ஆழ்நாளக் குருதியடைப்பு தடுக்க அளவுகோடிட்ட அழுத்தக்காலுறைகள்
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study: RCT | |
| Participants | Country: UK Sex: male and female | |
| Interventions | Type of treatment: GCS length not stated | |
| Outcomes | DVT PE: not mentioned | |
| Notes | Benign and malignant patients were differentiated: Malignant Incidence of proximal DVT not reported No adverse events were reported Kendall Co supplied the stockings and fibrinogen in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients allocated using random number series |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding (performance bias and detection bias) | Low risk | "The scans were assessed without reference to patient or group." Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 11/211 patients not analysed, but were accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes were reported |
| Other bias | Low risk | No other source of bias identified |
| Methods | Study: RCT | |
| Participants | Country: USA Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT (all) Proximal DVT Symptomatic PE (confirmed by lung perfusion scan)* | |
| Notes | Some had aspirin during the study, some had previous DVT, some had previous leg injuries, some had varicose veins, some with venous skin changes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not mentioned |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | All 18 patients were accounted for and included in analysis |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | High risk | This study was terminated early. "It was considered medically unjustifiable to continue this study when a significantly greater incidence of major deep vein thrombosis developed in the patients not wearing stockings." Also, authors of this study were awarded a grant from the Kendall Research Centre who manufacture stockings. |
| Methods | Study: RCT | |
| Participants | Country: Sweden | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT (all) Proximal DVT | |
| Notes | This study included the infusion of Dextran 70 as prophylactic measure in addition to stockings in both groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | "When analysing the data from the fibrinogen test, it was not clear which leg was stockinged." |
| Incomplete outcome data (attrition bias) | Low risk | 8/80 patients were not analysed, but were accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Unclear risk | The study was partly funded by a grant from Beiersdorf AB, who also supplied the compression stockings |
| Methods | Study: RCT | |
| Participants | Country: Singapore Inclusion criteria: low‐risk patients undergoing elective total knee arthroplasty with no known predisposition to thromboembolism Exclusion criteria: use of anticoagulants or aspirin, history of PE or DVT in the previous year, body mass index > 30 kg/m2, pre‐operative prolonged immobilization or being wheelchair bound, bleeding tendency or a history of gastro‐intestinal bleeding, surgery in the previous six months, cerebrovascular accident in the previous three months, uncontrolled hypertension, congestive cardiac failure, renal or liver impairment, allergy to heparin or heparin‐induced thrombocytopaenia, varicose veins or CVI, PVD, skin ulcers, dermatitis or wounds, and malignancy | |
| Interventions | Type of treatment: GCS applied to legs (length unspecified) Control: 110 (group 1) Treatment: 110 (group 2) Duration: applied until day five to seven or stopped earlier if patients were suspected to have DVT or PE based on bilateral duplex ultrasonography | |
| Outcomes | DVT (all) Proximal DVT Symptomatic PE | |
| Notes | Patients were divided into four experimental groups consisting of 110 patients each ‐ no prophylaxis, GCS only, IPC only, and LWMH only. Only patients at low risk of developing VTE were included Standardised rehabilitation protocols were used, with continuous passive movements initiated on day two and ambulation on day three post‐operatively Adverse events: no adverse effects such as skin rash, swelling above the appliance, pressure necrosis of the skin or peroneal nerve palsy related to the use of GCS and IPC. At one month follow up, two patients each in the control and GCS group were re‐admitted due to superficial wound infections. Source of funding was not disclosed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Single blinded. "Bilateral duplex ultrasonography (carried out by one of 3 dedicated ultrasonographers blinded to the prophylactic method used)." |
| Incomplete outcome data (attrition bias) | Low risk | All 440 patients were accounted for and analysed |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: Sweden Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT (all, unit of analysis = individual legs) Proximal DVT (unit of analysis = individual legs) Symptomatic PE (confirmed by scintigraphy) | |
| Notes | All patients had regular Dextran 70 prophylaxis Individual patients were randomised to the three treatment groups. However, only the non‐operated leg's values were included for our analysis because in these orthopaedic patients thrombotic process may have already been initiated during surgery Incidence of symptomatic DVT not reported Adverse events: two patients reported discomfort and discontinued wearing stockings after two days. Incidence of bleeding reported, which might be associated with low dose heparin ‐ three wound haematomas, minor bleeding from gastric drainage in one patient. Further seven patients withdrawn from the trial due to bleeding, which might have also been due to heparin Funded by Swedish Medical Research Council | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | "The data was analysed blindly concerning the type of prophylaxis." |
| Incomplete outcome data (attrition bias) | Low risk | 6/150 patients were not included in analysis, but were accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: UK Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT Symptomatic PE* (confirmed by lung scanning) | |
| Notes | Adverse events: not mentioned Incidence of proximal DVT not reported The source of funding was not disclosed. However, stockings were provided by the Kendall Company, who manufacture stockings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were allocated randomly" Comment: No mention of how this was achieved |
| Allocation concealment (selection bias) | Low risk | "Patients were allocated randomly to a stocking group or control group by instructions in sealed envelopes." |
| Blinding (performance bias and detection bias) | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 3/98 patients were not included in the analysis, but were accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes were reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: UK Sex: male and female | |
| Interventions | Type of treatment: GCS thigh and knee length | |
| Outcomes | DVT | |
| Notes | Analysis of patients was performed between those who received above‐knee and below‐knee stockings. Both operated and non‐operated legs were analysed separately. Method of randomisation is not clear although it appears appropriate Incidence of proximal PE not reported One fatal PE in knee replacement control group. Patients were not routinely assessed for presence of PE Incidence of adverse events not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not mentioned. Patients were randomised in a ratio of 1:1 in the thigh‐length GCS group and 1:4 in the knee‐length GCS group |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | 39/177 patients were not analysed, but were accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes were reported |
| Other bias | Unclear risk | The control group of the thigh‐length GCS group was also used as control for the knee‐length GCS group. Partly funded by Brevet Hospital Products who manufacture stockings |
| Methods | Study: RCT | |
| Participants | Country: UK Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT (all) Proximal DVT PE | |
| Notes | All patients in the treatment and control groups had enoxaparin 40 mg 12 hours before the operation, and then once daily until discharge An additional group of 14 patients who received no prophylaxis (placebo group) was excluded from this review Adverse effects: there were no differences in haemorrhagic complications between the three groups, and no adverse events were recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned consecutive numbers |
| Allocation concealment (selection bias) | Low risk | Using sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | "Venograms and V/Q scans were reported blindly by an independent panel of 3 and 1 radiologist respectively." |
| Incomplete outcome data (attrition bias) | Low risk | 15/93 patients could not be evaluated because 10 declined venography and 5 did not have it for technical reasons |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Unclear risk | This study was supported by Rhone‐Poulenc‐Rorer |
| Methods | Study: RCT | |
| Participants | Country: Sweden Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT (all) Proximal DVT Symptomatic DVT | |
| Notes | One limb was randomised to act as control. Aspirin was used in all patients Incidence of PE not mentioned Funded by Halmstad Hospital Foundation for Medical Research, the TRYGG‐HANSA Foundation for Medical Research and the Faculty of Medicine, Lund University. Stockings were supplied by The Kendall Health Products Company, who manufacture stockings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | "Results were calculated without knowledge of which leg had stockings and which leg developed a positive fibrinogen uptake test." Comment: insufficient detail |
| Incomplete outcome data (attrition bias) | Low risk | All 80 patients were accounted for and analysed |
| Selective reporting (reporting bias) | Low risk | Results for all outcome measures were reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: Sweden Sex: male and female | |
| Interventions | Type of treatment: GCS length not stated | |
| Outcomes | DVT | |
| Notes | All had Dextran 70 infusion at induction of anaesthesia and two days following operation. Dose: 500 ml per day Incidence of proximal DVT not reported Incidence of PE and adverse events not mentioned Stockings were supplied by AKLA AB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | 1/63 patients was not analysed, but was accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: UK Sex: male and female Exclusion criteria: none mentioned | |
| Interventions | Type of treatment: GCS thigh length. | |
| Outcomes | DVT | |
| Notes | Incidence of proximal DVT, PE and adverse events was not mentioned Stockings were supplied by Kendall Co | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By "tossing a coin" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | 5/75 patients were not analysed, but were accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: UK Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT Proximal DVT PE | |
| Notes | Left and right legs were randomised to receive treatment or control. The control group had only a sequential compression device fitted on the day of the operation. The treatment group had both GCS and sequential compression devices fitted Incidence of symptomatic DVT not reported Incidence of adverse events was not mentioned. All patients continued to wear stockings for the whole study period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | All 78 patients were accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Unclear risk | The study was supported in part by the Kendall Research Centre |
| Methods | Study: RCT | |
| Participants | Country: Japan Sex: male and female | |
| Interventions | Type of treatment: thigh‐length GCS | |
| Outcomes | DVT | |
| Notes | This trial was published in Japanese which made it difficult to extract information about the methodology accurately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not stated |
| Blinding (performance bias and detection bias) | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Japanese study, difficult to determine |
| Selective reporting (reporting bias) | Unclear risk | Japanese study, difficult to determine |
| Other bias | Unclear risk | Japanese study, difficult to determine |
| Methods | Study: RCT | |
| Participants | Country: Sweden Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT Incidence of proximal DVT not reported Incidence of PE was not routinely assessed | |
| Notes | All patients had heparin 5000 iu 12 hourly None of the patients developed fatal PE or reported side effects. However, bleeding complications were reported, likely associated with the use of heparin Study supported by Karolinksa Institutet, Stockholm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was achieved "...depending on the date of birth of the patient" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | 12/110 patients were excluded from analysis, but were accounted for |
| Selective reporting (reporting bias) | Low risk | Frequency of DVT in both groups was reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: USA Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length | |
| Outcomes | DVT | |
| Notes | Numbers in each group calls into question if this was properly randomised Two of six patients who developed DVT in the control arm, were noted to have developed clinical signs of DVT One patient developed a proximal DVT, though group allocation for this patient was not reported Incidence of PE and adverse events were not mentioned. Patients were not routinely assessed for presence of PE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By a random allocation table |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. However, discrepancy between the number of patients randomised to the treatment and control groups |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | All 95 patients were accounted for and analysed |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Unclear risk | Patients in the treatment group were given extra recommendations regarding exercise which were not given to patients in the control group, also received stockings |
| Methods | Study: RCT | |
| Participants | Country: UK Sex: female | |
| Interventions | Type of treatment: GCS length not stated. Control: 92 | |
| Outcomes | DVT PE | |
| Notes | Although randomised, method not made explicit. Losses to follow up, or loss to randomisation not made explicit Incidence of proximal DVT was not reported Method of diagnosis of PE was not reported No adverse events were mentioned and stockings were acceptable to patients, the only adverse comment being that the stockings were hot Stockings were supplied by Kendall Co | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number chart was used |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | "The scans were assessed blindly" |
| Incomplete outcome data (attrition bias) | Low risk | All 196 patients who entered the study were analysed and accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Low risk | No other sources of bias were identified |
| Methods | Study: RCT | |
| Participants | Country: USA Sex: male and female | |
| Interventions | Type of treatment: GCS thigh length (one had knee length because of obesity) | |
| Outcomes | DVT (all) Proximal DVT | |
| Notes | Losses to follow up: 1, dead: 19 (none due to PE). It is not explicit if these patients were included or excluded in the study 1 patient developed PE Source of funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | By "...a prescribed randomised arrangement" |
| Allocation concealment (selection bias) | Low risk | "Using sealed envelopes" |
| Blinding (performance bias and detection bias) | Low risk | "The results of the tests were interpreted independently by a panel of experts blinded to the patient's treatment group." |
| Incomplete outcome data (attrition bias) | Low risk | All 236 patients accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes measures were reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: Denmark Sex: male and female | |
| Interventions | Treatment: GCS thigh length | |
| Outcomes | DVT PE | |
| Notes | Heparin 5000 iu was given to all patients every 12 hourly for 7 days or until discharge. Thromboembolic complications are not clear Incidence of proximal DVT not reported No mention of adverse events Heparin and thrombograph was supplied by Novo Diagnostics and Kendall supplied the stockings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "by random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | "Phlebogram evaluated by radiologist not aware of the patient's treatment group" and scintigraphy "read blindly" |
| Incomplete outcome data (attrition bias) | Low risk | 20/196 patients withdrew, but all were accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were addressed |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Study: RCT | |
| Participants | Country: Denmark Sex: male and female | |
| Interventions | Type of treatment: GCS length not stated | |
| Outcomes | DVT | |
| Notes | On a background of heparin 5000 iu prophylaxis. One group received Dextran and GCS, which is excluded in our analysis Incidence of proximal DVT was not reported Patients were not routinely assessed for PE. One patient from the treatment group developed a fatal PE on the 14th post‐operative day, though had been excluded from the study on the 2nd post‐operative day due to poor compliance Adverse events: bleeding complications, likely associated with heparin. No complications associated with the use of GCS were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "continuous random numbers" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | 31 patients withdrew, but were all accounted for |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | Unclear risk | Supported, in part, by grants from NOVO A/S, KabiVitrum A/S and the Kendall Company |
CVI: chronic venous insufficiency
DVT: deep vein thrombosis
FUT: 125‐I fibrinogen uptake test. A sustained difference of more than 20% between consecutive or opposite points or a raising count were considered diagnostic of DVT
GCS: graduated compression stockings (also called TED stockings: thrombo‐embolic deterrent stockings). Compression is graduated, 18 mm Hg, 14 mm Hg, 8 mm Hg, 10 mm Hg and 8 mm Hg from ankle to upper thigh
GI: gastrointestinal
IPC: intermittent pneumatic compression device
LMWH: low molecular weight heparin
NSAIDs: non‐steroidal anti‐inflammatory drugs
PAD: peripheral arterial disease
PE: pulmonary embolism
PVD: peripheral vascular disease
RCT: randomised controlled trial
UFH: unfractionated heparin
VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Lacks appropriate control group. Compares the following groups of patients: low pressure knee‐length stockings, low pressure thigh‐length stockings, and moderate pressure knee‐length stockings | |
| Method is not very clear with regard to randomisation. The cause of dropouts is not clear. The duration for which the stockings were worn is not mentioned. Medical or surgical need for admission of these patients is not clear. Method of monitoring the occurrence of DVT in the study is unclear | |
| Incidence of DVT not assessed. Measures venous flow | |
| Not randomised and not published as a paper. High risk group of patients were involved (malignant diseases). A significant reduction in DVT was noted in the treatment group compared with the control group. The method of analysis seems appropriate | |
| Both patients and controls were assigned to the study on a rotation basis. Not randomised | |
| Not published as a full paper. Abstract does not mention number of legs in each group (transplanted side versus non‐transplanted side) | |
| Control group is retrospective | |
| Incidence of DVT not assessed. Studies venous haemodynamics | |
| Lacks appropriate control group. Compares patients given coumadin only to those given compression stockings only | |
| Included only stroke patients. It was decided that this trial would better suit a systematic review conducted by the Stroke Group (Naccarato 2010) | |
| Asymptomatic DVT seem to have only been assessed proximally, as incidence of distal asymptomatic DVT was not reported. Proximal asymptomatic DVT was diagnosed using venography, however it was unclear whether symptomatic DVT was also confirmed objectively using this method, and whether this was standardised throughout the study | |
| Lacks appropriate control group. Compares patients given heparin only to those given compression stockings only | |
| Thick elastic compression stockings used not TED. This study was randomised and would have been suitable for analysis | |
| Lacks appropriate control group. Two groups of trials ‐ GCS only versus GCS + intermittent pneumatic compression | |
| Lacks appropriate control group, based on reported clinical protocol. Formal methodology not published. No mention of use of mechanical method of thromboprophylaxis in this trial | |
| Lacks appropriate control group. Three groups ‐ thromboembolic stocking only versus external sequential pneumatic compression stockings only versus heparin + dihydroergotamine | |
| This trial assessed the use of therapeutic anticoagulation in patients with confirmed acute distal DVTs. Does not assess effectiveness of stockings in preventing DVTs | |
| Not randomised | |
| Incidence of DVT not assessed. Studies venous haemodynamics | |
| Haemodynamic study of mean blood flow velocity in patients wearing GCS versus those not wearing GCS. Does not report incidence of GCS in these patient groups | |
| Good study but not randomised prospectively | |
| Study not randomised | |
| Compares GCS versus placebo in patients with confirmed acute DVT to prevent progression to post‐thrombotic syndrome. Does not assess the effect of stockings in preventing DVT | |
| Lacks appropriate control group. Compares three groups ‐ stockings only versus LMWH 7 days versus LMWH 14 days | |
| Haemodynamic study aiming to measure venous velocities using intermittent pneumatic compression. Does not assess effect of GCS in preventing DVT | |
| Lacks appropriate control group. Compares patients given heparin only to those given stockings only | |
| All patients wore elastic compression stockings (stockings only versus stockings + intermittent pneumatic compression). Therefore, lacks appropriate control group | |
| Compares three groups sodium heparin versus calcium heparin versus stockings. Lacks appropriate control group | |
| Incidence of DVT not assessed. Analyses venous clearance | |
| Not randomised | |
| Lacks appropriate control group. Compares the following two experimental groups: electrical calf stimulation + low dose unfractionated heparin + elastic compression, versus low dose unfractionated heparin + elastic compression | |
| All patients wore stockings. GCS + standard heparin versus GCS + dipyridamole + acetylsalicylic acid versus GCS + placebo. Lacks appropriate control group | |
| Incidence of DVT not assessed. Measures residual limb volume | |
| Not an RCT | |
| All patients wore stockings. Compares LMWH + stockings versus stockings alone. Lacks appropriate control group | |
| Lacks appropriate control group. Compares IPCS versus LWMH | |
| Not amenable to analysis as the figures are difficult to interpret | |
| Not randomised | |
| A sequential compression device was used, not graduated compression stockings. Compares two groups ‐ heparin + dihydroergot versus physiotherapy (IPCS + physical exercise) | |
| This is a randomised controlled trial but very poorly conducted. They have compared two types of stockings with the same control group, which is inappropriate. There is a great deal of discrepancy in the number of patients in each group for an adequate RCT. Also, this trial included stroke patients, making it better suited to a similar review conducted by the Stroke Group (Naccarato 2010) | |
| Lacks appropriate control group. LWMH versus GCS | |
| All patients wore stockings. Compares two groups ‐ TED + aspirin versus TED + aspirin + pneumatic compression stockings | |
| Compares patients wearing IPCS + GCS to patients on enoxaparin, rather than to a control group of patients wearing intermittent pneumatic compression stockings only | |
| All patients wore stockings. Compares patients wearing stockings and taking nandroparin versus patients wearing stockings alone, rather than a control group of patients not wearing stockings and on nandroparin as background prophylaxis | |
| Lacks appropriate control group. LWMH versus GCS | |
| Antithrombotic stockings but does not state compression graduated stockings. French paper | |
| Not published as a full paper, therefore difficult to analyse | |
| Not adequately randomised | |
| This study compared above knee stockings with below knee stockings, rather than to a control group of no stockings or another method of prophylaxis | |
| This studies aimed to assess effectiveness of thigh‐length vs lower‐leg compression in preventing post‐thrombotic syndrome in patients known to have proximal DVT | |
| This study used pneumatic compression stockings rather than graduated compression stockings | |
| This study solely relied on the Tc99m plasmin test which is associated with high frequency of false positives. Diagnosis of DVT was not confirmed using another objective test. Furthermore, the method of randomisation used in this trial does not appear to be reliable, due to substantial difference in number of patients allocated to the GCS only group (74 patients) and GCS + heparin group (89 patients) | |
| This study aimed to assess effectiveness of two different types of stockings in the treatment and prevention of venous ulcers. Does not assess incidence of DVT | |
| This trial used Tubigrip rather than graduated compression stockings | |
| This study compared two groups ‐ mechanical compression + aspirin versus GCS + aspirin. Lacks a control group with patients on aspirin with no stockings | |
| Lacks appropriate control group. Compares incidence of DVT in two groups of patients ‐ darexaban versus GCS | |
| Lacks appropriate control group. Compares incidence of DVT in two groups of patients ‐ LMWH versus GCS + IPC | |
| Lacks appropriate control group. Compared TED stockings versus crepe bandage | |
| This study compared two groups ‐ LMWH + IPC versus LMWH + GCS. Lacks a control group of patients on LMWH with no stockings | |
| Lacks appropriate control group. Compares incidence of DVT in two groups of patients ‐ GCS versus Venowave + GCS | |
| This study compared effectiveness of engineered compression stockings in the prevention of DVT following ankle fractures. Further information was sought from trialists as results were only published as a conference abstract. Not all trial participants had been hospitalised, and therefore this trial did not meet our inclusion criteria | |
| Lacks appropriate control group. Compared the following two experimental groups: IPC + GCS versus GCS alone | |
| This study used a pneumatic plantar compression device rather than graduated compression stockings | |
| Based solely on clinical diagnosis of DVT instead of using doppler/venography for confirmation, as set out in the criteria for this review | |
| Studies venous haemodynamics. Does not assess the incidence of DVT | |
| Lacks appropriate experimental groups. Compared the following three groups: IPC versus LMWH versus no thromboprophylaxis | |
| Haemodynamic study | |
| Lacks appropriate experimental groups. Compared the following two groups: IPC versus no thromboprophylaxis |
DVT: deep vein thrombosis
GCS: graduated compression stockings
IPC: intermittent pneumatic compression
LMWH: low molecular weight heparin
TED: graduated compression stockings also called TED stockings ‐ thrombo‐embolic deterrent stockings
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study: RCT |
| Participants | Country: Turkey |
| Interventions | Type of treatment: compression stockings |
| Outcomes | DVT |
| Notes | Turkish paper Low molecular weight heparin (nadroparine calcium 0.3 ml 2850 IU AXa) was given to both control and treatment groups This study included an additional group of patients who wore stockings but did not take LMWH. However, this group was not appropriate for this review |
| Methods | Study: RCT |
| Participants | Total no. of participants: 131 |
| Interventions | Type of treatment: graduated supportive stockings |
| Outcomes | (not specified in the abstract) |
| Notes | Danish paper English abstract does not specify the number of patients included in the series of patients investigating the effect of combination of LMWH and GCS Exact figures not specified in the abstract |
FUT: 125‐I fibrinogen uptake test
GCS: graduated compression stockings
LMWH: low molecular weight heparin
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All Specialties Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| Analysis 1.1  Comparison 1 Incidence of DVT with stockings and without stockings, Outcome 1 All Specialties. | ||||
| 1.1 General Surgery | 9 | 1378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.20, 0.38] |
| 1.2 Orthopaedic Surgery | 6 | 598 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 1.3 Other Specialties | 4 | 769 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.16, 0.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All Specialties Show forest plot | 8 | 1035 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| Analysis 2.1  Comparison 2 Incidence of proximal DVT with stockings and without stockings, Outcome 1 All Specialties. | ||||
| 1.1 General Surgery | 2 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 1.2 Orthopaedic Surgery | 4 | 398 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.12, 0.53] |
| 1.3 Other Specialties | 2 | 321 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.05, 5.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All Specialties Show forest plot | 5 | 569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.15, 0.96] |
| Analysis 3.1 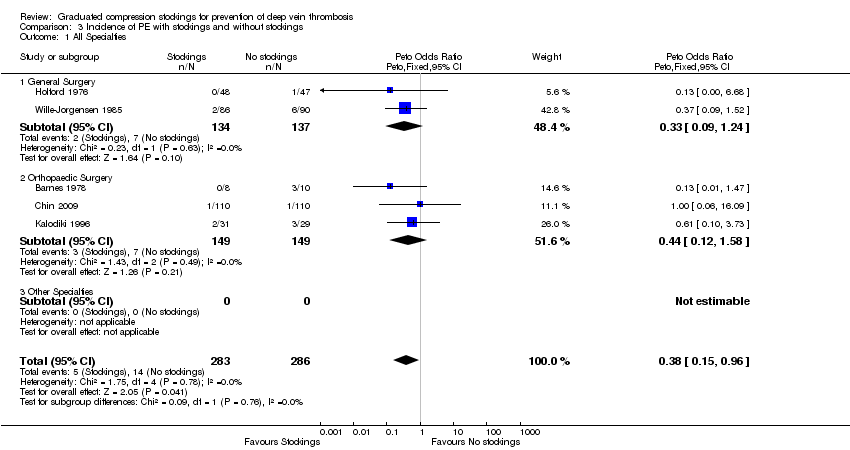 Comparison 3 Incidence of PE with stockings and without stockings, Outcome 1 All Specialties. | ||||
| 1.1 General Surgery | 2 | 271 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.09, 1.24] |
| 1.2 Orthopaedic Surgery | 3 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.12, 1.58] |
| 1.3 Other Specialties | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Method of randomisation Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| Analysis 4.1 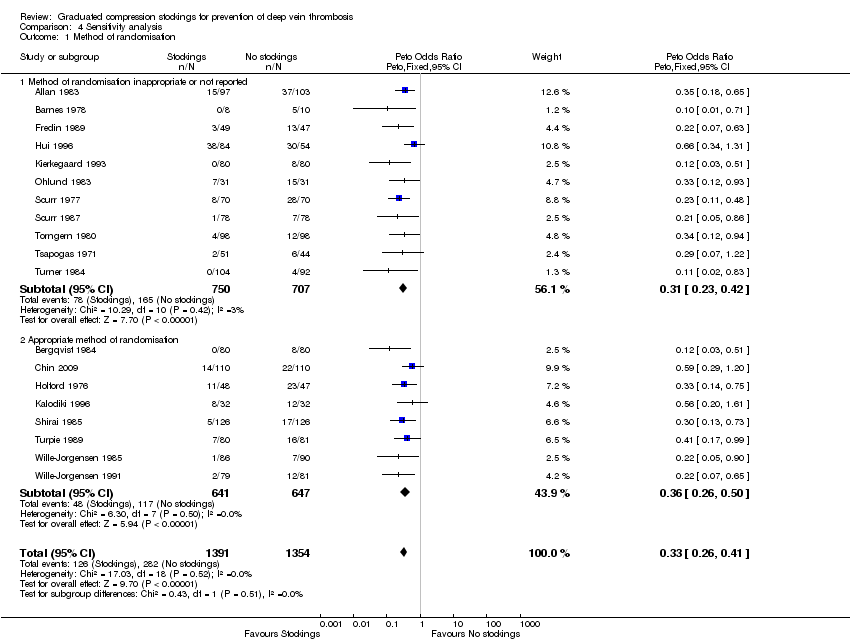 Comparison 4 Sensitivity analysis, Outcome 1 Method of randomisation. | ||||
| 1.1 Method of randomisation inappropriate or not reported | 11 | 1457 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.23, 0.42] |
| 1.2 Appropriate method of randomisation | 8 | 1288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.26, 0.50] |
| 2 Unit of Analysis for randomisation Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| Analysis 4.2 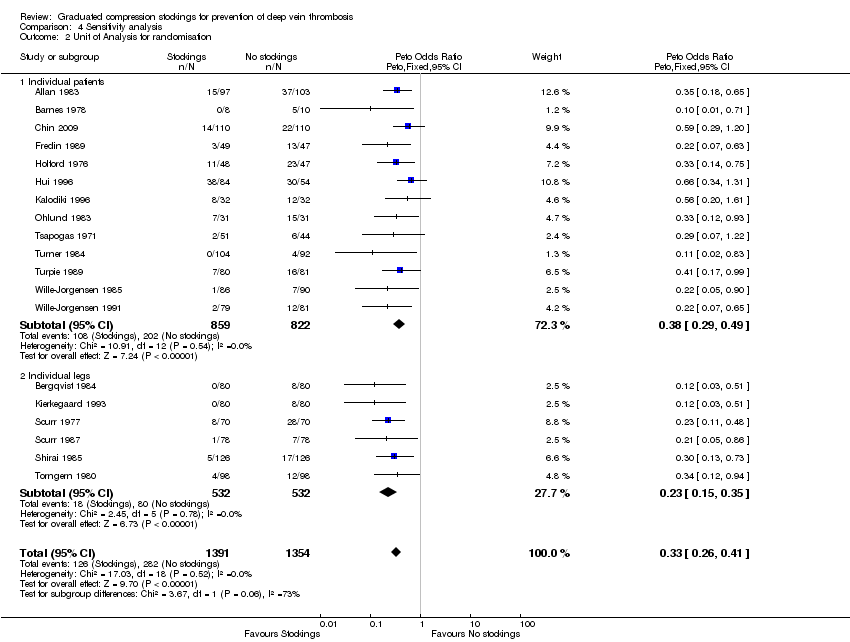 Comparison 4 Sensitivity analysis, Outcome 2 Unit of Analysis for randomisation. | ||||
| 2.1 Individual patients | 13 | 1681 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.29, 0.49] |
| 2.2 Individual legs | 6 | 1064 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.15, 0.35] |
| 3 Use of background method of thromboprophylaxis Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| Analysis 4.3 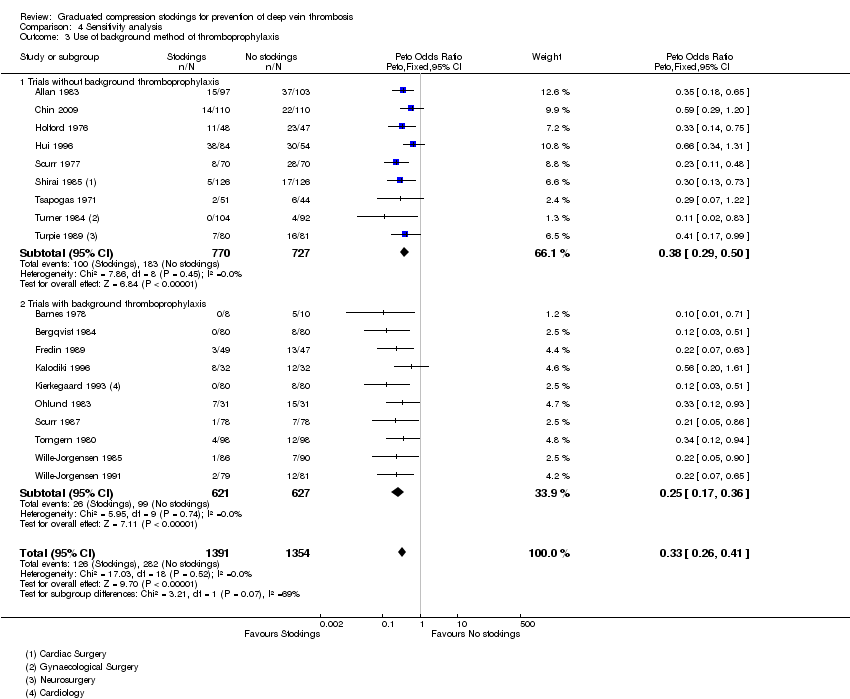 Comparison 4 Sensitivity analysis, Outcome 3 Use of background method of thromboprophylaxis. | ||||
| 3.1 Trials without background thromboprophylaxis | 9 | 1497 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.29, 0.50] |
| 3.2 Trials with background thromboprophylaxis | 10 | 1248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.17, 0.36] |
| 4 Method of diagnosis Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| Analysis 4.4  Comparison 4 Sensitivity analysis, Outcome 4 Method of diagnosis. | ||||
| 4.1 Fibrogen uptake test alone | 7 | 1101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.18, 0.39] |
| 4.2 Fibrinogen uptake test & phlebography | 6 | 1013 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.19, 0.43] |
| 4.3 Ultrasonography | 2 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
| 4.4 Phlebography | 4 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.29, 0.75] |

Study flow diagram.
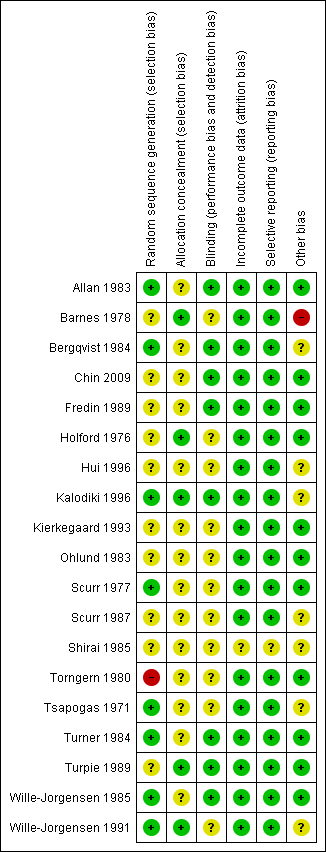
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
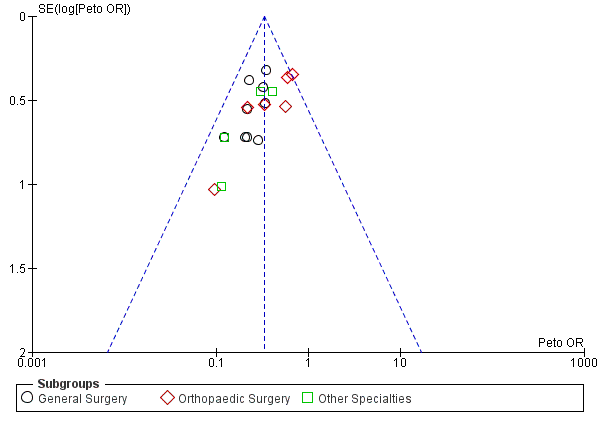
Funnel plot of comparison: Incidence of DVT with stockings and without stockings (all specialties).

Pie chart depicting the number of participants from each specialty included in the meta‐analysis.
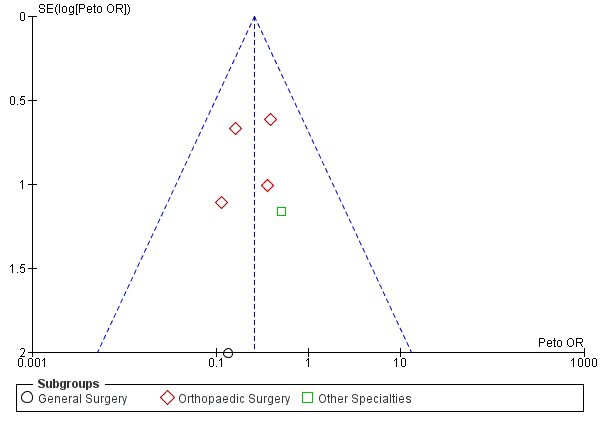
Funnel plot of comparison: Incidence of proximal DVT with stockings and without stockings (all specialties).
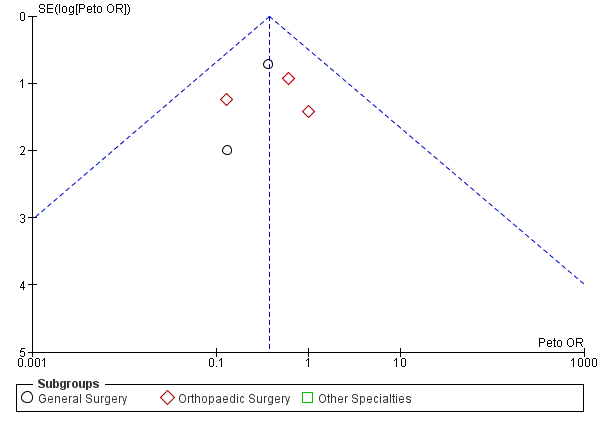
Funnel plot of comparison: Incidence of PE with stockings and without stockings (all specialties).
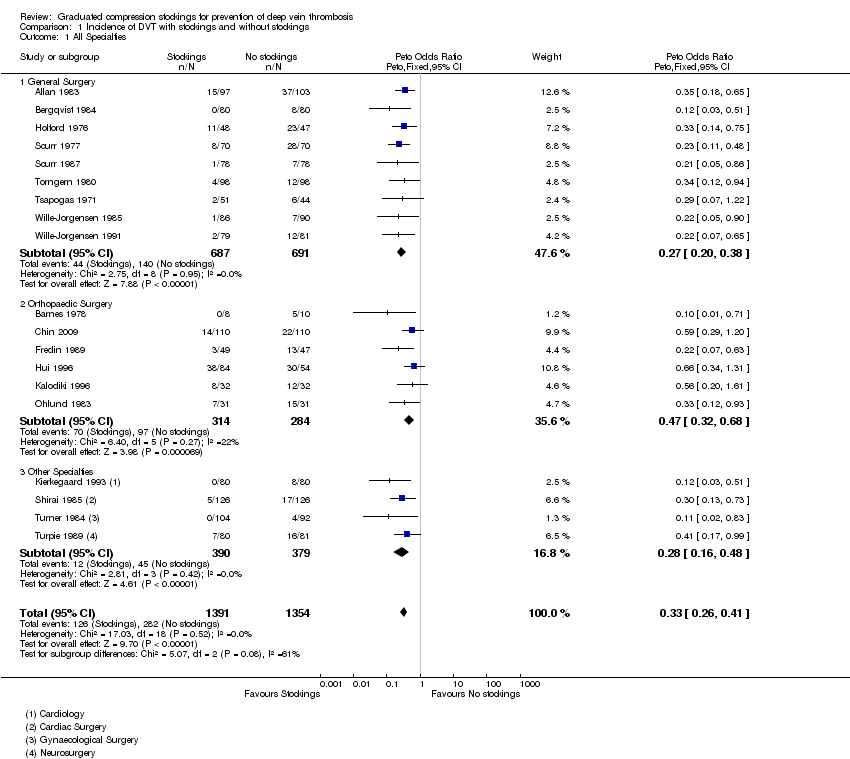
Comparison 1 Incidence of DVT with stockings and without stockings, Outcome 1 All Specialties.

Comparison 2 Incidence of proximal DVT with stockings and without stockings, Outcome 1 All Specialties.

Comparison 3 Incidence of PE with stockings and without stockings, Outcome 1 All Specialties.

Comparison 4 Sensitivity analysis, Outcome 1 Method of randomisation.

Comparison 4 Sensitivity analysis, Outcome 2 Unit of Analysis for randomisation.

Comparison 4 Sensitivity analysis, Outcome 3 Use of background method of thromboprophylaxis.
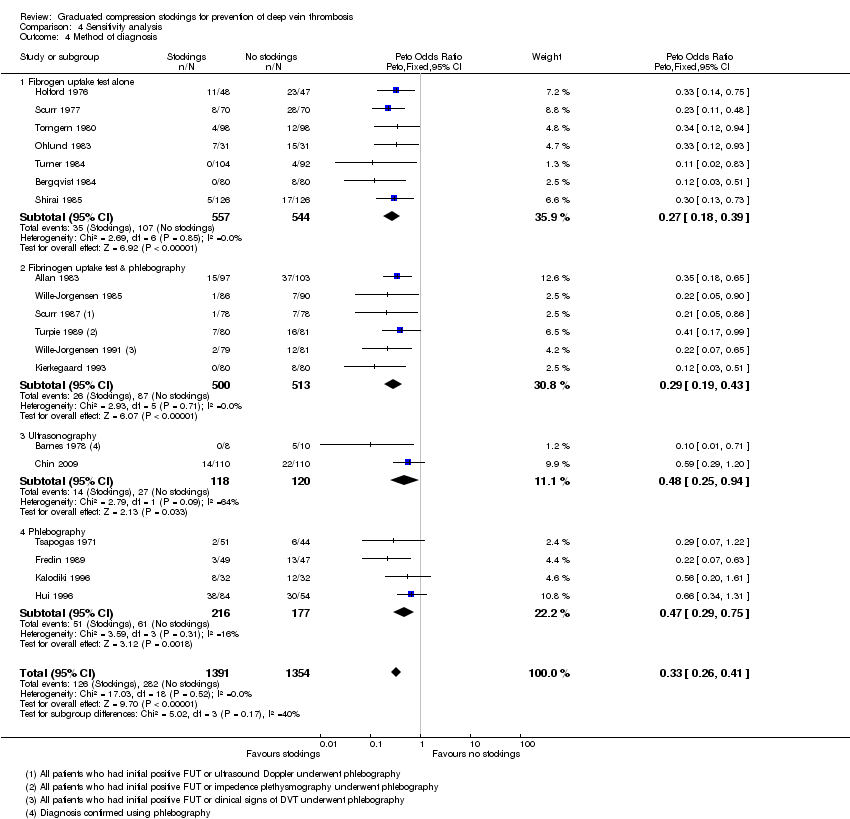
Comparison 4 Sensitivity analysis, Outcome 4 Method of diagnosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All Specialties Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| 1.1 General Surgery | 9 | 1378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.20, 0.38] |
| 1.2 Orthopaedic Surgery | 6 | 598 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 1.3 Other Specialties | 4 | 769 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.16, 0.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All Specialties Show forest plot | 8 | 1035 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 1.1 General Surgery | 2 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 1.2 Orthopaedic Surgery | 4 | 398 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.12, 0.53] |
| 1.3 Other Specialties | 2 | 321 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.05, 5.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All Specialties Show forest plot | 5 | 569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.15, 0.96] |
| 1.1 General Surgery | 2 | 271 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.09, 1.24] |
| 1.2 Orthopaedic Surgery | 3 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.12, 1.58] |
| 1.3 Other Specialties | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Method of randomisation Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| 1.1 Method of randomisation inappropriate or not reported | 11 | 1457 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.23, 0.42] |
| 1.2 Appropriate method of randomisation | 8 | 1288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.26, 0.50] |
| 2 Unit of Analysis for randomisation Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| 2.1 Individual patients | 13 | 1681 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.29, 0.49] |
| 2.2 Individual legs | 6 | 1064 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.15, 0.35] |
| 3 Use of background method of thromboprophylaxis Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| 3.1 Trials without background thromboprophylaxis | 9 | 1497 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.29, 0.50] |
| 3.2 Trials with background thromboprophylaxis | 10 | 1248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.17, 0.36] |
| 4 Method of diagnosis Show forest plot | 19 | 2745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.26, 0.41] |
| 4.1 Fibrogen uptake test alone | 7 | 1101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.18, 0.39] |
| 4.2 Fibrinogen uptake test & phlebography | 6 | 1013 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.19, 0.43] |
| 4.3 Ultrasonography | 2 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
| 4.4 Phlebography | 4 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.29, 0.75] |

