| Trial | Adverse event data¹ | Summary of reported results |
| Carbamazepine (CBZ) | Phenobarbitone (PB) |
| Banu 2007² | Reported list of 'problems' at the last visit (provided as IPD) | CBZ (n = 54): speech/learning delay (n = 6), headaches (n = 3), restlessness/hyperactivity/poor attention/irritability (n = 6), psychomotor deterioration/delay (n = 2), sleep disturbances (n = 2), fatigue (n = 1), hydrocephalus (build up of fluid on the brain) (n = 1), CBZ hypersensitivity (n = 1), aggression (n = 1), temper tantrums (n = 1), other behavioural problems (n = 5), poor cognition (n = 1), mild stroke (n = 1), mild right‐sided weakness (n = 1), intolerable behavioural problems (n = 6) | PB (n = 54): speech/learning delay (n = 7), restlessness/hyperactivity/poor attention/irritability (n = 8), sleep disturbances (n = 1), fatigue (n = 1), poor cognition (n = 2), aggression (n = 1), temper tantrums (n = 3), breath‐holding attacks (n = 1), other behavioural problems (n = 3), facial twitching (n = 1), left‐sided weakness (n = 1), leg pain (n = 1), vomiting (n = 1), intolerable behavioural problems (n = 4) |
| Bidabadi 2009³ | Rate of drug side‐effects | No statistical significant difference was seen after treatment between 2 groups in the rate of drug side‐effects | No statistical significant difference was seen after treatment between 2 groups in the rate of drug side‐effects |
| Cereghino 1974²,⁴ | Most frequently observed side‐effects | Gastrointestinal side‐effects and "impaired function" (general malaise). Frequency not clearly stated | Gastrointestinal side‐effects and "impaired function" (general malaise). Frequency not clearly stated |
| Chen 1996 | Withdrawal from the study due to 'allergic reactions' | CBZ (n = 24): 1 participant withdrew due to an allergic reaction | PB (n = 23): 2 participants withdrew due to allergic reactions |
| Cossu 1984 | No adverse events reported | Not reported | Not reported |
| Czapinski 1997³ | "Exclusions due to adverse events or no efficacy" | Proportion "excluded": 30% (out of 30 randomised to CBZ) | Proportion "excluded": 33.3% (out of 30 randomised to PB) |
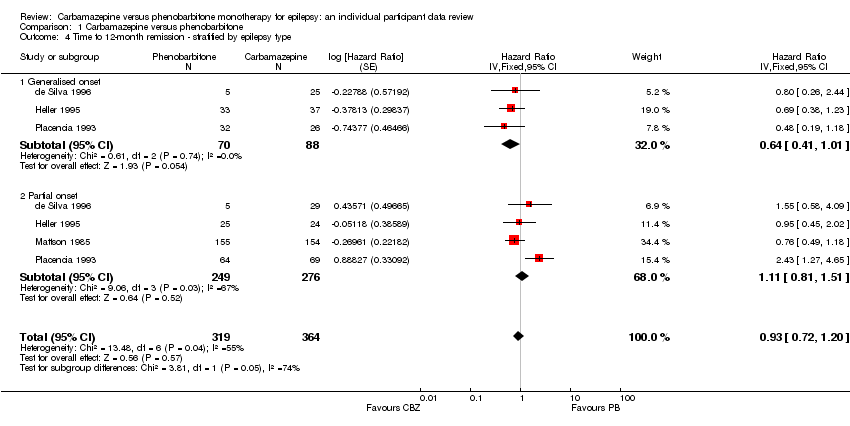
| de Silva 1996⁵,⁶ | "Unacceptable" adverse events leading to drug withdrawal | CBZ (n = 54): drowsiness (n = 1), blood dyscrasia (n = 1) | PB (n = 10): drowsiness (n = 1), behavioural (n = 5) |
| Feksi 1991 | Reports of minor adverse events and side‐effects leading to drug withdrawal | CBZ (n = 150): withdrawals due to side‐effects: skin rash (n = 4), psychosis (n = 1), aggressive behaviour (n = 1). Minor adverse events: CBZ: 46 participants reported 68 adverse events | PB (n = 152): withdrawals due to side‐effects: skin rash (n = 1), psychosis (n = 1), hyperactivity (n = 3). Minor adverse events: 58 participants reported 86 adverse events |
| Heller 1995⁵ | "Unacceptable" adverse events leading to drug withdrawal | CBZ (n = 61): drowsiness (n = 3), rash (n = 2), headache (n = 1), depression (n = 1) | PB (n = 58): drowsiness (n = 4), lethargy (n = 4), rash (n = 1), dizziness (n = 2), headaches (n = 1), nausea and vomiting (n = 1) |
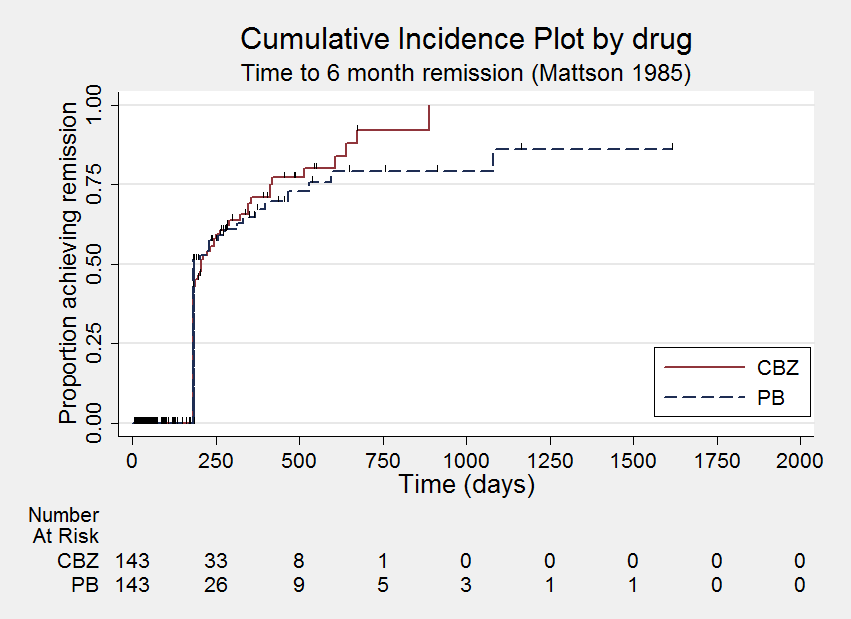
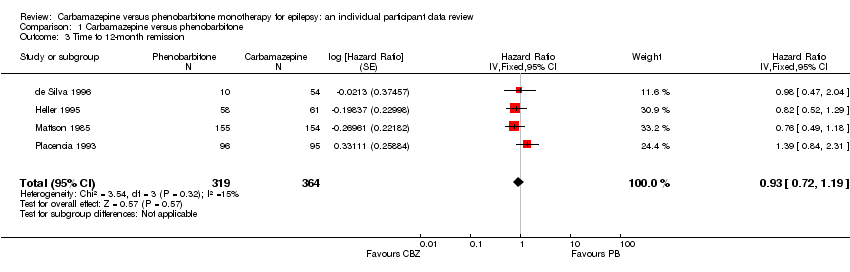
| Mattson 1985² | Narrative report of 'adverse effects' and 'serious side‐effects' | CBZ (n = 155): motor disturbance (ataxia, incoordination, nystagmus, tremor ‐ 33%), dysmorphic and idiosyncratic side‐effects (gum hypertrophy, hirsutism, acne, and rash ‐ 14%), gastrointestinal problems (27%), decreased libido or impotence (13%). No serious side‐effects | PB (n = 155): motor disturbance (ataxia, incoordination, nystagmus, tremor ‐ 24%), dysmorphic and idiosyncratic side‐effects (gum hypertrophy, hirsutism, acne, and rash ‐11 %), gastrointestinal problems (13%), decreased libido or impotence (16%). No serious side‐effects |
| Mitchell 1987 | Systemic side‐effects and side‐effects leading to drug change | CBZ (n = 15): 4 participants switched from CBZ to PB; 3 due to systemic side‐effects (1 with persistent rashes and 1 with marked granulocytopenia (decrease of granulocytes (white blood cells)) and 1 due to behavioural changes | PB (n = 18): 1 participant switched from PB to CBZ due to substantial behavioural side‐effects |
| Ogunrin 2005² | Participant‐reported symptomatic complaints (provided as IPD) | CBZ (n = 19), memory impairment (n = 9), psychomotor retardation (n = 1), inattention (n = 1), transient rash (n = 1), CBZ‐induced cough (n = 1) | PB (n = 18), memory impairment (n = 13), psychomotor retardation (n = 8), inattention (n = 9) |
| Placencia 1993 | Number of participants reporting side‐effects | CBZ (n = 95): 53 participants reported at least 1 side‐effect | PB (n = 97): 50 participants reported at least 1 side‐effect |