Fenobarbital posnatal para la prevención de la hemorragia intraventricular en neonatos prematuros
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Open randomised controlled trial. | |
| Participants | Preterm infants with a birthweight below 1500g with no congenital malformations and no maternal phenobarbitone administration. N = 58 | |
| Interventions | Two loading doses of phenobarbital 10 mg/kg intravenously starting before 6 hours of age and the second loading dose 12 hours later, followed by a maintenance dose of 2.5 mg/kg every 12 hours for 7 days. Maintenance doses were adjusted to achieve trough phenobarbitone concentrations of 20 ‐ 30 mg/l | |
| Outcomes | Papile grade of intraventricular hemorrhage by ultrasound on days 1,3,7, posthemorrhagic hydrocephalus, death. It is not clear that the ultrasonographers were blind to treatment allocation. | |
| Notes | Cerebral ultrasound was not carried out prior to trial entry so it was not possible to exclude babies who already had IVH before the first dose of phenobarbitone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Open randomised controlled trial. | |
| Participants | Infants less than 24 hours old with birthweights < 1500g or gestation < 33 weeks were all eligible. Infants with gestational ages between 33 and 36 weeks or birthweight > 1500g, were eligible if they required mechanical ventilation for RDS. Another requirement was a cranial ultrasound scan showing no haemorrhage. N = 42. | |
| Interventions | Two intravenous loading doses of phenobarbital 10 mg/kg 12 hours apart, followed by maintenance doses of 2.5 mg/kg i.v.or orally every 12 hours for 6 days. | |
| Outcomes | Ultrasound diagnosis of grade of intraventricular hemorrhage as mild (grade I or II on Papile scale) or medium/severe (grade III or IV on Papile scale). Death. Mechanical ventilation, pneumothorax, hypotension (< 2 SD below mean), pH < 7.2, pCO2 > 60 mm Hg, pCO2 < 25 mm HG, Bicarbonate administration (for metabolic acidosis). | |
| Notes | Of 95 potential trial participants, 42 were excluded because of IVH on the initial ultrasound scan. The control group were, on average, 1.1 weeks less mature and 220g lighter than the phenobarbitone group. No infants excluded after enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Open randomised controlled trial. Randomisation is described as by lottery but there is no description of how allocation concealment was achieved. | |
| Participants | Infants with birthweights below 1500g, admitted to the NICU within 6 hours, without congenital malformations and where the mother had not received barbiturates during pregnancy. N = 60. No information on infants excluded or lost after enrolment. | |
| Interventions | Two loading doses of 10 mg/kg phenobarbital each administered intravenously 12 hours apart. Maintenance does of 2.5 mg/hr every 12 hours were begun 12 hours after. Doses were adjusted to maintain serum concentrations in the 20‐30 micrograms/ml range for 7 days. | |
| Outcomes | Papile grade of intraventricular hemorrhage on ultrasound, ventriculomegaly, mechanical ventilation, pneumothorax requiring drainage, hypercapnia (pCO2 > 60 mm Hg), hypotension ( systolic blood pressure 10 mm Hg below expected value or impaired perfusion), bicarbonate therapy, death. | |
| Notes | Cerebral ultrasound was not carried out prior to trial entry so it was not possible to exclude babies who already had IVH before the first dose of phenobarbitone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, double‐blind, controlled trial. Identical numbered ampoules were prepared by the pharmacy. | |
| Participants | Inclusion criteria were a) birthweight <1751g b) endotracheal intubation before 12 hours c) absence of congenital anomaly d) no evidence of intracranial hemorrhage on ultrasound scan e) neonatal phenobarbital level < 5 micrograms/ml. N = 280. Of 291 enrolled, 11 had to be withdrawn and were excluded from analysis. 48 infants were excluded from enrolment because IVH was already present. | |
| Interventions | Two loading doses of phenobarbital 10 mg/kg or placebo intravenously with a half hour interval. Twelve hours later, the baby received the first of nine maintenance doses of 2.5 mg/kg or placebo at 12 hour intervals. | |
| Outcomes | Papile grade of intraventricular hemorrhage on ultrasound scan (any hemorrhage or severe grade III or IV) hemorrhage, acidosis (pH< 7.2 on day 1), pneumothorax/pulmonary interstitial emphysema, hypotension (< 30 mm Hg on day 1. Mortality data were by personal communication between Dr Kuban and Dr Horbar although age at death is not clear. | |
| Notes | The randomisation failed to give a similar gestational age in the two treatment groups. Thus 52.4 % of the phenobarbitone group had gestational age < 30 weeks but this was true of only 41.5 % of the control group. The authors attempted to allow for this imbalance by analysis within weight groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Open controlled trial. The method of randomisation is not described nor is any means of allocation concealment. | |
| Participants | Newborn infants with gestational ages between 27 and 34 weeks and who were ventilator dependent. N = 60. No information on infants excluded or lost after enrolment. | |
| Interventions | Phenobarbital 20 mg/kg i.v. as a loading dose within 12 hours of birth followed by phenobarbitone 2.5 mg/kg every 12 hours for the next 5 days. | |
| Outcomes | Cerebral ultrasound every 48 hours for 14 days. Intraventricular hemorrhage graded as I/II or III/IV on the Papile scale. Death. It is not clear whether the ultrasonographers were blind to treatment allocation. | |
| Notes | Cerebral ultrasound was not carried out prior to trial entry so it was not possible to exclude babies who already had IVH before the first dose of phenobarbitone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | An open controlled trial using alternate allocation to phenobarbitone or no injection. | |
| Participants | Infants with birthweights below 1250g and infants with birthweights 1250‐1500g who required mechanical ventilation in the first 24 hours. An ultrasound scan showing absence of intraventricular haemorrhage was also a requirement. N = 60. No information on infants excluded or lost after enrolment. | |
| Interventions | A loading dose of 20 mg/kg phenobarbital intramuscularly at a median time of 2 hours after birth (range 1 ‐ 22 hours). | |
| Outcomes | Papile grade of intraventricular hemorrhage on ultrasound, death, pneumothorax, hypercapnia (pCO2 >8 kPa), acidosis (pH< 7.15). The age limit for death is not specified but "one cot death". occurred at home at 4 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Open randomised controlled trial. The method of randomisation is not described. | |
| Participants | Newborn infants with birthweight below 1500g with a normal cerebral ultrasound scan before 6 hours of birth and receiving respiratory supportt. N = 19. No information on infants excluded after enrolment. | |
| Interventions | A loading dose of phenobarbital 30 mg/kg i.v. within 6 hours of birth, followed by a maintenance dose of 5 mg/kg per day for 72 hours. | |
| Outcomes | Cerebral ultrasound scans were carried out daily by sonographers who were blind to the initial treatment allocation. Intraventricular hemorrhage was graded according to the Papile scale. Mechanical ventilation, pneumothorax, hypercapnia (> 60 mm Hg), acidosis (pH < 7.15). Death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Open randomised controlled trial. Randomisation was by "lottery". Blinding of randomization: can't tell. | |
| Participants | Infants with birthweights below 1501g and gestational age 25 weeks or more, less than 4 hours old. Infants with malformations or maternal barbiturate treatment were excluded. N = 101. 111 infants were originally enrolled but 10 were excluded (7 in the phenobarbitone group and 3 in the control group) either because the gestational age was < 25 weeks or because of congenital anomaly. | |
| Interventions | 2 loading doses of phenobarbital 15 mg/kg i.v. were given 4 hours apart. Maintenance treatment with phenobarbitone 5 mg/kg per day was started 24 hours after the first dose and continued for 5 days. | |
| Outcomes | Cerebral ultrasound scans were carried out on days 1,3,5 and 7 and then weekly. Intraventricular hemorrhage was graded according to the Papile scale. Neurodevelopmental assessment at 27 months of age. Neonatal death, postnatal death, mechanical ventilation (total and > 7days), pneumothorax. | |
| Notes | Cerebral ultrasound was not carried out prior to trial entry so it was not possible to exclude babies who already had IVH before the first dose of phenobarbitone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised controlled trial | |
| Participants | Infants with birthweights below 1500g and under 32 weeks gestation. | |
| Interventions | 5mg/kg/day dose of phenobarbital i.v. for the first 5 days | |
| Outcomes | Cerebral ultrasound scans were carried out on days 1, 3, 5 and 10. Intraventricular hemorrhage was graded according to the Papile scale. Neonatal death, pulmonary haemorrage, oxygen requirement, respiratory rate, and patent ductus arterious up to 10 days of age. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised double‐blind controlled trial. The infants received numered, identical ampoules for injection. | |
| Participants | Infants under 1500g with a normal cerebral ultrasound scan in the first 4 hours. N = 60. Two infants were excluded after randomisation because of congenital malformations and they were replaced. | |
| Interventions | Phenobarbital 20 mg/kg or isotonic saline given i.v. or i.m. within 4 hours of birth. No maintenance doses given. | |
| Outcomes | Intraventricular hemorrhage on cerebral ultrasound scans carried out daily for the two weeks and then weekly. Grading 1,2,3 according to Levene initially, subsequently reclassified to be compatible with Papile grading. Mechanical ventilation after injection, pneumothorax, hypercapnia (pCO2 > 8 kPa), acidosis (pH < 7.2), Death before discharge from hospital. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomized or quasi‐randomised trial. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All intraventricular hemorrhage Show forest plot | 9 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.25] |
| Analysis 1.1  Comparison 1 Phenobarbital v control, Outcome 1 All intraventricular hemorrhage. | ||||
| 2 Severe intraventricular hemorrhage Show forest plot | 10 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.24] |
| Analysis 1.2  Comparison 1 Phenobarbital v control, Outcome 2 Severe intraventricular hemorrhage. | ||||
| 3 Ventricular dilatation or hydrocephalus Show forest plot | 3 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.08] |
| Analysis 1.3  Comparison 1 Phenobarbital v control, Outcome 3 Ventricular dilatation or hydrocephalus. | ||||
| 4 Hypotension Show forest plot | 3 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| Analysis 1.4  Comparison 1 Phenobarbital v control, Outcome 4 Hypotension. | ||||
| 5 Pneumothorax/interstitial emphysema Show forest plot | 8 | 682 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.92, 1.77] |
| Analysis 1.5  Comparison 1 Phenobarbital v control, Outcome 5 Pneumothorax/interstitial emphysema. | ||||
| 6 Hypercapnia Show forest plot | 5 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| Analysis 1.6  Comparison 1 Phenobarbital v control, Outcome 6 Hypercapnia. | ||||
| 7 Acidosis Show forest plot | 6 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.51] |
| Analysis 1.7  Comparison 1 Phenobarbital v control, Outcome 7 Acidosis. | ||||
| 8 Use of mechanical ventilation Show forest plot | 5 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.06, 1.32] |
| Analysis 1.8  Comparison 1 Phenobarbital v control, Outcome 8 Use of mechanical ventilation. | ||||
| 9 Mild neurodevelopmental impairment Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.15, 2.17] |
| Analysis 1.9  Comparison 1 Phenobarbital v control, Outcome 9 Mild neurodevelopmental impairment. | ||||
| 10 Severe neurodevelopmental impairment Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.41, 5.04] |
| Analysis 1.10  Comparison 1 Phenobarbital v control, Outcome 10 Severe neurodevelopmental impairment. | ||||
| 11 Death before discharge Show forest plot | 9 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| Analysis 1.11  Comparison 1 Phenobarbital v control, Outcome 11 Death before discharge. | ||||
| 12 All deaths during study Show forest plot | 10 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.21] |
| Analysis 1.12  Comparison 1 Phenobarbital v control, Outcome 12 All deaths during study. | ||||
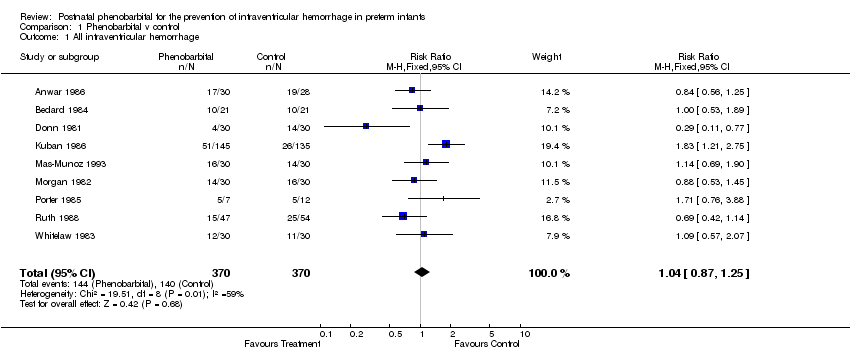
Comparison 1 Phenobarbital v control, Outcome 1 All intraventricular hemorrhage.

Comparison 1 Phenobarbital v control, Outcome 2 Severe intraventricular hemorrhage.
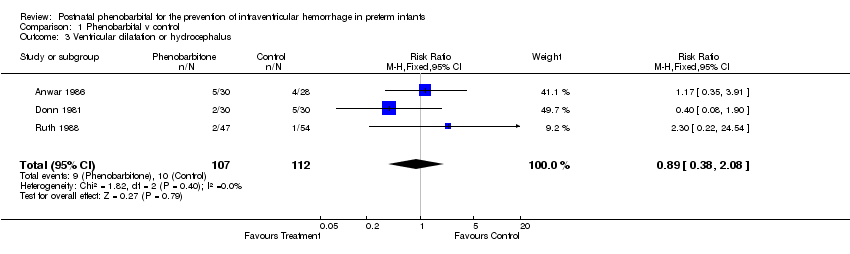
Comparison 1 Phenobarbital v control, Outcome 3 Ventricular dilatation or hydrocephalus.

Comparison 1 Phenobarbital v control, Outcome 4 Hypotension.
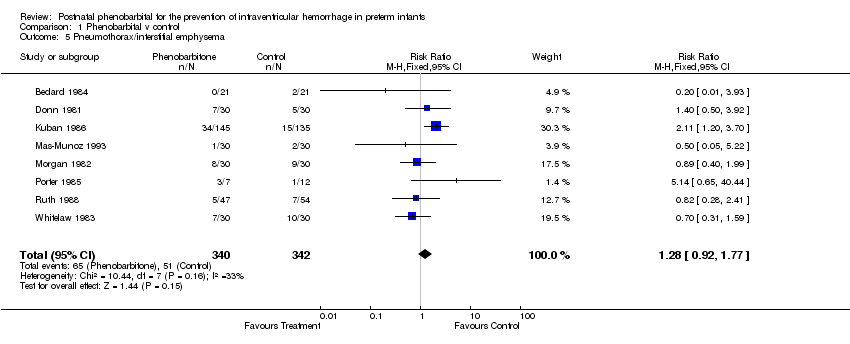
Comparison 1 Phenobarbital v control, Outcome 5 Pneumothorax/interstitial emphysema.

Comparison 1 Phenobarbital v control, Outcome 6 Hypercapnia.
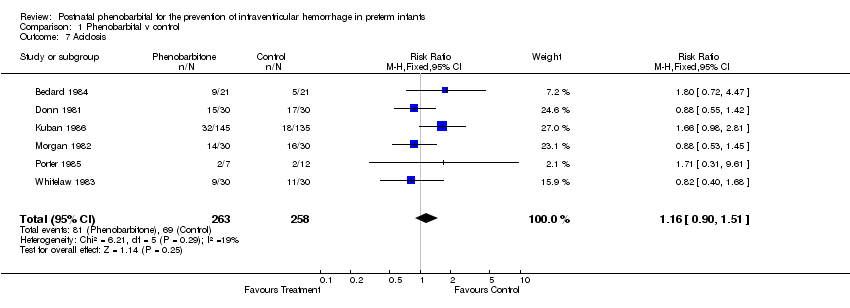
Comparison 1 Phenobarbital v control, Outcome 7 Acidosis.
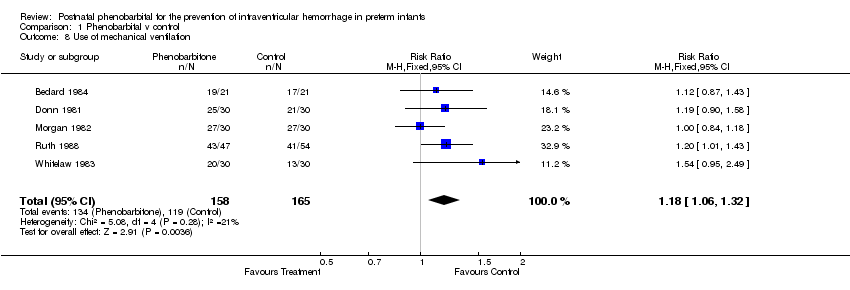
Comparison 1 Phenobarbital v control, Outcome 8 Use of mechanical ventilation.
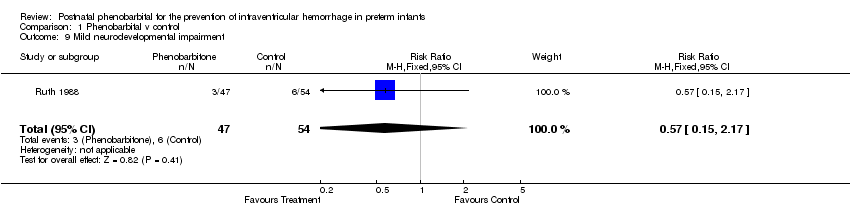
Comparison 1 Phenobarbital v control, Outcome 9 Mild neurodevelopmental impairment.
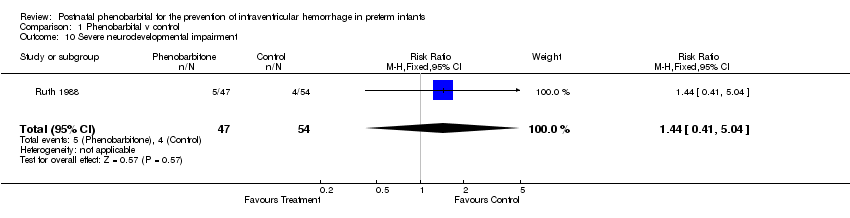
Comparison 1 Phenobarbital v control, Outcome 10 Severe neurodevelopmental impairment.

Comparison 1 Phenobarbital v control, Outcome 11 Death before discharge.

Comparison 1 Phenobarbital v control, Outcome 12 All deaths during study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All intraventricular hemorrhage Show forest plot | 9 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.25] |
| 2 Severe intraventricular hemorrhage Show forest plot | 10 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.24] |
| 3 Ventricular dilatation or hydrocephalus Show forest plot | 3 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.08] |
| 4 Hypotension Show forest plot | 3 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| 5 Pneumothorax/interstitial emphysema Show forest plot | 8 | 682 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.92, 1.77] |
| 6 Hypercapnia Show forest plot | 5 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 7 Acidosis Show forest plot | 6 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.51] |
| 8 Use of mechanical ventilation Show forest plot | 5 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.06, 1.32] |
| 9 Mild neurodevelopmental impairment Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.15, 2.17] |
| 10 Severe neurodevelopmental impairment Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.41, 5.04] |
| 11 Death before discharge Show forest plot | 9 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 12 All deaths during study Show forest plot | 10 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.21] |

