Фенитонин в сравнении с вальпроатом в монотерапии при парциальных и генерализированных тонико‐клонических припадках: обзор индивидуальных данных участников
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel study design, outpatient setting Study conducted in Eire (Republic of Ireland) Randomisation based on two Latin squares and the preference of drug for the participant An independent person selected “drug of first preference” from randomisation list | |
| Participants | Adults and children with a minimum of 2 untreated generalised or partial seizures in the 6 months preceding the trial Number randomised: PHT = 58; SV = 64 48 participants (39%) with partial epilepsy. 67 (55%) men Age range: 5‐71. Duration of treatment (range in months):3‐48 | |
| Interventions | Monotherapy with PHT or SV Mean daily dose achieved: PHT: 5.4 mg/kg; SV: 15.6 mg/kg | |
| Outcomes | Seizure control: | |
| Notes | Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation based on 2 Latin Squares without stratification. The first, second and third preference of drug for the participant appears to have been taken into account in the process. Unclear if assignment was completely random |
| Allocation concealment (selection bias) | High risk | An independent person (department secretary) selected the “drug of first preference” from randomisation list on a sequential basis. Allocation not adequately concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attirition rates reported. ITT approach taken, all randomised participants analysed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes (seizure control) and secondary outcomes (side effects) reported sufficiently. No protocol available, outcomes for this review not reported |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel study design Study conducted in the UK Participants randomised using computerised stratified minimisation program by age group, sex and seizure type Allocation was pharmacy‐controlled The main investigator performing cognitive testing was blinded to allocation. Participants and personnel unblinded | |
| Participants | Participants over 60 years of age with newly onset seizures (1 or more generalised tonic‐clonic seizures or 2 or more partial seizures) Number randomised: PHT = 81; SV = 85 80 participants (48%) with partial epilepsy, 71 (44%) men Mean age (range): 78 (61‐95 years). Range of follow‐up: 1‐20 months | |
| Interventions | Monotherapy with PHT or SV Starting doses: PHT: 200 mg/day, SV: 400 mg/day Median daily dose achieved: PHT 247 mg (range 175‐275); SV: 688 mg (range 400‐1000) | |
| Outcomes | Psychological tests (cognitive function, anxiety and depression) Adverse event frequency Seizure control | |
| Notes | Trial paper reports on a subset of 38 participants. Full IPD set provided and used for this review includes all 166 participants randomised in the trial. IPD provided for 3/4 outcomes of this review ('withdrawal from allocated treatment' not available) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised stratified minimisation programme, stratified for age group, gender and seizure type |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled allocation, prescription disclosed to general practitioner and consultant |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | The main investigator performing cognitive testing was blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported. ITT analysis undertaken with all randomised participants from IPD (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported in published report or provided in IPD (see footnote 2) |
| Other bias | Low risk | No other bias detected |
| Methods | 36‐month randomised comparative trial Parallel study design Study conducted in Poland Method of generation of random list and allocation concealment not stated | |
| Participants | Adults with newly diagnosed epilepsy Number randomised: PHT = 30; SV = 30 100% partial epilepsy, age range: 18 to 40 years Percentage men and range of follow‐up not mentioned | |
| Interventions | Monotherapy with PHT or SV Starting doses: PHT: 200 mg/day, SV: 600 mg/day. Dose achieved not stated | |
| Outcomes | Proportion achieving 24‐month remission at 3 years | |
| Notes | Abstract only. Outcomes chosen for this review were not reported. IPD pledged but not received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial "randomised" but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | "Exclusion rates" (interpreted as withdrawal rates) reported for all treatment groups, no further information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available and trial reported only in abstract form, outcomes for this review not available |
| Other bias | Unclear risk | Insufficient detail provided in abstract to allow judgement |
| Methods | Parallel study design, outpatient setting Study conducted at two centres in the UK Random list generated using random permuted blocks Allocation concealed using sealed opaque envelopes Unblinded | |
| Participants | Children with newly diagnosed epilepsy (2 or more untreated partial or generalised tonic‐clonic seizures in the 12 months preceding the trial) Number randomised: PHT = 54; SV = 49 55 children (53%) with partial epilepsy. 52 (50%) boys Mean age (range): 10 (3‐16) years. Range of follow‐up (months): 3‐88 | |
| Interventions | Monotherapy with PHT or SV Median daily dose achieved: PHT: 175 mg/day, SV: 600 mg/day | |
| Outcomes | Time to first seizure recurrence after start of therapy | |
| Notes | IPD provided for all outcomes of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via 4 batches of sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded, authors state masking of treatment would not be “practicable or ethical” and would “undermine compliance” |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded, authors state masking of treatment would not be “practicable or ethical” and would “undermine compliance” |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel study design, outpatient setting Study conducted in the UK Patients randomly allocated using quota allocation allowing for gender, age, seizure type and current treatment Outcome assessors were single‐blinded for cognitive testing | |
| Participants | Children with at least 3 newly diagnosed generalised or partial seizures within a period of 6 months Number randomised: PHT = 20; SV = 21 No information on epilepsy type, gender or range of follow‐up Age range: 5‐14 years. Trial duration: 12 months | |
| Interventions | Monotherapy with PHT or SV Mean dose achieved: PHT: 6.1 mg/day, SV: 25.3 mg/day | |
| Outcomes | Cognitive assessments | |
| Notes | Outcomes chosen for this review were not reported. IPD not available, but could be constructed from the publication for the outcome 'Time on allocated drug' (without stratification by seizure type) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quota allocation by gender, age, seizure type and current treatment is an inadequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Personnel and participants (and parents) unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors single‐blinded for cognitive testing |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, results reported and analysed for all participants randomised and all who completed various stages of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes described in methods section well reported in results section. Adverse events reported, no seizure outcomes reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel study design, outpatient setting Study conducted at two centres in the UK Random list generated using random permuted blocks Allocation concealed using sealed opaque envelopes Unblinded | |
| Participants | Adults with newly diagnosed epilepsy (2 or more untreated partial or generalised tonic‐clonic seizures in the 12 months preceding the trial) Number randomised: PHT = 63; SV = 61 53 participants (43%) with partial epilepsy. 62 (48%) men Mean age (range): 33 (14‐72) years Range of follow‐up (months): 1‐91 | |
| Interventions | Monotherapy with PHT or SV Median daily dose achieved: PHT: 300 mg/day, SV: 800 mg/day | |
| Outcomes | Time to first seizure recurrence after start of therapy | |
| Notes | IPD provided for all outcomes of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via 4 batches of concealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded, authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large drop‐out rate” |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded, authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large drop‐out rate” |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analyses from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel trial Study conducted at 16 centres in the United States Participants assigned via randomisation tables within each centre in a 2:1 ratio (SV:PHT) Method of allocation concealment not stated Unblinded | |
| Participants | Participants with at least 2 newly diagnosed and previously untreated primary generalised tonic‐clonic seizures within 14 days of starting the trial Number randomised: PHT = 50; SV = 86 0% participants with partial epilepsy, 73 (54%) men Mean age (range): 21 (3‐64 years). Participants followed up for up to 6 months | |
| Interventions | Monotherapy with PHT or SV Starting doses PHT: 3‐5 mg/kg/day, SV: 10‐15 mg/kg/day, doses gradually increased Doses achieved not stated | |
| Outcomes | Time to first generalised tonic‐clonic seizure 6‐month seizure recurrence rates Adverse events | |
| Notes | IPD provided for 3/4 outcomes of this review (maximum follow‐up 6 months, therefore trial cannot contribute to outcome 'Time to achieve 12‐month remission') | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised on a 2:1 ratio SV:PHT using randomisation tables in each centre (information provided by trial author) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial; authors state that differences in adverse events of PHT and SV would "quickly unblind" the trial anyway |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial, authors state that differences in adverse events of PHT and SV would "quickly unblind" the trial anyway |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel study design, outpatient setting Study conducted in Meerut, India No information provided on method of generation of random list, allocation concealment or blinding | |
| Participants | Participants with at least 2 partial or generalised tonic‐clonic seizures per month Unclear if participants were newly diagnosed Number randomised: PHT = 45; SV = 49 27 participants (29%) partial epilepsy, 70 (74%) men Age range: PHT: 12‐42 years; SV: 8‐52 years Participants were evaluated after 4, 12 and 24 weeks of treatment No information on range of follow‐up | |
| Interventions | Monotherapy with PHT or SV Average daily dose achieved: PHT: 5.6 mg/kg/day, SV: 18.8 mg/kg/day | |
| Outcomes | Reduction in frequency of seizures: Seizure control | |
| Notes | Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "randomly allocated irrespective of seizure type," no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Frequency of seizures reported for all randomised participants, no information provided on withdrawal rates/attrition rates etc. |
| Selective reporting (reporting bias) | Low risk | Frequency of seizures during treatment well reported, most common adverse events reported No protocol available to compare with a priori analysis plan, outcomes for this review not reported |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel study design, outpatient setting Study conducted in two centres (Glasgow, Scotland and Wellington, New Zealand) Participants allocated using telephone randomisation within the two centres (information provided by trial author) No information provided on method of allocation concealment or blinding | |
| Participants | 21 (64%) participants previously untreated, 12 (36%) participants continued to have seizures on previous drug therapies Original treatments gradually withdrawn before PHT or SV treatment introduced Number randomised: PHT = 15; SV = 18 19 participants (58%) with partial epilepsy, 12 (36%) men Mean age (range): 23 (7‐55 years). Mean follow‐up (range): 30 (9‐48 months) | |
| Interventions | Monotherapy with PHT or SV Starting doses: PHT: < 12 years 150 mg/day, older participants: 300 mg/day SV: < 12 years 300‐400 mg/day, older participants: 800‐1200 mg/day. Doses achieved not stated | |
| Outcomes | Seizures during treatment | |
| Notes | Outcomes chosen for this review were not reported IPD not available but could be constructed from the publication for the outcome 'Time to withdrawal of allocated treatment' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants "randomly divided", using telephone randomisation (information provided by trial author) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Results reported for all randomised participants, time on treatment reported for all randomised participants. No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | No protocol available, outcomes chosen for this review not reported. Seizure outcomes and adverse events well reported |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel study design, outpatient setting Study conducted in Madras (Chennai), India Random list generated using computer‐generated random numbers Method of concealment not mentioned Double‐blind achieved by providing additional placebo tablets | |
| Participants | Children with more than 1 previously untreated generalised tonic‐clonic (afebrile) seizure Number randomised: PHT = 52; SV = 48 0% partial epilepsy. 52 (52%) men. Age range: 4‐12 years Range of follow‐up (months): 22‐36 | |
| Interventions | Monotherapy with PHT or SV Starting doses: PHT: 5‐8 mg/kg/day, SV: 15‐50 mg/kg/day Dose achieved not stated | |
| Outcomes | Proportion with recurrence of seizures | |
| Notes | Outcomes chosen for this review were not reported. IPD not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised via a computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double–blinded using additional placebo tablets; unclear who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double–blinded using additional placebo tablets; unclear who was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported; all randomised participants analysed |
| Selective reporting (reporting bias) | Low risk | No protocol available; outcomes chosen for this review not reported |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel study design, outpatient setting Study conducted in the UK Participants allocated to treatment stratified by age group, gender and seizure type No information provided on method of generation of random list, allocation concealment or blinding | |
| Participants | Participants with 2 or more partial or generalised tonic‐clonic seizure in the past 3 years Participants were previously untreated but started on AED treatment within 3 months of their most recent seizure Number randomised: PHT = 70; SV = 70 63 participants (45%) with partial onset seizures, 73 (52%) men Mean age (range): 35 (14‐70 years). Range of follow‐up: 24‐48 months | |
| Interventions | Monotherapy with PHT or SV Starting doses: PHT 300 mg/day, SV 600 mg/day. Dose achieved not stated | |
| Outcomes | Time to 2‐year remission Time to first seizure Adverse events | |
| Notes | IPD provided for all outcomes included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised with stratification for age group, gender and seizure type. Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, ITT approach, all randomised participants analysed from IPD provided (see footnote 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided (see footnote 2) |
| Other bias | Unclear risk | No other bias detected |
1 Abbreviations:
AED: antiepileptic drug; IPD: individual participant data; ITT: Intention‐to‐treat; PHT: phenytoin; SV: sodium valproate.
2 For studies which provided IPD, attrition and reporting bias are reduced as attrition rates and unpublished outcome data are requested (Craig 1994; De Silva 1996; Heller 1995; Ramsay 1992; Turnbull 1985).
3 See Figure 2 and Figure 3 for 'Risk of bias' summary and graph.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Reports the same trial as Forsythe 1991, but more relevant information given in the Forsythe publication | |
| Abstract only. Preliminary results of the trial reported in Callaghan 1985 | |
| Abstract only. Preliminary results of the trial reported in Callaghan 1985 | |
| Preliminary results of the trial reported in Callaghan 1985 | |
| Abstract only. Preliminary results of the trial reported in Craig 1994 | |
| Reports the same abstract as Czapinski 1997a | |
| Reports the same abstract as Czapinski 1997a | |
| Abstract only. Preliminary results of the trial reported in Callaghan 1985 | |
| Reports the same trial as Callaghan 1985, but more relevant information given in the Callaghan publication | |
| No randomised comparison of phenytoin and valproate (participants randomised to a dose adjustment method rather than to a treatment) | |
| No randomised comparison of phenytoin and valproate (study of lamotrigine versus 'standard' AED treatment) | |
| Not fully randomised: "The treatment was chosen at random unless the individual diagnoses required a specific drug" | |
| No randomised comparison of phenytoin and valproate (post‐hoc analysis of 5 studies of oxcarbazepine versus another AED) | |
| Reports the same trial as Shakir 1981. There are some differences between the results in the 2 publications. The reason for this could not be established | |
| Abstract only. Reports the same trial as Craig 1994 | |
| Abstract only. Reports the same trial as Craig 1994 | |
| Preliminary results of the trial reported in Turnbull 1985 | |
| Preliminary results of the trial reported in Turnbull 1985 | |
| Not randomised |
AED: antiepileptic drug
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to withdrawal of allocated treatment Show forest plot | 6 | 569 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.73, 1.42] |
| Analysis 1.1 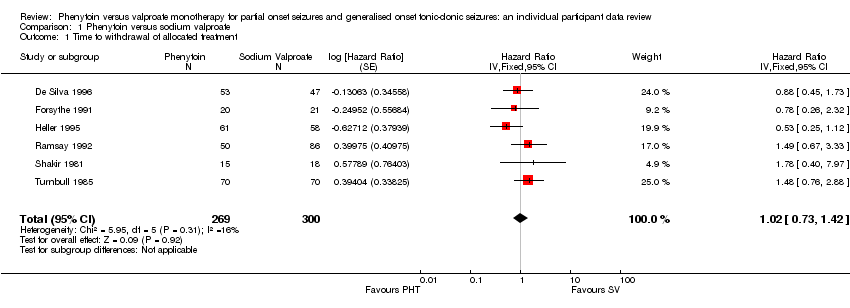 Comparison 1 Phenytoin versus sodium valproate, Outcome 1 Time to withdrawal of allocated treatment. | ||||
| 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type Show forest plot | 5 | 528 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.76, 1.55] |
| Analysis 1.2  Comparison 1 Phenytoin versus sodium valproate, Outcome 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type. | ||||
| 2.1 Generalised onset seizures (tonic‐clonic only) | 5 | 341 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.59, 1.64] |
| 2.2 Partial onset seizures | 4 | 187 | Hazard Ratio (Fixed, 95% CI) | 1.20 [0.74, 1.95] |
| 3 Time to achieve 12‐month remission Show forest plot | 4 | 514 | Hazard Ratio (Fixed, 95% CI) | 0.97 [0.77, 1.22] |
| Analysis 1.3 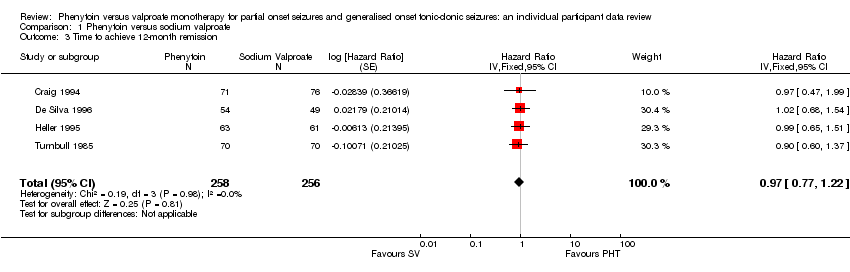 Comparison 1 Phenytoin versus sodium valproate, Outcome 3 Time to achieve 12‐month remission. | ||||
| 4 Time to achieve 12‐month remission ‐ stratified by epilepsy type Show forest plot | 4 | 514 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| Analysis 1.4 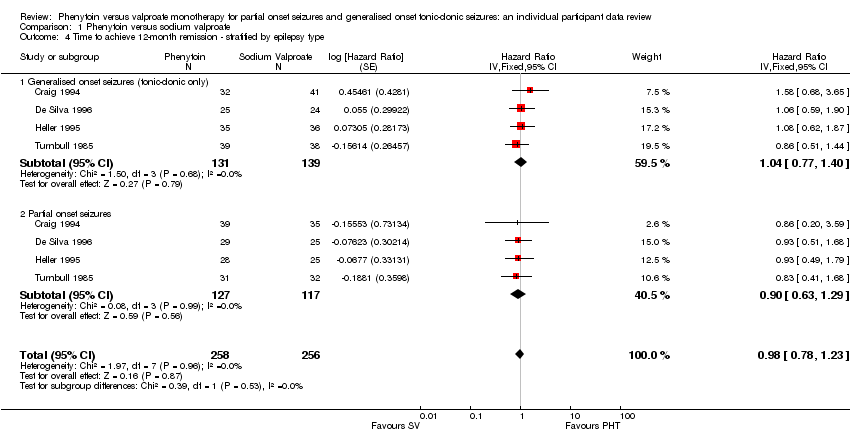 Comparison 1 Phenytoin versus sodium valproate, Outcome 4 Time to achieve 12‐month remission ‐ stratified by epilepsy type. | ||||
| 4.1 Generalised onset seizures (tonic‐clonic only) | 4 | 270 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.77, 1.40] |
| 4.2 Partial onset seizures | 4 | 244 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.63, 1.29] |
| 5 Time to achieve six‐month remission Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.76, 1.12] |
| Analysis 1.5 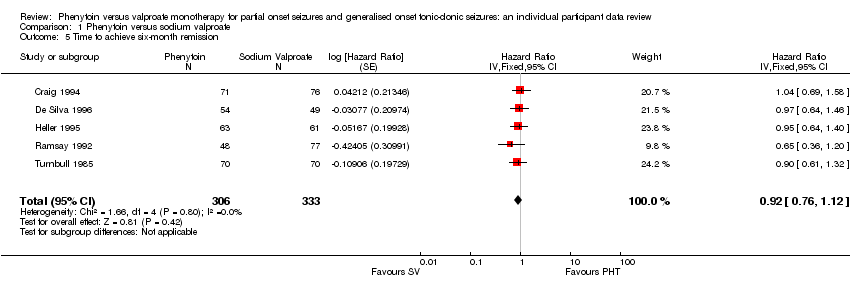 Comparison 1 Phenytoin versus sodium valproate, Outcome 5 Time to achieve six‐month remission. | ||||
| 6 Time to achieve six‐month remission ‐ stratified by epilepsy type Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.78, 1.15] |
| Analysis 1.6  Comparison 1 Phenytoin versus sodium valproate, Outcome 6 Time to achieve six‐month remission ‐ stratified by epilepsy type. | ||||
| 6.1 Generalised onset seizures (tonic‐clonic only) | 5 | 395 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.72, 1.18] |
| 6.2 Partial onset seizures | 4 | 244 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.73, 1.35] |
| 7 Time to first seizure Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.78, 1.18] |
| Analysis 1.7 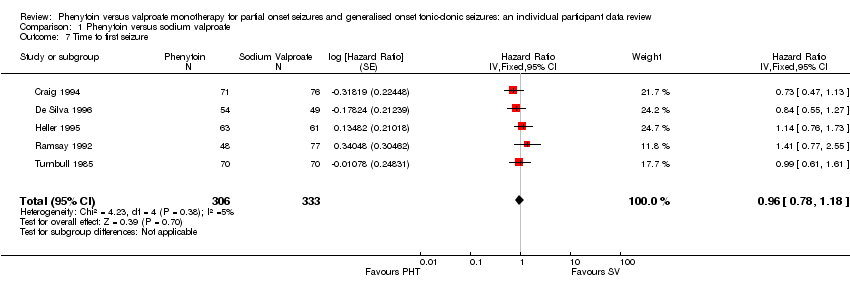 Comparison 1 Phenytoin versus sodium valproate, Outcome 7 Time to first seizure. | ||||
| 8 Time to first seizure ‐ stratified by epilepsy type Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.75, 1.14] |
| Analysis 1.8 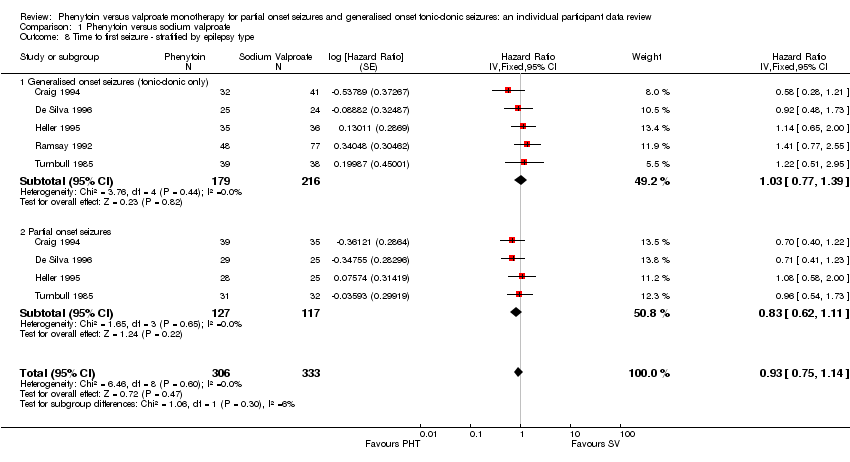 Comparison 1 Phenytoin versus sodium valproate, Outcome 8 Time to first seizure ‐ stratified by epilepsy type. | ||||
| 8.1 Generalised onset seizures (tonic‐clonic only) | 5 | 395 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.77, 1.39] |
| 8.2 Partial onset seizures | 4 | 244 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.62, 1.11] |
| 9 Time to first seizure ‐ epilepsy type reclassified to uncertain for generalised and age of onset > 30 years Show forest plot | 5 | 649 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.76, 1.15] |
| Analysis 1.9 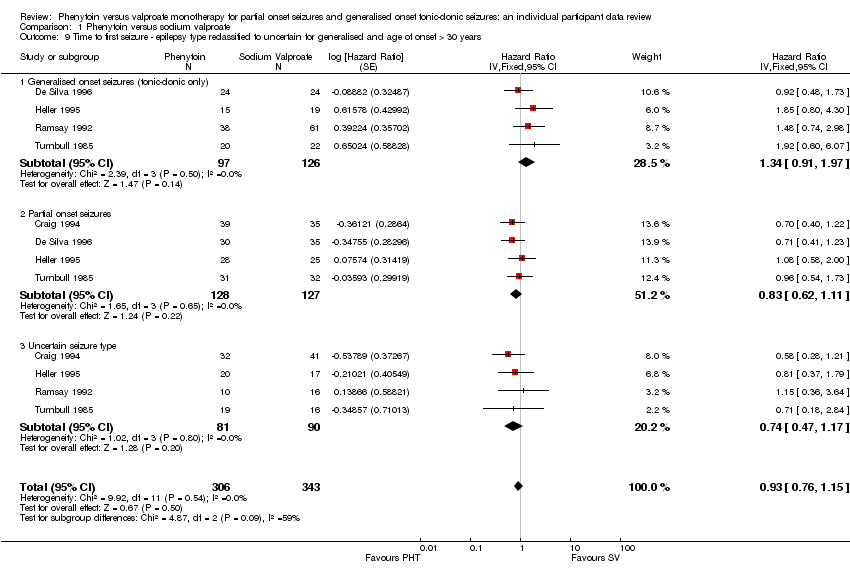 Comparison 1 Phenytoin versus sodium valproate, Outcome 9 Time to first seizure ‐ epilepsy type reclassified to uncertain for generalised and age of onset > 30 years. | ||||
| 9.1 Generalised onset seizures (tonic‐clonic only) | 4 | 223 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.91, 1.97] |
| 9.2 Partial onset seizures | 4 | 255 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.62, 1.11] |
| 9.3 Uncertain seizure type | 4 | 171 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.47, 1.17] |
| 10 Time to first seizure ‐ epilepsy type reclassified to partial for generalised and age of onset > 30 years Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.76, 1.15] |
| Analysis 1.10  Comparison 1 Phenytoin versus sodium valproate, Outcome 10 Time to first seizure ‐ epilepsy type reclassified to partial for generalised and age of onset > 30 years. | ||||
| 10.1 Generalised onset seizures (tonic‐clonic only) | 4 | 223 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.91, 1.97] |
| 10.2 Partial onset seizures | 5 | 416 | Hazard Ratio (Fixed, 95% CI) | 0.81 [0.64, 1.04] |
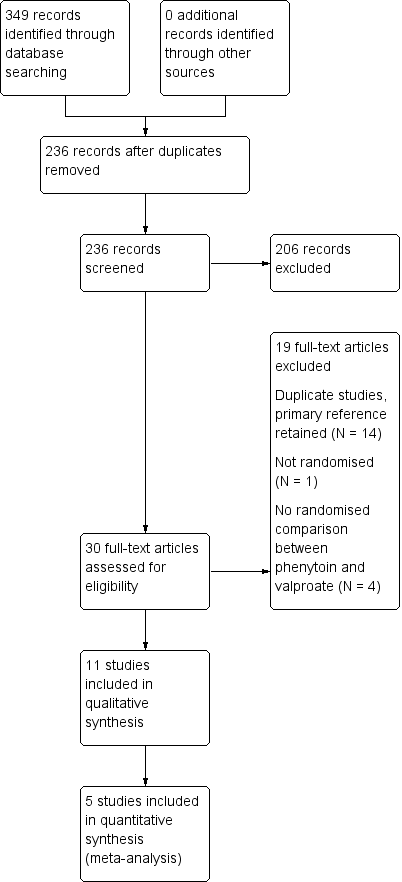
Study flow diagram.
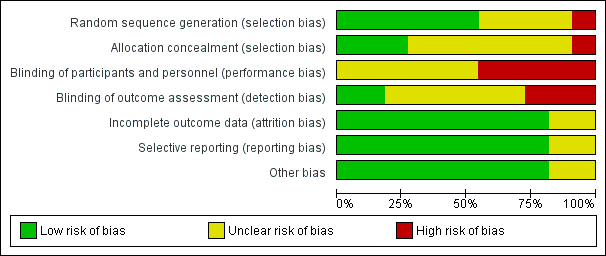
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
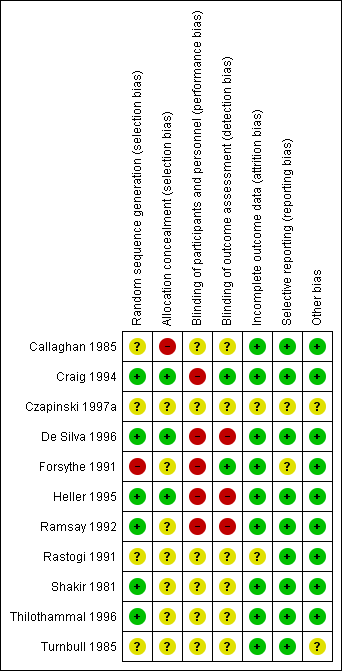
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

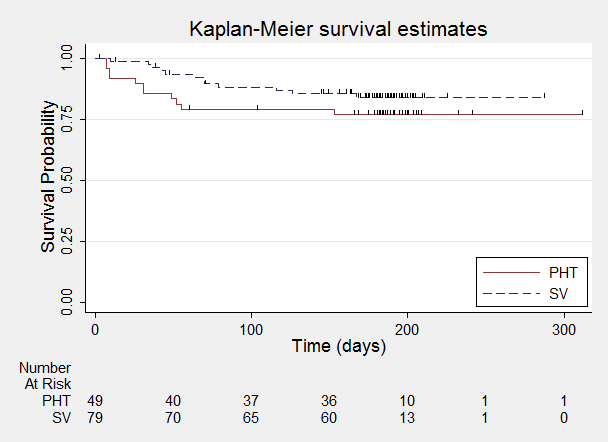
Time to withdrawal of allocated treatment.
One participant randomised to phenytoin (PHT) and nine participants randomised to valproate (SV) had time to withdrawal of zero days, and are therefore not included in "Number at Risk".

Time to withdrawal of allocated treatment ‐ stratified by epilepsy type.
One participant with generalised epilepsy randomised to phenytoin (PHT) and nine participants with generalised epilepsy randomised to valproate (SV) had time to withdrawal of zero days, and are therefore not included in "Number at Risk".

Time to achieve 12‐month remission.
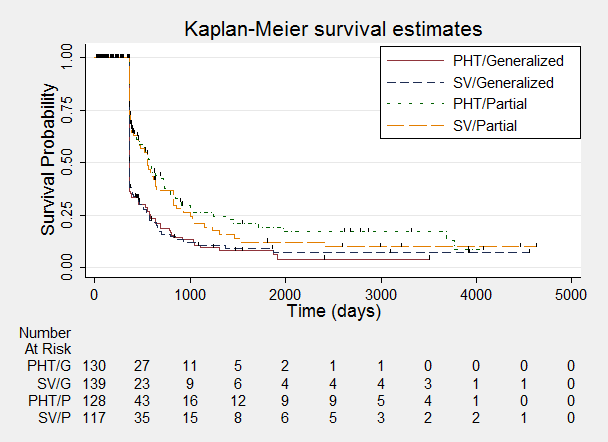
Time to achieve 12‐month remission ‐ stratified by epilepsy type.

Time to achieve six‐month remission.

Time to achieve six‐month remission ‐ stratified by epilepsy type.

Time to first seizure.

Time to first seizure ‐ stratified by epilepsy type.

Comparison 1 Phenytoin versus sodium valproate, Outcome 1 Time to withdrawal of allocated treatment.

Comparison 1 Phenytoin versus sodium valproate, Outcome 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type.

Comparison 1 Phenytoin versus sodium valproate, Outcome 3 Time to achieve 12‐month remission.

Comparison 1 Phenytoin versus sodium valproate, Outcome 4 Time to achieve 12‐month remission ‐ stratified by epilepsy type.

Comparison 1 Phenytoin versus sodium valproate, Outcome 5 Time to achieve six‐month remission.

Comparison 1 Phenytoin versus sodium valproate, Outcome 6 Time to achieve six‐month remission ‐ stratified by epilepsy type.
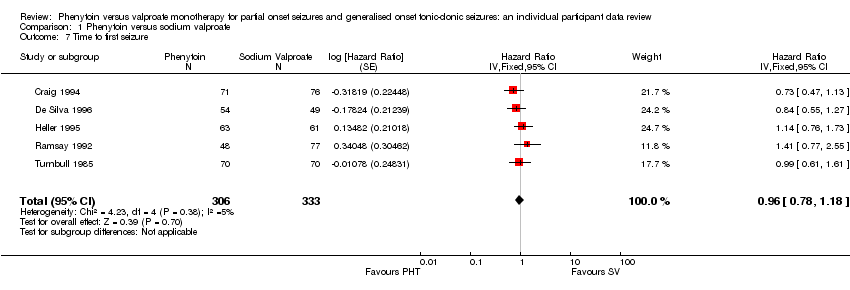
Comparison 1 Phenytoin versus sodium valproate, Outcome 7 Time to first seizure.

Comparison 1 Phenytoin versus sodium valproate, Outcome 8 Time to first seizure ‐ stratified by epilepsy type.

Comparison 1 Phenytoin versus sodium valproate, Outcome 9 Time to first seizure ‐ epilepsy type reclassified to uncertain for generalised and age of onset > 30 years.

Comparison 1 Phenytoin versus sodium valproate, Outcome 10 Time to first seizure ‐ epilepsy type reclassified to partial for generalised and age of onset > 30 years.
| Phenytoin compared with valproate for partial onset seizures and generalised onset tonic‐clonic seizures | ||||||
| Patient or population: Adults and children with newly‐onset partial or generalised tonic‐clonic seizures Settings: Outpatients Intervention: Valproate Comparison: Phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenytoin | Valproate | |||||
| Time to withdrawal of allocated treatment (retention time) ‐ stratified by epilepsy type Range of follow‐up (all participants): 1‐91 months | 27 per 100 | 25 per 100 (18 to 33) | HR 1.09 (0.76 to 1.55)1 | 528 | ⊕⊕⊕⊝ | HR > 1 indicates a clinical |
| Time to withdrawal of allocated treatment (retention time) ‐ stratified by epilepsy type: generalised onset seizures (tonic‐clonic only) Range of follow‐up (all participants): 1‐91 months | 18 per 100 | 19 per 100 (12 to 29) | HR 0.98 (0.59 to 1.64) | 341 | ⊕⊕⊕⊝ | HR > 1 indicates a clinical |
| Time to withdrawal of allocated treatment (retention time) ‐ stratified by epilepsy type: partial onset seizures Range of follow‐up (all participants): 1‐91 months | 39 per 100 | 34 per 100 (23 to 49) | HR 1.20 (0.74 to 1.95) | 187 (4 studies) | ⊕⊕⊕⊝ | HR > 1 indicates a clinical |
| Time to achieve 12‐month remission (seizure‐free period) ‐ stratified by epilepsy type Range of follow‐up (all participants): 1‐91 months | 67 per 100 | 67 per 100 (58 to 75) | HR 0.98 (0.78 to 1.23)1 | 514 (4 studies) | ⊕⊕⊕⊝ | HR > 1 indicates a clinical |
| Time to achieve 12‐month remission (seizure‐free period) ‐ stratified by epilepsy type: generalised onset seizures (tonic‐clonic only) Range of follow‐up (all participants): 1 ‐ 91 months | 67 per 100 | 69 per 100 (58 to 79) | HR 1.04 (0.77 to 1.40) | 270 (4 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a clinical |
| Time to achieve 12‐month remission (seizure‐free period) ‐ stratified by epilepsy type: partial onset seizures Range of follow up (all participants): 1‐91 months | 67 per 100 | 63 per 100 (50 to 76) | HR 0.90 (0.63 to 1.29) | 244 (4 studies) | ⊕⊕⊕⊝ | HR > 1 indicates a clinical |
| Time to achieve 6‐month remission (seizure‐free period) ‐ stratified by epilepsy type Range of follow‐up (all participants): 1 ‐ 91 months | 60 per 100 | 58 per 100 (51 to 65) | HR 0.95 (0.78 to 1.15)1 | 639 (5 studies) | ⊕⊕⊕⊝ | HR > 1 indicates a clinical |
| Time to achieve 6‐month remission (seizure‐free period) ‐ stratified by epilepsy type: generalised onset seizures (tonic‐clonic only) Range of follow‐up (all participants): 1 ‐ 91 months | 69 per 100 | 66 per 100 (57 to 75) | HR 0.92 (0.72 to 1.18) | 395 (5 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a clinical |
| Time to achieve 6‐month remission (seizure‐free period) ‐ stratified by epilepsy type: partial onset seizures Range of follow‐up (all participants): 1‐91 months | 51 per 100 | 50 per 100 (40 to 62) | HR 0.99 (0.73 to 1.35) | 244 (4 studies) | ⊕⊕⊕⊝ | HR > 1 indicates a clinical |
| Time to first seizure (post‐randomisation) ‐ stratified by epilepsy type Range of follow‐up (all participants): 1‐91 months | 59 per 100 | 62 per 100 (55 to 70) | HR 0.93 (0.75 to 1.14)1 | 639 (5 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a clinical |
| Time to first seizure (post‐randomisation) ‐ stratified by epilepsy type: generalised onset seizures (tonic‐clonic only) Range of follow‐up (all participants): 1‐91 months | 48 per 100 | 47 per 100 (38 to 58) | HR 1.03 (0.77 to 1.39) | 395 (5 studies) | ⊕⊝⊝⊝ | HR > 1 indicates a clinical |
| Time to first seizure (post‐randomisation) ‐ stratified by epilepsy type: partial onset seizures Range of follow‐up (all participants): 1‐91 months | 75 per 100 | 81 per 100 (71 to 89) | HR 0.83 (0.62 to 1.11) | 244 (4 studies) | ⊕⊕⊝⊝ | HR > 1 indicates a clinical |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The assumed risk is calculated as the event rate in the phenytoin treatment group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Pooled HR for all participants adjusted for seizure type. | ||||||
| Trial | Outcomes reported | Summary of results |
| 1. Seizure control: (a) excellent (seizure‐free) (b) good (> 50% reduction) (c) poor (< 50% reduction) 2. Adverse events | 1. PHT (n = 58); SV (n = 64) (a) 39 (67%) 34 (53%) (b) 7 (12%) 16 (25%) (c) 12 (21%) 14 (22%) 2. 6 (10%) 7 (11%) | |
| 1. Proportion achieving 24‐month remission at 3 years 2. Proportion excluded after randomisation due to adverse events or no efficacy | 1. PHT: 59%; SV: 64% 2. PHT: 23%; SV: 23% | |
| 1. Cognitive assessments 2. Withdrawals from randomised drug | 1. Significant difference favouring SV test of speed of information processing (P < 0.01) No significant differences between treatment groups for any other cognitive tests 2. PHT: 6/20 (30%); SV: 7/21 (33%) | |
| 1. Reduction in frequency of seizures at 24 weeks: (a) excellent (100% reduction) (b) good (75%‐99% reduction) (c) fair (50%‐74% reduction) (d) poor (< 50% reduction) 2. Adverse events | 1. PHT (n = 45); SV (n = 49) (a) 23 (51%) 24 (49%) (b) 13 (24%) 17 (35%) (c) 8 (18%) 5(10%) (d) 1 (2%) 3 (6%) 2. All reported adverse events were minor PHT: gum hyperplasia (18%), nystagmus (13%), gastrointestinal symptoms (4%), drowsiness (4%), ataxia (2%) SV: gastrointestinal symptoms (12%), drowsiness (6%), weight gain (2%) | |
| 1.Seizures during treatment 2. Adverse events | 1. PHT: 5 (33%); SV: 7 (39%) 2. PHT: 1 case of ataxia, 5 cases of acne. SV: 2 cases of gastrointestinal symptoms, 2 cases of hair loss, 4 cases of weight gain | |
| 1. Recurrence of seizures 2. Adverse events | 1. PHT: 14/52 (27%) SV: 10/48 (21%) 2. PHT: 33/52 (63%) SV: 15/48 (31%) | |
| PHT: phenytoin; SV: sodium valproate | ||
| Trial | Number randomised | Time to withdrawal of allocated treatment | Time to achieve 12‐month remission | Time to achieve 6‐month remission | Time to first seizure | ||||||||||
| PHT | SV | Total | PHT | SV | Total | PHT | SV | Total | PHT | SV | Total | PHT | SV | Total | |
| 81 | 85 | 166 | 0 | 0 | 0 | 71 | 76 | 147 | 71 | 76 | 147 | 71 | 76 | 147 | |
| 54 | 49 | 103 | 53 | 47 | 100 | 54 | 49 | 103 | 54 | 49 | 103 | 54 | 49 | 103 | |
| 20 | 21 | 41 | 20 | 21 | 41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 63 | 61 | 124 | 61 | 58 | 119 | 63 | 61 | 124 | 63 | 61 | 124 | 63 | 61 | 124 | |
| 50 | 86 | 136 | 50 | 86 | 136 | 0 | 0 | 0 | 48 | 77 | 125 | 48 | 77 | 125 | |
| 70 | 70 | 140 | 70 | 70 | 140 | 70 | 70 | 140 | 70 | 70 | 140 | 70 | 70 | 140 | |
| 15 | 18 | 33 | 15 | 18 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 353 | 390 | 743 | 269 | 300 | 569 | 258 | 256 | 514 | 306 | 333 | 639 | 306 | 333 | 639 |
| 1Withdrawal information not provided for Craig 1994, so cannot contribute to 'Time to withdrawal of allocated treatment'. PHT: phenytoin; SV: sodium valproate | |||||||||||||||
| Statistic | Time to withdrawal of allocated treatment | Time to achieve 12‐month remission | Time to achieve six‐month remission | Time to first seizure | |
| Test for heterogeneity | Chi² | (df = 5) 5.95 | (df = 3) 0.19 | (df = 4) 1.66 | (df = 4) 4.23 |
| P value | 0.31 | 0.98 | 0.80 | 0.38 | |
| I² | 16% | 0% | 0% | 5% | |
| Overall effect | HR (95% CI) | 1.02 (0.73 to 1.49) | 0.97 (0.77 to 1.22) | 0.92 (0.76 to 1.12) | 0.96 (0.78 to 1.18) |
| P value | 0.92 | 0.81 | 0.42 | 0.70 | |
| Test for interaction between treatment and epilepsy type | Chi² | (df = 1) 0.31 | (df = 1) 0.39 | (df = 1) 0.13 | (df = 1) 1.06 |
| P value | 0.58 | 0.53 | 0.72 | 0.3 | |
| I² | 0% | 0% | 0% | 5.6% | |
| Overall effect adjusted for epilepsy type | HR (95% CI) | 1.09 (0.76 to 1.55) | 0.98 (0.78 to 1.23) | 0.95 (0.78 to 1.15) | 0.93 (0.75 to 1.14) |
| P value | 0.19 | 0.87 | 0.60 | 0.47 | |
| CI: confidence interval; df: degrees of freedom of Chi² distribution; HR: Hazard ratio; P < 0.05 is classified as statistically significant | |||||
| Reason for early termination | Classification | De Silva 19962 | Heller 19952,3 | Ramsey 1992 | Turnbull 1985 | Total1 | |||||
| PHT n = 53 | SV n = 47 | PHT n = 63 | SV n = 58 | PHT n = 50 | SV n = 86 | PHT n = 70 | SV n = 70 | PHT n = 236 | SV n = 261 | ||
| Adverse events/intoxication | Event | 2 | 2 | 1 | 4 | 5 | 7 | 14 | 7 | 22 | 20 |
| Poor seizure control/lack of efficacy | Event | 10 | 11 | 8 | 9 | 2 | 1 | 0 | 2 | 20 | 23 |
| Both adverse events and lack of efficacy | Event | 5 | 4 | 2 | 6 | 0 | 0 | 2 | 1 | 9 | 11 |
| Non‐compliance | Event | 0 | 0 | 0 | 0 | 1 | 7 | 2 | 2 | 3 | 9 |
| Participant went into remission | Censored | 24 | 16 | 14 | 13 | 0 | 0 | 0 | 0 | 38 | 29 |
| Lost to follow‐up | Censored | 0 | 0 | 0 | 0 | 4 | 10 | 7 | 7 | 11 | 17 |
| Death4 | Censored | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 3 |
| Other5 | Censored | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 1 |
| Completed the study/did not withdraw | Censored | 12 | 14 | 38 | 26 | 36 | 60 | 42 | 48 | 128 | 148 |
| n = number of individuals contributing to the outcome 'Time to withdrawal of allocated treatment'; PHT: phenytoin; SV: sodium valproate | |||||||||||
| Trial | Adverse event data1 | Summary of reported results | |
| Phenytoin (PHT) | SV (Sodium Valproate) | ||
| All adverse events developed (by drug) and adverse events leading to discontinuation of treatment | PHT (n = 58): gum hypertrophy (n = 2), rash (n = 2), ataxia (n = 2)
| SV (n = 64): weight gain (n = 4 – all discontinued treatment), drowsiness (n = 2), aggressive behaviour (n = 1 – discontinued treatment) | |
| Adverse event frequency (spontaneous reports)2 Discontinuations due to adverse events3 | PHT (n = 25): unsteadiness (n = 9), sleepiness (n = 7), drowsiness (n = 2), impaired concentration (n = 2), confusion (n = 1), constipation (n = 1), diarrhoea (n = 1), dysarthria (n = 1), lethargy (n = 1), nystagmus (n = 1), rash (n = 1), tired legs (n = 1) PHT discontinuations (n = 6): rash (n =1), diarrhoea (n = 1), confusion (n = 1), unsteadiness (n = 1), constipation (n = 1), sleepiness (n = 1) | SV (n = 17): unsteadiness (n = 2), sleepiness (n = 3), tremor (n = 5), oedema (n = 3), alopecia (n = 2), depression (n = 2), weight gain (n = 2) SV discontinuations (n = 2): weight gain and depression (n = 1), unsteadiness (n =1) | |
| “Exclusions” due to adverse events or no efficacy4 | Proportion “excluded”: PHT: 33.3% | Proportion “excluded”: SV: 23.3% | |
| “Unacceptable” adverse events leading to drug withdrawal5 | PHT (n = 54): drowsiness (n = 2), skin rash (n = 1) blood dyscrasia (n = 1), hirsutism (n = 1) | SV (n = 49): behavioural (n = 1), tremor (n = 1) | |
| No adverse event data reported (Withdrawal data only reported) | 1 participant (PHT) withdrew from the study due to depression and anorexia | No adverse event data (or withdrawals due to adverse events) reported | |
| “Unacceptable” adverse events leading to drug withdrawal5 | PHT (n = 63): myalgia (n = 1), irritability (n = 1)
| SV (n = 61): dizziness (n = 2) abnormal liver function test (n = 1) | |
| Most common adverse events (by treatment group)6 | PHT (n = 50): dyspepsia (n = 1), nausea (n = 2), dizziness (n = 2), somnolence (n = 5), tremor (n = 2), rash (n = 4)
| SV (n = 86): dyspepsia (n = 7), nausea (n = 10), dizziness (n = 5), somnolence (n = 8), tremor (n = 5), rash (n = 3) | |
| Commonest adverse events (reported as percentages by treatment group)6 | PHT (n = 45): gum hyperplasia (17.7%), nystagmus (13.33%), ataxia (2.2%), gastrointestinal disturbances (4.44%), drowsiness (4.44%) | SV (n = 49): gastrointestinal disturbances (12%), drowsiness (6.12%), weight gain (2.04%) | |
| Adverse events (narrative description)2 | PHT (n = 15): 1 case of ataxia, 5 cases of acne | SV (n = 18): 2 cases of gastrointestinal symptoms, 2 cases of hair loss, 4 cases of weight gain | |
| Assessment of adverse events2 | PHT (n = 52): 33 participants reported at least one side effect Reported frequencies: gingival hypertrophy (n = 30), ataxia (n = 13), sedation (n = 12), nausea and vomiting (n = 1) Other reported adverse events (no frequencies): nystagmus, confusion | SV (n = 48): 15 participants reported at least one side effect Reported frequencies: hyperactivity (n = 6), impaired school performance (n = 4), severe skin allergy (n = 1) | |
| Withdrawals due to dose‐related and idiosyncratic adverse events | PHT (n = 70): 11 withdrawals due to dose‐related adverse events (nystagmus, ataxia, tremor, diplopia and mental change) 5 withdrawals due to idiosyncratic adverse events (skin eruption, erythroderma and jaundice) | SV (n = 70): 9 withdrawals due to dose‐related adverse events (tremor, irritability, restlessness and alopecia) No withdrawals due to idiosyncratic adverse events | |
| 1Adverse event data, as reported narratively in the publications. Adverse event data were not requested in original IPD requests but will be for all future IPD requests. For numbers of withdrawals due to adverse events in studies for which IPD were provided (De Silva 1996; Heller 1995; Ramsay 1992; Turnbull 1985) see Table 4. | |||
| Time to withdrawal of allocated treatment | Time to achieve 12‐month remission | Time to achieve 6‐month remission | Time to first seizure | |
| (i) Original analysis | P: 1.20 (0.76 to 1.95) G: 0.98 (0.59 to 1.64) O: 1.09 (0.76 to 1.55) | P: 0.90 (0.63 to 1.29) G: 1.04 (0.77 to 1.40) O: 0.98 (0.78 to 1.23) | P: 0.99 (0.73 to 1.35) G: 0.92 (0.72 to 1.18) O: 0.95 (0.78 to 1.15) | P: 0.83 (0.62 to 1.11) G: 1.03 (0.77 to 1.39) O: 0.93 (0.75 to 1.14) |
| (i) Test for interaction | Chi² = 0.31, df = 1, P = 0.58, I² = 0% | Chi² = 0.39, df = 1, P = 0.53, I² = 0% | Chi² = 0.13, df = 1, P = 0.72, I² = 0% | Chi² = 1.06, df = 1, P = 0.30, I² = 5.6% |
|
| ||||
| (ii) Generalised onset and age at onset > 30 classified as uncertain seizure type | P: 1.20 (0.76 to 1.95) G: 1.33 (0.74 to 2.38) U: 0.47 (0.12 to 1.85) O: 1.17 (0.82 to 1.67) | P: 0.90 (0.63 to 1.29) G: 0.93 (0.63 to 1.39) U: 1.36 (0.85 to 2.17) O: 1.01 (0.80 to 1.27) | P: 0.99 (0.73 to 1.35) G: 0.88 (0.62 to 1.25) U: 1.11 (0.76 to 1.61) O: 0.99 (0.81 to 1.20) | P: 0.83 (0.62 to 1.11) G: 1.34 (0.91 to 1.97) U: 0.74 (0.47 to 1.17) O: 0.93 (0.76 to 1.15) |
| (ii) Test for interaction | Chi² = 1.88, df = 2, P = 0.39, I² = 0% | Chi² = 2.07, df = 2, P = 0.36, I² = 3.3% | Chi² = 0.78, df = 2, P = 0.68, I² = 0% | Chi² = 4.87, df = 2, P = 0.09, I²= 58.9% |
|
| ||||
| (iii) Generalised onset and age at onset > 30 reclassified as partial onset | P: 0.98 (0.63 to 1.53) G: 1.33 (0.74 to 2.38) O: 1.10 (0.77 to 1.56) | P: 1.01 (0.76 to 1.34) G: 0.93 (0.63 to 1.39) O: 0.98 (0.78 to 1.24) | P: 1.00 (0.79 to 1.27) G: 0.88 (0.62 to 1.25) O: 0.97 (0.80 to 1.18) | P: 0.81 (0.64 to 1.04) G: 1.34 (0.91 to 1.97) O 0.94 (0.76 to 1.15) |
| (iii) Test for interaction | Chi² = 0.67, df = 1, P = 0.41, I² = 0% | Chi² = 0.10, df = 1, P = 0.75, I² = 0% | Chi² = 0.36, df = 1 P = 0.55, I² = 0% | Chi² = 4.55, df = 1 P = 0.03, I² = 78% |
| P: partial epilepsy; G: generalised epilepsy; O: overall (all participants); U: uncertain epilepsy. Results are presented as pooled HR (95% CI) with fixed‐effect See Analysis 1.2, Analysis 1.4, Analysis 1.6, and Analysis 1.8 for original analyses of 'Time to withdrawal of allocated treatment', 'Time to achieve 12‐month remission', 'Time to achieve 6‐month remission', and 'Time to first seizure' respectively. See Analysis 1.9 and Analysis 1.10 for forest plots of 'Time to first seizure' sensitivity analyses for generalised and age at onset > 30 reclassified as uncertain epilepsy type and partial epilepsy, respectively. Forest plots are not presented for 'Time to withdrawal of allocated treatment', 'Time to achieve 6‐month remission', 'Time to achieve 12‐month remission' sensitivity analyses, as results were similar for partial onset and generalised onset subgroups, and conclusions are unchanged. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to withdrawal of allocated treatment Show forest plot | 6 | 569 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.73, 1.42] |
| 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type Show forest plot | 5 | 528 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.76, 1.55] |
| 2.1 Generalised onset seizures (tonic‐clonic only) | 5 | 341 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.59, 1.64] |
| 2.2 Partial onset seizures | 4 | 187 | Hazard Ratio (Fixed, 95% CI) | 1.20 [0.74, 1.95] |
| 3 Time to achieve 12‐month remission Show forest plot | 4 | 514 | Hazard Ratio (Fixed, 95% CI) | 0.97 [0.77, 1.22] |
| 4 Time to achieve 12‐month remission ‐ stratified by epilepsy type Show forest plot | 4 | 514 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 4.1 Generalised onset seizures (tonic‐clonic only) | 4 | 270 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.77, 1.40] |
| 4.2 Partial onset seizures | 4 | 244 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.63, 1.29] |
| 5 Time to achieve six‐month remission Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.76, 1.12] |
| 6 Time to achieve six‐month remission ‐ stratified by epilepsy type Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.78, 1.15] |
| 6.1 Generalised onset seizures (tonic‐clonic only) | 5 | 395 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.72, 1.18] |
| 6.2 Partial onset seizures | 4 | 244 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.73, 1.35] |
| 7 Time to first seizure Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.78, 1.18] |
| 8 Time to first seizure ‐ stratified by epilepsy type Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.75, 1.14] |
| 8.1 Generalised onset seizures (tonic‐clonic only) | 5 | 395 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.77, 1.39] |
| 8.2 Partial onset seizures | 4 | 244 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.62, 1.11] |
| 9 Time to first seizure ‐ epilepsy type reclassified to uncertain for generalised and age of onset > 30 years Show forest plot | 5 | 649 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.76, 1.15] |
| 9.1 Generalised onset seizures (tonic‐clonic only) | 4 | 223 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.91, 1.97] |
| 9.2 Partial onset seizures | 4 | 255 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.62, 1.11] |
| 9.3 Uncertain seizure type | 4 | 171 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.47, 1.17] |
| 10 Time to first seizure ‐ epilepsy type reclassified to partial for generalised and age of onset > 30 years Show forest plot | 5 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.76, 1.15] |
| 10.1 Generalised onset seizures (tonic‐clonic only) | 4 | 223 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.91, 1.97] |
| 10.2 Partial onset seizures | 5 | 416 | Hazard Ratio (Fixed, 95% CI) | 0.81 [0.64, 1.04] |