Stosowanie ogrzewanego i nawilżanego powietrza podczas przeziębienia
Información
- DOI:
- https://doi.org/10.1002/14651858.CD001728.pub6Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 29 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Meenu Singh was the sole author of this review and subsequent updates until 2005. The 2011 and 2013 updates were conducted by Meenu Singh and Manvi Singh.
The current update (2017) was conducted by Meenu Singh (MeS), Manvi Singh (MaS), Nishant Jaiswal (NJ), and Anil Chauhan (AC).
| Roles and responsibilities | |
| Task | Undertaken by |
| Review stage: select which trials to include | NJ, AC, MeS |
| Review stage: extract data from trials | MaS, MeS |
| Review stage: enter data into Review Manager 5 | NJ, AC |
| Review stage: carry out the analysis | MaS, MeS |
| Review stage: interpret the analysis | MaS, MeS |
| Review stage: draft the final review | MeS, AC, NJ, MaS |
| Update stage: update the review | MeS, AC, NJ, MaS |
Sources of support
Internal sources
-
Post Graduate Institute of Medical Education and Research, Chandigarh, India.
External sources
-
eHealth Project, Ministry of Health and Family Welfare, Government of India, India.
Financial Support to Poonam Chaudhary, B. Lib. Information specialist.
Declarations of interest
Meenu Singh: none known.
Manvi Singh: none known.
Nishant Jaiswal: none known.
Anil Chauhan: none known.
Acknowledgements
The Library Service, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. We thank Cochrane Acute Respiratory Infections Group Information Specialist David Honeyman for database searching and Managing Editor Liz Dooley and Assistant Managing Editor Ann Jones for editorial assistance. We would also like to acknowledge Michael Macknin for sharing study funding details.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Aug 29 | Heated, humidified air for the common cold | Review | Meenu Singh, Manvi Singh, Nishant Jaiswal, Anil Chauhan | |
| 2013 Jun 04 | Heated, humidified air for the common cold | Review | Meenu Singh, Manvi Singh | |
| 2011 May 11 | Heated, humidified air for the common cold | Review | Meenu Singh, Manvi Singh | |
| 2006 Jul 19 | Heated, humidified air for the common cold | Review | Meenu Singh | |
| 2004 Apr 19 | Heated, humidified air for the common cold | Review | Meenu Singh | |
| 2001 Jul 27 | Heated, humidified air for the common cold | Review | Meenu Singh | |
Differences between protocol and review
In this 2017 update, we moved the secondary outcome "Number of participants with no symptoms" to the primary outcomes and rephrased it as "Number of participants with subjective response: therapy did not help" because we believed it addressed an important question about the efficacy of heated, humidified air. We also rephrased the secondary outcome "Decrease in viral culture titre in the nasal secretions" to " Number of participants with a positive viral culture in the nasal washings", as it added clarity for the readers about what exactly is reported in the review.
Notes
The original version of this review, Singh 1999, was submitted to the Cochrane Library as a review; no Cochrane Review protocol was published. The initial protocol and review were conducted for presentation at an acute respiratory infection conference held in Canberra, Australia.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram for 2017 update.
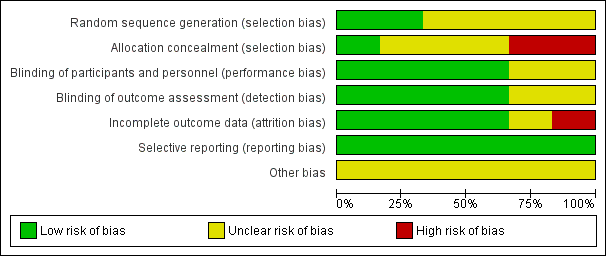
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Rhinothermy versus control, Outcome 1 Number of participants with persistent symptoms.

Comparison 1 Rhinothermy versus control, Outcome 2 Number of participants with subjective response to therapy: therapy did not help.
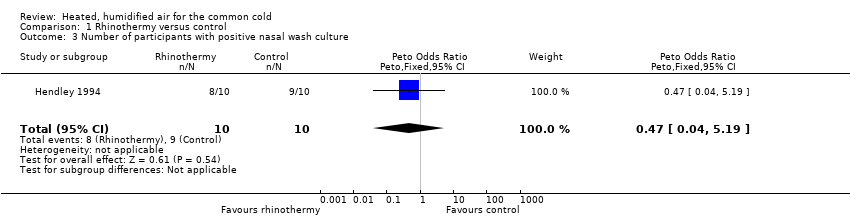
Comparison 1 Rhinothermy versus control, Outcome 3 Number of participants with positive nasal wash culture.
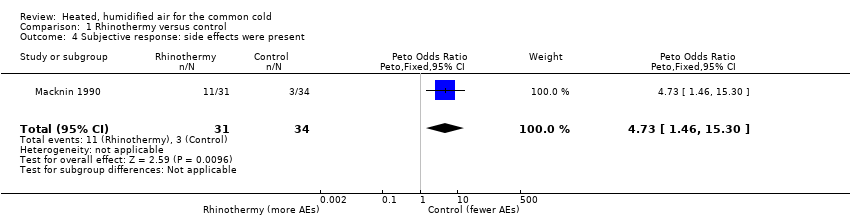
Comparison 1 Rhinothermy versus control, Outcome 4 Subjective response: side effects were present.
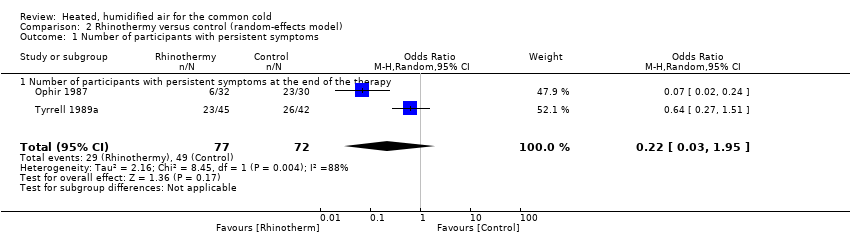
Comparison 2 Rhinothermy versus control (random‐effects model), Outcome 1 Number of participants with persistent symptoms.
| Heated, humidified air compared to control for treating the common cold | ||||||
| Patient or population: People with the common cold | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with rhinothermy | |||||
| Reduction in the clinical severity of the common cold (measured by decrease in the symptom score index) | Study population | Fixed‐effect model OR 0.30 Random‐effects model OR 0.22 (0.03 to 1.95) | 149 | ⊕⊕⊝⊝ | The significance of the effect is uncertain because use of the fixed‐effect model produces a different result than use of the random‐effects model. | |
| 681 per 1000 | Fixed‐effect model 390 per 1000 Random‐effects model 319 per 1000 (60 to 806) | |||||
| Number of participants with the subjective response: therapy did not help | Study population | OR 0.58 | 124 | ⊕⊕⊝⊝ | We downgraded the evidence for risk of bias and imprecision. | |
| 524 per 1000 | 389 per 1000 | |||||
| Number of participants with positive nasal wash cultures | Study population | OR 0.47 | 20 | ⊕⊕⊝⊝ | We downgraded the evidence for risk of bias and imprecision. | |
| 900 per 1000 | 809 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Ophir 1987 had high attrition rates; Tyrrell 1989b did not perform allocation concealment. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with persistent symptoms Show forest plot | 2 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.16, 0.56] |
| 1.1 Number of participants with persistent symptoms at the end of therapy | 2 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.16, 0.56] |
| 2 Number of participants with subjective response to therapy: therapy did not help Show forest plot | 2 | 124 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.28, 1.18] |
| 3 Number of participants with positive nasal wash culture Show forest plot | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.04, 5.19] |
| 4 Subjective response: side effects were present Show forest plot | 1 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.73 [1.46, 15.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with persistent symptoms Show forest plot | 2 | 149 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.95] |
| 1.1 Number of participants with persistent symptoms at the end of the therapy | 2 | 149 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.95] |

