Stosowanie ogrzewanego i nawilżanego powietrza podczas przeziębienia
Abstract
Background
Heated, humidified air has long been used by people with the common cold. The theoretical basis is that steam may help congested mucus drain better and that heat may destroy the cold virus as it does in vitro. This is an update of a review last published in 2013.
Objectives
To assess the effects of inhaling heated water vapour (steam) in the treatment of the common cold by comparing symptoms, viral shedding, and nasal resistance.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (to February 2017), MEDLINE (1966 to 24 February 2017), Embase (1990 to 24 February 2017), and Current Contents (1998 to 24 February 2017). We also searched World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (8 March 2017) and ClinicalTrials.gov (8 March 2017) as well as reference lists of included studies.
Selection criteria
Randomised controlled trials using heated water vapour in participants with the common cold or experimentally induced common cold were eligible for inclusion.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Three review authors independently screened titles and abstracts for inclusion of potential studies identified from the search. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram. We used a data collection form for study characteristics and outcome data that was developed and used for previous versions of this review. Two review authors independently extracted data, and a third review author resolved any disagreements. We used Review Manager 5 software to analyse data.
Main results
We included six trials from five publications involving a total of 387 participants. We included no new studies in this 2017 update. The 'Risk of bias' assessment suggested an unclear risk of bias in the domain of randomisation and a low risk of bias in performance, detection, attrition, and reporting.
It was uncertain whether heated, humidified air provides symptomatic relief for the common cold, as the fixed‐effect analysis showed evidence of an effect (odds ratio (OR) 0.30, 95% confidence interval (CI) 0.16 to 0.56; 2 studies, 149 participants), but the random‐effects analysis showed no significant difference in the results (OR 0.22, 95% CI 0.03 to 1.95). There is an argument for using either form of analysis. No studies demonstrated an exacerbation of clinical symptom scores. One study conducted in the USA demonstrated worsened nasal resistance, but an earlier Israeli study showed improvement. One study examined viral shedding in nasal washings, finding no significant difference between treatment and placebo groups (OR 0.47, 95% CI 0.04 to 5.19). As judged by the subjective response to therapy (i.e. therapy did not help), the number of participants reporting resolution of symptoms was not significantly higher in the heated humidified group (OR 0.58, 95% CI 0.28 to 1.18; 2 studies, 124 participants). There was significant heterogeneity in the effects of heated, humidified air on different outcomes, therefore we graded the quality of the evidence as low. Some studies reported minor adverse events (including discomfort or irritation of the nose).
Authors' conclusions
The current evidence does not show any benefits or harms from the use of heated, humidified air delivered via the RhinoTherm device for the treatment of the common cold. There is a need for more double‐blind, randomised trials that include standardised treatment modalities.
PICO
Streszczenie prostym językiem
Stosowanie ogrzewanego i nawilżanego powietrza podczas przeziębienia
Pytanie badawcze
Przeanalizowaliśmy skutki inhalacji ogrzanym i nawilżonym powietrzem dostarczanym przez urządzenie (RhinoTherm) u osób z przeziębieniem.
Wprowadzenie
Przeziębienie jest jedną z najczęstszych infekcji u ludzi. Zwykle nie powoduje powikłań, ale może prowadzić do nieobecności w pracy lub szkole ze względu na dyskomfort spowodowany objawami. Przeziębienie rozpoznaje się na podstawie objawów, a w terapii stosuje się głównie leki objawowe. Do objawów należą: gorączka, utrata apetytu, złe samopoczucie, uczucie zimna oraz ból głowy, mięśni i o różnej lokalizacji. Wiele z tych objawów jest spowodowanych nieżytem wynikającym z obrzęku błony śluzowej i gęstego śluzu wewnątrz nosa. Przez dziesięciolecia przeziębienie leczono inhalacjami pary wodnej, aby ułatwić usuwanie śluzu. Istnieją dane laboratoryjne wskazujące, że wirus wywołujący przeziębienie może być wrażliwy na ciepło, ale skuteczność tej interwencji nie została sprawdzona w badaniach klinicznych przeprowadzonych na dużą skalę. Inhalacje pary wodnej są nadal stosowane, ponieważ dają subiektywne odczucie zmniejszenia objawów przeziębienia.
Data wyszukiwania
Dane są aktualne do 24 lutego 2017 roku.
Charakterystyka badań
Włączyliśmy sześć badań klinicznych z randomizacją, przeprowadzonych metodą podwójnie ślepej próby z pięciu publikacji, obejmujących łącznie 387 uczestników, opublikowanych w latach 1987‐1995 w języku angielskim. We wszystkich badaniach w leczeniu objawów przeziębienia używano urządzenia RhinoTherm, które dostarczało ogrzewanego, nawilżonego powietrza przez różny czas i przy różnym tempie przepływu. Trzy badania były przeprowadzone w USA, dwa w Wielkiej Brytanii i jedno w Izraelu. Do większości badań włączano osoby z naturalnie występującym przeziębieniem, natomiast w jednym uczestników zarażano wirusem wywołującym przeziębienie.
Źródła finansowania badań
Urządzenie RhinoTherm było dostarczone przez Netzer Sereni w czterech badaniach i przez A Beacham w dwóch badaniach. Jedno badanie było finansowane z wewnętrznych środków finansowych Cleveland Clinic, a inne było wspierane z funduszy dostępnych dla autorów. W pozostałych badaniach nie podano informacji na temat źródeł finansowania.
Główne wyniki
W żadnym z włączonych badań nie zgłoszono nasilenia objawów klinicznych po inhalacji ogrzanym i nawilżonym powietrzem. U uczestników dwóch badań nie obserwowano utrzymywania się objawów, jednak wyniki były niespójne. W dwóch badaniach zgłoszono łagodne działania niepożądane. Nie stwierdzono wpływu leczenia na wydalanie rynowirusa (wirus będący najczęstszą przyczyną zakażenia górnych dróg oddechowych ‐ przyp. tłum.).
Jakość danych naukowych
Korzystając z kryteriów GRADE, oceniliśmy jakość dowodów naukowych jako niską dla następujących danych: zmniejszenie nasilenia klinicznych objawów przeziębienia (mierzonych przez zmniejszenie liczby punktów w skali oceny objawów); liczba uczestników z subiektywną odpowiedzią: leczenie nie pomogło i liczba uczestników, u których wyhodowano wirusa w posiewie z nosa, z powodu ryzyka wystąpienia błędu systematycznego i niespójności wyników badania.
Authors' conclusions
Summary of findings
| Heated, humidified air compared to control for treating the common cold | ||||||
| Patient or population: People with the common cold | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with rhinothermy | |||||
| Reduction in the clinical severity of the common cold (measured by decrease in the symptom score index) | Study population | Fixed‐effect model OR 0.30 Random‐effects model OR 0.22 (0.03 to 1.95) | 149 | ⊕⊕⊝⊝ | The significance of the effect is uncertain because use of the fixed‐effect model produces a different result than use of the random‐effects model. | |
| 681 per 1000 | Fixed‐effect model 390 per 1000 Random‐effects model 319 per 1000 (60 to 806) | |||||
| Number of participants with the subjective response: therapy did not help | Study population | OR 0.58 | 124 | ⊕⊕⊝⊝ | We downgraded the evidence for risk of bias and imprecision. | |
| 524 per 1000 | 389 per 1000 | |||||
| Number of participants with positive nasal wash cultures | Study population | OR 0.47 | 20 | ⊕⊕⊝⊝ | We downgraded the evidence for risk of bias and imprecision. | |
| 900 per 1000 | 809 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Ophir 1987 had high attrition rates; Tyrrell 1989b did not perform allocation concealment. | ||||||
Background
Description of the condition
The common cold is an acute, self limiting viral infection of the upper respiratory tract involving, sneezing, nasal congestion and discharge (rhinorrhoea), sore throat, cough, low‐grade fever, headache, and malaise. Different viruses can cause the common cold, the most common of which are the more than 100 serotypes of rhinoviruses (Sahin 2015).
"Life is made up of sobs, sniffles and smiles, with sniffles predominating" (Adams 1967). Sniffles, the common cold, and other acute respiratory infections account for about 40% of employee absenteeism and about 30% of absenteeism from school (Predy 2005). Separate studies of families have shown that the average preschool child has six to 10 colds per year, and the average adult has two to four colds per year (Monto 1974). Care for people with the common cold imposes significant economic burden.
Description of the intervention
Many remedial measures such as antihistamines, decongestants, intranasal ipratropium bromide, vitamin C, interferon, and traditional remedies such as Chinese herbs, garlic, and ginseng are used in the treatment of the common cold (AlBalawi 2013; De Sutter 2012; Karsch‐Volk 2014; Smith 1993; Zhang 2009). Inhaling warm, damp air is thought to offer relief from symptoms of the common cold. Hot water, hot soup, and tea have been used for centuries for this purpose and have been subject to scientific investigation (Saketkhoo 1978; Sanu 2008).
How the intervention might work
Lwoff 1969 suggested that raising the mucosal temperature to 43 °C for three 30‐minute periods can block rhinoviral replication and stop the common cold. Studies of the effect of heated, humidified air suggest that raising nasal mucosal temperature may inhibit rhinoviral replication (Forstall 1994). A device to raise nasal mucosal temperature (RhinoTherm) has been developed. It has been claimed that 80% of participants who used RhinoTherm in the early stages of common cold felt better the next day (Ophir 1987; Yerushalmi 1980).
Why it is important to do this review
A multimillion dollar industry thrives on treatments for alleviating symptoms of the common cold (Fendrick 2003). Treatments range from antihistamines, decongestants, antibiotics, vitamins, and minerals from the conventional medical system to several physical therapies ranging from inhaling steam with herbs, 'neti' treatment, and 'Pranayaam' from the complementary systems of medicine. The common cold and allergic rhinitis constitute a global health problem that affects social life, sleep, school and work performance, and imposes a substantial economic burden on society due to absence from work and reduced working capacity (Hellgren 2010). Two studies estimated the productivity lost to the common cold by using a telephone‐administered survey that measured three sources of loss: absenteeism, on‐the‐job productivity, and caregiver absenteeism (Bramley 2002; Fendrick 2003). Each cold experienced by a working adult caused an average of 8.7 work hours lost (2.8 absenteeism hours and 5.9 hours of on‐the‐job loss) and 1.2 work hours lost due to caring for children aged under 13 years who had colds. The economic cost of lost productivity due to the common cold approaches nearly USD 25,000 million, of which USD 16,600 million is attributed to on‐the‐job productivity loss, USD 8000 million is attributed to absenteeism, and USD 230 million is attributed to caregiver absenteeism (Bramley 2002).
It was therefore important to review the evidence to provide a scientific foundation for the safety and efficacy of heated, humidified air to treat common cold symptoms.
Objectives
To assess the effects of inhaling heated water vapour (steam) in the treatment of the common cold by comparing symptoms, viral shedding, and nasal resistance.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials investigating rhinothermy. "Rhinothermy involves the application of heated and humidified air to the nasal passages" to treat common cold symptoms (Goodall 2016).
Types of participants
The treatment group consisted of people of all ages with natural or experimentally induced common cold or acute viral rhinopharyngitis, receiving warm vapour inhalation via a RhinoTherm device. The control group included people with natural or experimentally induced cold who received room temperature air or room temperature humidified air.
We excluded people who did not meet the predefined inclusion criteria.
Types of interventions
We included trials that compared inhalation from a device delivering warm, humidified air (40 °C to 47 °C) that raised intranasal temperature with breathing from a similar device that delivered humidified or ambient temperature air at up to 30 °C.
Types of outcome measures
Primary outcomes
-
Reduction in the clinical severity of the common cold (i.e. a decrease in the symptom score index). This was measured based on symptoms of nasal blockage, sneezing, and nasal drainage, which were scored on a four‐point scale (0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe).
-
Number of participants with the subjective response: therapy did not help.
Secondary outcomes
-
Decrease in the weight of nasal secretions.
-
Decrease in nasal resistance measured using a rhinomanograph (ICS Medical Corporation, Schaumburgh, IL).
-
Number of participants with a positive viral culture in the nasal washings.
-
Adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases:
-
Cochrane Central Register of Controlled Trials, which contains the Cochrane Acute Respiratory Infection Group's Specialised Register, in the Cochrane Library searched on 24 February 2017 using the strategy in Appendix 1;
-
MEDLINE (Ovid) (from 1966 to 24 February 2017) using the strategy in Appendix 2;
-
Embase (Elsevier) (from 1990 to 24 February 2017) using the strategy in Appendix 3; and
-
Current Contents (Thomson Reuters) (from 1998 to 24 February 2017) (Appendix 4).
We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). Where applicable, it was modified appropriately for other databases.
We searched the following clinical trials registries:
-
the World Health Organization (WHO) ICTRP (8 March 2017); and
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov, 8 March 2017).
We did not restrict results by language or publication status (published, unpublished, in press, or in progress). Details of search strategies used for previous versions of this review are presented in Appendix 5.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We planned to contact experts in the field to identify any additional unpublished materials.
Data collection and analysis
Selection of studies
Three review authors (MaS, NJ, AC) independently screened titles and abstracts of the studies identified by the search for potential inclusion in the review. We retrieved the full‐text reports of the studies deemed potentially eligible, and the same two review authors independently screened the full texts to identify studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion, or by consulting a third review author (MeS) when necessary. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009). We did not impose any language restrictions.
Data extraction and management
We used a data collection form for study characteristics and outcome data that was developed for previous versions of this review. One review author (MaS or MeS) extracted study characteristics from included studies. We extracted the following study characteristics.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (MaS, MeS) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus. Two review authors (NJ, AC) transferred data into the Review Manager 5 file (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A third review author (MeS) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
All review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We assessed the risk of bias according to the following domains.
-
Random sequence generation
-
Allocation concealment
-
Blinding of participants and personnel
-
Blinding of outcome assessment
-
Incomplete outcome data
-
Selective outcome reporting
-
Other bias
We graded each potential source of bias as high, low, or unclear and provided quotes from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each domain listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with an author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed data to that outcome.
Measures of treatment effect
We entered outcome data for each study into data tables in Review Manager 5 to calculate the treatment effects (RevMan 2014). We used odds ratio for dichotomous outcomes, and planned to use mean differences or standardised mean differences for continuous outcomes.
Dealing with missing data
All of the included studies provided dropout rates; none conducted intention‐to‐treat analyses (Higgins 2011a). We contacted trial authors of two included studies to verify key study characteristics.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis (Deeks 2011).
Assessment of reporting biases
If we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We planned to pool data from studies judged to be clinically homogeneous using Review Manager 5 (RevMan 2014). We planned to perform meta‐analysis where more than one study provided usable data in any single comparison using fixed‐effect and random‐effects models.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: reduction in the clinical severity of the common cold (measured by decrease in the symptom score index); number of participants with the subjective response: therapy did not help; and number of participants with a positive viral culture in the nasal washings. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the evidence quality as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We employed methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011b, using GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
No subgroup analyses were planned for this review.
Sensitivity analysis
We conducted sensitivity analysis for the number of participants with persistent symptoms using the Mantel‐Haenszel random‐effects model.
Results
Description of studies
See Characteristics of included studies.
Results of the search
Of the 499 records identified in the searches for the 2017 update, we excluded 496 after title and abstract screening. We obtained full‐text copies of three studies (Murdoch 2014; Varricchio 2013; Yu 2013), which we excluded following assessment. We included no new studies in this 2017 update. The previous version of this review included five randomised controlled trials, which we retained in this update (Figure 1).

Study flow diagram for 2017 update.
Included studies
We included six trials reported in five publications involving a total of 387 participants (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987; Tyrrell 1989a; Tyrrell 1989b). Tyrrell 1989 included the general population and volunteer arms, which were analysed separately as Tyrrell 1989a and Tyrrell 1989b, respectively. All included studies were randomised and double‐blinded. All trials were conducted in the 1980s to 1990s, with small sample sizes.
Tyrrell 1989a and Tyrrell 1989b used a RhinoTherm invented by A. Beacham, and four trials used RhinoTherm devices manufactured by Netzer Sereni (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987). RhinoTherm is a microprocessor‐controlled device that delivers warm, humidified air at a controlled temperature.
Forstall 1994 was conducted in the USA in 1992 and included 75 participants, of whom seven were excluded from the final analysis. Of the included participants, 81% and 61% were females in the intervention and control groups, respectively. Participants were aged over 18 years and had symptoms of moderate to severe cold, nasal congestion, discharge or sneezing at the time of enrolment. The intervention was administered to 32 participants via RhinoTherm delivering 40 L per minute of saturated constant air flow at 47 °C for one hour. The control group (N = 36) received 2 L per minute of ambient air at 20 °C to 24 °C for one hour. This study was funded by Cleveland Clinic internal funding.
Hendley 1994 was conducted in the USA with 20 healthy participants whose colds were an experimentally induced rhino viral infection. The average age of participants was 20 years, and 80% were female. Participants with fever, respiratory illness, or taking antihistamines, decongestants, steroids, or nasal spray were excluded. The intervention was delivered to 10 participants via RhinoTherm to provide 38 L to 40 L per minute humidified air at 42 °C to 44 °C after 24 hours and 48 hours. The control group (N = 10) received 2 L per minute of ambient air at 22 °C to 23 °C. The study was supported by authors' discretionary funds.
Ophir 1987 was conducted in Israel and included 62 participants with a mean age of 34 years. The trial randomised 70 participants, of whom eight dropped out (3 and 5 from the intervention and control groups, respectively). The intervention arm included 32 participants with common cold who were treated with heated vapour at 42 °C to 44 °C at a flow rate of 40 L per minute delivered via RhinoTherm in two 20‐minute sessions at 60‐ to 90‐minute intervals. The control group (N = 30) were treated with 22 °C to 24 °C at 2 L per minute of ambient air.
Macknin 1990 was conducted in the USA in 1989 and included a total of 66 participants. The mean ages of participants were 36 years for the intervention group and 32 years for the control group. The percentage of female participants was 75% in the intervention group and 79% in the control group. The intervention group (N = 32) received heated, distilled vapour at 42 °C to 45 °C at 40 L per minute in two 20‐minute sessions at 60‐ to 90‐minute intervals. The control group (N = 34) received 2 L per minute at 20 °C to 24 °C.
The trial by Tyrell 1989 included two study arms.
Tyrrell 1989a was conducted in a UK general practice in 1989 and included 96 participants, of which data records for 87 participants were obtained. Of these 87 participants, 45 received humidified air at 43 °C, and 42 received humidified air at 30 °C at a rate of 40 L for 20 minutes. The ratio of male to female was 48% to 52% in the intervention group and 60% to 40% in the control group. The participants in this study arm did not cross‐over from Tyrrell 1989b.
Tyrrell 1989b was a separate study arm. After two days of quarantine, 75 participants aged 18 to 50 years were experimentally induced with intranasal drops of human rhinovirus type 14. Volunteers were randomised to receive water vapour at 43 °C or 30 °C for 30 minutes. Participants received three 30‐minute sessions with 90‐minute intervals.
Excluded studies
See Characteristics of excluded studies.
We excluded six studies that did not meet the inclusion criteria; three were previously excluded (Baroody 2000; Grübber 2003; Yerushalmi 1980), and three were excluded from the 2017 search (Murdoch 2014; Varricchio 2013; Yu 2013).
Three studies assessed populations that were not relevant to this review (Baroody 2000; Yerushalmi 1980; Yu 2013); two investigated interventions that this review did not assess (Grübber 2003; Varricchio 2013); and one reported on an intervention and population not relevant to this review (Murdoch 2014).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 2 and summarised in Figure 3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
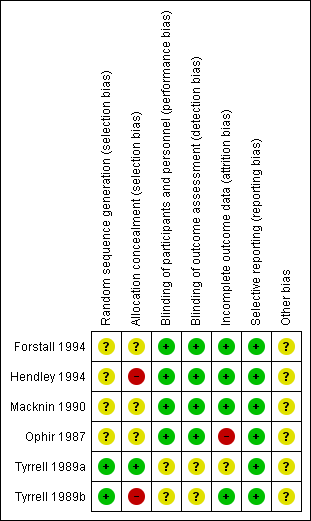
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four of six included studies randomised participants. However, we assessed these studies as at unclear risk of bias for random sequence generation because they did not describe randomisation methods (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987). We assessed two studies as at low risk of bias (Tyrrell 1989a; Tyrrell 1989b).
Allocation concealment was not stated clearly in three trials, which were assessed as at unclear risk of bias for this domain (Forstall 1994; Macknin 1990; Ophir 1987). The other three studies did not mention allocation concealment and were assessed as at high risk of bias (Hendley 1994; Tyrrell 1989a; Tyrrell 1989b).
Blinding
We assessed four studies as at low risk for performance bias (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987). We assessed two studies as at unclear risk of bias because it was not clear who was blinded or how blinding was done (Tyrrell 1989a; Tyrrell 1989b).
We assessed four studies as at low risk of detection bias (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987). We judged two studies to be at unclear risk of detection bias (Tyrrell 1989a; Tyrrell 1989b).
Incomplete outcome data
We assessed one study as at high risk of bias because the attrition rate in the placebo group was more than the event rate in the placebo group (Ophir 1987). In Tyrrell 1989a, data were presented for analysis for 87 of 96 enrolled participants with no reason given, hence it was classified as at unclear risk of bias. The remaining four included studies were at low risk of bias for this domain (Forstall 1994; Hendley 1994; Macknin 1990; Tyrrell 1989b).
Selective reporting
The protocols for the published studies were not available for review. We compared the outcomes listed in the methods section to reported outcomes for all included studies. We found that all outcomes listed in the methods sections were reported in the results of the studies. We therefore assessed all studies as at low risk of bias for this domain (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987; Tyrrell 1989a; Tyrrell 1989b).
Other potential sources of bias
None known.
Effects of interventions
Primary outcomes
1. Reduction in the clinical severity of the common cold (measured by decrease in the symptom score index)
The included studies did not provide unequivocal evidence supporting the use of warm vapour inhalations for treatment of the common cold. No studies demonstrated a worsening of clinical symptom scores, but two studies reported the persistence of symptoms (odds ratio (OR) 0.30, 95% confidence interval (CI) 0.16 to 0.56; 2 studies, 149 participants, fixed‐effect model) (Analysis 1.1). There was significant heterogeneity (I² = 87%).
Three studies used similar symptom index scores (Forstall 1994; Macknin 1990; Ophir 1987). Ophir 1987 showed a significant improvement in symptom index score; however, Forstall 1994 and Macknin 1990 did not show any improvements. Macknin 1990 showed a greater change towards symptom improvement from baseline in the placebo group. We could not pool these studies because standard deviations were unavailable. Tyrrell 1989a in their general practice study and Tyrrell 1989b in the volunteer study, showed more improvements in the participants given the hot humidified air at 43 C by the rhinotherm device. A different symptom score used by Hendley 1994 showed no significant difference between study and control interventions.
2. Number of participants with subjective response: therapy did not help
As judged by subjective response to therapy (therapy did not help), there was a statistically non‐significant resolution of symptoms in the heated, humidified group (OR 0.58, 95% CI 0.28 to 1.18; 2 studies, 124 participants, I² = 22%) (Analysis 1.2).
Secondary outcomes
1. Decrease in the weight of nasal secretions
None of the included studies commented on weight of nasal secretions of participants.
2. Decrease in nasal resistance as measured by a rhinomanograph
Three studies showed improvement in nasal resistance (Ophir 1987; Tyrrell 1989a; Tyrrell 1989b). Forstall 1994 demonstrated increased nasal resistance one week after steam inhalation (data skewed at entry). This contrasted with an earlier study that showed improvement in nasal resistance (Macknin 1990). We could not pool data for analysis.
3. Number of participants with a positive viral culture in the nasal washings
There was no statistically significant difference in number of participants with positive nasal wash cultures (OR 0.47, 95% CI 0.04 to 5.19; 1 study, 20 participants) (Analysis 1.3).
4. Adverse events
Macknin 1990 reported that adverse events were statistically significant in the rhinothermy group (OR 4.73, 95% CI 1.46 to 15.30; 1 study, 65 participants, P = 0.010) (Analysis 1.4). Minor adverse events included nasal and lip irritation, lightheadedness, increased congestion, and discomfort from the mask delivering heated, humidified air (Macknin 1990). Forstall 1994 reported episodes of nasal congestion, minor mucosal burns, and discomfort from condensation in the mask. One participant was reported to have experienced temporary dizziness for two to three minutes following treatment with saturated, hot air (Ophir 1987). However, the studies reporting adverse events related to thermal discomfort used the treatment for longer durations.
Sensitivity analysis
We conducted a sensitivity analysis for the number of participants with persistent symptoms using the Mantel‐Haenszel random‐effects model, which showed no significant difference in results (OR 0.22, 95% CI 0.03 to 1.95) (Analysis 2.1).
Discussion
Summary of main results
We included six trials from five publications (one publication included two trials that we assessed separately) that investigated heated, humidified air for the treatment of people with the common cold. The RhinoTherm devices were provided by Netzer Sereni in four studies (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987), and by A Beacham in two studies (Tyrrell 1989a; Tyrrell 1989b). One study was funded by Cleveland Clinic internal funding (Forstall 1994), while another study was supported by authors' discretionary funds (Hendley 1994). The remaining studies did not mention funding sources (Macknin 1990; Ophir 1987; Tyrrell 1989a; Tyrrell 1989b).
Three trials reported benefits of heated, humidified air for symptom relief in people with the common cold (Ophir 1987; Tyrrell 1989a; Tyrrell 1989b). However, sample sizes were small. Results on symptom indices were equivocal. No studies demonstrated an exacerbation of clinical symptom scores (Analysis 1.1; Analysis 1.2). Forstall 1994 demonstrated worsened nasal resistance, but Ophir 1987 showed improvements. Hendley 1994 examined viral shedding in nasal washings through cultures, finding no difference between treatment and placebo groups. Two studies reported minor adverse events (including discomfort or nasal irritation) (Forstall 1994; Macknin 1990).
Overall completeness and applicability of evidence
We aimed to examine the evidence for heated, humidified air delivered using a RhinoTherm device for treating the common cold. The trials assessed participants that included healthy volunteers with experimentally‐induced cold. This added an element of indirectness. The small sample sizes and the fact that all outcomes were not addressed made the effect estimates susceptible to change. The included trials are now more than two decades old, and we identified no new studies (completed or ongoing) for this update. Hence, cautious interpretation of the findings is suggested.
Quality of the evidence
We used GRADE (GRADEpro GDT 2014), as described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011), to assess the quality and certainty of the evidence (summary of findings Table for the main comparison). The existing evidence was of low quality, which implies that further research is very likely to change our effect estimates.
We downgraded the evidence for potential risk of bias, inconsistency among the trials, imprecision and indirectness of the trials. We attributed imprecision and inconsistency to the small sample sizes of the trials.
Potential biases in the review process
We were unable to pool the data for most outcomes due to a lack of included studies reporting the outcome. We could not estimate publication bias due to the small number of included studies. We aimed to reduce bias in the review process by using a standardised search strategy and including studies up to the most recent search date. We performed searching and data extraction according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The strength of this review is in the inclusion of studies using similar methodologies and instrument for the intervention. There was a difference in the duration of warm vapour inhalation, with a longer period (30 minutes) associated with no benefit and increased resistance of the nasal passages. We found potential biases in most included studies (unclear risk of bias for randomisation and allocation concealment).
Agreements and disagreements with other studies or reviews
A review of non‐antibiotic treatments for upper respiratory tract infections (the common cold), based on seven Cochrane Reviews and presenting risk ratios for outcomes, drew similar conclusions (Arroll 2005).

Study flow diagram for 2017 update.
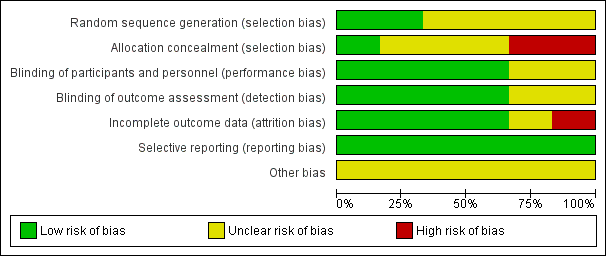
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Rhinothermy versus control, Outcome 1 Number of participants with persistent symptoms.

Comparison 1 Rhinothermy versus control, Outcome 2 Number of participants with subjective response to therapy: therapy did not help.
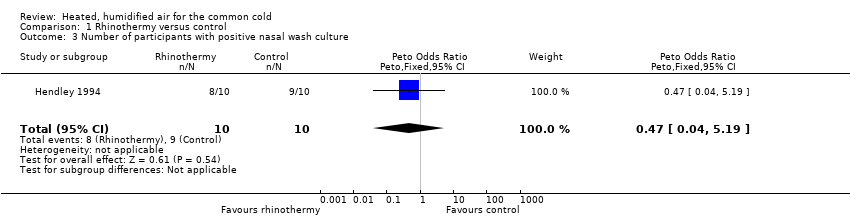
Comparison 1 Rhinothermy versus control, Outcome 3 Number of participants with positive nasal wash culture.
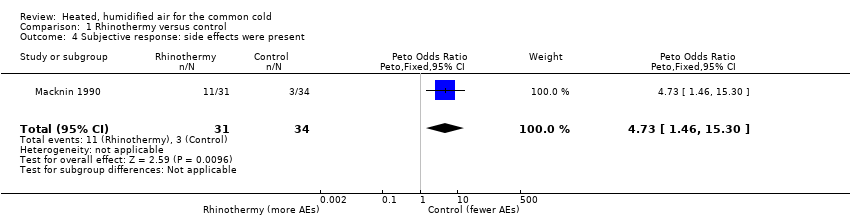
Comparison 1 Rhinothermy versus control, Outcome 4 Subjective response: side effects were present.
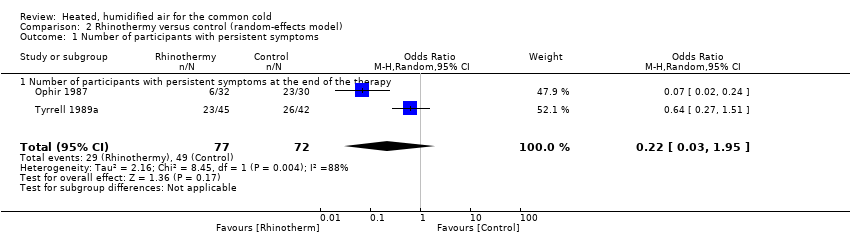
Comparison 2 Rhinothermy versus control (random‐effects model), Outcome 1 Number of participants with persistent symptoms.
| Heated, humidified air compared to control for treating the common cold | ||||||
| Patient or population: People with the common cold | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with rhinothermy | |||||
| Reduction in the clinical severity of the common cold (measured by decrease in the symptom score index) | Study population | Fixed‐effect model OR 0.30 Random‐effects model OR 0.22 (0.03 to 1.95) | 149 | ⊕⊕⊝⊝ | The significance of the effect is uncertain because use of the fixed‐effect model produces a different result than use of the random‐effects model. | |
| 681 per 1000 | Fixed‐effect model 390 per 1000 Random‐effects model 319 per 1000 (60 to 806) | |||||
| Number of participants with the subjective response: therapy did not help | Study population | OR 0.58 | 124 | ⊕⊕⊝⊝ | We downgraded the evidence for risk of bias and imprecision. | |
| 524 per 1000 | 389 per 1000 | |||||
| Number of participants with positive nasal wash cultures | Study population | OR 0.47 | 20 | ⊕⊕⊝⊝ | We downgraded the evidence for risk of bias and imprecision. | |
| 900 per 1000 | 809 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Ophir 1987 had high attrition rates; Tyrrell 1989b did not perform allocation concealment. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with persistent symptoms Show forest plot | 2 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.16, 0.56] |
| 1.1 Number of participants with persistent symptoms at the end of therapy | 2 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.16, 0.56] |
| 2 Number of participants with subjective response to therapy: therapy did not help Show forest plot | 2 | 124 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.28, 1.18] |
| 3 Number of participants with positive nasal wash culture Show forest plot | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.04, 5.19] |
| 4 Subjective response: side effects were present Show forest plot | 1 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.73 [1.46, 15.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with persistent symptoms Show forest plot | 2 | 149 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.95] |
| 1.1 Number of participants with persistent symptoms at the end of the therapy | 2 | 149 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.95] |

