Estrógenos locales para la atrofia vaginal en mujeres posmenopáusicas
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation on a 2:1 basis, via a random number‐generating computer programme using sequential envelopes (sealed, opaque) at each trial centre | |
| Participants | Inclusion criteria: postmenopausal, symptoms of urogenital atrophy (vaginal dryness with or without dyspareunia, pruritus, dysuria and/or urgency and signs of atrophic vaginitis, including pallor, petechiae, friability and/or dryness) | |
| Interventions | Treatment: oestradiol vaginal ring (Estring), inserted high in the vagina by the investigator at the inclusion visit with instructions to remain in situ continuously for 12 weeks. Women were allowed to remove the ring for a short time if coitus desired. Ring: silicone core contains 2 mg of micronised 17β oestradiol, uniform sustained release of 5‐10 mcg per 24 hours of oestradiol for 3 months | |
| Outcomes | Cure and response rate, maturation value and vaginal pH, vaginal and vulval irritation/ulceration, intercurrent vaginal bleeding (indication of endometrial thickness), adherence to treatment, discomfort, acceptance of treatment delivery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence generation |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by sealed opaque envelope |
| Blinding (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Some outcome assessors (pathologist) were blinded; unclear whether participants were blinded as some outcomes were self assessed |
| Incomplete outcome data (attrition bias) | Low risk | Data analysed using ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Treatment groups were balanced at baseline |
| Methods | A 3‐arm parallel RCT | |
| Participants | 230 postmenopausal women with atrophic vaginitis Age (mean): Group A: 58.3 (7.4); Group B: 57.7 (6.5); Group C: 57.6 (4.8) Inclusion criteria: women aged 45 years or older with moderate to severe vaginal dryness and soreness were enrolled. All participants had serum E2 concentrations of 20 pg/mL or less, with 5% or less superficial vaginal cells. Participants were also required to be at least 12 months postmenopausal, with an endometrial thickness of 5 mm or less as determined by transvaginal ultrasonography Exclusion criteria: known or suspected history of breast carcinoma, hormone‐dependent tumour, genital bleeding of unknown cause, acute thrombophlebitis or thromboembolic disorder associated with oestrogen use, vaginal infection requiring treatment, allergy to the test drug or its constituents, or any serious disease or chronic condition that could interfere with study compliance were among the criteria for exclusion. The use of any investigational drug within the 30 days preceding screening, any homeopathic preparation within the 7 days preceding study drug initiation, and any exogenous corticosteroid or sex hormones within the 8 weeks preceding study drug initiation were prohibited | |
| Interventions | Group A: 25 mcg oestradiol tablet (n = 91). 1 tablet inserted into the vagina daily for 14 days, then twice per week Group B: 10 mcg tablet (n = 92). 1 tablet inserted into the vagina daily for 14 days, then twice per week Group C: placebo (n = 47). 1 tablet inserted into the vaginal daily for 14 days, then twice per week Duration: 12 weeks; follow up: 52 weeks | |
| Outcomes | Vaginal pH, maturation index, vaginal health, vaginal symptoms, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was not reported what was used in generating the randomisation code, however it was stated that, "A randomization code was generated and assigned |
| Allocation concealment (selection bias) | Low risk | It was reported that, "A sealed envelope with the randomization number and identity of the treatment for each participant was given to each investigator. Allocations were concealed in sealed envelope" |
| Blinding (performance bias) | Low risk | Trial was a "double‐blind, placebo‐controlled, parallel‐group 12‐week study" |
| Blinding of outcome assessment (detection bias) | Low risk | Some outcomes assessed by participants who were blinded. Adverse events, symptoms and other outcomes assessed by investigators who also were blinded. Also endometrial biopsy results assessed by “two independent pathologists who were masked to treatment group and each others' interpretation |
| Incomplete outcome data (attrition bias) | Low risk | Analysis of data was based on ITT |
| Selective reporting (reporting bias) | Low risk | All the outcomes specified in the methods section ware reported |
| Other bias | Low risk | It was reported that, "Demographic and baseline characteristics were similar across treatment groups, with the exception of a slightly higher percentage of white participants in the 25 mcg E2" group |
| Methods | A 4‐arm parallel RCT | |
| Participants | 423 postmenopausal women with moderate to severe symptoms of vaginal atrophy Age (mean, SD): Group A: 57.7 (5.8); Group B: 58.0 (5.8); Group C: 57.5 (5.5); Group D: 58.7 (5.8) Inclusion criteria: the trial enrolled generally healthy postmenopausal women (aged 45‐80) with an intact uterus and symptoms of moderate to severe vaginal atrophy. These were defined as the following: a baseline composite score (at the initial screening visit) of at least 5 (1 = mild, 2 = moderate, 3 = severe) for the 4 symptoms of vaginal dryness, itching, burning and dyspareunia (at least one of these symptoms had to be moderate or severe); a total score of 15 or less on the Genital Health Clinical Evaluation (GHCE), a tool used to evaluate six parameters (vaginal pH, fluid secretion, moisture, vaginal rugosity, mucosal colour, and epithelial mucosa) scored on a scale of 1‐4; vaginal pH of at least 5; and a clinical diagnosis of atrophic vaginitis, defined as 0% to 5% superficial cells on vaginal cytologic smear. Additional inclusion criteria included a serum oestradiol concentration of 30 pg/mL or less and a serum follicle stimulating hormone level greater than the lower limit of normal for postmenopausal women at the given laboratory Exclusion criteria: the use of an intrauterine device within 3 months of screening or the use of any oral, vaginal, or transdermal medication containing oestrogens, androgens, or progestins within 8 weeks of screening. Women who had used vaginal moisturisers, lubricants, jellies, ointments, douches, herbal medications, over‐the‐counter preparations, home remedies, or natural oestrogen products (ie, soy products) for the treatment of menopausal symptoms agreed to refrain from their use for a minimum of 7 days before screening. Participants who currently used more than two antihypertensive medications, had used any investigational drug or device within 30 days of screening, or had urogynecologic surgery within 3 months of screening were also excluded. | |
| Interventions | Group A: conjugated oestrogen (21/7) (n = 143). 0.3 mg cream applied once daily (21 days on/7 days off) Group B: placebo (21/7) (n = 72). 0.3 mg cream applied once daily (21 days on/7 days off) Group C: conjugated oestrogen (2 x /wk) (n = 140). 0.3 mg cream applied twice weekly Group D: placebo (2 x /wk) (n = 68). 0.3 mg cream applied twice weekly Duration: 12 weeks | |
| Outcomes | Participant‐reported symptoms, vaginal health, vaginal pH, maturation index, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported; it was only stated that, "participants were randomly assigned to one of four treatment regimens" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on method used in allocation concealment |
| Blinding (performance bias) | Low risk | It was reported that, "The first phase was double‐blind, randomized, and placebo‐controlled" |
| Blinding of outcome assessment (detection bias) | Low risk | Some outcomes were assessed by participants (adverse events, symptom scores) and participants were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Rates and reasons for withdrawals were similar across treatment groups; data was analysed using the modified ITTA: "The primary efficacy analyses at week 12 were done using the modified intent‐to‐treat (MITT)" |
| Selective reporting (reporting bias) | Low risk | All the outcomes specified in the methods section were reported |
| Other bias | Low risk | "There were no differences between treatment groups in demographic and clinical characteristics at baseline" |
| Methods | Women were allocated to one of two treatment schedules via a central randomisation list | |
| Participants | Inclusion criteria: 2 years after spontaneous or surgical menopause (bilateral oophorectomy) and symptoms of atrophic vaginitis including vaginal dryness | |
| Interventions | Treatment: oestradiol ring (Estring) containing 2 mg micronised 17β oestradiol with a constant release of 7.5 mcg oestradiol/24 hours for 90 days | |
| Outcomes | Maturation value, vaginal pH, cured or improved symptoms, responders (parabasal cells decreased > 25%), adverse events, vaginal irritation administration form, preference | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomisation but no further details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias) | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single blinding but some outcomes were self assessed |
| Incomplete outcome data (attrition bias) | Low risk | Data analysis based on ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient details to make a conclusive judgement |
| Methods | Open label randomisation method | |
| Participants | Inclusion criteria: postmenopausal women complaining of vaginal dryness, natural menopause oophorectomy | |
| Interventions | Treatment: a non‐hormonal local bio adhesive vaginal gel composed of purified water enmeshed in a carbomer‐polycarbophil system (Replens) one vaginal application 3 times a week for 3 months | |
| Outcomes | Vaginal pH, vaginal dryness index, pruritis, dyspareunia, overall feeling, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Blinding (performance bias) | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Open label trial and some of the outcomes were self‐assessed; but unclear whether outcome assessors were blinded or not |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant withdrew from the trial |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | 2‐arm parallel RCT | |
| Participants | 167 postmenopausal women with vaginal atrophy Age (mean): Group A: 56.5; Group B: 57.2 Inclusion criteria: postmenopausal with at least 2 years of amenorrhoea caused by either natural or surgical menopause (bilateral oophorectomy). They also presented symptoms and signs of atrophy of the vaginal mucosa including as a minimum, vaginal dryness and at least one sign of the condition verified by the investigator Exclusion criteria: history of malignant or pre‐malignant lesions of the breasts or endometrium, malignant colon or hepatic tumours, malignant melanoma, venous thromboembolic disorders (deep vein thrombosis, pulmonary embolism) or arterial thromboembolic disorders (ischaemic heart disease, myocardial infarction, cerebrovascular accident), peripheral arterial disease, mesenteric artery thrombosis, renal artery thrombosis, or coagulopathies | |
| Interventions | Group A: low‐dose estriol vaginal gel (n = 114). 1 g of vaginal gel containing 50 µg of estriol Group B: placebo (n = 53). 1 g of placebo vaginal gel Duration: 12 weeks | |
| Outcomes | Changes in vaginal atrophy symptomatology, vaginal pH, maturation index, adverse event, adherence to treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported, it was only stated that, "Eligible women were randomized in a ratio of 2:1....." |
| Blinding (performance bias) | Low risk | Study was described as, "randomized, doubleblind, placebo‐controlled study." |
| Blinding of outcome assessment (detection bias) | Low risk | Participant‐reported outcomes ‐ a double‐blind trial |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportions of and reasons for withdrawals differ between the two treatment groups and data was not analysed on the basis of ITT |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Participants similar in baseline demographic characteristics but numbers not balanced at randomisation |
| Methods | Method of randomisation not stated | |
| Participants | Inclusion criteria: at least 2 years after spontaneous or surgical menopause presenting with one or more signs and symptoms of atrophic vaginitis due to oestrogen deficiency, pruritus vulvae, dyspareunia, dysuria, urinary urgency; on examination; petechiae, friability or vaginal dryness | |
| Interventions | Treatment: oestradiol‐releasing silicone ring (Estring) core containing 2 mg 17β‐oestradiol releasing 7.5 mcg/24 hours for 90 days | |
| Outcomes | Vaginal pH, response, adverse events, adherence to treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single blinding but some outcomes were self‐assessed |
| Incomplete outcome data (attrition bias) | High risk | Proportions of withdrawals differed between the two treatment groups and data analysis was not based on ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Method of randomisation: not stated | |
| Participants | Inclusion and exclusion criteria as per study 1 | |
| Interventions | Treatment: oestradiol‐releasing silicone ring (Estring) per study 1 | |
| Outcomes | Maturation value, vaginal pH, endometrial thickness, freedom of symptoms (dyspareunia, pallor, petechiae, friability, vaginal dryness) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Low risk | Double‐blinded trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Some outcomes were self‐assessed but unclear whether other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Data analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Method of randomisation: not stated | |
| Participants | Inclusion criteria: participants presented with symptoms and signs of urinary stress incontinence, vaginal atrophy, and histories of recurrent urinary tract infections. None had received oestrogen before the study | |
| Interventions | Treatment: intravaginal oestradiol ovules (1 mg) once daily for 2 weeks and then 2 ovules once weekly for 6 months | |
| Outcomes | Vaginal dryness, dyspareunia, vaginal pH, KPI of vaginal epithelium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Low risk | Double‐blinded, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Some outcomes were self‐assessed but unclear whether other outcome assessors were blinded e.g. the pathologists |
| Incomplete outcome data (attrition bias) | Low risk | Data analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Randomisation method not stated | |
| Participants | Inclusion criteria: women with signs and symptoms of vaginal atrophy and did not require systemic oestrogen therapy for treatment of vasomotor symptoms or prophylaxis of osteoporosis and had not experienced vaginal bleeding for 1 year | |
| Interventions | Treatment: oestradiol vaginal tablet 25 mcg 17β oestradiol | |
| Outcomes | Vaginal dryness, maturation, adverse effects, leakage of medicine, requirement of sanitary wear, ease of use, hygenic | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single‐blinded study but some outcomes were self‐assessed |
| Incomplete outcome data (attrition bias) | Low risk | Data analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Treatment groups were balanced at baseline |
| Methods | Randomisation method not stated | |
| Participants | Inclusion criteria: women suffering from vaginal symptoms related to postmenopausal atrophy | |
| Interventions | Treatment: 25 mcg 17β‐oestradiol tablet (Vagifem) once daily for 2 weeks, then twice a week for 10 weeks | |
| Outcomes | Moderate‐severe symptoms of vaginal atrophy; dryness, itching and burning, dyspareunia, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Low risk | Double‐blinded trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blinded trial but unclear whether other outcome assessors were blinded e.g. the pathologists |
| Incomplete outcome data (attrition bias) | High risk | Proportion of withdrawals differs between the two treatment groups and analysis was not on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | A 4‐arm parallel RCT | |
| Participants | 80 postmenopausal women with symptoms of vaginal atrophy Age (mean, SD): Group A: 57.0 (5.4); Group B: 56.2 (5.3); Group C: 56.4 (4.8); Group D: 57.7 (4.7) Inclusion criteria: women aged 40–70 years with physiological menopause and a history of amenorrhoea for > 3 years with a follicle‐stimulating hormone level of > 30 mIU/mL. They had not taken hormonal treatment for menopausal symptoms in the past 6 months, had shown normal Pap smears and mammograms for the past 12 months, and had complaints compatible with the symptoms of vaginal atrophy (vaginal dryness, vulvovaginal irritation/itching, and pain at sexual activity 6 months ago) Exclusion criteria: women who were expected to undergo an oophorectomy or hysterectomy and those with a body mass index < 18.5 kg/m2 or > 30 kg/m2. "We excluded those women with a contraindication for the use of estrogen or testosterone, namely those with a history of myocardial infarction, severe hypertension, diabetes mellitus, thromboembolic disease, liver failure, ulcerative colitis, Crohn’s disease, breast or endometrial cancer, fibrocystic breast disease with atypical hyperplasia, genital bleeding of unknown origin, a family history of breast cancer, endometrial hyperplasia, or positive serology for human immunodeficiency virus, hepatitis B, or C". Finally, women were excluded if they had a vaginal infection at the time of their gynecological examination. | |
| Interventions | Group A: acid polyacrylic (n = 20) Vaginal cream with polyacrylic acid (Vagidrat®, Myralis Pharma Ltd, Aguai, Sao Paulo, Brazil) one vaginal applicator with 3 g cream per application. Group B: testosterone (n = 20) Vaginal cream with testosterone propionate: 1 vaginal applicator with 1 g of cream per application containing 300 μg testosterone propionate prepared using testosterone micronised powder in an emollient cream with silicone to keep the cream iso‐osmolar Group C: estrogen vaginal cream (n = 20) Vaginal cream with conjugated estrogens (Premarin®, Wyeth Pharmaceuticals, Itapevi, São Paulo, Brazil): one vaginal applicator with 1 g of cream per application containing 0.625 mg conjugated estrogens Group D: lubricant (placebo; n = 20) Lubricant with glycerin gel (K‐Y jelly Johnson & Johnson, São José dos Campos, São José dos Campos, Brazil) 3 g in one applicator per application adjusted to maintain similarity with the polyacrylic acid application. This group was used as a control for the 3 other treatment groups Duration: 12 weeks | |
| Outcomes | Adverse event (allergic vaginitis) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | It was stated that, "All participants were given a number (1–80) according to their order of inclusion in the study." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on method used in allocation concealment |
| Blinding (performance bias) | Low risk | It was reported that, "Dispensation of the topical agent was done by a gynecologist who was not part of the selection/interview team" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was reported on the blinding of outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | It was reported that, "Data were analyzed according to intention to treat, including all participants in each group" |
| Selective reporting (reporting bias) | Low risk | All the outcomes specified in the methods section were reported |
| Other bias | Unclear risk | It was reported that, "There were no significant differences between groups in terms of age, time after menopause, skin color, smoking habits, numbers of pregnancies, or socioeconomic status" |
| Methods | Randomised to control or placebo group according to randomisation code Parallel group design with double blinding at 2 centres | |
| Participants | Inclusion criteria: moderate to severe urogenital and systemic postmenopausal complaints and follicle stimulating and oestradiol (E2) serum levels within postmenopausal range, last menses one year prior to treatment, score at least 6 points on UGI (urogenital scale) and 20 on KI (Kupperman Index) | |
| Interventions | Treatment: vaginal suppository containing 3.5 mg oestriol (E3) or placebo, twice weekly for 3 weeks followed by 1 suppository weekly for 6 months | |
| Outcomes | Efficacy: UGI score, vaginal pH | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence was said to have been generated through randomisation code, no further details were reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias) | Low risk | Double‐blinded trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether outcome assessors other than the participants were blinded e.g. the pathologists |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawals were not reported and unclear whether data were analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Randomisation not specified, list used consecutive numbers. Double‐blinding, single‐centre, parallel group design | |
| Participants | Inclusion criteria: > 1 year menopausal symptoms, vasomotor instability | |
| Interventions | Treatment: vaginal ovules of oestradiol 3.5 mg. First 3 weeks 2 per week and remainder 1 per week | |
| Outcomes | Improvement in symptoms (none or light) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence was said to have been generated using consecutive numbers, no further details were reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Low risk | Double‐blinded trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether other outcome assessors other than the participants were blinded e.g. the pathologists |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of withdrawals per treatment group was not reported and data were not analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | 3‐arm parallel design RCT | |
| Participants | 436 postmenopausal women with vaginal atrophy Age (mean, SD): Group A: 64.9 (8.1); Group B:65.4 (7.3); Group C: 64.8 (7.8) Inclusion criteria: postmenopausal women (last menstrual period more than 12 months ago or having undergone bilateral ovariectomy) aged 18 years or older with a clinical diagnosis of vaginal atrophy, a vaginal maturation index (VMI) < 40% and a vaginal pH value > 5 were eligible for inclusion. At least one subjective symptom of vaginal atrophy (dryness, pain/burning sensation, pruritus, discharge, dyspareunia) had to be rated at a score of ≥ 65 on the visual analogue scale (VAS) Exclusion criteria: hormone replacement therapy, therapy with phytoestrogens or local vaginal hormonal therapy during 12 weeks preceding baseline, as well as current or suspected estrogen‐dependent malignant tumour, a Pap smear ≥ III, endometrial thickness > 5 mm, current or suspected vaginal infection, current symptomatic urinary tract infection, existing or previous breast cancer or suspicion thereof, undiagnosed bleeding in the genital area, current venous thromboembolic disease, known severe renal insufficiency or hypersensitivity to estriol or any of the excipients (hard fat and emulsifiers) of the study medication | |
| Interventions | Group A: estriol pessary 0.2 mg (n = 142). Estriol pessary 0.2 mg once‐daily application or 20 days, followed by twice‐weekly administration for a further 9 weeks as a maintenance therapy Group B: estriol pessary 0.03 mg (n = 147) (same administration as in Group A) Group C: placebo (n = 147). Treatment protocol as described for Group A Duration: 12 weeks | |
| Outcomes | Improvement in atrophy symptoms, vaginal pH, adverse events, tolerability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Low risk | Stated as “double blind” |
| Blinding of outcome assessment (detection bias) | Low risk | Stated as “double blind” (for participant‐ and clinician‐assessed outcomes) |
| Incomplete outcome data (attrition bias) | Low risk | Proportions of and reasons for withdrawals fairly balanced between the treatment groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Baseline demographic characteristic (age) was similar between the groups |
| Methods | Open, parallel‐group, single‐blinded comparative trial with active control, randomised in the proportions 2:1 | |
| Participants | Inclusion criteria: postmenopausal women at least 2 years after spontaneous or surgical (bilateral oophorectomy) menopause, complaining of oestrogen deficiency symptoms of atrophic vaginitis, signs of atrophic vaginal mucosa | |
| Interventions | Treatment: silicone rubber vaginal ring (silastic) containing 2 mg of micronised 17β‐oestradiol releasing 6.5 to 9.5 mcg per 24 hours over a 3‐month period | |
| Outcomes | Cured/improved subjects' symptoms, physician assessment of mucosa, maturation value, vaginal pH, adverse events, withdrawals, adherence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single‐blinded trial but some of the outcomes were participant‐assessed |
| Incomplete outcome data (attrition bias) | Unclear risk | Data were analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Treatment groups were balanced at baseline |
| Methods | 2‐arm parallel RCT Number of centres: 1 Number of women randomised: 160 Number of women analysed: ?160 Number of withdrawals: ?0 | |
| Participants | Inclusion criteria: 160 postmenopausal women with clinical diagnosis of vaginal atrophy Age (mean, SD): 50‐70 years: Group A: 56.55 ± 8.63; Group B: 55.28 ± 6.12 BMI (kg/m2): Group A: 23.21 ± 3; Group B: 25 22.48 ± 2.56 Exclusion criteria: history of carcinoma of the breast or endometrium, abnormal genital bleeding, acute thrombophlebitis, or thromboembolic disorders associated with previous oestrogen therapy, treated with systemic or vaginal oestrogen within 6 months of the study, or had any contraindication for oestrogen therapy | |
| Interventions | Group A: vaginal oestrogen cream (1 tube per night for 14 nights, then 1 tube 2 nights in 1 week for 10 weeks) Group B: Vagifem (oestrogen tablet) 25 mcg tablets (with similar treatment plan) | |
| Outcomes | Severity of vaginal atrophy (assessed by gynaecologist), 4 main symptoms of atrophic vaginitis: dysuria, dyspareunia, vaginal itching and dryness (participant self report), Satisfaction with treatment, acceptability of treatment (pain, vaginal leakage, need for sanitary towels, user friendliness), adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "women were randomly divided into Vagifem (from Novo Nordisk) or vaginal estrogen cream (Equin from Actoverco) treatment groups (80 women in each group)" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Unclear risk | Not reported but participants were likely to be unblinded since the 2 treatments required different administration |
| Blinding of outcome assessment (detection bias) | Unclear risk | Gynaecologists were “unaware of the treatment group” and so there is a low risk of bias for the gynaecologist assessment of vaginal atrophy but other outcomes were answered by participant self‐report |
| Incomplete outcome data (attrition bias) | Low risk | It appears that there were no dropouts after randomisation |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods section were reported |
| Other bias | Low risk | Baseline demographic characteristics similar in both treatment groups |
| Methods | 3‐arm parallel design RCT | |
| Participants | 65 women undergoing vaginal reconstructive surgery and also with evidence of symptomatic vaginal atrophy and pelvic organ prolapse Age (mean, SD): Group A: 65 (7.4); Group B: 66 (7.9); Group C: 65 (7.8) Inclusion criteria: postmenopausal women at least 2 years after spontaneous or surgical menopause with symptomatic urogenital atrophy and pelvic organ prolapse who opted to undergo reconstructive vaginal surgery. Eligible candidates had to have at least one symptom (vaginal dryness, vulvar pruritus, dyspareunia, dysuria, or urinary urgency) and/or sign (vaginal pallor, petechiae, friability, or vaginal dryness) of atrophic vaginitis Exclusion criteria: contraindications to oestrogen use (vaginal bleeding, oestrogen‐dependent cancers, hepatic or thrombotic disease), allergies to silicone and/or oestradiol, absence of vulvovaginal atrophy on examination and/or vaginal pH of less than or equal to 4.0, or use of vaginal or systemic oestrogen in the previous 6 months | |
| Interventions | Group A: oestradiol‐releasing vaginal ring (n = 22). This was applied immediately after surgery Group B: placebo ring (n = 21). As in Group A Group C: no ring (n = 22) Duration: 12 weeks | |
| Outcomes | Vaginal pH, maturation index, objective signs of atrophy, subjective bother of atrophy, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment via computer generation in "blocks of 20 to one of 3 groups" |
| Allocation concealment (selection bias) | Low risk | Allocation of randomisation group was made by the primary author via sealed envelopes on the day of surgery once the participant was under anaesthesia in the operating room |
| Blinding (performance bias) | Unclear risk | Participants were blinded but unclear whether the clinicians were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Some of the outcome assessors were blinded to the treatment protocols but unclear whether clinician‐assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition came from the 2 groups with vaginal rings in place. No attrition from the control group and analysis was not based on ITT |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Baseline demographic characteristic (age) was similar between the treatment groups |
| Methods | 3‐arm parallel design | |
| Participants | 90 postmenopausal women without a hysterectomy and with vaginal atrophy Age (mean): Group A: 56; Group B: 57; Group C: 57 Inclusion criteria: non‐hysterectomised, postmenopausal (2 or more years since final menstrual cycle) women who were 45 years of age or older with symptoms of vaginal dryness and/or pruritus, pain/soreness, vulvar or vaginal burning, and dyspareunia. All participants were required to have serum E2 levels less than 20 pg/mL, follicle‐stimulating hormone levels greater than 40 mIU/mL, no superficial cells on vaginal cytology, an endometrial thickness of less than 4.0 mm as assessed by transvaginal ultrasonography, and a normal mammography during the 6 months leading up to study entry Exclusion criteria: use of any investigational drug or exogenous sex hormones within the 6 months leading up to study drug initiation, or current use of corticosteroids, known or suspected history of hormone‐dependent tumour, breast carcinoma, genital bleeding of unknown cause, acute thromboembolic disorder associated with oestrogen use, vaginal infection requiring treatment, allergy to the test drug or its constituents, hot flushes, and any serious disease or chronic condition that could interfere with study compliance | |
| Interventions | Group A: conjugated equine estrogen cream (n = 30) 0.5 g corresponding to 0.3 mg administered vaginally at bedtime Group B: isoflavone vaginal gel (n = 30) 1 g of isoflavone gel or 1 g administered vaginally at bedtime Group C: placebo (n = 30) 1 g of placebo administered vaginally at bedtime Duration: 12 weeks | |
| Outcomes | Maturation index, vaginal atrophy symptomatology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Low risk | Reported as .."double‐blind, randomized, placebo‐controlled, clinical trial.." |
| Blinding of outcome assessment (detection bias) | Low risk | Reported as "double" blind and outcomes were either assessed by participants or clinicians |
| Incomplete outcome data (attrition bias) | High risk | 1/3 of participants discontinued from CEE cream group, 17% from placebo group and none from isoflavone group. Likely to cause bias and analysis was not based on ITT although it was reported that "All data reported at week 0 and week 12 are from the intent‐to‐treat analyses, with missing values for each individual computed using the last observation carried forward approach" |
| Selective reporting (reporting bias) | Unclear risk | Other than endometrial safety, no adverse events are reported |
| Other bias | Low risk | "No significant differences were observed among the three groups regarding age, age at and time since menopause, height, weight or BMI" |
| Methods | Randomisation by central office using numbers sequentially, no blinding, parallel design, multicentre (27) | |
| Participants | Inclusion criteria: women who report with one bothersome lower tract symptom appearing at least 2 years after spontaneous or surgical postmenopause | |
| Interventions | Treatment: oestradiol releasing vaginal ring (Estring) with constant release of 7.5 mg oestradiol per 24 hours for 3‐month period | |
| Outcomes | Urgency, frequency, urge incontinence, stress incontinence; nocturia, dysuria, vaginal dryness, dyspareunia, adverse events, assessment of administration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by central office using numbers sequentially no further details were reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | High risk | Open label (no blinding) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether other outcome assessors e.g. the pathologists were blinded or not |
| Incomplete outcome data (attrition bias) | Low risk | Data were analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Treatment groups were balanced at baseline |
| Methods | 2‐arm parallel RCT | |
| Participants | 38 postmenopausal women with bothersome symptoms of vulvo vaginal atrophy Inclusion criteria: healthy postmenopausal women with bothersome symptoms of vulvo vaginal atrophy Exclusion criteria: not reported | |
| Interventions | Group A: very low dose oestradiol vaginal cream (n = 19) applied to the vagina daily for 2 weeks followed by twice weekly for 10 weeks Group B: placebo (n = 19) applied as described for the active treatment group Duration: 12 weeks | |
| Outcomes | Vaginal pH, maturation index, symptoms relating to atrophy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information was reported on randomisation process |
| Allocation concealment (selection bias) | Unclear risk | No information was provided on allocation concealment |
| Blinding (performance bias) | Unclear risk | No information was given with respect to the blinding of participants and/or personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was provided on the blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information was given on withdrawals/losses to follow‐up per treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Although all the outcomes specified in the methods section ware reported no data were, however, available on any of the outcomes |
| Other bias | Unclear risk | Insufficient information was reported to make a conclusive judgement |
| Methods | Open label, randomised, no blinding | |
| Participants | Inclusion criteria: healthy, natural postmenopausal, urogenital symptoms, periods ceased for 1 year | |
| Interventions | Treatment: oestradiol vaginal tablet (25 mcg) daily for 2 weeks and then once a week for 10 weeks | |
| Outcomes | KPI index (karyopyknotic), maturation value, endometrial thickness, vaginal health index, vaginal pH, symptoms of vaginal atrophy (dryness, burning/pain, dyspareunia, frequency, nocturia, stress incontinence), endometrial proliferation, plasma oestradiol levels, satisfaction, adherence to treatment, pelvic discomfort | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | High risk | Open label (no blinding) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether other outcome assessors such as the pathologists were blinded or not |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportions and reasons for withdrawals differ between the two treatment groups and data were not analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Randomisation method not stated | |
| Participants | Inclusion criteria: > 1 year past last menstrual period, not on hormone therapy, cancer free and experiencing vaginal discomfort or dyspareunia | |
| Interventions | Treatment: non‐hormonal local bio adhesive vaginal moisturiser (Replens) 3 times per week | |
| Outcomes | Vaginal moisture, fluid volume, elasticity, pH | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | High risk | Open label (no blinding) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether outcome assessors such as the pathologists were blinded or not |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals were reported in the treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Women were randomly assigned in a 2:1 ratio using separate computer‐generated randomisation lists | |
| Participants | Inclusion criteria: naturally menopausal for at least a year, oophorectomised 1 year or more or post‐hysterectomy (with 1 or both ovaries remaining; 55 years or older; FSH level of at least 40 mIU/mL; have symptoms of vaginal dryness, one or more signs (pallor, petechiae, friability, or lack of vaginal moisture) | |
| Interventions | Treatment: oestradiol vaginal ring (Estring) 7.5 mcg of oestradiol per 24 hours inserted for 12 weeks followed by a 3‐week period of no medication | |
| Outcomes | Improvement in vaginal atrophy > 25% reduction in cells: basal/parabasal and intermediate, physician evaluation, vaginal pH, participant assessment, end tissue response, challenge test, sonography, end biopsies (for evidence of endometrial hyperplasia), adverse events, product comfort, ease of use and overall rating, adherence to treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single‐blinded trial (cytologist blinded) but some outcomes were participant‐assessed |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportions of withdrawals differ between treatment groups and data were not analysed on the basis of ITT for all outcomes |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Treatment groups were balanced at baseline |
| Methods | A 3‐arm parallel RCT but only 2 arms of the trial were relevant | |
| Participants | 50 postmenopausal women with urogenital and sexual dysfunction Age (mean, SD): Group A: 52.16 (7.53); Group B: 51.60 (5.66) Inclusion criteria: postmenopausal women in the age group of 40–65 years with symptoms of urogenital and sexual dysfunction, who have undergone spontaneous amenorrhoea at least 12 months prior to screening or have undergone surgical menopause at least 6 weeks prior, were included in the study. Urogenital disorders included vaginal atropy, vulvitis, urethritis, dyspareunia, recurrent urinary tract infections, and urinary incontinence symptoms. Sexual dysfunction disorders included sexual pain disorder or a problem with sexual desire, arousal, or orgasm that causes distress Exclusion criteria: women with any known contraindication to HRT as well as those using any hormonal product within 6 weeks of screening visit were excluded from the study | |
| Interventions | Group A: local oestrogen cream (n = 25). 25 women were given Premarin cream preparation locally once daily application of 1 gm of cream containing 0.625 mg of conjugated equine oestrogen for 2 weeks followed by twice weekly application for further 10 weeks Group B: non‐hormonal lubricant (n = 25). 25 women were given non‐hormonal lubricant gel locally, once‐daily application of 1 g of gel for 2 weeks followed by twice weekly application for further 10 weeks | |
| Outcomes | Adverse event (total events) | |
| Notes | Most outcome data were reported in non‐usable form e.g. mean percentage, etc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on method used in allocation concealment |
| Blinding (performance bias) | Unclear risk | No information was reported on performance bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was reported on the blinding of outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | It was stated that "All women analyzed at completion of 12 weeks of therapy" |
| Selective reporting (reporting bias) | Low risk | All the outcomes specified in the methods section ware reported |
| Other bias | Low risk | "All the study parameters in study groups 1 and 2 and in the control group were comparable with each other at the initiation of therapy" |
| Methods | Open‐label, parallel, multi‐centre (6 centres) study. Women randomised using a predetermined, computer‐generated scheme. No blinding | |
| Participants | Inclusion criteria: intact uteri and two or more vaginal symptoms (dryness, soreness, or irritation) rated as moderate to severe. 1 year past menopause and have serum E2 concentrations of 30 pg/ml (110 pmol/L) and FSH 40 IU/L or more. | |
| Interventions | Treatment: 17β oestradiol vaginal tablets (25 mcg) (Vagifem) once daily for 2 weeks, then once a week | |
| Outcomes | Dryness, irritation and soreness; adverse events (e.g. evidence of endometrial thickness), plasma oestradiol levels, ease of administration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women randomised using a predetermined, computer‐generated scheme |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | High risk | Open label (no blinding) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether other outcome assessors such as the pathologists were blinded or not |
| Incomplete outcome data (attrition bias) | High risk | Proportions of withdrawals differed between the treatment groups and data were not analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Treatments groups balanced at baseline |
| Methods | A 2‐arm parallel RCT | |
| Participants | 309 postmenopausal women with vaginal atrophy Age (mean, SD): Group A: 57.5 (5.64); Group B: 57.7 (5.27) Inclusion criteria: all participants were required to have serum E2 levels less than 20 pg/mL, follicle stimulating hormone levels more than 40 mIU/mL, 5% or more superficial cells in vaginal cytology, vaginal pH more than 5.0, an endometrial thickness of less than 4.0 mm as assessed by transvaginal ultrasonography, and a normal mammogram within the 6 months before study entry Exclusion criteria: known or suspected history of breast carcinoma, hormone‐dependent tumour, genital bleeding of unknown cause, acute thrombophlebitis or thromboembolic disorder associated with oestrogen use, vaginal infection requiring treatment, allergy to the test drug or its constituents, or any serious disease or chronic condition that could interfere with study compliance. The use of any investigational drug within the 30 days preceding screening, exogenous sex hormones within 3 months before study drug initiation, or current use of corticosteroids were prohibited | |
| Interventions | Group A: oestrogen vaginal tablet (n = 205). one vaginal tablet (10 ug oestradiol) inserted daily for 14 days and subsequently one tablet twice per week Group B: placebo (n = 104). Placebo identical in appearance to the active drug was inserted as described for the treatment group Duration: 12 weeks | |
| Outcomes | Vaginal pH, adverse events | |
| Notes | Some outcome data were reported in non‐usable form e.g. mean without SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It was stated that "A central telephone system was used for randomization" |
| Allocation concealment (selection bias) | Low risk | It was reported that "Copies of the randomization codes were kept in sealed envelopes at the site as well as by the clinical trial sponsor" |
| Blinding (performance bias) | Low risk | Study was reported as "'double‐blind, randomized, parallel‐group, placebo controlled, multicenter trial" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was reported on the blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All data reported at week 12 and week 52 are from the ITT analyses, with missing values for each individual imputed using the last observation carried forward approach |
| Selective reporting (reporting bias) | Low risk | All the outcomes specified in the methods section ware reported |
| Other bias | Low risk | It was reported that "Demographics and baseline characteristics were comparable across treatment groups" |
| Methods | Multicentre, double‐blind, placebo‐controlled. Women randomised by the method of random number generator Number of women analysed: n = 1567. | |
| Participants | Inclusion criteria: urogenital complaints with at least 1 year's history of postmenopause. The women should not have been subjected to any oestrogen replacement treatment for at least 6 months Location: Croatia | |
| Interventions | Treatment: 25 ug of micronised 17β‐oestradiol (Vagifem) vaginal tablet | |
| Outcomes | Burning, recurrent vaginitis, petechiae, dyspareunia, vaginal atrophy, serum oestradiol, adverse events, success (participant and physician) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by the method of random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Low risk | Double‐blinded, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether other outcome assessors such as the pathologists were blinded |
| Incomplete outcome data (attrition bias) | High risk | Proportions of withdrawals differed between the 2 treatment groups and data were not analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Double‐blinded, multicentre (35 centres). Randomisation schedule generated with the SAS ProcPlan. Randomised in blocks of 6‐13 weeks of treatment | |
| Participants | Inclusion criteria: a postmenopausal woman, with or without a uterus who had had at least 7 moderate to severe hot flushes a day or an average of at least 56 moderate to severe vasomotor symptoms per week for the 2 weeks before randomisation. In addition, a woman with a uterus was required to have amenorrhoea for more than 12 months before randomisation; if she had amenorrhoea for less than 12 months but at least 6 months, she was also required to have a follicle stimulating hormone of at least 40 IU and an E2 level of no more than 20 pg/mL. A woman was also eligible if she had had a hysterectomy and bilateral oophorectomy performed more than 6 weeks before randomisation | |
| Interventions | Treatment: vaginal ring delivering 50 ug/day of E2 (n = 113) or 100 ug/day of E2 (n = 112) | |
| Outcomes | Maturation index, vaginal dryness; overall satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated SAS programme |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias) | Low risk | Double‐blinded trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether other outcome assessors such as the pathologists were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of withdrawals per treatment group not reported and number of women analysed differed from the number randomised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Open label, parallel, 2:1 distribution. Computer‐generated programme allocation. Opening sequential envelopes. Single blinding by independent cytopathologist | |
| Participants | Inclusion criteria: 2 years postmenopausal with significant symptoms or objective signs of urogenital atrophy (vaginal dryness, genital pruritus, dyspareunia, dysuria, urinary frequency, urgency or nocturia, have an endometrium equal or less than 5 mm thickness on USS and a negative progestogen challenge test | |
| Interventions | Treatment: oestradiol vaginal ring containing 2 mg micronised 17β oestradiol releasing 8 mcg per 24 hours; equally 0.7 mg over 3 months | |
| Outcomes | Oestradiol levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in envelopes |
| Blinding (performance bias) | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single‐blinded trial but some outcomes were participant‐assessed |
| Incomplete outcome data (attrition bias) | High risk | Proportions of withdrawals differed between the treatment groups and data were not analysed on the basis of ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
ITT: intention‐to‐treat
KPI index: karyopyknotic index
PP: per protocol
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Design not relevant: not a RCT | |
| Duration of intervention was less than 12 weeks | |
| Design not relevant: no control or placebo group | |
| Duration of intervention less than 12 weeks: study only 8 weeks in length | |
| Intervention not relevant | |
| Duration of treatment was less than 12 weeks | |
| Intervention not relevant: dose response study | |
| Design not relevant: survey | |
| Participants not relevant: included healthy postmenopausal women with no symptoms of vaginal atrophy | |
| Duration of intervention less than 12 weeks | |
| Design not relevant: no comparison group | |
| Intervention not relevant: dose response study | |
| Duration of intervention was less than 12 weeks | |
| Duration of treatment was less than 12 weeks | |
| Participants not relevant: hysterectomised postmenopausal women | |
| Intervention not relevant: oral oestrogen | |
| Design not relevant: not a true RCT | |
| Duration of intervention less than 12 weeks: study only 9 weeks in length | |
| Duration of intervention less than 12 weeks: study only 4 weeks in length | |
| Intervention not relevant: dose response | |
| Duration of intervention less than 12 weeks: study only 4 weeks in length | |
| Design not relevant: not a true RCT | |
| Design not relevant: no randomisation | |
| Outcomes not relevant | |
| Duration of interventions was less than 12 weeks | |
| Design not relevant: not a true RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | 2‐arm parallel RCT |
| Participants | 472 sexually active postmenopausal women experiencing dyspareunia |
| Interventions | Group A: 15 mcg oestradiol vaginal cream 0.003% once daily for 2 weeks followed by 3 times per week for an additional 10 weeks Group B: “vehicle vaginal cream” (placebo) with same administration as group A |
| Outcomes | Participant‐assessed severity of dyspareunia Vaginal pH Vaginal maturation indices Clinician assessment of vaginal health Self‐assessed severity of vaginal dryness and vaginal bleeding from sexual activity |
| Notes | This is a conference abstract, need to ask for the data for the reported outcomes (P values only given) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point). | ||||
| 1.1 Oestrogen ring versus oestrogen cream | 2 | 341 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.80, 2.19] |
| 1.2 Oestrogen ring versus oestrogen tablets | 3 | 567 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 1.3 Oestrogen ring versus placebo | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.67 [3.23, 49.66] |
| 2 Endometrial thickness Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 2 Endometrial thickness. | ||||
| 2.1 Oestrogen ring versus oestrogen cream | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.94] |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 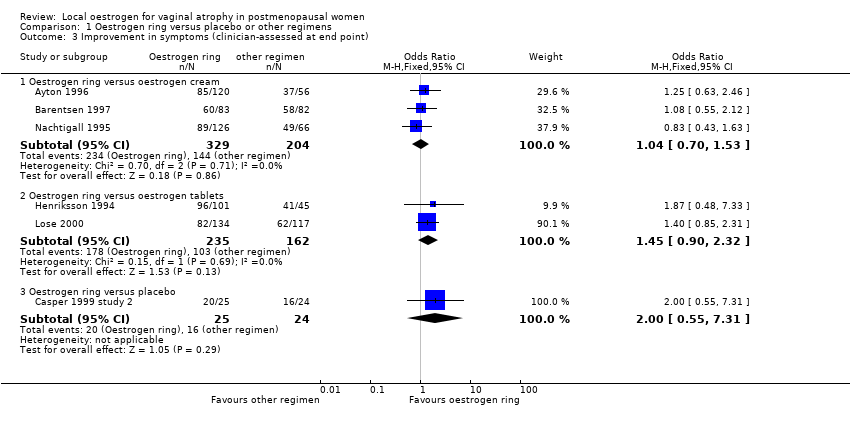 Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point). | ||||
| 3.1 Oestrogen ring versus oestrogen cream | 3 | 533 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.70, 1.53] |
| 3.2 Oestrogen ring versus oestrogen tablets | 2 | 397 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.90, 2.32] |
| 3.3 Oestrogen ring versus placebo | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.55, 7.31] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point). | ||||
| 4.1 Oestrogen ring versus oestrogen cream | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 4.2 Oestrogen ring versus oestrogen tablets | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 4.3 Oestrogen ring versus placebo | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐1.31 [‐1.82, ‐0.80] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 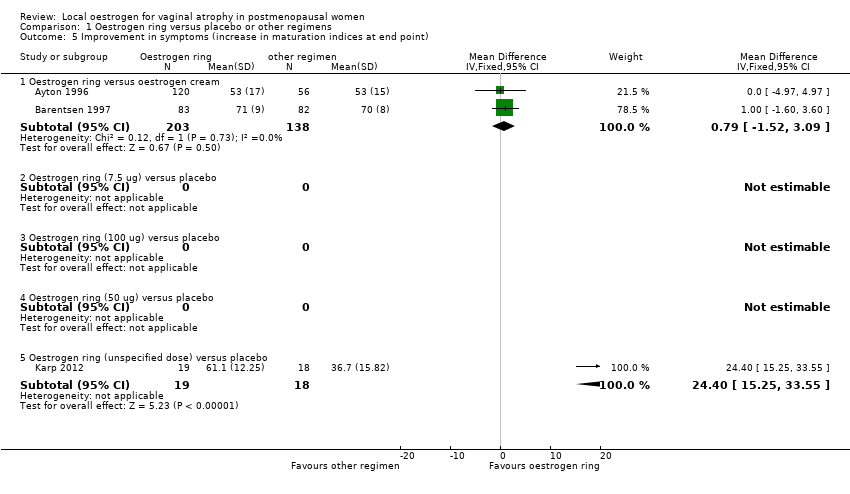 Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point). | ||||
| 5.1 Oestrogen ring versus oestrogen cream | 2 | 341 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [‐1.52, 3.09] |
| 5.2 Oestrogen ring (7.5 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Oestrogen ring (100 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Oestrogen ring (50 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Oestrogen ring (unspecified dose) versus placebo | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 24.4 [15.25, 33.55] |
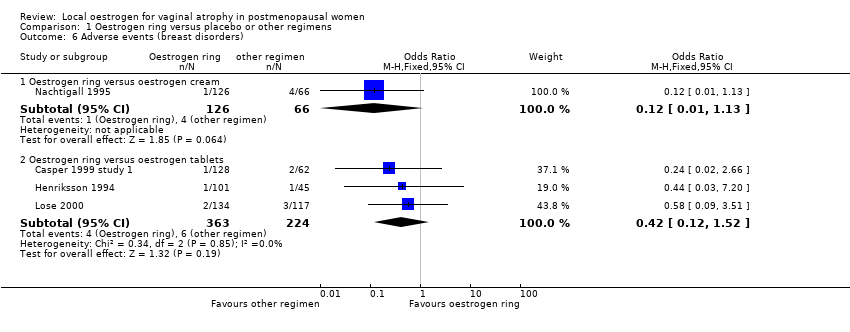
| 6 Adverse events (breast disorders) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 6 Adverse events (breast disorders). | ||||
| 6.1 Oestrogen ring versus oestrogen cream | 1 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 1.13] |
| 6.2 Oestrogen ring versus oestrogen tablets | 3 | 587 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.12, 1.52] |
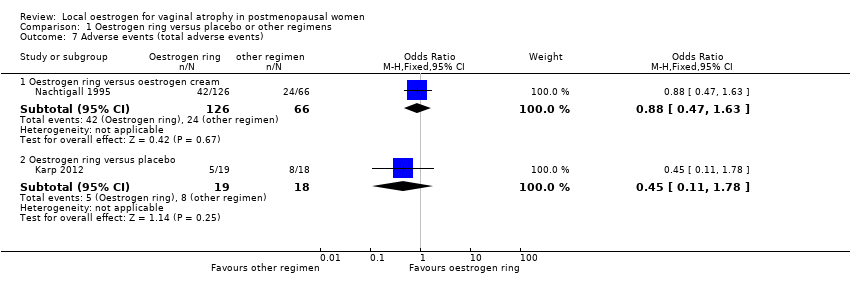
| 7 Adverse events (total adverse events) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7 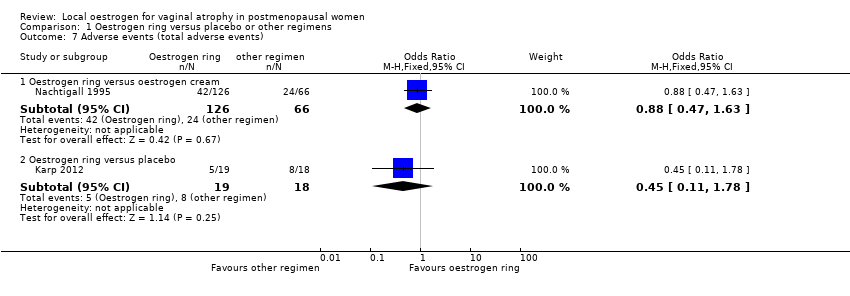 Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 7 Adverse events (total adverse events). | ||||
| 7.1 Oestrogen ring versus oestrogen cream | 1 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.63] |
| 7.2 Oestrogen ring versus placebo | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.11, 1.78] |
| 8 Adherence to treatment Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 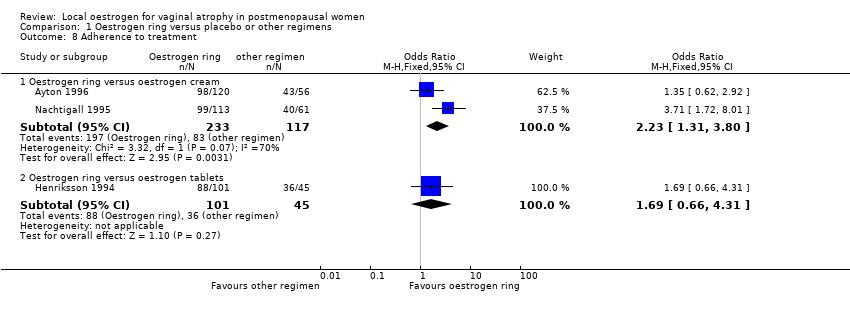 Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 8 Adherence to treatment. | ||||
| 8.1 Oestrogen ring versus oestrogen cream | 2 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.31, 3.80] |
| 8.2 Oestrogen ring versus oestrogen tablets | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.66, 4.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
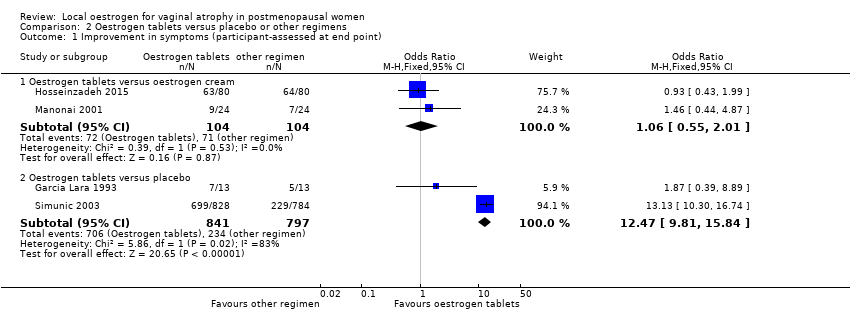
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point). | ||||
| 1.1 Oestrogen tablets versus oestrogen cream | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.55, 2.01] |
| 1.2 Oestrogen tablets versus placebo | 2 | 1638 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.47 [9.81, 15.84] |
| 2 Endometrial thickness Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 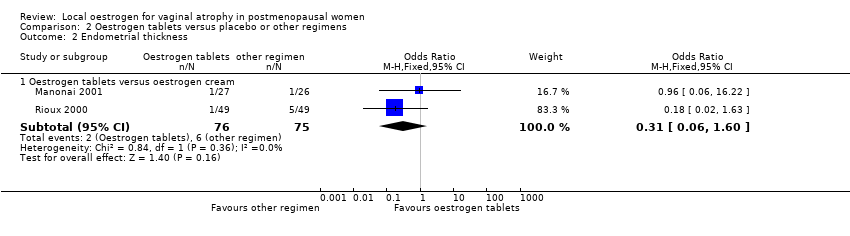 Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 2 Endometrial thickness. | ||||
| 2.1 Oestrogen tablets versus oestrogen cream | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.60] |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 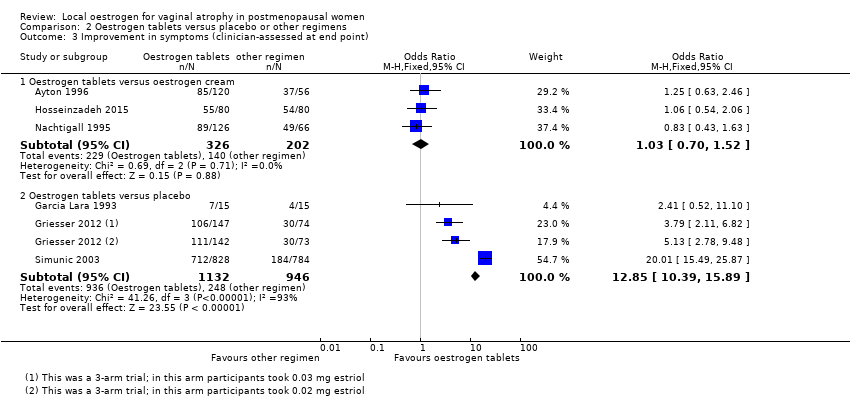 Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point). | ||||
| 3.1 Oestrogen tablets versus oestrogen cream | 3 | 528 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.52] |
| 3.2 Oestrogen tablets versus placebo | 3 | 2078 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.85 [10.39, 15.89] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point). | ||||
| 4.1 Oestrogen tablets versus oestrogen cream | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.12, 0.52] |
| 4.2 Oestrogen tablets versus placebo | 2 | 524 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.10, ‐0.80] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point). | ||||
| 5.1 Oestrogen tablets versus oestrogen cream | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐4.69 [‐13.58, 4.20] |
| 5.2 Oestrogen tablets versus placebo | 1 | 436 | Mean Difference (IV, Fixed, 95% CI) | 18.63 [14.57, 22.69] |
| 6 Adverse events (breast disorders) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 6 Adverse events (breast disorders). | ||||
| 6.1 Oestradiol tablets versus oestriol tablets | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.12, 77.09] |
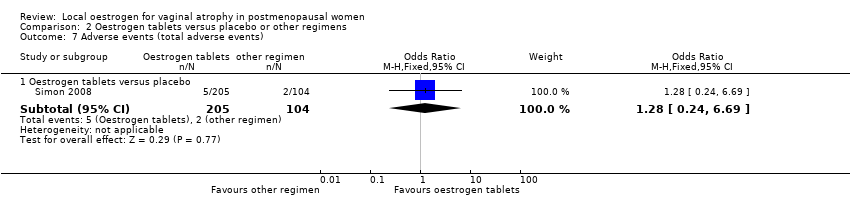
| 7 Adverse events (total adverse events) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 7 Adverse events (total adverse events). | ||||
| 7.1 Oestrogen tablets versus placebo | 1 | 309 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.24, 6.69] |
| 8 Adherence to treatment Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 8 Adherence to treatment. | ||||
| 8.1 Oestrogen tablets versus oestrogen cream | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.41, 8.94] |
| 8.2 Oestradiol tablets versus oestriol tablets | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.15, 6.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1 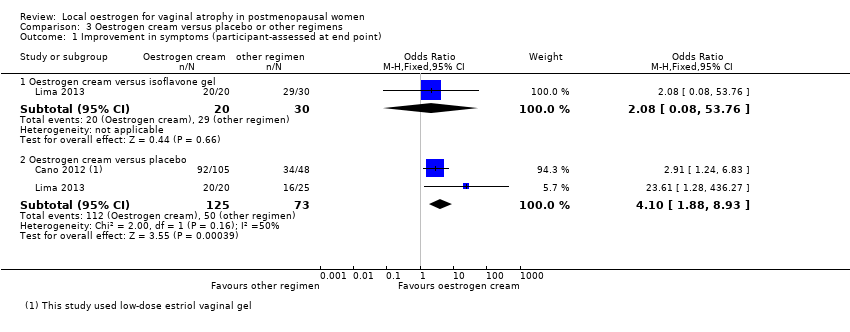 Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point). | ||||
| 1.1 Oestrogen cream versus isoflavone gel | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.08, 53.76] |
| 1.2 Oestrogen cream versus placebo | 2 | 198 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.10 [1.88, 8.93] |
| 2 Endometrial thickness | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point). | ||||
| 3.1 Oestrogen cream versus placebo | 1 | 153 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.47, 7.36] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point). | ||||
| 4.1 Oestrogen cream versus non‐hormonal local bio adhesive vaginal moisturiser | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.52, ‐0.21] |
| 4.2 Oestrogen cream (21 days) versus placebo (21 days) | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.47, ‐0.93] |
| 4.3 Oestrogen cream (twice weekly) versus placebo (twice weekly) | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.58, ‐1.02] |
| 4.4 Oestriol gel (50 ug) versus placebo gel | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.23, ‐0.37] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point). | ||||
| 5.1 Oestrogen cream versus placebo | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | 23.7 [17.25, 30.15] |
| 6 Adverse events (breast disorders) | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Adverse events (total adverse events) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7 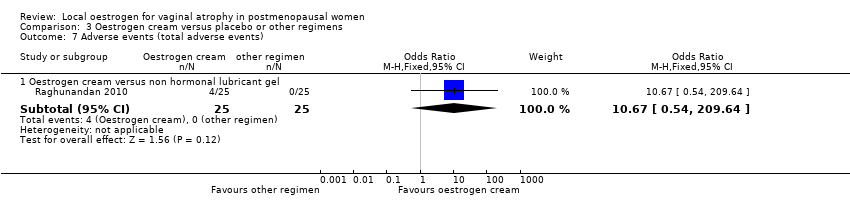 Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 7 Adverse events (total adverse events). | ||||
| 7.1 Oestrogen cream versus non hormonal lubricant gel | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.67 [0.54, 209.64] |
| 8 Adherence to treatment | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
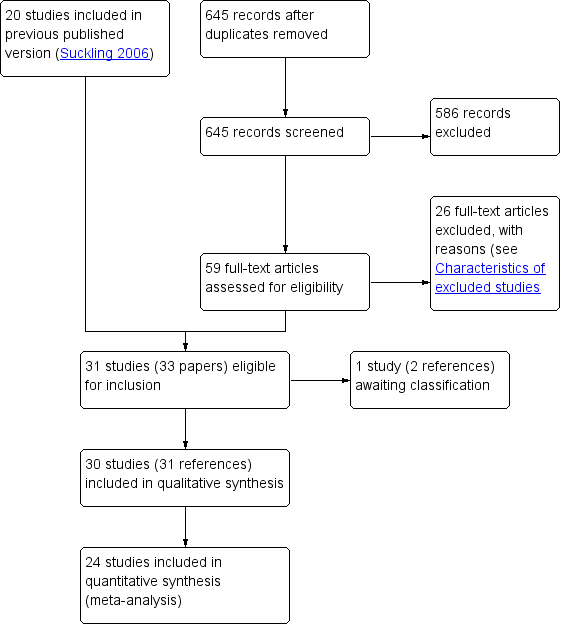
Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
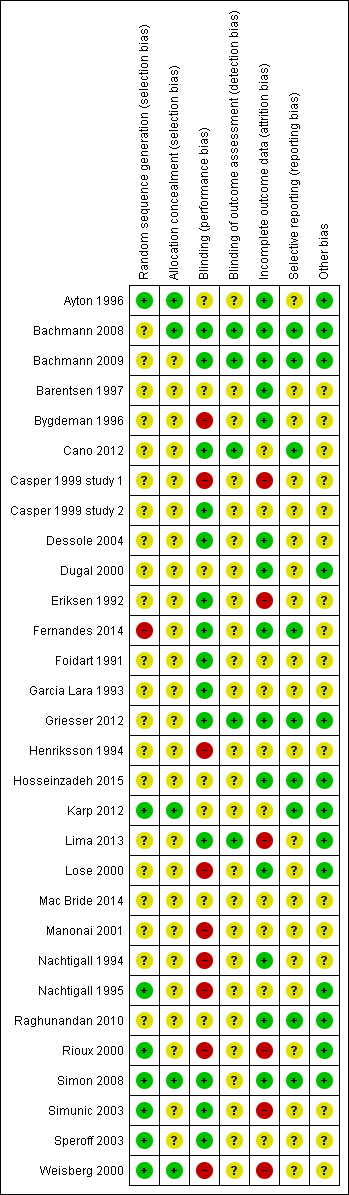
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 Oestrogen ring versus placebo or other regimens, outcome: 1.1 Improvement in symptoms (participant‐assessed at end point).

Forest plot of comparison: 2 Oestrogen tablets versus placebo or other regimens, outcome: 2.1 Improvement in symptoms (participant‐assessed at end point).

Forest plot of comparison: 3 Oestrogen cream versus placebo or other regimens, outcome: 3.1 Improvement in symptoms (participant‐assessed at end point).

Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point).
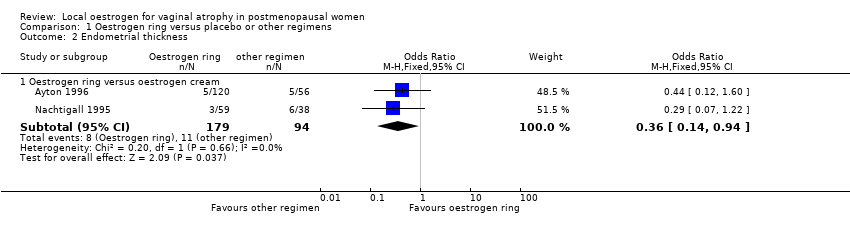
Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 2 Endometrial thickness.

Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point).
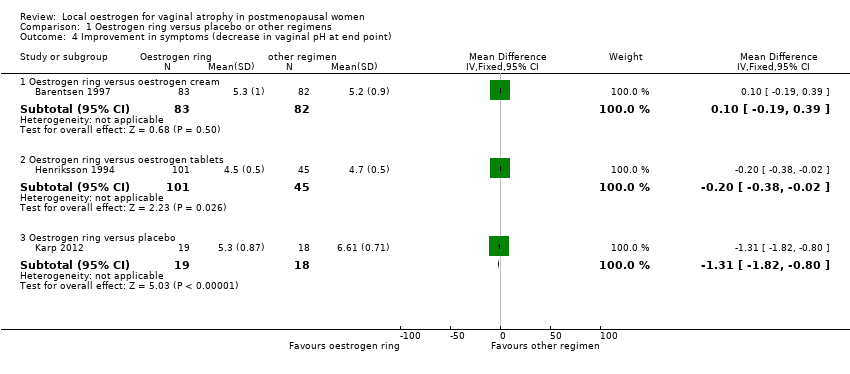
Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point).

Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point).
Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 6 Adverse events (breast disorders).
Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 7 Adverse events (total adverse events).

Comparison 1 Oestrogen ring versus placebo or other regimens, Outcome 8 Adherence to treatment.
Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 2 Endometrial thickness.

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 6 Adverse events (breast disorders).
Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 7 Adverse events (total adverse events).

Comparison 2 Oestrogen tablets versus placebo or other regimens, Outcome 8 Adherence to treatment.

Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 1 Improvement in symptoms (participant‐assessed at end point).
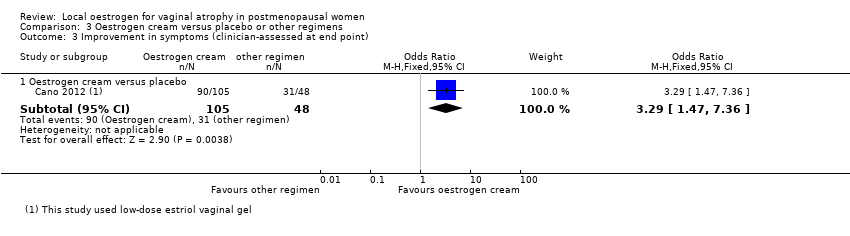
Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 3 Improvement in symptoms (clinician‐assessed at end point).
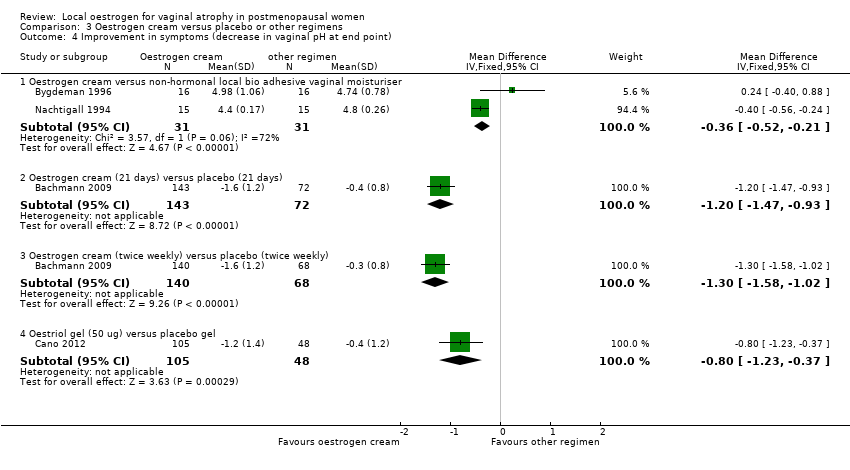
Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 4 Improvement in symptoms (decrease in vaginal pH at end point).

Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 5 Improvement in symptoms (increase in maturation indices at end point).
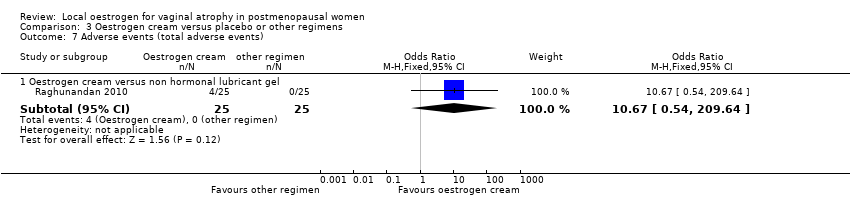
Comparison 3 Oestrogen cream versus placebo or other regimens, Outcome 7 Adverse events (total adverse events).
| Oestrogen ring compared to other regimens for vaginal atrophy in postmenopausal women | ||||||
| Patient or population: postmenopausal women with vaginal atrophy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other regimen | Oestrogen ring | |||||
| Improvement in symptoms (participant‐assessed) (oestrogen ring vs oestrogen cream) | 717 per 1000 | 771 per 1000 | OR 1.33 | 341 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (participant‐assessed) (oestrogen ring vs oestrogen tablets) | 582 per 1000 | 521 per 1000 | OR 0.78 | 567 | ⊕⊕⊝⊝ | |
| Endometrial thickness (oestrogen ring vs oestrogen cream) | 117 per 1000 | 46 per 1000 | OR 0.36 | 273 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen ring vs oestrogen cream) | 706 per 1000 | 714 per 1000 | OR 1.04 | 533 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen ring vs oestrogen tablets) | 636 per 1000 | 717 per 1000 | OR 1.45 | 397 | ⊕⊕⊝⊝ | |
| Adverse events (total adverse events) (oestrogen ring vs oestrogen cream) | 364 per 1000 | 335 per 1000 | OR 0.88 | 192 | ⊕⊕⊝⊝ | |
| Adverse events (total adverse events) (oestrogen ring vs placebo) | 444 per 1000 | 264 per 1000 | OR 0.45 | 37 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by 1 level as most risk of bias domains were rated either as unclear or high | ||||||
| Oestrogen tablets compared to other regimens for vaginal atrophy in postmenopausal women | ||||||
| Patient or population: postmenopausal women with vaginal atrophy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other regimen | Oestrogen tablets | |||||
| Improvement in symptoms (participant‐assessed) (oestrogen tablets vs oestrogen cream) | 683 per 1000 | 695 per 1000 | OR 1.06 | 208 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (participant‐assessed) (oestrogen tablets vs placebo) | 294 per 1000 | 839 per 1000 | OR 12.47 | 1638 | ⊕⊕⊝⊝ | Using a random effects model, there was no evidence of a difference in effect: OR 5.80, 95% CI 0.88 to 38.29 |
| Endometrial thickness (oestrogen tablets vs oestrogen cream) | 80 per 1000 | 26 per 1000 | OR 0.31 | 151 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen tablets vs oestrogen cream) | 697 per 1000 | 699 per 1000 | OR 1.03 | 528 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen tablets vs placebo) | 262 per 1000 | 820 per 1000 | OR 12.85 | 2078 | ⊕⊕⊝⊝ | |
| Adverse events (total adverse events) (oestrogen tablets vs placebo) | 19 per 1000 | 24 per 1000 | OR 1.27 | 309 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by 1 level as most risk of bias domains were assessed either as unclear or high | ||||||
| Oestrogen cream compared to other regimens for vaginal atrophy in postmenopausal women | ||||||
| Patient or population: postmenopausal women with vaginal atrophy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other regimen | Oestrogen cream | |||||
| Improvement in symptoms (participant‐assessed) (oestrogen cream vs isoflavone gel) | 967 per 1000 | 984 per 1000 | OR 2.08 | 50 | ⊕⊕⊝⊝ | |
| Improvement in symptoms (participant‐assessed) oestrogen cream vs placebo) | 685 per 1000 | 899 per 1000 | OR 4.10 | 198 | ⊕⊕⊝⊝ | |
| Endometrial thickness not reported | ‐ | ‐ | Not estimable | ‐ | ‐ | |
| Improvement in symptoms (clinician‐assessed) (oestrogen cream vs placebo) | 646 per 1000 | 857 per 1000 | OR 3.29 | 153 | ⊕⊕⊝⊝ | |
| Adverse events (total adverse events) (oestrogen cream vs non‐hormonal lubricant gel) | ‐ | ‐ | OR 10.67 | 50 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by 1 level as most risk of bias domains were assessed either as unclear or high | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen ring versus oestrogen cream | 2 | 341 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.80, 2.19] |
| 1.2 Oestrogen ring versus oestrogen tablets | 3 | 567 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 1.3 Oestrogen ring versus placebo | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.67 [3.23, 49.66] |
| 2 Endometrial thickness Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestrogen ring versus oestrogen cream | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.94] |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestrogen ring versus oestrogen cream | 3 | 533 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.70, 1.53] |
| 3.2 Oestrogen ring versus oestrogen tablets | 2 | 397 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.90, 2.32] |
| 3.3 Oestrogen ring versus placebo | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.55, 7.31] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestrogen ring versus oestrogen cream | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.19, 0.39] |
| 4.2 Oestrogen ring versus oestrogen tablets | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 4.3 Oestrogen ring versus placebo | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐1.31 [‐1.82, ‐0.80] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestrogen ring versus oestrogen cream | 2 | 341 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [‐1.52, 3.09] |
| 5.2 Oestrogen ring (7.5 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Oestrogen ring (100 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Oestrogen ring (50 ug) versus placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Oestrogen ring (unspecified dose) versus placebo | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 24.4 [15.25, 33.55] |
| 6 Adverse events (breast disorders) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Oestrogen ring versus oestrogen cream | 1 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 1.13] |
| 6.2 Oestrogen ring versus oestrogen tablets | 3 | 587 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.12, 1.52] |
| 7 Adverse events (total adverse events) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestrogen ring versus oestrogen cream | 1 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.63] |
| 7.2 Oestrogen ring versus placebo | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.11, 1.78] |
| 8 Adherence to treatment Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestrogen ring versus oestrogen cream | 2 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.31, 3.80] |
| 8.2 Oestrogen ring versus oestrogen tablets | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.66, 4.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen tablets versus oestrogen cream | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.55, 2.01] |
| 1.2 Oestrogen tablets versus placebo | 2 | 1638 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.47 [9.81, 15.84] |
| 2 Endometrial thickness Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestrogen tablets versus oestrogen cream | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.60] |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestrogen tablets versus oestrogen cream | 3 | 528 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.52] |
| 3.2 Oestrogen tablets versus placebo | 3 | 2078 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.85 [10.39, 15.89] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestrogen tablets versus oestrogen cream | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.12, 0.52] |
| 4.2 Oestrogen tablets versus placebo | 2 | 524 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.10, ‐0.80] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestrogen tablets versus oestrogen cream | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐4.69 [‐13.58, 4.20] |
| 5.2 Oestrogen tablets versus placebo | 1 | 436 | Mean Difference (IV, Fixed, 95% CI) | 18.63 [14.57, 22.69] |
| 6 Adverse events (breast disorders) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Oestradiol tablets versus oestriol tablets | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.12, 77.09] |
| 7 Adverse events (total adverse events) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestrogen tablets versus placebo | 1 | 309 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.24, 6.69] |
| 8 Adherence to treatment Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestrogen tablets versus oestrogen cream | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.41, 8.94] |
| 8.2 Oestradiol tablets versus oestriol tablets | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.15, 6.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in symptoms (participant‐assessed at end point) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen cream versus isoflavone gel | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.08, 53.76] |
| 1.2 Oestrogen cream versus placebo | 2 | 198 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.10 [1.88, 8.93] |
| 2 Endometrial thickness | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Improvement in symptoms (clinician‐assessed at end point) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestrogen cream versus placebo | 1 | 153 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.47, 7.36] |
| 4 Improvement in symptoms (decrease in vaginal pH at end point) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestrogen cream versus non‐hormonal local bio adhesive vaginal moisturiser | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.52, ‐0.21] |
| 4.2 Oestrogen cream (21 days) versus placebo (21 days) | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.47, ‐0.93] |
| 4.3 Oestrogen cream (twice weekly) versus placebo (twice weekly) | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.58, ‐1.02] |
| 4.4 Oestriol gel (50 ug) versus placebo gel | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.23, ‐0.37] |
| 5 Improvement in symptoms (increase in maturation indices at end point) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestrogen cream versus placebo | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | 23.7 [17.25, 30.15] |
| 6 Adverse events (breast disorders) | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Adverse events (total adverse events) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestrogen cream versus non hormonal lubricant gel | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.67 [0.54, 209.64] |
| 8 Adherence to treatment | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

