary outcomes and reported outcomes. Where study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. Studies using the interventions in a potentially eligible infant population but not reporting on any of the primary and secondary outcomes were documented in the Characteristics of included studies tables. We used the funnel plots to screen for publication bias where there were a sufficient number of studies (at least 10) reporting the same outcome. If publication bias was suggested by substantial asymmetry of the funnel plot on visual assessment, we planned to assess this statistically use Harbord's modification of Egger's test (Harbord 2006).
Data synthesis
We used a fixed‐effect model inverse variance meta‐analysis for combining data where trials examined the same intervention and the populations and methods of the trials were judged to be similar.
Subgroup analysis and investigation of heterogeneity
We pre‐specified subgroup analyses for primary outcomes to compare effects in trials:
-
in which most infants were exclusively formula‐fed versus trials in which most infants were exclusively or partially fed with human milk (maternal or donor);
-
in which most participants were extremely low birth weight (ELBW; < 1000 g) or extremely preterm (< 28 weeks' gestation at birth) versus trials in which most infants were ≥ 28 weeks' gestation at birth or of birth weight ≥ 1000 g; and
-
which restricted participation to infants with intrauterine growth restriction or absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery versus trials which did not do so.
Sensitivity analysis
We planned to perform sensitivity analyses if:
-
there was unexplained high heterogeneity (I² > 75%) (explored by removing the outlying trial or trials);
-
a trial with high risk of bias (including high level of missing outcome data) was included in the meta‐analysis of an outcome where the other studies had low risk of bias (removed the study with high risk of bias).
Summary of findings and assessment of the certainty of the evidence
Two review authors (WM and LY) used the GRADE approach, as outlined in the GRADE handbook (Schünemann 2013; Walsh 2021), to assess the certainty of the evidence for effects on infant‐ and family‐important outcomes, principally NEC, all‐cause mortality, feed intolerance, and invasive infection. We included these four outcomes in summary of findings Table 1.
Two review authors (WM and LY) independently assessed the certainty of the evidence for each of the outcomes above. We initially considered evidence from RCTs to be of high certainty. We downgraded this certainty by one level for serious limitations or by two levels for very serious limitations based on five GRADE criteria: design weakness (risk of bias), inconsistency across studies, indirectness, imprecision of estimates, and presence of publication bias. We used the GRADEpro GDT software to create summary of findings Table 1 for reporting the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence for a given outcome as one of four grades.
-
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
After the removal of duplicates from the search results, we screened 6450 titles and abstracts, which included forward and backward citation searches, clinical trials registers and grey literature. We evaluated 21 new articles sourced as full‐text reports. We included five of these new trials alongside the nine previously included trials (Figure 1).
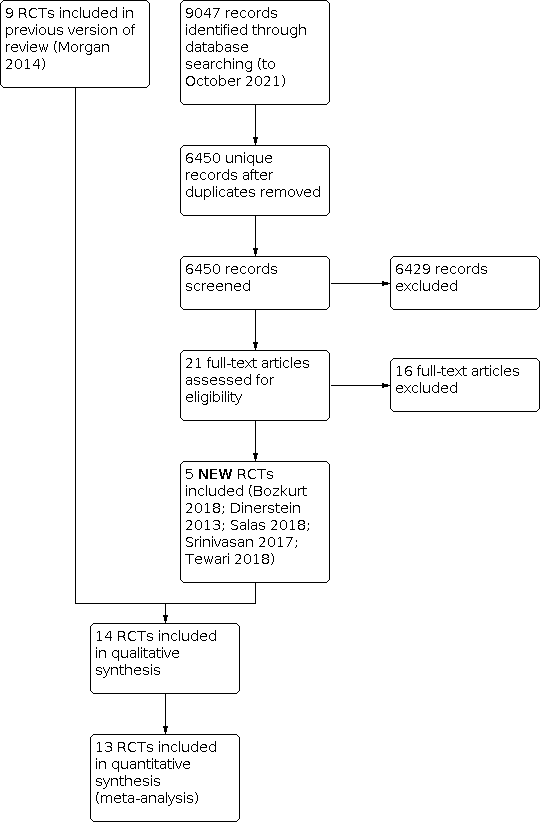
Study flow diagram: review update.
Included studies
Fourteen trials fulfilled the review eligibility criteria (Abdelmaaboud 2012; Armanian 2013; Arnon 2013; Bozkurt 2020; Davey 1994; Dinerstein 2013; Karagianni 2010; Khayata 1987; Leaf 2012; Ostertag 1986; Pérez 2011; Salas 2018; Srinivasan 2017; Tewari 2018). See Characteristics of included studies.
Population
A total of 1551 infants participated in the included trials.
Three small trials were undertaken in neonatal care centres in North America during the 1980s and early 1990s.
-
Davey 1994: 62 clinically stable preterm infants of birth weight less than 2000 grams who had a low umbilical artery catheter in place (most participants were of birth weight less than 1500 grams or gestational age less than 32 weeks).
-
Khayata 1987: 12 VLBW infants.
-
Ostertag 1986: 38 VLBW infants assessed to be at high risk of developing NEC.
The more recent trials were performed in neonatal care centres in various countries during the 2000s to 2010s.
-
Abdelmaaboud 2012: single‐centre study in Qatar, 125 preterm infants with intrauterine growth restriction and abnormal Doppler flow patterns on ultrasound of the umbilical artery (most participants were of birth weight less than 1500 grams).
-
Armanian 2013: single‐centre study in Iran, 82 VLBW infants.
-
Arnon 2013: single‐centre study in Israel, 60 small for gestational age preterm infants (most participants were of birth weight less than 1500 grams).
-
Bozkurt 2020: single‐centre study in Turkey, 229 preterm infants with birth weight less than 1251 grams.
-
Dinerstein 2013: single‐centre study in Argentina, 62 appropriate for gestation preterm infants (< 31 weeks' gestation).
-
Karagianni 2010: single‐centre study in Greece, 84 infants less than 35 weeks' gestation with a birth weight less than 10th percentile and evidence of abnormal fetal blood flow patterns on Doppler ultrasound of the umbilical artery.
-
Leaf 2012: 54‐centre trial in the UK and Ireland, 404 infants: (a) less than 35 weeks' gestation, (b) birth weight less than 10th percentile and (c) evidence of abnormal fetal blood flow patterns on Doppler ultrasound studies. Since most participants were of birth weight less than 1500 grams, we made a consensus decision to include the trial.
-
Pérez 2011: single‐centre study in Colombia, 239 very preterm or VLBW infants.
-
Salas 2018: single‐centre study in the USA, 60 preterm infants (< 29 weeks' gestation), appropriate weight for gestation.
-
Srinivasan 2017: single centre study in India, 32 preterm infants with evidence of intrauterine growth restriction associated with absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery.
-
Tewari 2018: single centre study in India, 62 preterm (27 to 32 weeks' gestation) infants with absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery.
In six trials (accounting for about half of the total participants), all participating infants had evidence of intrauterine growth restriction or circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery (Abdelmaaboud 2012; Arnon 2013; Davey 1994; Karagianni 2010; Leaf 2012; Srinivasan 2017). We included these trials since most (> 50%) of the infant were of < 32 weeks' gestational age at birth or of birth weight < 1500 g.
Interventions/comparisons
Trials typically defined delayed introduction of progressive enteral feeds as later than day four to day seven after birth. Early feeding varied from day one to day four after birth.
In nine trials, infants received expressed human milk or artificial formula or both (Abdelmaaboud 2012; Armanian 2013; Arnon 2013; Bozkurt 2020; Davey 1994; Karagianni 2010; Leaf 2012; Pérez 2011; Salas 2018). Infants in three of the trials received only expressed maternal milk or donor human milk (Dinerstein 2013; Srinivasan 2017; Tewari 2018). In two trials, infants received only formula (Ostertag 1986; Khayata 1987).
Infants received enteral feeds by gavage at one‐ or two‐hourly intervals in all the trials except Ostertag 1986, where infants received feeds by continuous intragastric infusion. Most trials specified criteria and indications for advancing (daily increments of 15 to 30 mL/kg) or interrupting enteral feed (e.g. residual gastric contents not greater than 3 to 5 mL or one‐third to one‐half of the previous feed volume, frequent vomiting, abdominal distention or detection of blood in the stools).
Outcomes
All the trials except Khayata 1987 reported NEC (stage II/III modified Bell criteria; confirmed radiologically or at surgery or autopsy). Other reported outcomes included mortality, time to establish full enteral feeding, invasive infection and duration of hospital stay. None of the trials assessed long term growth or neurodevelopment.
Excluded studies
We excluded 16 reports after full‐text screening (see Characteristics of excluded studies table). Several studies were excluded for design reasons (not randomised), and most trials were excluded either because both groups received early introduction of progressive enteral feeds, or the primary comparison was rate of feed volume advancement rather than timing of introduction.
Characteristic of studies awaiting classification
There is one study awaiting classification (Li 2016).
Risk of bias in included studies
'Risk of bias' assessments are described in the Characteristics of included studies table. The methodological quality of the included trials was generally high, but the nature of the intervention meant that parents, caregivers, or clinical investigators were aware of each infant's allocated feeding group (Figure 2).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In half of the trial reports, methods to ensure adequate allocation concealment were not described. The other trials employed adequate methods to generate random sequences (typically computer‐generated) and to ensure adequate allocation concealment (typically using sealed opaque envelopes).
Blinding
None of the included trials was able to mask feeding strategies from parents, caregivers, or clinical investigators (though some may have masked assessment of abdominal radiographs for diagnosis of NEC). All the trials were assessed as being at high risk of performance or detection bias.
Incomplete outcome data
All trials reported complete or near‐complete assessments of primary outcomes (low risk of attrition bias).
Selective reporting
Although trial protocols were not available for most trials, selective reporting bias was not considered a major threat given that all relevant clinical outcomes were reported.
Other potential sources of bias
We did not find evidence of important between‐group baseline differences in participant characteristics or demographics in any other trials.
Effects of interventions
See summary of findings Table 1.
Primary outcomes
Necrotising enterocolitis
Meta‐analysis of data from 13 trials (1507 infants) showed that delayed introduction of progressive enteral feeds may not reduce the risk of NEC: RR 0.81, 95% CI 0.58 to 1.14 (I² = 0%); RD ‐0.02, 95% CI ‐0.04 to 0.01 (Analysis 1.1). The funnel plot was not markedly asymmetrical (Figure 3). We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations (risk of bias) and imprecision.

Funnel plot‐ Necrotising enterocolitis
Subgroup analyses
-
We found no evidence of subgroup differences by type of milk: test for subgroup differences: Chi² = 2.45, degrees of freedom (df) = 2 (P = 0.29), I² = 18.3% (Figure 4)
-
None of the trials recruited predominantly ELBW or extremely preterm infants
-
We found no evidence of subgroup differences by trials that restricted participation to growth‐restricted infants or infants with absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery versus trials that did not: test for subgroup differences: Chi² = 0.02, df = 1 (P = 0.90), I² = 0% (Analysis 1.2 )

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.1 Necrotising enterocolitis.
Mortality
Meta‐analysis of data from 12 trials (1399 infants) showed that delayed introduction of progressive enteral feeds may not affect the risk of death before hospital discharge: RR 0.97, 95% CI 0.70 to 1.36 (I² = 0%); RD ‐0.00, 95% CI ‐0.03 to 0.03 (Analysis 1.3).
The funnel plot was not markedly asymmetrical (Figure 5). We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations and imprecision.

Funnel plot‐ mortality prior to discharge
Subgroup analyses
-
We found no evidence of subgroup differences by type of milk: test for subgroup differences: Chi² = 2.05, df = 2 (P = 0.36), I² = 2.2% (Figure 6).
-
None of the trials recruited predominantly ELBW or extremely preterm infants.
-
We found no evidence of subgroup differences by trials that restricted participation to growth‐restricted infants or infants with absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery versus trials that did not: test for subgroup differences: Chi² = 0.84, df = 1 (P = 0.36), I² = 0% (Analysis 1.4).

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding (all trials), outcome: 1.3 Mortality prior to discharge.
Secondary outcomes
Growth
Four trials reported the median time to regain birth weight, and none of these showed a between‐group difference (Abdelmaaboud 2012; Bozkurt 2020; Davey 1994; Tewari 2018). The data available were insufficient for meta‐analysis. Three trials reported rate of weight gain during the trial period. Two did not show a between‐group difference (Khayata 1987; Pérez 2011). Bozkurt 2020 reported that infants in the delayed introduction group had a slower rate of weight gain (15 g/day versus 19 g/day). The data available were insufficient for meta‐analysis.
None of the other trials reported growth parameters.
Neurodevelopment
None of the trials assessed neurodevelopmental outcomes.
Time to establish full enteral feeding
The median time to establish full enteral feeding was longer in the delayed introduction group in the included trials:
-
Abdelmaaboud 2012: two days
-
Armanian 2013: five days
-
Arnon 2013: three days
-
Bozkurt 2020: two days
-
Davey 1994: three days
-
Dinerstein 2013: two days
-
Karagianni 2010: three days
-
Khayata 1987: data not reported
-
Leaf 2012: three days
-
Ostertag 1986: data not reported
-
Pérez 2011: four days
-
Salas 2018: two days
-
Srinivasan 2017: four days
-
Tewari 2018: 5.5 days (extremely preterm), four days (very preterm)
The reports did not provide data (mean and SD) in a form to allow meta‐analysis.
Time to establish full oral feeding
None of the trials assessed time to establish full oral feeding.
Feed intolerance
Meta‐analysis of data from six trials (581 infants) showed that delayed introduction of progressive enteral feeds slightly reduces the risk of feed intolerance: RR 0.81, 95% CI 0.68 to 0.97 (I² = 18%); RD ‐0.09, 95% CI ‐0.17 to ‐0.02; NNTB 11, 95% CI 6 to 50 (Analysis 1.5). We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations and imprecision.
One trial did not detect a difference, but the report did not provide data to allow quantitative synthesis (Davey 1994).
Invasive infection
Meta‐analysis of data from seven trials (872 infants) showed that delayed introduction of progressive enteral feeds probably increases the risk of invasive infection: RR 1.44, 95% CI 1.15 to 1.80 (I² = 0%); RD 0.10, 95% CI 0.04 to 0.15; NNTH 10, 95% CI 7 to 25 (Analysis 1.6). We assessed the certainty of evidence as moderate using GRADE methods, downgraded for serious study design limitations.
Duration of hospital stay
Meta‐analysis from four trials (368 infants) showed a longer duration of hospitalisation in the delayed feeding group: MD 4.57 days, 95% CI 1.53 to 7.61; I² = 24% (Analysis 1.7).
Another three trials did not show an effect, but the reports did not provide data to allow quantitative synthesis (Abdelmaaboud 2012; Leaf 2012; Tewari 2018).
Sensitivity analyses for heterogeneity or risk of bias
We had planned sensitivity analyses for high heterogeneity (I² > 75%) and for risk of bias. However, none of the pre‐specified meta‐analyses contained high levels of heterogeneity, nor did any include data from a trial with high risk of bias where the other studies had low risk of bias.
Discussion
Summary of main results
The trial data included in this review provide low‐certainty evidence that delaying the introduction of progressive enteral feeds beyond about four days after birth may not reduce the risk of NEC in very preterm or VLBW infants. The boundaries of the 95% CI for the estimate of effect are consistent with either two fewer cases or three more cases of NEC in every 100 infants who have delayed introduction of progressive enteral feeds. Meta‐analysis of data from these trials did not show evidence of an effect on all‐cause mortality, with the 95% CI boundaries being consistent with either three fewer or three more deaths in every 100 infants who had delayed introduction of progressive enteral feeds. Prespecified analyses did not show subgroup effects on risk of NEC or death among infants with growth restriction or evidence of absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery. Meta‐analysis showed that delayed introduction of progressive enteral feeds may result in a slight reduction in feed intolerance. Data from seven trials showed a higher risk of late‐onset infection among infants who had delayed introduction of progressive enteral feeds. The point estimate suggests that an extra episode of late‐onset infection occurs for every 10 infants who have delayed rather than early introduction of progressive enteral feeds. None of the included trials has reported effects on long term growth or neurodevelopmental outcomes.
Overall completeness and applicability of evidence
These data are relevant to current practice since most of the included trials were conducted since the year 2000. Six of these trials (767 infants) specifically recruited infants thought to be at high risk of developing NEC due to intrauterine growth restriction and abnormal fetal circulatory distribution or flow. This widens the applicability of the findings since this is the population for which most clinical uncertainty and variation in practice with regard to early feeding strategies exists (Klingenberg 2012). Previously, this population of infants has been specifically excluded from participating in many trials of early enteral feeding practices (Tyson 2007).
Artificial formula feeding increases the risk NEC (Quigley 2019). The risk‐benefit balance of enteral feeding strategies may differ between human milk‐fed and formula‐fed very preterm or VLBW infants (Young 2020). Subgroup analyses at trial‐level by type of milk did not show evidence of differences in effect. Most trials, however, included infants who received either human milk or cow milk formula or both, and subgroup data were not reported. If such subgroup data were available, an individual participant‐level meta‐analysis could be conducted to explore this issue further. It is also unclear whether the findings can be applied to infants who receive continuous infusion of intragastric feeds, as most of the infants in the included trials received enteral feeds as interval gastric boluses. Randomised controlled trials have reported conflicting findings about the effect on continuous enteral infusion on feed tolerance in VLBW (and especially ELBW) infants (Premji 2021).
The included trials were mainly undertaken in neonatal care centres in middle‐ or high‐income countries. It is less clear how applicable this evidence is to neonatal care practices in low‐income countries. Conservative strategies, such as delayed introduction of enteral feeds, may confer substantial nutritional disadvantage in settings with less technologically developed healthcare provision where adjunctive parenteral nutrition is not readily and safely available (Akindolire 2020). In some low‐ or middle‐income countries where severe infection is a much more important cause of mortality and morbidity, the nutritional and immunological advantages of early feeding, particularly with breast milk, may outweigh any risks associated with enteral feeding for very preterm or VLBW infants (de Silva 2004). A recent Cochrane Review has assessed the data from six trials undertaken in India since the late 2000s that compared exclusive enteral feeding (no parenteral fluid) from birth with gradual introduction of enteral feeds over several days in VLBW infants with birth weight greater than 1000 grams (Walsh 2020). While these trials were not eligible for inclusion in this review, none found evidence of an effect on NEC or other adverse outcomes.
Quality of the evidence
The GRADE‐assessed certainty (quality) of evidence for primary outcomes was downgraded because of lack of masking in the included trials and imprecision of estimates of effect (summary of findings Table 1). Although these trials were otherwise of good methodological quality, in common with other trials of feeding interventions in this population, it was not possible to mask parents, caregivers and clinical assessors to the nature of the intervention. Lack of masking may have resulted in surveillance and ascertainment biases. It is more likely, however, to have caused an overestimation of feed intolerance and NEC among infants whose feed volumes were advanced faster. Assessment of abdominal radiographs for signs of NEC was masked in some trials to try to ensure that the diagnosis of severe NEC (confirmed by radiological detection of gas in the bowel wall or portal tract) was not prone to bias. As microbial generation of gas in the bowel wall is substrate dependent, however, infants who received more enteral milk (substrate) may have been more likely to demonstrate this radiological sign than infants with equally severe bowel disease who had less intraluminal substrate. This 'substrate effect' is also more likely to cause over‐ascertainment of NEC among infants who had faster rates of feed volume advancement (Tyson 2007).
The other reason for downgrading the certainty of evidence was the existence of substantial imprecision in estimates of effect, with meta‐analyses generating 95% CI that included benefit as well as no benefit or harm. Although the total number of participants in the 14 included trials was more than 1500, not all trials contributed data to all outcome estimates, and estimates of effect were consequently imprecise, especially for less common outcomes including NEC and mortality.
The definition of delayed introduction of progressive feeds varies between subpopulations of very preterm or VLBW infants who have different empiric risks for developing feed intolerance and NEC. The effects of enteral feeding are likely to be very different, for example, for an inotrope‐supported infant of birth weight less than 750 grams compared with a clinically stable infant of birth weight greater than 1000 grams. For this Cochrane Review, we defined delayed introduction as later than four days after birth since some observational studies have found the risk of NEC to be lower when feeds are introduced five to seven days after birth (Patole 2005). For ELBW or extremely preterm infants, it may be more appropriate to define delayed introduction as more than seven days after birth (or even later) since small‐intestinal motility is poorly organised before about 28 weeks' gestation resulting in a high risk of feed intolerance. In addition, enteral feeds are often delayed in this population because of respiratory or metabolic instability or because of other putative risk factors for NEC, such as the existence of a patent ductus arteriosus, the use of non‐steroidal anti‐inflammatory drugs or the presence of an umbilical arterial catheter (McGuire 2004).
Potential biases in the review process
The main concern with the review process is the possibility that findings are subject to publication and other reporting biases (Hopewell 2009). Data from trials which show statistically significant or potentially important effects tend to be more readily available for inclusion in meta‐analyses (Gale 2020). Publication bias, as well as other sources of small‐study bias, can inflate effect size estimates in meta‐analyses of interventions to improve outcomes in very preterm or VLBW infants (Young 2021). The Cochrane Review of probiotics to prevent NEC in very preterm or VLBW infants, for example, shows a large reduction in the risk of NEC, but the funnel plot and regression analysis indicate that publication bias is likely to have inflated the pooled effect size estimate (Sharif 2020). We attempted to minimise this threat by screening the reference lists of included trials and related reviews and searching the proceedings of major international perinatal conferences to identify trial reports that are not published in full form in academic journals. Inspection of funnel plots of meta‐analyses that included at least 10 data points did not show sufficient asymmetry to raise concerns about possible publication or small study bias.
In six trials (accounting for about half of all the participants), all participating infants needed to have evidence of intrauterine growth restriction or circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery (Abdelmaaboud 2012; Arnon 2013; Davey 1994; Karagianni 2010; Leaf 2012; Srinivasan 2017). Most infants who participated in these trials were very preterm or VLBW infants, but subgroup data were not available. We included data from these trials since intrauterine growth restriction or circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery have been associated with a high risk of developing NEC and associated complications, and the findings are applicable to enteral feeding policies and practices (Embleton 2017).
Agreements and disagreements with other studies or reviews
This review focused specifically on the comparison of delayed versus early introduction of progressive enteral feeds. Other Cochrane Reviews have assessed how (i) enteral fasting versus trophic feeding (minimal enteral nutrition), (ii) slow versus faster rates of feed volume advancement, and (iii) early full enteral feeding versus gradual introduction of feeds affects important outcomes in very preterm or VLBW infants (Morgan 2013; Oddie 2017; Walsh 2020). These reviews, consistent with the findings of this review, have found evidence that conservative feeding strategies probably do not reduce the risk of NEC, mortality, or associated morbidity.

Study flow diagram: review update.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot‐ Necrotising enterocolitis

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.1 Necrotising enterocolitis.

Funnel plot‐ mortality prior to discharge

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding (all trials), outcome: 1.3 Mortality prior to discharge.

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 1: Necrotising enterocolitis (NEC)

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 2: NEC (subgroup analysis of infants growth‐restricted or with AREDFV)

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 3: Mortality prior to discharge

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 4: Mortality (subgroup analysis of infants growth‐restricted or with AREDFV)

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 5: Feed intolerance

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 6: Invasive infection

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 7: Duration of hospital admission (days)
| Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very preterm or very low birth weight infants | |||||
| Patient or population: very preterm (< 32 weeks' gestation) or very low birth weight (< 1500 g) infants | |||||
| Outcomes | Anticipated absolute effects* | Relative effect | No. of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with early introduction | Risk with delayed introduction | ||||
| Necrotising enterocolitis prior to hospital discharge | 85 per 1000 | 69 per 1000 (95% CI 49 to 97) | RR 0.81 (95% CI 0.58 to 1.14] | 1507 (13) | ⊕⊕⊝⊝ |
| Mortality prior to hospital discharge | 84 per 1000 | 81 per 1000 (95% CI 59 to 114) | RR 0.97 (95% CI 0.70 to 1.36) | 1399 (12) | ⊕⊕⊝⊝ |
| Feed intolerance prior to hospital discharge | 461 per 1000 | 374 per 1000 (95% CI 314 to 447) | RR 0.81 (95% CI 0.68 to 0.97) | 581 (6) | ⊕⊕⊝⊝ |
| Invasive infection prior to hospital discharge | 266 per 1000 | 383 per 1000 (95% CI 306 to 479) | RR 1.44 (95% CI 1.15 to 1.80) | 872 (7) | ⊕⊕⊕⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group certainty of evidence
| |||||
| aDowngraded one level for serious study limitations (risk of bias due to lack of masking of clinicians, caregivers, and investigators in trials) bDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial benefit or harm) cDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial benefit or slight/no benefit) | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Necrotising enterocolitis (NEC) Show forest plot | 13 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.14] |
| 1.1.1 Human milk or formula‐fed (or mixed) infants | 9 | 1313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.59, 1.24] |
| 1.1.2 Only human milk‐fed infants | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.04, 1.28] |
| 1.1.3 Only formula‐fed infants | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.40, 2.94] |
| 1.2 NEC (subgroup analysis of infants growth‐restricted or with AREDFV) Show forest plot | 13 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.14] |
| 1.2.1 Trials including only growth‐restricted/AREDFV infants | 6 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.32] |
| 1.2.2 Trials including all infants | 7 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.30] |
| 1.3 Mortality prior to discharge Show forest plot | 12 | 1399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.36] |
| 1.3.1 Human milk or formula‐fed (or mixed) infants | 9 | 1267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.27] |
| 1.3.2 Only human milk‐fed infants | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.55, 5.57] |
| 1.3.3 Only formula‐fed infants | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.57, 3.61] |
| 1.4 Mortality (subgroup analysis of infants growth‐restricted or with AREDFV) Show forest plot | 12 | 1399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.36] |
| 1.4.1 Trials including only growth‐restricted/AREDFV infants | 5 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.68, 2.12] |
| 1.4.2 Trials including all infants | 7 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.31] |
| 1.5 Feed intolerance Show forest plot | 6 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.68, 0.97] |
| 1.5.1 Human milk or formula‐fed (or mixed) infants | 4 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.02] |
| 1.5.2 Only human milk‐fed infants | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 1.6 Invasive infection Show forest plot | 7 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.15, 1.80] |
| 1.6.1 Human milk or formula‐fed (or mixed) infants | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.06, 1.68] |
| 1.6.2 Only human milk‐fed infants | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.21, 5.46] |
| 1.7 Duration of hospital admission (days) Show forest plot | 4 | 378 | Mean Difference (IV, Fixed, 95% CI) | 4.57 [1.53, 7.61] |
| 1.7.1 Human milk or formula‐fed (or mixed) infants | 3 | 346 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [1.62, 8.17] |
| 1.7.2 Only human milk‐fed infants | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [‐5.76, 10.76] |

