Administración temprana de corticosteroides inhalados para la prevención de la enfermedad pulmonar crónica en neonatos prematuros de muy bajo peso al nacer
Appendices
Appendix 1. Standard search methodology ‐ 2016 update
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. 2011 Search Strategy
PubMed:
((bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) AND (("2007"[PDat] : "3000"[PDat]))
CINAHL:
( (bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate) ) and ( ( infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND ( randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) ) 2007 ‐ Present
Cochrane Central Register of Controlled Trials
(bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate) and (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW), from 2007 to 2011
Embase:
1 ((bronchopulmonary dysplasia or lung diseases or chronic lung disease) and (anti‐inflammatory agents or steroids or dexamethasone or inhalation or aerosols or budesonide or beclomethasone dipropionate or flunisolide or fluticasone propionate)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1849)
2 (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (603948)
3 (human not animal).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (11849457)
4 (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1256505)
5 1 and 2 and 3 and 4 (336)
6 limit 5 to yr="2007 ‐Current" (76)
ClinicalTrials.gov:
(infant OR newborn) AND (bronchopulmonary dysplasia OR lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)
Controlled‐trials.com:
(infant OR newborn) AND (bronchopulmonary dysplasia OR lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)
Appendix 3. Risk of bias tool
The following issues were evaluated and entered into the 'Risk of bias' table:
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
a. low risk (any truly random process e.g. random number table; computer random number generator);
b. high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number);
c. unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
a. low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
b. high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
c. unclear risk.
3. Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
a. low risk, high risk or unclear risk for participants;
b. low risk, high risk or unclear risk for personnel;
c. low risk, high risk or unclear risk for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
a. low risk (< 20% missing data);
b. high risk (≥ 20% missing data);
c. unclear risk.
5. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
a. low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
b. high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
c. unclear risk.
6.Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
a. low risk;
b. high risk;
c. unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
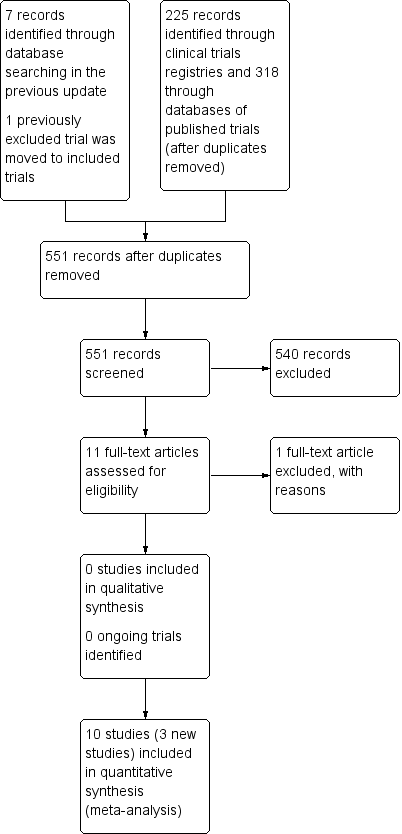
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
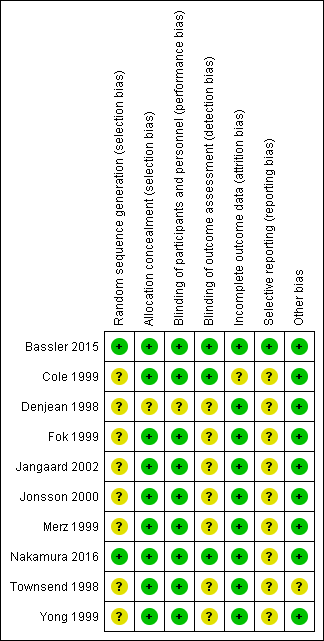
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), outcome: 1.1 CLD at 36 weeks' PMA.

Forest plot of comparison: 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), outcome: 1.6 Death by or CLD at 36 weeks' PMA.

Forest plot of comparison: 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), outcome: 2.1 CLD at 36 weeks' PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 1 CLD at 36 weeks PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 2 CLD at 28 days of age.
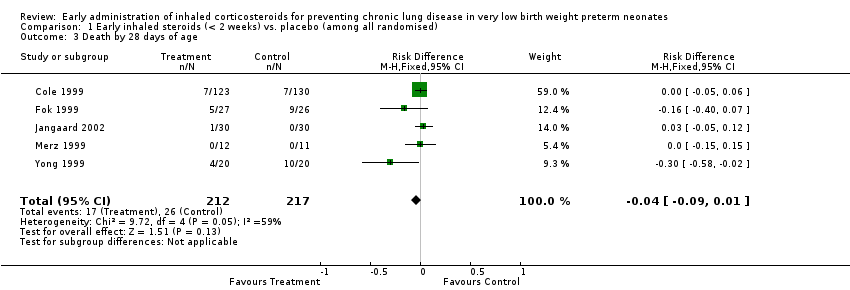
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 3 Death by 28 days of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 4 Death by 36 weeks PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 5 Death by or CLD at 28 days of age.
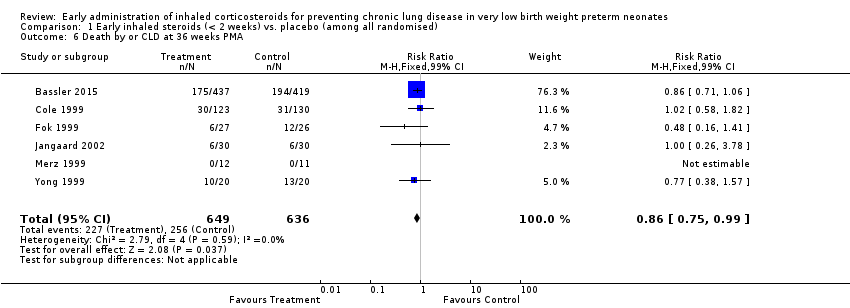
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 6 Death by or CLD at 36 weeks PMA.
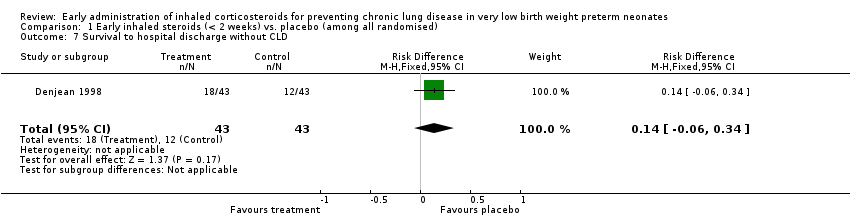
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 7 Survival to hospital discharge without CLD.
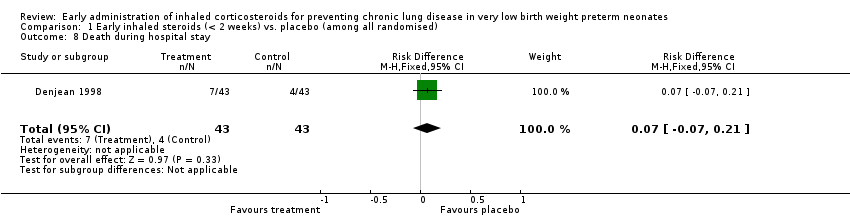
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 8 Death during hospital stay.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 9 Culture proven infection during hospital stay.
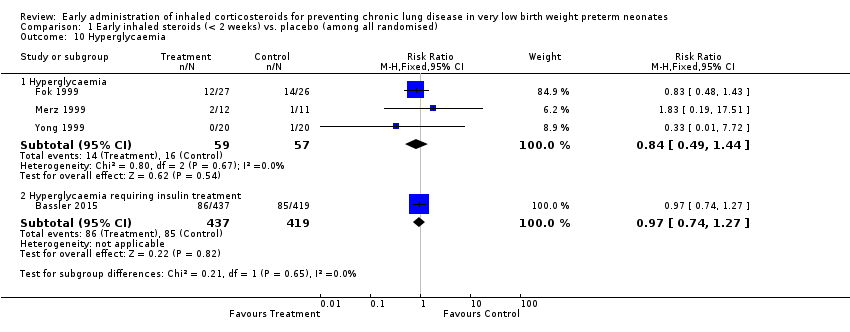
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 10 Hyperglycaemia.
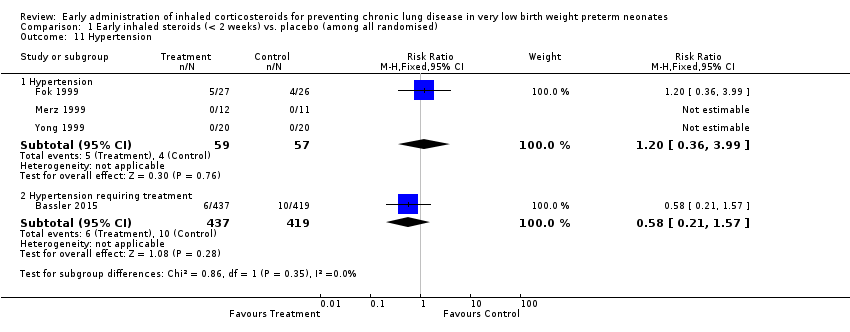
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 11 Hypertension.
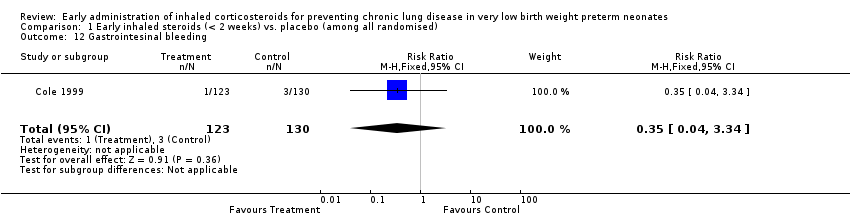
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 12 Gastrointesinal bleeding.
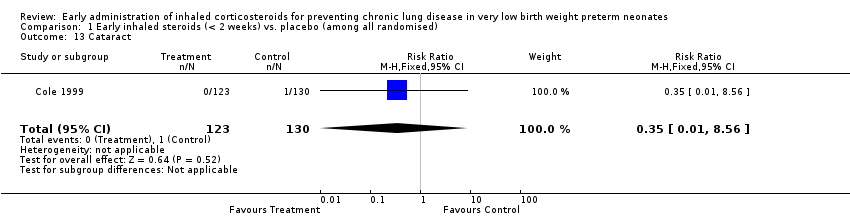
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 13 Cataract.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 14 Intraventricular haemorrhage.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 15 Periventricular leukomalacia.
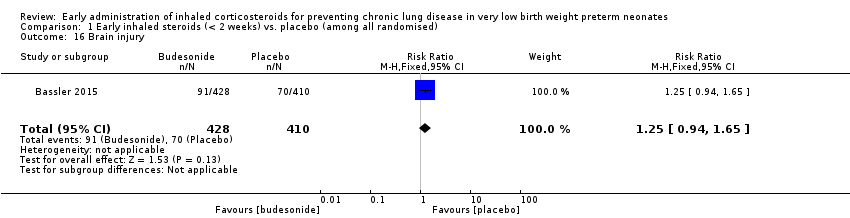
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 16 Brain injury.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 17 Necrotizing enterocolitis.
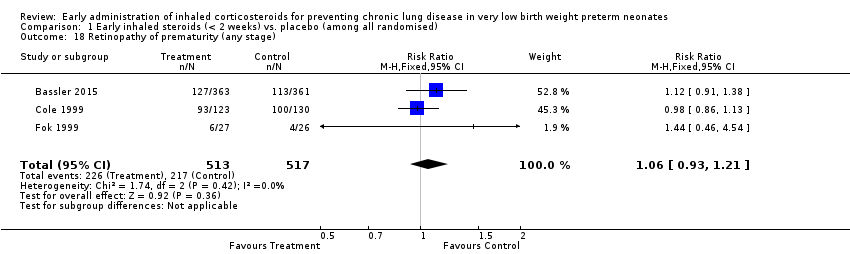
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 18 Retinopathy of prematurity (any stage).

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 19 Patent ductus arteriosus.
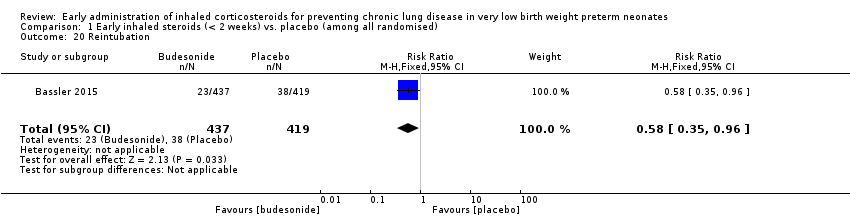
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 20 Reintubation.
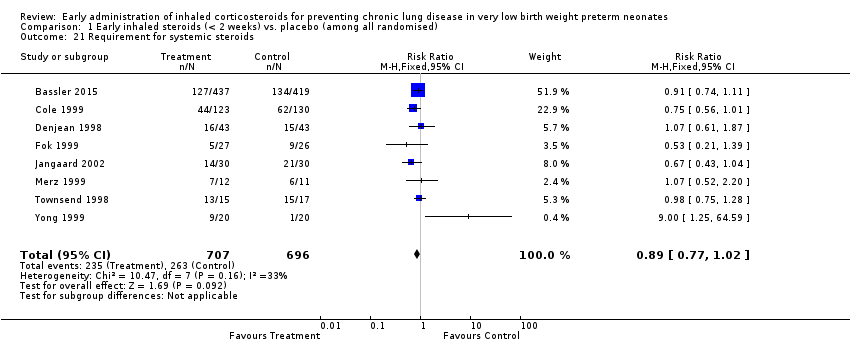
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 21 Requirement for systemic steroids.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 22 Failure to extubate within 14 days.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 23 Death or oxygen dependency at discharge.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 24 Death or severe BPD.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 25 Death or grade 3 or 4 IVH.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 26 Death or PVL.
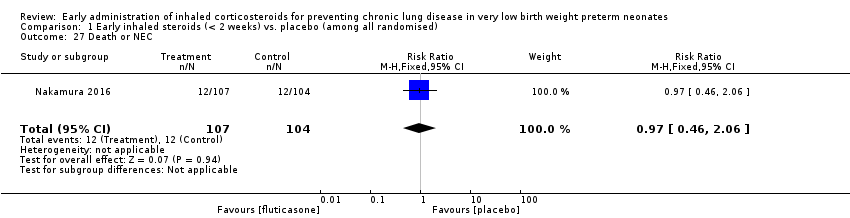
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 27 Death or NEC.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 28 Death or sepsis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 29 Death or ROP (stage not stated).

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 30 Death or neurodevelopmental impairment at 18 months PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 31 Death or neurodevelopmental impairment at 3 years of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 32 Death or cerebral palsy at 3 years of age.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 1 CLD at 36 weeks PMA.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 2 CLD at 28 days of age.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 3 Cerebral palsy.
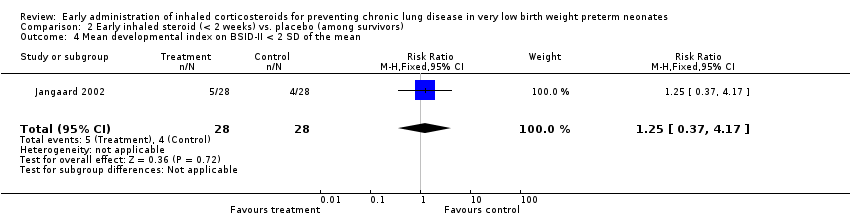
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 4 Mean developmental index on BSID‐II < 2 SD of the mean.
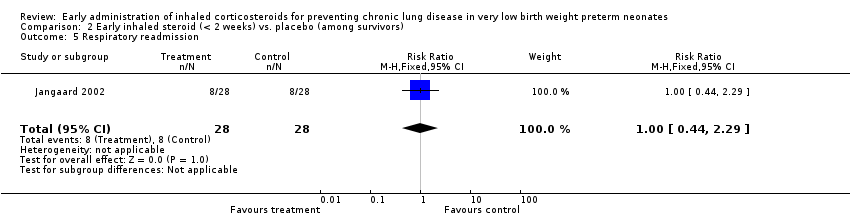
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 5 Respiratory readmission.
| Early inhaled steroids (< 2 weeks) compared to placebo (among all randomised) for preventing chronic lung disease in very low birth weight preterm neonates | ||||||
| Patient or population: very low birth weight preterm neonates | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (among all randomised) | Early inhaled steroids (< 2 weeks) | |||||
| CLD at 36 weeks' PMA | Study population | RR 0.97 | 429 | ⊕⊕⊕⊝ | ||
| 152 per 1000 | 148 per 1000 | |||||
| Moderate | ||||||
| 115 per 1000 | 112 per 1000 | |||||
| Death by, or CLD at, 36 weeks' PMA | Study population | RR 0.86 | 1285 | ⊕⊕⊕⊝ | ||
| 403 per 1000 | 346 per 1000 | |||||
| Moderate | ||||||
| 350 per 1000 | 301 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Method of sequence generation was unclear in all included studies except for the study by Bassler 2015. In the studies by Fok 1999, Jangaard 2002, Merz 1999 and Yong 1999 blinding of outcome assessment was unclear. Except for the study by Bassler 2015, none of the included studies were registered and we were unable to identify whether there was selective reporting or not. | ||||||
| Early inhaled steroid (< 2 weeks) compared to placebo (among survivors) for preventing chronic lung disease in very low birth weight preterm neonates | ||||||
| Patient or population: Very low birth weight preterm neonates | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (among survivors) | Early inhaled steroid (< 2 weeks) | |||||
| CLD at 36 weeks' PMA | Study population | RR 0.76 | 1088 | ⊕⊕⊕⊝ | ||
| 314 per 1000 | 239 per 1000 | |||||
| Moderate | ||||||
| 188 per 1000 | 143 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CLD at 36 weeks PMA Show forest plot | 5 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.52] |
| 2 CLD at 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.14] |
| 3 Death by 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 4 Death by 36 weeks PMA Show forest plot | 6 | 1285 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 5 Death by or CLD at 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 6 Death by or CLD at 36 weeks PMA Show forest plot | 6 | 1285 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.86 [0.75, 0.99] |
| 7 Survival to hospital discharge without CLD Show forest plot | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [‐0.06, 0.34] |
| 8 Death during hospital stay Show forest plot | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 9 Culture proven infection during hospital stay Show forest plot | 6 | 1121 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.00, 0.11] |
| 9.1 Positive blood or CSF culture | 2 | 896 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 9.2 Positive blood culture | 4 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.06, 0.16] |
| 10 Hyperglycaemia Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Hyperglycaemia | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.44] |
| 10.2 Hyperglycaemia requiring insulin treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 11 Hypertension Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Hypertension | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.36, 3.99] |
| 11.2 Hypertension requiring treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.57] |
| 12 Gastrointesinal bleeding Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.34] |
| 13 Cataract Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.56] |
| 14 Intraventricular haemorrhage Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.41] |
| 15 Periventricular leukomalacia Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.59, 3.46] |
| 16 Brain injury Show forest plot | 1 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.65] |
| 17 Necrotizing enterocolitis Show forest plot | 3 | 1162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.24] |
| 18 Retinopathy of prematurity (any stage) Show forest plot | 3 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 19 Patent ductus arteriosus Show forest plot | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.17] |
| 20 Reintubation Show forest plot | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.96] |
| 21 Requirement for systemic steroids Show forest plot | 8 | 1403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 22 Failure to extubate within 14 days Show forest plot | 5 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 23 Death or oxygen dependency at discharge Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.15] |
| 24 Death or severe BPD Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.25] |
| 25 Death or grade 3 or 4 IVH Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.68] |
| 26 Death or PVL Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 27 Death or NEC Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.06] |
| 28 Death or sepsis Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.40] |
| 29 Death or ROP (stage not stated) Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 30 Death or neurodevelopmental impairment at 18 months PMA Show forest plot | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.70, 1.70] |
| 31 Death or neurodevelopmental impairment at 3 years of age Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.56] |
| 32 Death or cerebral palsy at 3 years of age Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.64, 1.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CLD at 36 weeks PMA Show forest plot | 6 | 1088 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.02] |
| 2 CLD at 28 days of age Show forest plot | 5 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 3 Cerebral palsy Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.42] |
| 4 Mean developmental index on BSID‐II < 2 SD of the mean Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.17] |
| 5 Respiratory readmission Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.29] |

