Carbamazepine versus phenobarbitone monotherapy for epilepsy: an individual participant data review
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Single‐centre, double‐blind randomised controlled trial of participants recruited from clinical referral to a multidisciplinary child development centre at a children's hospital in Dhaka, Bangladesh 2 treatment arms: CBZ and PB | |
| Participants | 108 children between the ages of 2 to 15 with 2 or more generalised tonic‐clonic, partial, or secondarily generalised seizures in the previous year Number randomised: CBZ = 54, PB = 54 61 male children (56%) 59 with partial seizures (55%) 26 had previous AED treatment (24%) Mean age (range): 6 (2 to 15 years) Study duration: 12 months Range of follow‐up: 0 to 20.5 months | |
| Interventions | Monotherapy with CBZ (immediate release) or PB Starting daily dose: CBZ = 1.5 mg/kg/day, PB = 5 mg/kg/day Maximum daily dose: CBZ = 4 mg/kg/day, PB = 16 mg/kg/day | |
| Outcomes |
| |
| Notes | We received IPD for all randomised participants. We received reasons for withdrawal of allocated treatment as well as the date of the last follow‐up visit, but withdrawal of allocated treatment did not always coincide with the date of the last follow‐up visit (i.e. several participants had the allocated treatment substituted for the other trial drug and continued to be followed up). Dates of withdrawal of allocated treatment could not be provided; therefore, we could not calculate 'time to withdrawal of allocated treatment'. We received the date of first seizure after randomisation, but dates of other seizures in the follow‐up time could not be provided; therefore, we calculated 'time to first seizure' for all participants, but we could not calculate the time to six‐ and 12‐month remission. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were 'randomly assigned to treatment'; the method of randomisation was not stated and not provided by the authors. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by sealed envelopes prepared on a different site to the site of recruitment of participants. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, a psychologist, and a therapist were blinded throughout the trial. The treating physician was unblinded for practical and ethical reasons. |
| Blinding of outcome assessment (detection bias) | Unclear risk | A researcher performing outcome assessment was blinded throughout the trial but unblinded for analysis. It was unclear if this could have influenced the results. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were reported. We analysed all randomised participants from the IPD provided². |
| Selective reporting (reporting bias) | Low risk | We calculated 1 outcome for this review from the IPD provided². We could not calculate other outcomes for this review as the appropriate data were not recorded/not available. All cognitive outcomes from the study were well reported. |
| Other bias | High risk | There were inconsistencies between rates of seizure recurrence between the data provided and the published paper, which the authors could not resolve (see Sensitivity analysis). |
| Methods | Six‐month, systematic, simple randomised trial of children referred to a child neurology clinic (the author was from Guilan University of Medical Sciences, Iran, so it was likely that the study was also conducted there) 2‐arm trial: CBZ and PB | |
| Participants | Children aged 2 to 12 years with partial seizures with secondary generalisation Number randomised: CBZ = 36, PB = 35 36 male children (53%) 100% partial seizures, the per cent newly diagnosed was not stated Age range: 2 to 12 years Study duration: 6 months Mean follow‐up: not stated | |
| Interventions | Monotherapy with PB or CBZ. Doses started or achieved not stated | |
| Outcomes |
| |
| Notes | The trial was reported in abstract form only with very limited information. Outcomes chosen for this review were not reported; IPD were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as a 'systematic simple randomised study'; no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information was provided on blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was provided on blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | No attrition rates were reported; it was unclear if all participants were analysed. |
| Selective reporting (reporting bias) | Unclear risk | There was no protocol available; the study was available in abstract format only. Outcomes for this review were not available. |
| Other bias | Low risk | We detected no other bias. |
| Methods | Randomised, double‐blind cross‐over trial with 3, 21‐day treatment periods and a 2‐week washout period (regular medications used) 3 treatment arms: CBZ, phenytoin, and PB | |
| Participants | Institutionalised adult participants with uncontrolled seizures on current medication Number randomised: PB = 45, CBZ = 45 41 participants (91%) with partial epilepsy 28 (62%) male participants Age range: 18 to 51 years Study duration: 13 weeks (3 x 21‐day treatment periods plus 2 x 2‐week washout periods) | |
| Interventions | Monotherapy with PB or CBZ | |
| Outcomes |
| |
| Notes | The outcomes chosen for this review were not reported due to the cross‐over design of the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of groups from random number tables (confirmed by author). |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided on blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided on blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawal rates reported, no further information provided. |
| Selective reporting (reporting bias) | Low risk | All efficacy and tolerability outcomes specified in the methods sections reported well in the results section. No protocol available, outcomes for this review not available due to trial cross‐over design. |
| Other bias | High risk | Cross‐over design may not be appropriate for monotherapy designs, likely carryover effects from one period to another so the comparison may not be entirely monotherapy. |
| Methods | Randomised, parallel group study conducted in Taiwan 3 treatment arms: CBZ, PB, sodium valproate | |
| Participants | Children with 2 or more previously untreated unprovoked epileptic seizures Number randomised: PB = 25, CBZ = 26; number analysed: PB = 23, CBZ = 25 (see notes) Mean age (range): PB = 9.9 (7 to 15 years), CBZ = 10.8 (7 to 15 years) CBZ versus PB: 26 (54%) participants with partial epilepsy 25 (52%) male participants Study duration: 12 months Range of follow‐up: not stated | |
| Interventions | Monotherapy with PB or CBZ. Dose started or achieved not stated | |
| Outcomes |
| |
| Notes | 2 children from the PB group and 1 child from the CBZ group withdrew from the study because of allergic reactions. Published results were presented for children who completed the study only. Outcomes chosen for this review were not reported; IPD were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated with "simple randomisation of block size 3." |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The cognitive assessor was 'single‐blinded', implying that participants and personnel were unblinded, but no further information was provided. |
| Blinding of outcome assessment (detection bias) | Low risk | The cognitive assessor was single‐blinded. |
| Incomplete outcome data (attrition bias) | High risk | Withdrawal rates were reported; results were presented only for those who completed the study (CBZ versus PB: 3/51 (6%) excluded from analysis). An ITT approach was not taken. |
| Selective reporting (reporting bias) | Low risk | All cognitive, efficacy, and tolerability outcomes specified in the methods sections were reported well in the results section. No protocol was available. Outcomes chosen for this review were not reported. |
| Other bias | Low risk | We detected no other bias |
| Methods | Randomised, double‐blind study to assess short‐term therapy of CBZ and PB on cognitive and memory function conducted in Italy 3 treatment arms: CBZ, PB, and placebo | |
| Participants | Participants with newly diagnosed and untreated temporal lobe epilepsy with no seizures in the previous month Number randomised: CBZ = 6, PB = 6 100% partial (temporal lobe epilepsy), 100% newly diagnosed Mean age (SD): CBZ = 26.33 (9.73) years, PB = 18.5 (2.56) years Age range: 15 to 45 years 1 male and 5 females in each group Study duration: 3 weeks; all participants completed in 3 weeks | |
| Interventions | Monotherapy with CBZ or PB, Dose started and achieved not stated | |
| Outcomes |
| |
| Notes | The trial was published in Italian; the characteristics and outcomes were translated. Outcomes chosen for this review were not reported; IPD were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised ('randomizzazione' in Italian); no further information was available. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Trial is described as double‐blind ('condizioni di doppia cecità' in Italian), we assume this refers to participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed this short study and contribute to analysis. |
| Selective reporting (reporting bias) | Unclear risk | Cognitive and memory outcomes described in methods section well reported in results section. No seizure outcomes or adverse events reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori. |
| Other bias | High risk | Very small participant numbers and very short‐term follow‐up. Unclear if this study was adequately powered and of sufficient duration to detect differences. |
| Methods | 36‐month randomised comparative study 4 treatment arms: CBZ, sodium valproate, phenytoin, PB | |
| Participants | Adults with newly diagnosed epilepsy with partial complex seizures Number randomised: PB = 30, CBZ = 30 100% partial epilepsy (partial complex seizures) Age range: 18 to 40 years Percentage male and range of follow‐up: not mentioned | |
| Interventions | Monotherapy with PB or CBZ Starting doses CBZ = 400 mg/day, PB = 100 mg/day. Dose achieved not stated | |
| Outcomes |
| |
| Notes | This was an abstract only. Outcomes chosen for this review were not reported. IPD were pledged but not received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomised, but no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information was provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | "Exclusion rates" were reported for all treatment groups; no further information was provided. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available; the study was available in abstract format only. Outcomes for this review were not available. |
| Other bias | Low risk | We detected no other bias. |
| Methods | Randomised, parallel group, open‐label paediatric study conducted in 2 centres in the UK 4 treatment arms: CBZ, sodium valproate, phenytoin, PB | |
| Participants | Children with newly diagnosed epilepsy (2 or more untreated partial or generalised tonic‐clonic seizures in the 12 months preceding the study) Number randomised: PB = 10, CBZ = 54 (see notes) 35 children (55%) with partial epilepsy 34 (53%) male children Mean age (range): 9 (3 to 16) years Range of follow‐up: 3 to 88 (months) | |
| Interventions | Monotherapy with PB or CBZ Median daily dose achieved: PB = not stated; CBZ = 400 mg/day | |
| Outcomes |
| |
| Notes | 6 of the first 10 children assigned to PB had unacceptable adverse effects, so no further children were assigned to PB. The 10 children randomised to PB were retained in analysis. We received IPD for all outcomes of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type, and presence of neurological signs. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed via 4 batches of concealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded ‐ the authors stated that masking of treatment would not have been "practicable or ethical" and would have "undermine[d] compliance". Lack of masking could have led to early withdrawal of the PB arm from the trial. |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded ‐ the authors stated masking of treatment would not have been "practicable or ethical" and would have "undermine[d] compliance". Lack of masking could have led to early withdrawal of the PB arm from the trial, which was likely to have influenced the overall results. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were reported; we analysed all randomised participants from the IPD provided² |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported or calculated with the IPD provided² |
| Other bias | Low risk | We detected no other bias |
| Methods | Randomised parallel group trial conducted among residents of the Nakuru district, a semi‐urban population of rural Kenya 2 treatment arms: CBZ and PB | |
| Participants | Participants had a history of generalised tonic‐clonic seizures and at least 2 generalised tonic‐clonic seizures within the preceding year (with or without other seizure types) and untreated in the 3 months prior to the study. 79 (26%) participants had been treated in the past with AEDs Number randomised: PB = 150, CBZ = 152 115 (38%) of participants had experienced partial seizures 173 (57%) male participants Mean age (range): 21 (6 to 65 years) Range of follow‐up: participants followed up for up to 1 year | |
| Interventions | Monotherapy with CBZ or PB Starting doses: PB: 6 to 10 years of age: 30 mg/day, 11 to 15 years of age: 45 mg/day, 16+ years of age: 60 mg/day CBZ: 6 to 10 years of age: 400 mg/day, 11 to 15 years of age: 500 mg/day, 16+ years of age: 600 mg/day Dose achieved not stated | |
| Outcomes |
| |
| Notes | IPD were made available but not used because of inconsistencies and problems with the data provided (see Included studies for further details). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised with random number list, no information provided on method of generating random list. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via sealed opaque envelopes (information provided by study author). |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | High risk | Attrition rates reported, results presented only for participants completing 12 months follow‐up (results not presented for 53 (17.5%) participants out of 302 who withdrew from treatment), approach is not ITT. |
| Selective reporting (reporting bias) | Low risk | No protocol available, outcomes chosen for this review not reported. Seizure outcomes and adverse events well reported. |
| Other bias | High risk | Inconsistencies with IPD and published results so IPD could not be used (see Included studies for further details). |
| Methods | Randomised, parallel group, open‐label study conducted in 2 centres in the UK 4 treatment arms: CBZ, sodium valproate, phenytoin, PB | |
| Participants | Adults with newly diagnosed epilepsy (2 or more untreated partial or generalised tonic‐clonic seizures in the 12 months preceding the study) Number randomised: PB = 58, CBZ = 61 49 participants (41%) with partial epilepsy 55 (46%) male participants Mean age (range): 32 (13 to 77) years Range of follow‐up: 1 to 91 months | |
| Interventions | Monotherapy with PB or CBZ. Median daily dose achieved: PB = 105 mg/day; CBZ = 600 mg/day | |
| Outcomes |
| |
| Notes | We received IPD for all outcomes of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via 4 batches of concealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded, authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large dropout rate.” Lack of blinding may have lead to more withdrawals of PB. |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded, authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large dropout rate.” Lack of blinding may have lead to more withdrawals of PB which is likely to have influenced the overall results. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analyses from IPD provided² |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided² |
| Other bias | Low risk | No other bias detected |
| Methods | Multicentre, randomised, parallel group, double‐blinded study over 10 centres in the USA with separate randomisation schemes used for each seizure type 4 treatments: CBZ, phenytoin, PB, primidone | |
| Participants | Adults with previously untreated or under‐treated simple or complex partial or secondary generalised tonic‐clonic seizures Number randomised: CBZ = 155, PB = 155 100% partial epilepsy 268 (88%) male participants Mean age (range): 41 (18 to 82) years Range of follow‐up: 1 to 177 months | |
| Interventions | Monotherapy with PB or CBZ Median daily dose achieved: PB = 160 mg/day; CBZ = 800 mg/day | |
| Outcomes |
| |
| Notes | We received IPD for all outcomes of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised with stratification for seizure type. The method of randomisation was not stated and not provided by the authors. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided in the publication or by the study authors. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was double‐blind (participants and personnel), which was achieved using an additional blank tablet. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was unclear if outcome assessment was blinded; no information was provided. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were reported; we analysed all randomised participants from the IPD provided². |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported or calculated with the IPD provided². |
| Other bias | Unclear risk | We detected no other bias. |
| Methods | Randomised, double‐blind, single‐centre, parallel paediatric study conducted in Los Angeles, USA 2 treatment arms: CBZ and PB | |
| Participants | Children with newly diagnosed epilepsy Number randomised: PB = 18, CBZ = 15 100% partial epilepsy, 100% newly diagnosed 20 (61%) male children Mean age (range): PB = 7.89 (2 to 12 years), CBZ = 6.07 (2 to 12 years) Study duration: 12 months Range of follow‐up: not reported | |
| Interventions | Monotherapy with PB or CBZ. Doses started and achieved not stated | |
| Outcomes |
| |
| Notes | 33 participants were randomised to PB (18) and CBZ (15) in this study; 6 children were enrolled into a six‐month pilot study (PB (4) CBZ (2)) prior to the randomised study. The 6 children were included in six‐month follow‐up psychometric data. Outcomes for this review were not reported; IPD were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 33 children were "randomised using a scheme that balanced drug distribution by age and sex"; no further details were provided on the randomisation scheme. 6 non‐randomised children were also used in some analyses. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial blinded participants (and parents); clinicians were unblinded for clinical follow‐up. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial blinded psychometric (cognitive) testers blinded for clinical follow‐up. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were reported; results were reported for all children who completed each stage of follow‐up. |
| Selective reporting (reporting bias) | Low risk | Cognitive/behavioural outcomes, seizure control outcomes, and adverse events were all well reported. No protocol was available; outcomes for this review were not reported. |
| Other bias | High risk | There was evidence that the study may have been underpowered to detect differences (e.g. 55% power to find a 5‐point difference in IQ score). The behavioural questionnaire was not fully validated. Non‐randomised children from a pilot study were included in the results for psychometric outcomes and medical outcomes. |
| Methods | Double‐blinded, parallel group, randomised study conducted in a single‐centre in Nigeria. 3 treatment arms: carbamazepine, phenytoin, phenobarbitone | |
| Participants | Consectuive newly diagnosed participants aged 14 or over presenting at the outpatient neurology clinic of the University Teaching Hopsital, Benin City, Nigeria, with recurrent, untreated afebrile seizures 22 male participants (59%) Mean age (range): 23.62 years (14 to 38 years) | |
| Interventions | Monotherapy with PB or CBZ. Median daily dose (range): PB = 120 mg (60 to 180 mg), CBZ = 600 mg (400 mg to 1200 mg) | |
| Outcomes |
| |
| Notes | We received IPD for all randomised participants. The study duration was 12 weeks; all participants completed the study without withdrawing; therefore, we could not calculate the outcomes 'time to withdrawal of allocated drug', 'time to six‐month remission', and 'time to 12‐month remission'. We calculated 'time to first seizure' from the IPD provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study randomised participants using simple randomisation: Each participant was asked to pick 1 from a table of numbers (1 to 60); the numbers corresponded to allocation of 1 of 3 drugs (the author provided information). |
| Allocation concealment (selection bias) | Low risk | Recruitment/randomisation of participants and allocations of treatments took place on different sites (the author provided information). |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were single‐blinded. The study did not blind the research assistant recruiting participants and counselling on medication adherence. |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators performing cognitive assessments were single‐blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants completed the study. We analysed all randomised participants from the IPD provided². |
| Selective reporting (reporting bias) | Low risk | We calculated 1 outcome for this review from the IPD provided². Other outcomes for this review were not available because of short study length. All cognitive outcomes from the study were well reported. |
| Other bias | Low risk | We detected no other bias. |
| Methods | Randomised parallel group study conducted in the context of existing community health care in a rural highland area of a developing country (Ecuador) | |
| Participants | Participants with a history of at least 2 afebrile seizures and no previous AED treatment in the 4 weeks preceding the study were eligible Number randomised: PB = 97, CBZ = 95 133 participants (69%) with partial epilepsy 67 (35%) male participants Mean age (range): PB = 28.6 (2 to 68 years), CBZ = 29.2 (2 to 68 years) Study duration: 12 months Range of follow‐up: 0 to 53.4 months | |
| Interventions | Monotherapy with PB or CBZ. Minimum maintenance doses by age groups: 2 to 5 years: PB: 15 mg/day, CBZ: 150 mg/day; 6 to 10 years: PB: 30 mg/day, CBZ: 300 mg/day; 11 to 15 years: PB: 45 mg/day, CBZ: 500 mg/day; > 16 PB: 60 mg/day, CBZ: 600 mg/day. Doses gradually increased Doses achieved not stated | |
| Outcomes |
| |
| Notes | We received IPD for all outcomes used in this review. Results in the published paper were given for 139 participants who completed 6 months' follow‐up, but we received IPD for all 192 participants randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised with random number list, no information provided on method of generating random list. |
| Allocation concealment (selection bias) | High risk | Allocation concealed used sealed opaque envelopes but method not used for all participants (information provided by study author). |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided². |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported or calculated with the IPD provided². |
| Other bias | High risk | Inconsistencies between number and reasons of withdrawals between the data and the published paper which could not be resolved by the authors (see Sensitivity analysis). |
AED: antiepileptic drug
CBZ: carbamazepine
IPD: individual participant data
IQ: intelligence quotient
ITT: intention‐to‐treat
PB: phenobarbitone
WISC‐R scale: the Wechsler Intelligence Scale for Children
²For studies for which we received IPD (Banu 2007; de Silva 1996; Heller 1995; Mattson 1985; Ogunrin 2005; Placencia 1993), attrition and reporting bias were reduced as we requested attrition rates and unpublished outcome data.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| It was unclear whether this trial was randomised and whether participants received either CBZ or PB as monotherapy. | |
| The trial was not randomised, and the treatment choice was made based on types of seizures. | |
| This was a preliminary analysis of Cereghino 1974. | |
| The trial was not randomised; participants were already on CBZ or PB monotherapy upon entry into the study. | |
| The trial was not randomised; participants were already on CBZ or PB monotherapy upon entry into the study. | |
| CBZ or PB therapy were added to current treatment. We could not make a comparison between CBZ monotherapy and PB monotherapy. | |
| We could not make a comparison between CBZ monotherapy and PB monotherapy. This was a cross‐over trial, but some participants were receiving treatment at the start of the first period, which had to be withdrawn slowly. | |
| The trial was not fully randomised: "The treatment was chosen at random unless the individual diagnoses required a specific drug." | |
| This reported the same trial as Mattson 1985, and Mattson 1985 gave more relevant information. |
CBZ: carbamazepine
PB: phenobarbitone
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to withdrawal of allocated treatment Show forest plot | 4 | 676 | Hazard Ratio (Fixed, 95% CI) | 1.49 [1.15, 1.94] |
| Analysis 1.1  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 1 Time to withdrawal of allocated treatment. | ||||
| 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type Show forest plot | 4 | 676 | Hazard Ratio (Fixed, 95% CI) | 1.50 [1.15, 1.95] |
| Analysis 1.2  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type. | ||||
| 2.1 Generalised onset | 3 | 156 | Hazard Ratio (Fixed, 95% CI) | 1.53 [0.81, 2.88] |
| 2.2 Partial onset | 4 | 520 | Hazard Ratio (Fixed, 95% CI) | 1.49 [1.12, 2.00] |
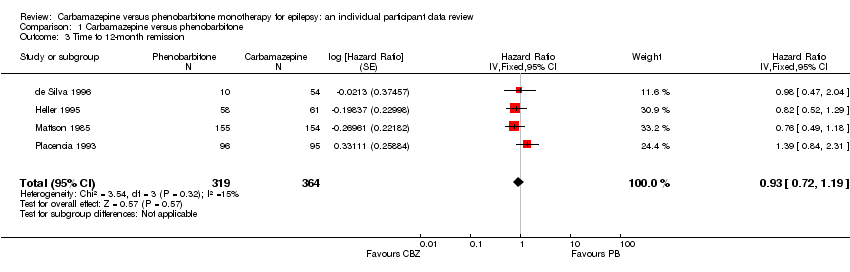
| 3 Time to 12‐month remission Show forest plot | 4 | 683 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.72, 1.19] |
| Analysis 1.3  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 3 Time to 12‐month remission. | ||||
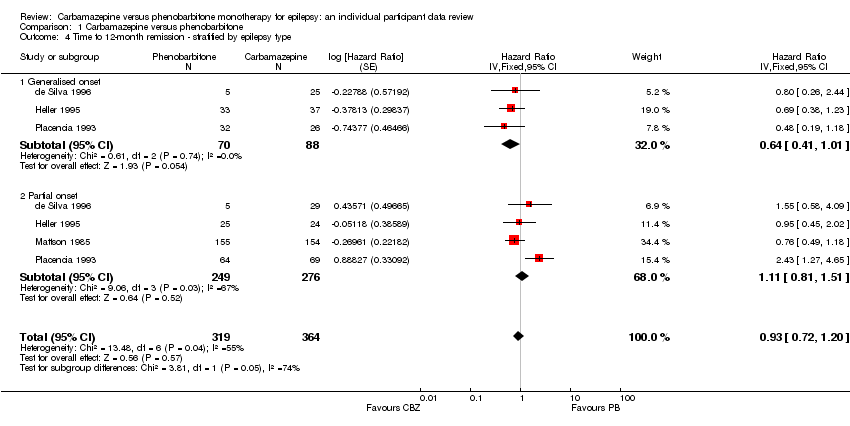
| 4 Time to 12‐month remission ‐ stratified by epilepsy type Show forest plot | 4 | 683 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.72, 1.20] |
| Analysis 1.4  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 4 Time to 12‐month remission ‐ stratified by epilepsy type. | ||||
| 4.1 Generalised onset | 3 | 158 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.41, 1.01] |
| 4.2 Partial onset | 4 | 525 | Hazard Ratio (Fixed, 95% CI) | 1.11 [0.81, 1.51] |
| 5 Time to six‐month remission Show forest plot | 4 | 683 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.83, 1.26] |
| Analysis 1.5  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 5 Time to six‐month remission. | ||||
| 6 Time to six‐month remission ‐ stratified by epilepsy type Show forest plot | 4 | 683 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| Analysis 1.6  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 6 Time to six‐month remission ‐ stratified by epilepsy type. | ||||
| 6.1 Generalised onset | 3 | 158 | Hazard Ratio (Fixed, 95% CI) | 0.69 [0.47, 1.01] |
| 6.2 Partial onset | 4 | 525 | Hazard Ratio (Fixed, 95% CI) | 1.17 [0.90, 1.50] |
| 7 Time to first seizure Show forest plot | 6 | 822 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.71, 1.04] |
| Analysis 1.7  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 7 Time to first seizure. | ||||
| 8 Time to first seizure ‐ stratified by epilepsy type Show forest plot | 6 | 822 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.72, 1.06] |
| Analysis 1.8  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 8 Time to first seizure ‐ stratified by epilepsy type. | ||||
| 8.1 Generalised onset | 5 | 238 | Hazard Ratio (Fixed, 95% CI) | 1.23 [0.86, 1.77] |
| 8.2 Partial onset | 6 | 584 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| 9 Time to first seizure ‐ sensitivity analysis Show forest plot | 6 | 822 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| Analysis 1.9  Comparison 1 Carbamazepine versus phenobarbitone, Outcome 9 Time to first seizure ‐ sensitivity analysis. | ||||
| 9.1 Generalised onset | 5 | 173 | Hazard Ratio (Fixed, 95% CI) | 1.39 [0.90, 2.13] |
| 9.2 Partial onset | 6 | 584 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| 9.3 Uncertain seizure type | 3 | 65 | Hazard Ratio (Fixed, 95% CI) | 1.22 [0.59, 2.51] |

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
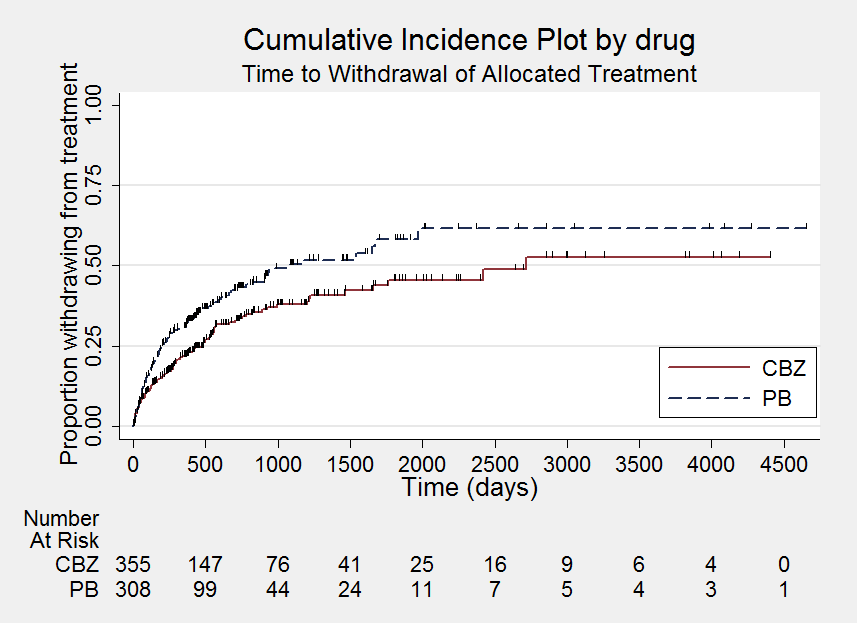
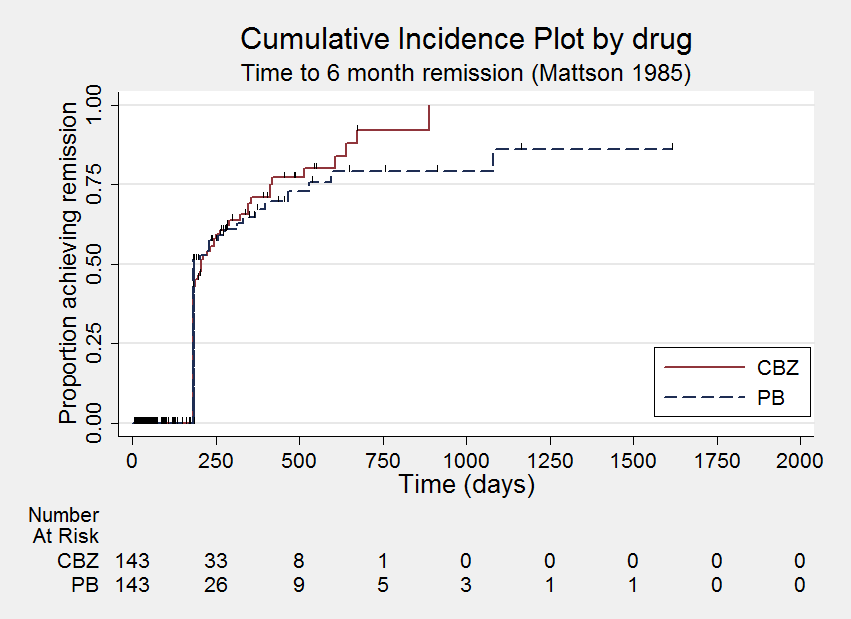
Time to withdrawal of allocated treatment

Time to withdrawal of allocated treatment ‐ stratified by epilepsy type

Time to 12‐month remission

Time to 12‐month remission ‐ stratified by epilepsy type
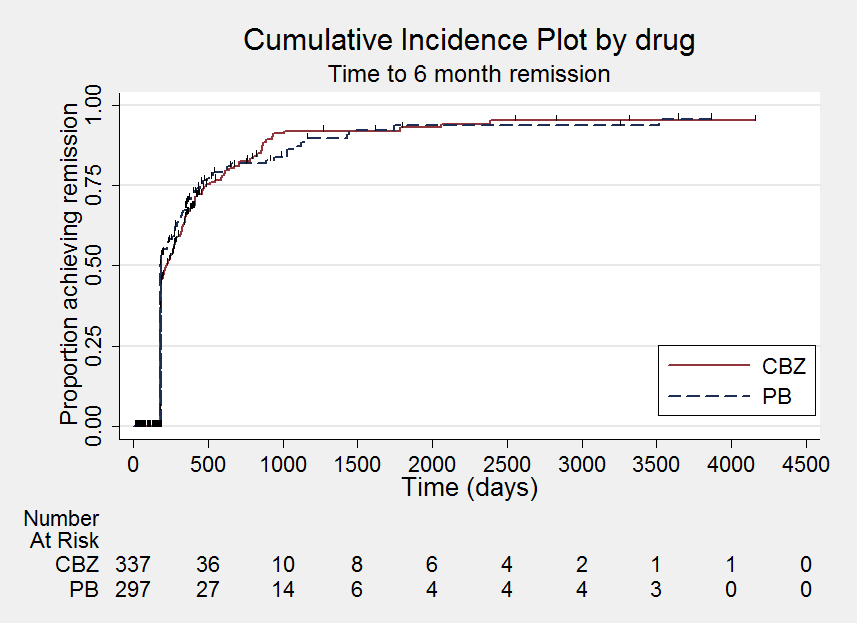
Time to six‐month remission
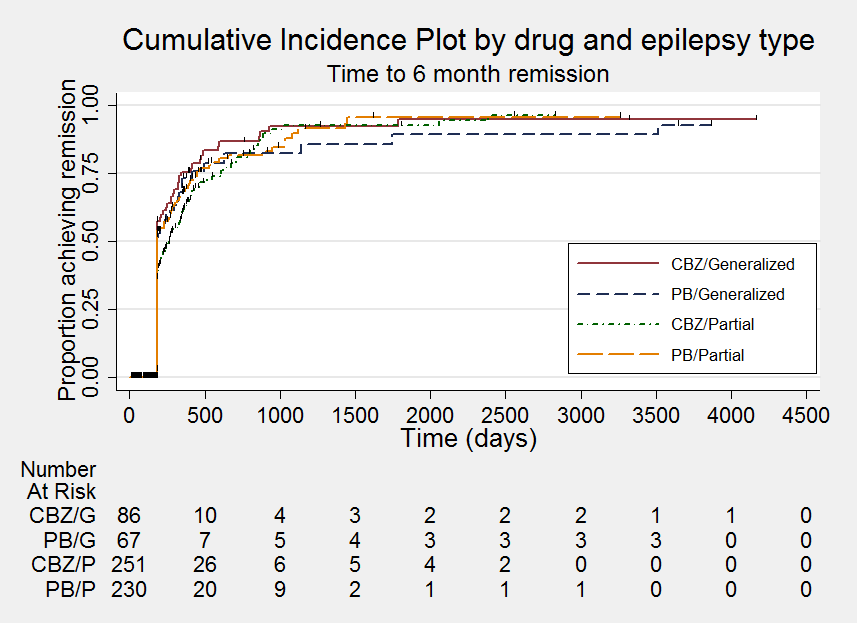
Time to six‐month remission ‐ stratified by epilepsy type

Time to first seizure
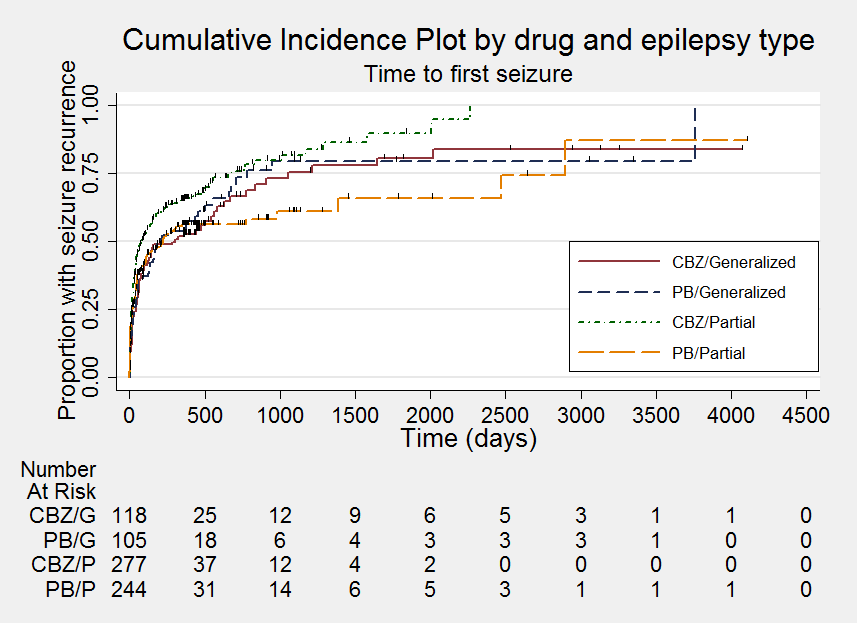
Time to first seizure ‐ stratified by epilepsy type

Comparison 1 Carbamazepine versus phenobarbitone, Outcome 1 Time to withdrawal of allocated treatment.

Comparison 1 Carbamazepine versus phenobarbitone, Outcome 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type.
Comparison 1 Carbamazepine versus phenobarbitone, Outcome 3 Time to 12‐month remission.
Comparison 1 Carbamazepine versus phenobarbitone, Outcome 4 Time to 12‐month remission ‐ stratified by epilepsy type.

Comparison 1 Carbamazepine versus phenobarbitone, Outcome 5 Time to six‐month remission.

Comparison 1 Carbamazepine versus phenobarbitone, Outcome 6 Time to six‐month remission ‐ stratified by epilepsy type.

Comparison 1 Carbamazepine versus phenobarbitone, Outcome 7 Time to first seizure.

Comparison 1 Carbamazepine versus phenobarbitone, Outcome 8 Time to first seizure ‐ stratified by epilepsy type.

Comparison 1 Carbamazepine versus phenobarbitone, Outcome 9 Time to first seizure ‐ sensitivity analysis.
| Carbamazepine compared with phenobarbitone for epilepsy | ||||||
| Patient or population: adults and children with newly onset partial or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenobarbitone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenobarbitone | Carbamazepine | |||||
| Time to withdrawal of allocated treatment ‐ all participants, stratified by epilepsy type Range of follow‐up (all participants): 0 to 4653 days | 390 per 1000 | 281 per 1000 | HR 1.50 (1.15 to 1.95) | 676 (4 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a |
| Time to withdrawal of allocated treatment Subgroup: generalised onset seizures Range of follow‐up (all participants): 0 to 4653 days | 286 per 1000 | 197 per 1000 | HR 1.53 (0.81 to 2.88) | 156 (3 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a |
| Time to withdrawal of allocated treatment Subgroup: partial onset seizures Range of follow‐up (all participants): 0 to 4272 days | 420 per 1000 | 307 per 1000 | HR 1.49 (1.12 to 2.00) | 520 (4 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The assumed risk is calculated as the event rate in the phenobarbitone treatment group. The corresponding risk in the carbamazepine treatment group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Pooled HR for all participants adjusted for seizure type. | ||||||
| Carbamazepine compared with phenobarbitone for epilepsy | ||||||
| Patient or population: adults and children with newly onset partial or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenobarbitone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenobarbitone | Carbamazepine | |||||
| Time to achieve 12‐month remission ‐ all participants, stratified by epilepsy type Range of follow‐up (all participants): 0 to 4222 days | 367 per 1000 | 346 per 1000 | HR 0.93 (0.72 to 1.20) | 683 | ⊕⊕⊝⊝ | HR > 1 indicates a |
| Time to achieve 12‐month remission Subgroup: generalised onset seizures Range of follow‐up (all participants): 0 to 4163 days | 500 per 1000 | 358 per 1000 | HR 0.64 (0.41 to 1.01) | 158 | ⊕⊕⊝⊝ | HR > 1 indicates a |
| Time to achieve 12‐month remission Subgroup: partial onset seizures Range of follow‐up (all participants): 0 to 4222 days | 329 per 1000 | 358 per 1000 | HR 1.11 (0.81 to 1.51) | 525 | ⊕⊕⊝⊝ | HR > 1 indicates a |
| Time to first seizure ‐ all participants, stratified by epilepsy type Range of follow‐up (all participants): 0 to 4108 days | 487 per 1000 | 536 per 1000 | HR 0.87 (0.72 to 1.06) | 822 (6 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a |
| Time to first seizure ‐ Subgroup: generalised onset seizures Range of follow‐up (all participants): 0 to 4108 days | 548 per 1000 | 475 per 1000 | HR 1.23 (0.86 to 1.77) | 238 (5 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a |
| Time to first seizure ‐ Subgroup: partial onset seizures Range of follow‐up (all participants): 0 to 4108 days | 462 per 1000 | 557 per 1000 | HR 0.76 (0.60 to 0.96) | 584 (6 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The assumed risk is calculated as the event rate in the phenobarbitone treatment group. The corresponding risk in the carbamazepine treatment group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Pooled HR for all participants adjusted for seizure type. | ||||||
| Trial | Outcomes reported | Summary of results |
|
|
| |
|
|
| |
|
|
| |
| Changes in memory function from baseline after 3 weeks of treatment (verbal, visual, (visual‐verbal and visual‐non‐verbal), acoustic, tactile, and spatial) |
| |
|
|
| |
|
| PB (n = 123), CBZ (n = 126)
| |
|
|
| |
| CBZ: carbamazepine | ||
| Trial | Number randomised | Time to withdrawal of allocated treatment | Time to 12‐month remission | Time to six‐month remission | Time to first seizure | ||||||||||
| CBZ | PB | Total | CBZ | PB | Total | CBZ | PB | Total | CBZ | PB | Total | CBZ | PB | Total | |
| 54 | 54 | 108 | Information not available | Information not available | Information not available | 54 | 54 | 108 | |||||||
| 54 | 10 | 64 | 53 | 10 | 63 | 54 | 10 | 64 | 54 | 10 | 64 | 54 | 10 | 64 | |
| 61 | 58 | 119 | 60 | 55 | 115 | 61 | 58 | 119 | 61 | 58 | 119 | 61 | 58 | 119 | |
| 155 | 155 | 310 | 154 | 155 | 309 | 154 | 155 | 309 | 154 | 155 | 309 | 151 | 151 | 302 | |
| 19 | 18 | 37 | Information not available | Information not available | Information not available | 19 | 18 | 37 | |||||||
| 95 | 97 | 192 | 94 | 95 | 189 | 95 | 96 | 191 | 95 | 96 | 191 | 95 | 97 | 192 | |
| Total | 438 | 392 | 830 | 361 | 315 | 676 | 364 | 319 | 683 | 364 | 319 | 683 | 434 | 388 | 822 |
| CBZ: carbamazepine | |||||||||||||||
| Reason for early termination | Classification | Total⁴ | |||||||||||
| CBZ n = 53 | PB = 10 | CBZ n = 60 | PB = 55 | CBZ n = 154 | PB = 155 | CBZ = 94 | PB = 95 | CBZ = 54 | PB = 54 | CBZ = 415 | PB = 369 | ||
| Adverse events | Event | 3 | 2 | 8 | 12 | 11 | 5 | 5 | 5 | 0 | 0 | 27 | 24 |
| Seizure recurrence | Event | 12 | 2 | 5 | 7 | 3 | 7 | 0 | 0 | 1 | 2 | 21 | 18 |
| Both seizure recurrence and adverse events | Event | 6 | 4 | 4 | 3 | 30 | 26 | 0 | 0 | 0 | 0 | 40 | 33 |
| Non‐compliance/participant choice | Event | 0 | 0 | 0 | 0 | 11 | 19 | 13 | 9 | 6 | 0 | 30 | 28 |
| Another AED added/AED changed | Event | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 7 | 4 | 7 | 7 |
| Participant went into remission | Censored | 18 | 1 | 6 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 24 | 6 |
| Lost to follow‐up | Censored | 0 | 0 | 0 | 0 | 26 | 26 | 11 | 5 | 7 | 15 | 44 | 46 |
| Death⁵ | Censored | 0 | 0 | 0 | 0 | 4 | 2 | 2 | 1 | 0 | 0 | 6 | 3 |
| Other⁶ | Censored | 0 | 0 | 0 | 0 | 16 | 13 | 0 | 0 | 0 | 0 | 16 | 13 |
| Completed the study (did not withdraw) | Censored | 14 | 1 | 37 | 30 | 53 | 54 | 63 | 75 | 33 | 31 | 200 | 191 |
| AED: antiepileptic drug | |||||||||||||
| Analysis | Time to withdrawal of allocated treatment | Time to 12‐month remission | Time to six‐month remission | Time to first seizure¹ | |
| Original analysis | Participants | 676 (Analysis 1.2) | 683 (Analysis 1.4) | 683 (Analysis 1.6) | 822 (Analysis 1.8) |
| Pooled HR (95% CI) P value | 1.50 (1.15 to 1.95) P = 0.003 | 0.93 (0.72 to 1.20) P = 0.57 | 0.99 (0.80 to 1.23) P = 0.95 | 0.87 (0.72 to 1.06) P = 0.18 | |
| Heterogeneity | I² statistic = 35% | I² statistic = 55% | I² statistic = 58% | I² statistic = 44% | |
| Sensitivity analysis for Placencia 1993² | Participants | 487 | 492 | 492 | 630 |
| Pooled HR (95% CI) P value | 1.66 (1.25 to 2.20) P = 0.0005 | 0.82 (0.61 to 1.09) P = 0.15 | 0.88 (0.68 to 1.14) P = 0.34 | 0.87 (0.71 to 1.08) P = 0.22 | |
| Heterogeneity | I² statistic = 35% | I² statistic = 0% | I² statistic = 0% | I² statistic = 34% | |
| Sensitivity analysis for de Silva 1996³ | Participants | 633 | 640 | 640 | 779 |
| Pooled HR (95% CI) P value | 1.42 (1.08 to 1.86) P = 0.01 | 0.90 (0.69 to 1.17) P = 0.42 | 0.97 (0.78 to 1.21) P = 0.79 | 0.87 (0.71 to 1.06) P = 0.17 | |
| Heterogeneity | I² statistic = 0% | I² statistic = 57% | I² statistic = 60% | I² statistic = 39% | |
| CI: confidence interval | |||||
| Trial | Adverse event data¹ | Summary of reported results | |
| Carbamazepine (CBZ) | Phenobarbitone (PB) | ||
| Reported list of 'problems' at the last visit (provided as IPD) | CBZ (n = 54): speech/learning delay (n = 6), headaches (n = 3), restlessness/hyperactivity/poor attention/irritability (n = 6), psychomotor deterioration/delay (n = 2), sleep disturbances (n = 2), fatigue (n = 1), hydrocephalus (build up of fluid on the brain) (n = 1), CBZ hypersensitivity (n = 1), aggression (n = 1), temper tantrums (n = 1), other behavioural problems (n = 5), poor cognition (n = 1), mild stroke (n = 1), mild right‐sided weakness (n = 1), intolerable behavioural problems (n = 6) | PB (n = 54): speech/learning delay (n = 7), restlessness/hyperactivity/poor attention/irritability (n = 8), sleep disturbances (n = 1), fatigue (n = 1), poor cognition (n = 2), aggression (n = 1), temper tantrums (n = 3), breath‐holding attacks (n = 1), other behavioural problems (n = 3), facial twitching (n = 1), left‐sided weakness (n = 1), leg pain (n = 1), vomiting (n = 1), intolerable behavioural problems (n = 4) | |
| Rate of drug side‐effects | No statistical significant difference was seen after treatment between 2 groups in the rate of drug side‐effects | No statistical significant difference was seen after treatment between 2 groups in the rate of drug side‐effects | |
| Most frequently observed side‐effects | Gastrointestinal side‐effects and "impaired function" (general malaise). Frequency not clearly stated | Gastrointestinal side‐effects and "impaired function" (general malaise). Frequency not clearly stated | |
| Withdrawal from the study due to 'allergic reactions' | CBZ (n = 24): 1 participant withdrew due to an allergic reaction | PB (n = 23): 2 participants withdrew due to allergic reactions | |
| No adverse events reported | Not reported | Not reported | |
| "Exclusions due to adverse events or no efficacy" | Proportion "excluded": 30% (out of 30 randomised to CBZ) | Proportion "excluded": 33.3% (out of 30 randomised to PB) | |
| "Unacceptable" adverse events leading to drug withdrawal | CBZ (n = 54): drowsiness (n = 1), blood dyscrasia (n = 1) | PB (n = 10): drowsiness (n = 1), behavioural (n = 5) | |
| Reports of minor adverse events and side‐effects leading to drug withdrawal | CBZ (n = 150): withdrawals due to side‐effects: skin rash (n = 4), psychosis (n = 1), aggressive behaviour (n = 1). Minor adverse events: CBZ: 46 participants reported 68 adverse events | PB (n = 152): withdrawals due to side‐effects: skin rash (n = 1), psychosis (n = 1), hyperactivity (n = 3). Minor adverse events: 58 participants reported 86 adverse events | |
| "Unacceptable" adverse events leading to drug withdrawal | CBZ (n = 61): drowsiness (n = 3), rash (n = 2), headache (n = 1), depression (n = 1) | PB (n = 58): drowsiness (n = 4), lethargy (n = 4), rash (n = 1), dizziness (n = 2), headaches (n = 1), nausea and vomiting (n = 1) | |
| Narrative report of 'adverse effects' and 'serious side‐effects' | CBZ (n = 155): motor disturbance (ataxia, incoordination, nystagmus, tremor ‐ 33%), dysmorphic and idiosyncratic side‐effects (gum hypertrophy, hirsutism, acne, and rash ‐ 14%), gastrointestinal problems (27%), decreased libido or impotence (13%). No serious side‐effects | PB (n = 155): motor disturbance (ataxia, incoordination, nystagmus, tremor ‐ 24%), dysmorphic and idiosyncratic side‐effects (gum hypertrophy, hirsutism, acne, and rash ‐11 %), gastrointestinal problems (13%), decreased libido or impotence (16%). No serious side‐effects | |
| Systemic side‐effects and side‐effects leading to drug change | CBZ (n = 15): 4 participants switched from CBZ to PB; 3 due to systemic side‐effects (1 with persistent rashes and 1 with marked granulocytopenia (decrease of granulocytes (white blood cells)) and 1 due to behavioural changes | PB (n = 18): 1 participant switched from PB to CBZ due to substantial behavioural side‐effects | |
| Participant‐reported symptomatic complaints (provided as IPD) | CBZ (n = 19), memory impairment (n = 9), psychomotor retardation (n = 1), inattention (n = 1), transient rash (n = 1), CBZ‐induced cough (n = 1) | PB (n = 18), memory impairment (n = 13), psychomotor retardation (n = 8), inattention (n = 9) | |
| Number of participants reporting side‐effects | CBZ (n = 95): 53 participants reported at least 1 side‐effect | PB (n = 97): 50 participants reported at least 1 side‐effect | |
| CBZ: carbamazepine; PB: phenobarbitone | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to withdrawal of allocated treatment Show forest plot | 4 | 676 | Hazard Ratio (Fixed, 95% CI) | 1.49 [1.15, 1.94] |
| 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type Show forest plot | 4 | 676 | Hazard Ratio (Fixed, 95% CI) | 1.50 [1.15, 1.95] |
| 2.1 Generalised onset | 3 | 156 | Hazard Ratio (Fixed, 95% CI) | 1.53 [0.81, 2.88] |
| 2.2 Partial onset | 4 | 520 | Hazard Ratio (Fixed, 95% CI) | 1.49 [1.12, 2.00] |
| 3 Time to 12‐month remission Show forest plot | 4 | 683 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.72, 1.19] |
| 4 Time to 12‐month remission ‐ stratified by epilepsy type Show forest plot | 4 | 683 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.72, 1.20] |
| 4.1 Generalised onset | 3 | 158 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.41, 1.01] |
| 4.2 Partial onset | 4 | 525 | Hazard Ratio (Fixed, 95% CI) | 1.11 [0.81, 1.51] |
| 5 Time to six‐month remission Show forest plot | 4 | 683 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.83, 1.26] |
| 6 Time to six‐month remission ‐ stratified by epilepsy type Show forest plot | 4 | 683 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 6.1 Generalised onset | 3 | 158 | Hazard Ratio (Fixed, 95% CI) | 0.69 [0.47, 1.01] |
| 6.2 Partial onset | 4 | 525 | Hazard Ratio (Fixed, 95% CI) | 1.17 [0.90, 1.50] |
| 7 Time to first seizure Show forest plot | 6 | 822 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.71, 1.04] |
| 8 Time to first seizure ‐ stratified by epilepsy type Show forest plot | 6 | 822 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.72, 1.06] |
| 8.1 Generalised onset | 5 | 238 | Hazard Ratio (Fixed, 95% CI) | 1.23 [0.86, 1.77] |
| 8.2 Partial onset | 6 | 584 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| 9 Time to first seizure ‐ sensitivity analysis Show forest plot | 6 | 822 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| 9.1 Generalised onset | 5 | 173 | Hazard Ratio (Fixed, 95% CI) | 1.39 [0.90, 2.13] |
| 9.2 Partial onset | 6 | 584 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| 9.3 Uncertain seizure type | 3 | 65 | Hazard Ratio (Fixed, 95% CI) | 1.22 [0.59, 2.51] |