Vacunas antineumocócicas conjugadas para la prevención de la otitis media
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised ‐ yes, at individual level Design ‐ standard parallel‐group design Intention‐to‐treat (ITT) ‐ yes Follow‐up ‐ 6 to 31 months | |
| Participants | N ‐ 37,868 healthy infants Setting ‐ 23 medical centres within Northern California Kaiser Permanente (NCKP), USA Inclusion criteria ‐ healthy children aged 2 months Exclusion criteria ‐ children with sickle cell disease, known immunodeficiency, any serious chronic or progressive disease, a history of seizures or a history of either pneumococcal or meningococcal disease Baseline characteristics ‐ not described | |
| Interventions | Children were randomly allocated to either a 7‐valent pneumococcal conjugate vaccine (PCV7) or a meningococcus type C conjugate vaccine (MenC) at 2, 4, 6 and 12 to 15 months of age Tx ‐ PCV7 (containing the polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to carrier protein CRM197); N = 18,927 received 1 dose or more of the vaccine (unclear how many children were included in otitis media analyses) | |
| Outcomes | Primary outcome ‐ protective efficacy of PCV7 against invasive pneumococcal disease caused by vaccine serotypes Secondary outcomes ‐ effect of PCV7 on (a) number of otitis media episodes in fully vaccinated per‐protocol; (b) number of otitis media visits; (c) time to frequent otitis media (defined as 3 or more episodes in 6 months or 4 or more in 12 months); (d) number of tympanostomy tubes placements; (e) number of cases of spontaneous draining ruptured tympanic membranes with culture of a vaccine serotype pneumococcus Clinical diagnoses of acute otitis media were obtained from computerised data sources using diagnoses registered by emergency physicians and paediatricians in the NCKP population. Each clinic visit constituted a new episode unless it was classified as a follow‐up visit. A visit < 21 days after another otitis media visit was always considered a follow‐up visit. A visit 42 days or more after the most recent otitis media visit was considered a new episode. Visits occurring between 21 and 42 days, if the appointment was made < 3 days in advance, were considered new episodes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Indicated to be double‐blind study but insufficient details provided to ensure blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Clinical diagnoses of AOM were obtained from computerised data sources using diagnoses registered by emergency physicians and paediatricians (non‐trialists) |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how many children were included in otitis media analyses |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. Otitis media endpoint (efficacy against otitis media episodes) is reported as a secondary endpoint |
| Other bias | Low risk | Control group was vaccinated against MenC disease, but meningococci are not a causative pathogen in otitis media. Study enrolment was stopped as a result of prespecified interim analysis |
| Methods | Randomised ‐ yes, at individual level Design ‐ standard parallel‐group design Intention‐to‐treat (ITT) ‐ no, per‐ protocol analysis Follow‐up ‐ 2 years starting 1 month after complete immunisation | |
| Participants | N ‐ 264 healthy infants (261 children were included in clinical follow‐up) Setting ‐ 8 day‐care centres in Beer‐Sheva, Israel Inclusion criteria ‐ healthy children aged 12 to 35 months Exclusion criteria ‐ children that received any vaccine within a 4‐week period before, or were scheduled to receive any vaccine during the 4 weeks after the administration of the study vaccines, or received immunoglobulin within 8 weeks of study vaccination, known or suspected impairment of immunologic functions, major congenital malformation or serious chronic disease, known hypersensitivity to any components of the study vaccine, previous severe vaccine‐associated adverse reaction, previous vaccination with any pneumococcal or meningococcal vaccine, febrile illness (rectal temperature, 38 °C) within 72 h before vaccination Baseline characteristics ‐ described and balanced (Table 1) | |
| Interventions | Children were randomly allocated to either a 9‐valent pneumococcal conjugate vaccine (PCV9) or a meningococcus type C conjugate vaccine (MenC). Children aged 12 to 17 months at time of enrolment received 2 intramuscular injections 2 to 3 months apart and those 18 to 35 months at time of enrolment received 1 intramuscular injection Tx ‐ PCV9 (containing the polysaccharides of serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F and 23F conjugated to carrier protein CRM197); N = 131 | |
| Outcomes | Primary outcome ‐ effect of PCV9 on nasopharyngeal carriage of S. pneumoniae of the serotypes found in the vaccines in general and antibiotic‐resistant S. pneumoniae in particular Secondary outcomes ‐ effect of PCV9 on parent‐reported respiratory infections including otitis media 18 encounters were planned for each child during the 2‐year follow‐up period. During the first year encounters were planned to take place monthly and during the second year bimonthly. At each visit the parents were questioned about illness and antibiotic use since the last visit. Illness episodes were divided into 4 categories: | |
| Notes | Participants lost to follow‐up during first 12 months ‐ total: 32/261 (12.3%) Participants lost to follow‐up during first 12 months ‐ Tx: 16/131 (12.2%) Participants lost to follow‐up during first 12 months ‐ C: 16/130 (12.3%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described. Block randomisation (n = 6) stratified by DCC and age |
| Allocation concealment (selection bias) | Low risk | Randomisation list provided in a sealed envelope by Wyeth‐Lederle Vaccines |
| Blinding of participants and personnel (performance bias) | Low risk | Appearance of PCV9 and MenC vaccines was not similar. 2 nurses not belonging to the study team injected the vaccines. They were not allowed reveal the child's allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Parental interview. A positive report of OM was defined as an episode |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up rates reported in Table 1. 12% of children followed up for < 12 months |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available |
| Other bias | Low risk | |
| Methods | This trial was part of a study including Kilpi 2003 (FinOM Vaccine Trial). Both Eskola 2001 and Kilpi 2003 used the same control group (hepatitis B vaccine) but a different treatment group, each with a different type of 7‐valent pneumococcal conjugate vaccine. Eskola 2001 used a PCV7 containing the polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to carrier protein CRM197, while Kilpi 2003 used a PCV7 containing polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to the outer membrane protein complex of N. meningitidis serogroup B Randomised ‐ yes, at individual level Design ‐ standard parallel‐group design Intention‐to‐treat (ITT) ‐ yes, both ITT and per‐protocol analysis described Follow‐up ‐ 22 consecutive months (children were followed up to 24 months of age) | |
| Participants | N ‐ 1662 healthy infants Setting ‐ 8 study clinics in the communities of Tampere, Kangsala and Nokia, Finland Inclusion criteria ‐ healthy children aged 2 months Exclusion criteria ‐ not described Baseline characteristics ‐ described and balanced (Table 1) | |
| Interventions | Children were randomly allocated to either a 7‐valent pneumococcal conjugate vaccine (PCV7) or a hepatitis B at 2, 4, 6 and 12 to 15 months of age Tx ‐ PCV7 (containing the polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to carrier protein CRM197); N = 831 (N = 786 completed the follow‐up as specified in the protocol) | |
| Outcomes | Primary outcome ‐ effect of PCV7 on the number of acute otitis media (AOM) episodes due to the pneumococcal serotypes included in the vaccine Secondary outcomes ‐ effect of PCV7 on the number of all‐cause AOM episodes, culture‐confirmed AOM episodes and pathogen‐specific AOM episodes, preventing first and subsequent AOM episodes, number of children with recurrent AOM episodes (defined as 3 or more AOM episodes in last 6 months or 4 or more in the last 12 months), serious adverse events All children attended 1 of the study clinics for enrolment at 2 months of age and thereafter at 4, 6, 7, 12, 13, 18 and 24 months. Parents were encouraged to bring their child to the study clinic for evaluation of symptoms suggesting respiratory infection or AOM. AOM was diagnosed by otoscopy (visibly abnormal tympanic membrane in terms of colour, position or mobility, suggesting middle ear effusion) and the presence of at least 1 of the following symptoms or signs of acute infection: fever, earache, irritability, diarrhoea, vomiting, acute otorrhoea not caused by otitis externa and other symptoms of respiratory infection | |
| Notes | Participants lost to follow‐up ‐ total: 82/1662 (4.9%) did not complete the follow‐up period according to protocol Participants lost to follow‐up ‐ Tx: 45/831 (5.4%) did not complete the follow‐up period according to protocol Participants lost to follow‐up ‐ C: 37/831 (4.5%) did not complete the follow‐up period according to protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 6 letters corresponding to the 3 treatment options were randomly allocated to consecutive subject identification numbers, using an allocation of 1:1:1 and a block size of 12 (see Kilpi 2003) |
| Allocation concealment (selection bias) | Low risk | Individual treatment assignments were kept in sealed envelopes until vaccination (see Kilpi 2003) |
| Blinding of participants and personnel (performance bias) | Low risk | Use of vaccinators who were not otherwise involved in the trial follow‐up. Letter code was destroyed immediately after vaccination (see Kilpi 2003) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment of the outcome was done according to a strict definition of AOM. Assessment was done by other personnel than those that vaccinated the children (vaccinators were not otherwise involved in the trial follow‐up) (see Kilpi 2003) |
| Incomplete outcome data (attrition bias) | Low risk | No reporting of reasons for drop‐out and/or lost to follow‐up. Not expected to have major impact on outcome since 94.6% in the PCV7 and 95.5% in the control group completed the follow‐up as specified in the protocol |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov (although uploaded after study end) |
| Other bias | Low risk | |
| Methods | This study is an extension of Black 2000 (data updated to 1999). Follow‐up continued until children left Northern California Kaiser Permanente (NCKP) or until 20 April 1999, when the study was unblinded and the control group was offered PCV7. For a detailed description of the characteristics of this study, see Black 2000 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This study is an extension of Black 2000 Method of random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Indicated to be double‐blind study but insufficient details provided to ensure blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Clinical diagnoses of AOM were obtained from computerised data sources using diagnoses registered by emergency physicians and paediatricians (non‐trialists) |
| Incomplete outcome data (attrition bias) | High risk | Substantial number of randomised children did not stay in the Kaiser Permanente healthcare database to the end of follow‐up (April 1999), i.e. 27% in the PCV group and 26% in the control group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available but trial includes all expected outcomes (including OM visits, frequent OM, tube procedures and Ab prescriptions) |
| Other bias | Low risk | Control group was vaccinated against MenC disease, but meningococci are not a causative pathogen in otitis media. Study enrolment was stopped as a result of prespecified interim analysis |
| Methods | Randomised ‐ yes, at individual level Design ‐ standard parallel‐group design Intention‐to‐treat (ITT) ‐ yes Follow‐up ‐ follow‐up started 14 days after the second set of vaccinations and continued for 6 to 18 months, depending on the year of inclusion | |
| Participants | N ‐ 597 children with a previously diagnosed respiratory tract infection (RTI) Setting ‐ general practitioners (GPs) in the centre of the Netherlands selected children Inclusion criteria ‐ children aged 18 to 72 months with a previously diagnosed respiratory tract infection (RTI) registered according to the International Classification of Primary Care (ICPC), i.e. acute otitis media (AOM); cough (with fever); acute upper RTI; acute laryngitis/tracheitis; acute bronchitis/bronchiolitis; influenza; pneumonia; pleurisy/pleural effusion Exclusion criteria ‐ children with chronic asthma or recurrent wheezing (for longer than 3 months) treated with corticosteroids; craniofacial abnormalities; clinically significant hypersensitivity to eggs; previous serious adverse reactions to vaccines; previous influenza, pneumococcal or hepatitis B vaccinations and those with conditions for which these vaccinations are already recommended, such as chronic cardiac and respiratory conditions Baseline characteristics ‐ described and balanced (Table 1) | |
| Interventions | Children were randomly allocated to either trivalent influenza plus 7‐valent pneumococcal conjugate vaccination (TIV/PCV7), trivalent influenza plus placebo vaccination (TIV/placebo) or hepatitis B virus vaccination plus placebo vaccination (HBV/placebo) Children received 2 vaccinations 4 to 8 weeks apart in the first year of inclusion and the first 2 cohorts of children received a subsequent vaccination in the subsequent year Tx ‐ TIV (strains in the 2003‐2004 formulation were H1N1, H3N2 and B/HongKong/330/01; strains in the 2004‐2005 formulation were H1N1, H3N2 and B/Shanghai/361/2002; strains in the 2005‐2006 formulation included H1N1, H3N2 and B/Shanghai/361/2002/PCV7 (containing the polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to carrier protein CRM197); N = 197 (N = 163 completed; 67,867 person‐days analysed, 14% missing) C2 ‐ HBV (recombinant HBV vaccine; Engerix‐B Junior)/placebo; N = 195 (N = 160 completed; 67,679 person‐days analysed, 15% missing) | |
| Outcomes | Primary outcome ‐ effect of the TIV/PCV7 on febrile RTI, defined as fever (tympanic temperature 38.0 °C) for at least 2 consecutive days accompanied by 1 or more of the aforementioned signs or symptoms of RTI with a moderate or severe severity score Secondary outcomes ‐ effect of the TIV/PCV7 on febrile RTI–related polymerase chain reaction (PCR)‐confirmed influenza, GP visits, antibiotic prescriptions or a physician‐diagnosed episode of AOM Each parent was instructed to keep a daily diary, recording any clinical signs or symptoms associated with RTI and to characterise their severity on a scale of 1 (mild) to 3 (severe). The parent also was instructed to measure the child's body temperature using a validated electronic tympanic thermometer. The parent also was asked to record all GP visits due to their child's RTI‐related complaints. For each such visit, the GP was instructed to complete a form including information on the diagnosis and possible antibiotic prescriptions | |
| Notes | Participants lost to follow‐up ‐ total: 108/579 (18.7%) completely (n = 41) or partially (n = 67) lost to follow‐up Participants lost to follow‐up ‐ Tx: 34/197 (17.3%) completely (n = 8) or partially (n = 26) lost to follow‐up; 67,867 person‐days analysed, 14% missing Participants lost to follow‐up ‐ C1: 39/187 (20.8%) completely (n = 19) or partially (n = 20) lost to follow‐up; 60,515 person‐days analysed, 20% missing Participants lost to follow‐up ‐ C2: 35/195 (17.9%) completely (n = 14) or partially (n = 21) lost to follow‐up; 67,679 person‐days analysed, 15% missing 2 of the 3 treatment arms received an additional vaccination in the second year of the study. To evaluate blinding, parents of these cohorts of children were asked which vaccinations that they thought their child had received just after the vaccinations were given and at the end of the study. Just after the vaccination, 87% of the parents either did not know or identified the wrong set of vaccinations; at the end of the study, this percentage was 80%, indicating successful blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described; children were randomly assigned in blocks of 3 in a 1:1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was described |
| Blinding of participants and personnel (performance bias) | Low risk | The injections were administered by non‐blinded research nurses who were not involved in subsequent follow‐up and were instructed to not reveal the intervention allocation. The treatment group assignments were not revealed to parents, investigators, research personnel conducting the follow‐up or health care providers, all of whom remained blinded throughout the study |
| Blinding of outcome assessment (detection bias) | Low risk | The parents were asked to record all GP visits due to their child's RTI‐related complaints. For each such visit, the GP was instructed to complete a form including information on the diagnosis and possible antibiotic prescriptions. The treatment group assignments were not revealed to parents, investigators, research personnel conducting the follow‐up or health care providers, all of whom remained blinded throughout the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Substantial loss to follow‐up (< 14% in both groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov |
| Other bias | High risk | Co‐administered with influenza vaccine in influenza season. Pivotal role of influenza viruses acknowledged by the authors |
| Methods | This trial was part of a study including Eskola 2001 (FinOM Vaccine Trial). Both Eskola 2001 and Kilpi 2003 used the same control group (hepatitis B vaccine) but a different treatment group, each with a different type of 7‐valent pneumococcal conjugate vaccine. Eskola 2001 used a PCV7 containing the polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to carrier protein CRM197, while Kilpi 2003 used a PCV7 containing polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to the outer membrane protein complex of N. meningitidis serogroup B Randomised ‐ yes, at individual level Design ‐ standard parallel‐group design Intention‐to‐treat (ITT) ‐ no, per‐protocol analysis Follow‐up ‐ 22 consecutive months (children were followed up to 24 months of age) | |
| Participants | N ‐ 1666 healthy infants Setting ‐ 8 study clinics in the communities of Tampere, Kangsala and Nokia, Finland Inclusion criteria ‐ healthy children aged 2 months Exclusion criteria ‐ not described Baseline characteristics ‐ described and balanced (Table 1) | |
| Interventions | Children were randomly allocated to either a 7‐valent pneumococcal conjugate vaccine (PCV7) or a hepatitis B at 2, 4, 6 and 12 to 15 months of age. From 3 November 1997 onward, for the children randomised to receive OMPC‐PCV7, the fourth dose of the conjugate vaccine was replaced by a 23‐valent pneumococcal polysaccharide vaccine (PPV‐23) that consisted of serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F (Pneumovax23) Tx ‐ PCV7 (containing the polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to the outer membrane protein complex of N. meningitidis serogroup B (OMPC)); N = 835 (N = 805 completed the follow‐up as specified in the protocol) | |
| Outcomes | See Eskola 2001 | |
| Notes | Participants lost to follow‐up ‐ total: 67/1,666 (4.0%) did not complete the follow‐up period according to protocol Participants lost to follow‐up ‐ Tx: 30/835 (3.6%) did not complete the follow‐up period according to protocol Participants lost to follow‐up ‐ C: 37/831 (4.5%) did not complete the follow‐up period according to protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 6 letters corresponding to the 3 treatment options were randomly allocated to consecutive subject identification numbers, using an allocation of 1:1:1 and a block size of 12 |
| Allocation concealment (selection bias) | Low risk | Individual treatment assignments were kept in sealed envelopes until vaccination |
| Blinding of participants and personnel (performance bias) | Low risk | Use of vaccinators who were not otherwise involved in the trial follow‐up. Letter code was destroyed immediately after vaccination |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment of the outcome was done according to a strict definition of AOM. Assessment was done by other personnel than those who vaccinated the children (vaccinators were not otherwise involved in the trial follow‐up) |
| Incomplete outcome data (attrition bias) | Low risk | No reporting of reasons for drop‐out and/or loss to follow‐up. Not expected to have a major impact on outcome since 96.0% in the PCV7 OMPC and 95.5% in the control group completed the follow‐up as specified in the protocol |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov (although uploaded after study end) |
| Other bias | Unclear risk | Mixed schedule with 187 children boosted with PPV‐23. How was it known that only the children allocated to PCV7 OMPC should receive PPV‐23 after November 1997? |
| Methods | The design of this cluster‐randomised trial has been described extensively in Moulton 2001, while the findings on invasive pneumococcal disease (main outcome of the trial) have been published in O'Brien 2003 Randomised ‐ yes, at group level Design ‐ cluster‐randomised design Intention‐to‐treat (ITT) ‐ no, per‐protocol analysis Follow‐up ‐ depending on time of inclusion, maximum duration of follow‐up 40 months | |
| Participants | N ‐ 944 (944 of the 4476 children were randomly selected for chart review. This sample size was determined by logistic feasibility and expected frequency of healthcare events. Of these 944 children, 856 were found to have strictly met the chart review criteria) Setting ‐ Navajo and White Mountain Apache region Inclusion criteria ‐ Navajo and White Mountain Apache children below 2 years of age Exclusion criteria ‐ no exclusion criteria described Baseline characteristics ‐ balanced but data not shown | |
| Interventions | Children were randomly allocated to either a 7‐valent pneumococcal conjugate vaccine (PCV7) or a meningococcus type C conjugate vaccine (MenC). For each of the study and control vaccines, 3 immunisation schedules were designed according to age of entry into the trial: 6 weeks to 6 months (3 doses, ideally at 2, 4 and 6 months of age and a booster at 12 to 15 months of age), 7 months to 11 months (2 doses 1 month apart and a booster at 12 to 15 months of age) and 12 months to 23 months (2 doses separated by at least 2 months). Over the course of the trial, the great majority of new enrollees are in the first group, which is referred to as the primary efficacy cohort Tx ‐ PCV7 (containing the polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to carrier protein CRM197); N = unknown (N = 424 analysed in primary efficacy group) | |
| Outcomes | Primary outcome ‐ effect of PCV7 on clinically diagnosed episodes of OM A new OM episode was counted if any of the following were recorded as the diagnosis: OM, AOM, bilateral OM, chronic OM, OM with perforation, otorrhoea, pressure equalising tube placement, perforated tympanic membrane, serous OM and bullous myringitis An episode of AOM was categorised as either AOM or bilateral AOM. An OM episode was categorised as severe if there were 3 or more OM visits for the episode. A child's first medical visit for OM was considered their first episode. OM visits occurring less than 21 days after the immediately prior otitis‐related visit and visits noted as a follow‐up to a previous otitis‐related visit were counted as follow‐up visits, not as OM episodes | |
| Notes | Participants lost to follow‐up ‐ total: 88/944 (9.3%) not included in primary efficacy analysis Participants lost to follow‐up ‐ Tx: unknown Participants lost to follow‐up ‐ C: unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation using 38 independent randomisation units, stratified using 3 blocks of 4 units and 13 blocks of 2 units |
| Allocation concealment (selection bias) | Low risk | 6 labels were assigned to the vaccines (B, F, H, M, T, U), with 3 labels for PCV7 and 3 for MenC. The grouping of these codes was known only to a statistician employed by the manufacturer (who had no other responsibilities with respect to the trial other than handling treatment allocation and randomisation issues. No loss of clusters |
| Blinding of participants and personnel (performance bias) | Low risk | Masked treatment assignment (vaccines were labelled). In addition, field staff were blinded as to serotype of the invasive disease cases and thus did not know which ones would be likely to be prevented by an effective vaccine |
| Blinding of outcome assessment (detection bias) | Unclear risk | Every medical visit made by study children was evaluated through 2 years of age. OM visits, as documented by the patients' treating physician, were recorded. Treating physicians were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | 88 of the 944 children (9.3%) not included in primary efficacy analysis; no information provided on the distribution across treatment groups |
| Selective reporting (reporting bias) | Low risk | Study design was described extensively in Moulton 2001 and O'Brien 2003 |
| Other bias | Low risk | Study enrolment was stopped as a result of prespecified interim analysis |
| Methods | This study is an additional analysis of Eskola 2001, which is part of the FinOM Vaccine Trial and studies the effect of PCV7 containing the polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to carrier protein CRM197 as compared to a hepatitis B vaccine. For a detailed description of the characteristics of this study, see Eskola 2001 | |
| Participants | ||
| Interventions | ||
| Outcomes | Primary outcome ‐ effect of PCV7 on PCR‐positive AOM | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This is an additional analysis of Eskola 2001 6 letters corresponding to the 3 treatment options were randomly allocated to consecutive subject identification numbers, using an allocation of 1:1:1 and a block size of 12 |
| Allocation concealment (selection bias) | Low risk | Individual treatment assignments were kept in sealed envelopes until vaccination |
| Blinding of participants and personnel (performance bias) | Low risk | Use of vaccinators who were not otherwise involved in the trial follow‐up. Letter code was destroyed immediately after vaccination |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment of the outcome was done according to a strict definition of AOM. Assessment was done by other personnel than those that vaccinated the children (vaccinators were not otherwise involved in the trial follow‐up) |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how many children were lost to follow‐up. In Eskola 2001, 94.6% in the PCV7 and 95.5% in the control group completed the follow‐up as specified in the protocol |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov (although uploaded after study end) |
| Other bias | Low risk | |
| Methods | Randomised ‐ yes, at individual level Design ‐ standard parallel‐group design Intention‐to‐treat (ITT) ‐ yes, both ITT and per‐protocol analysis described Follow‐up ‐ efficacy follow‐up started on the day of the first dose of study vaccine (for ITT analysis) or 2 weeks after the third vaccine dose (for the per‐protocol analysis) and continued until 24 to 27 months of age | |
| Participants | N ‐ 4968 healthy infants Setting ‐ 27 paediatric centres in the Czech Republic and 23 in Slovakia Inclusion criteria ‐ healthy children aged between 6 weeks and 5 months with no acute illness Exclusion criteria ‐ use of any investigational or non‐registered drug or vaccine other than the study vaccines within 30 days preceding the study vaccines' first dose; previous vaccination against S. pneumoniae; fever (defined as a rectal temperature of 38 ºC or higher or temperature by other routes of 37.5 ºC or higher); history of allergic disease or reactions likely to be exacerbated by any component of the study vaccines; other conditions that might have potentially interfered with the interpretation of study outcomes according to the investigator Baseline characteristics ‐ described and balanced (Table 1) | |
| Interventions | Children were randomly allocated to either an 11‐valent pneumococcal conjugate vaccine (PCV11) or a hepatitis A at the ages of about 3, 4, 5 and 12 to 15 months of age Tx ‐ PCV11 (containing the polysaccharides of serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F conjugated to protein D (surface lipoprotein of H. influenzae)); N = 2489 (N = 2455 included in per‐protocol cohort for efficacy) | |
| Outcomes | Primary outcome ‐ effect of PCV11 on first episode of acute otitis media (AOM) caused by vaccine pneumococcal serotypes Secondary outcomes ‐ effect of PCV11 on first episode of AOM caused by non‐typeable Haemophilus influenzae There was no active surveillance. Unscheduled doctor visits could take place any time during follow‐up according to standard local practice (parents consulting their local paediatrician in case of illness of their child). Parents were advised to consult their paediatrician if their child was sick, had ear pain or had spontaneous ear discharge. Children with suspected AOM were immediately referred to ENT surgeons AOM was defined as either abnormal findings of the tympanic membrane at otoscopy (i.e. redness, bulging, loss of light reflex) or the presence of middle ear effusion as shown by simple or pneumatic otoscopy or by microscopy together with at least 2 of the following signs or symptoms: ear pain, ear discharge, hearing loss, fever, lethargy, irritability, anorexia, vomiting or diarrhoea. These signs or symptoms had to be present for a maximum of 14 days For patients with repeated doctor visits, a new episode of AOM was judged to have started if more than 30 days had elapsed since the beginning of the previous episode. Additionally, for categories defined according to bacterial pathogen or serotype, a new episode was judged to have started if any interval had elapsed since the beginning of an episode caused by a different bacterial pathogen or serotype Recurrent AOM was defined as 3 or more AOM episodes in the last 6 months or 4 or more in the last 12 months | |
| Notes | Participants lost to follow‐up ‐ total: 61/4968 (1.2%) did not complete the follow‐up period according to protocol Participants lost to follow‐up ‐ Tx: 34/2489 (1.4%) did not complete the follow‐up period according to protocol Participants lost to follow‐up ‐ C: 27/2479 (1.1%) did not complete the follow‐up period according to protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Randomisation (1:1) was done with a study‐specific central randomisation system via the Internet which, on receipt of the infant's initials and birth date, determined the vaccine number to be used |
| Blinding of participants and personnel (performance bias) | Unclear risk | Indicated to be double‐blinded study. Sponsor numbered the vaccine supplies. It was, however, unknown whether the appearance of the vaccines was similar at the time of administration |
| Blinding of outcome assessment (detection bias) | Low risk | Visits during efficacy follow‐up were according to standard local clinical practice. When AOM was suspected children were referred to ENT surgeons |
| Incomplete outcome data (attrition bias) | Low risk | No reporting of reasons for drop‐out and/or loss to follow‐up. Not expected to have a major impact on outcome since 98.6% in the PCV11 and 98.9% in the control group completed the follow‐up as specified in the protocol |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov |
| Other bias | Low risk | Study enrolment was stopped as a result of prespecified interim analysis |
| Methods | This study was performed in parallel with Veenhoven 2003, but analysed separately due to differences in study population Randomised ‐ yes, at individual level Design ‐ standard parallel‐group design Intention‐to‐treat (ITT) ‐ unclear Follow‐up ‐ 26 months | |
| Participants | N ‐ 74 children with a history of frequent acute otitis media (AOM) Setting ‐ ENT department of the Ghent University Hospital in Belgium Inclusion criteria ‐ children aged 1 to 7 years with a history of frequent AOM defined as at least 2 separate clinically diagnosed AOM episodes in the past year Exclusion criteria ‐ children with any underlying illnesses including immunocompromising conditions other than partial serum IgA and IgG2 deficiencies, craniofacial abnormalities, previous pneumococcal vaccination or documented hypersensitivity to any of the vaccine components Baseline characteristics ‐ described and balanced (Table 1) | |
| Interventions | Children were randomly allocated to either a 7‐valent pneumococcal conjugate vaccine (PCV7) or a hepatitis A vaccine. Children aged 12 to 24 months received 2 intramuscular injections with a 1‐month interval and those aged over 2 years received 1 intramuscular injection. Those allocated to PCV7 received an additional 23‐valent pneumococcal polysaccharide vaccination (PPSV‐23) respectively at 6 months (in children aged 12 to 24 months) and 7 months (in those aged above 2 years) later Tx ‐ PCV7 (containing polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to the carrier protein CRM197)/PPSV23 (containing polysaccharides of the serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F); N = 38 (N = 35 completed the vaccination scheme) | |
| Outcomes | Primary outcome ‐ effect of PCV7/PPSV23 on the number of AOM episodes during 18 months follow‐up Secondary outcomes ‐ effect of PCV7/PPSV23 on immunogenicity; nasopharyngeal carriage of conjugate vaccine related serotypes; and antibiotic‐resistant pneumococci At scheduled hospital visits at 7, 14, 20 and 26 months after randomisation, a medical history was taken, antibiotic usage noted and an otomicroscopic examination performed AOM was defined by an abnormal tympanic membrane on otomicroscopy (red, dull or bulging); plus at least 1 of the following symptoms or signs of acute infection: earache, acute otorrhoea, fever (> 38.5 °C rectally) or irritability | |
| Notes | Participants lost to follow‐up ‐ total: 6/74 (8.1%) did not complete the follow‐up period according to protocol Participants lost to follow‐up ‐ Tx: 3/38 (7.9%) did not complete the follow‐up period according to protocol Participants lost to follow‐up ‐ C: 3/36 (8.3%) did not complete the follow‐up period according to protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described, randomisation stratified according to age (12 to 24 months versus 25 to 84 months) and number of previous AOM episodes per year (2 to 3 versus 4 or more episodes) |
| Allocation concealment (selection bias) | Low risk | 2 study nurses immunised all children according to a randomisation list provided to them in a sealed envelope by a third party (the Julius Center for Health Sciences, Utrecht, The Netherlands) |
| Blinding of participants and personnel (performance bias) | Low risk | The nurses that vaccinated children were not allowed to reveal the child's allocation to either the study team or the parents |
| Blinding of outcome assessment (detection bias) | Low risk | When a new AOM episode was suspected, parents were asked to bring their sick child within 24 hours to the study centre for otoscopic diagnosis. In case of all other AOM episodes during follow‐up, participants were allowed to visit the study centre, their family physician or a paediatrician who was asked to report otoscopic findings, diagnosis and treatment on an AOM registration form |
| Incomplete outcome data (attrition bias) | Low risk | In total 6 of the 74 children (8.1%) did not complete the follow‐up period according to protocol (equally distributed across groups). Reasons for withdrawals are described in the Results section of the article |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | |
| Methods | Randomised ‐ yes, at individual level Design ‐ standard parallel‐group design Intention‐to‐treat (ITT) ‐ yes Follow‐up ‐ 18 months, starting 1 month after completion of the vaccination scheme | |
| Participants | N ‐ 383 children with a history of frequent acute otitis media (AOM) Setting ‐ a general hospital (Spaarne Hospital, Haarlem) and a tertiary care hospital (Wilhelmina Children's Hospital of the University Medical Centre Utrecht) in the Netherlands Inclusion criteria ‐ children aged 1 to 7 years with a history of frequent AOM defined as 2 or more AOM episodes in the year before study entry. The number of previous AOM episodes was based on parental report and on clinical confirmation of the diagnosis by a physician Exclusion criteria ‐ children with immunodeficiency, cystic fibrosis, immotile cilia syndrome, craniofacial abnormalities, chromosomal abnormalities such as Down's syndrome and severe adverse events during previous vaccinations Baseline characteristics ‐ described and balanced (Table 1) | |
| Interventions | Children were randomly allocated to either a 7‐valent pneumococcal conjugate vaccine (PCV7) followed by a 23‐valent pneumococcal polysaccharide vaccination (PPSV23) or a hepatitis A or B vaccine Children aged 12 to 24 months in the pneumococcal vaccination group received PCV7 twice with a 1‐month interval followed 6 months later by PPSV23. The control vaccine group received 3 hepatitis B vaccinations according to a similar time schedule Children aged 25 to 84 months in the pneumococcal vaccine group received 1 dose of PCV7 followed 7 months later by PPSV23. The control group received hepatitis A vaccine twice Tx ‐ PCV7 (containing polysaccharides of serotypes 4, 6B, 9V, 14, 18C, 19F and 23F conjugated to the carrier protein CRM197)/PPSV23 (containing polysaccharides of the serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F); N = 190 (N = 190 included in ITT analysis) | |
| Outcomes | Primary outcome ‐ effect of PCV7/PPSV23 on the number of clinical episodes of AOM during 18 months follow‐up Secondary outcomes ‐ effect of PCV7/PPSV23 on the number of AOM episodes due to the 7 pneumococcal serotypes included in the PCV7 vaccine and nasopharyngeal carriage of conjugate vaccine serotypes Parents were instructed to visit the study clinics or their family physician, otolaryngologist or paediatrician to assess symptoms suggesting AOM. Physicians registered signs and symptoms of every AOM episode on standard registration forms and were unaware of treatment allocation. AOM was defined according to the guideline issued by the Dutch College of General Practitioners, i.e. presence of an abnormal tympanic membrane on otoscopy (red, dull or bulging), or otorrhoea and at least 1 of these signs or symptoms of acute infection: acute earache, new‐onset otorrhoea, irritability or fever greater than 38.5 ºC rectally or 38.0 ºC axillary | |
| Notes | Participants lost to follow‐up ‐ total: 1/383 (0.3%); all children included in ITT analysis Participants lost to follow‐up ‐ Tx: 0/190 (0%) Participants lost to follow‐up ‐ C: 1/193(0.5%) Performed in parallel with the study of Van Kempen 2006, but analysed separately due to differences in study population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers that identified the vaccine scheme, randomisation stratified according to age (12 to 24 months versus 25 to 84 months) and number of previous AOM episodes per year (2 to 3 versus 4 or more episodes) |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was described |
| Blinding of participants and personnel (performance bias) | Low risk | Vaccine was administered to the child by a study nurse, so that parents and physicians were unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Parents were instructed to visit the study clinics or their family physician, otolaryngologist or paediatrician to assess symptoms suggesting AOM. Physicians registered signs and symptoms of every AOM episode on standard registration forms and were unaware of treatment allocation. AOM was defined according to the guideline issued by the Dutch College of General Practitioners |
| Incomplete outcome data (attrition bias) | Low risk | All randomised children were included in ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | |
Ab: antibiotics; AOM: acute otitis media; C: control; DCC: day‐care centre; DTaP: diphtheria‐tetanus toxoid‐acellular pertussis vaccine; DTP: diphtheria‐tetanus toxoid‐pertussis vaccine; DTwP: diphtheria‐tetanus toxoid‐whole cell pertussis vaccine; ENT: ear, nose and throat; IgA: immunoglobulin A; IgG: immunoglobulin G; GP: general practitioner; ITT: intention‐to‐treat; NaCl: sodium chloride; OM: otitis media; PCR: polymerase chain reaction; PCV: pneumococcal conjugate vaccine; PPV: pneumococcal polysaccharide vaccine; RTI: respiratory tract infection; TIV: trivalent influenza vaccine; Tx: treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No control vaccination. As such, parents were not blinded to treatment allocation (children received either PCV or no vaccination). However, for outcome assessment, parents were instructed to visit the ENT department whenever they suspected an episode of AOM. Parental threshold to consult ENT may be lower in children allocated to control treatment (no vaccination) than in those allocated to PCV, which may have introduced (detection) bias | |
| Re‐analysis of the Eskola 2001 study without new outcome data that could be used for our review | |
| RCT studying the effect of PCV on OME | |
| RCT studying the effect of PCV on suppurative otitis media (abstract of conference meeting) |
AOM: acute otitis media
ENT: ear, nose and throat
OME: otitis media with effusion
PCV: pneumococcal conjugate vaccine
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | 'COMPAS: Phase III, Double‐blind, Randomized Study to Demonstrate Efficacy of GSK Biologicals' Pneumococcal Conjugate Vaccine (GSK1024850A) Against Community Acquired Pneumonia and Acute Otitis Media (AOM)' |
| Methods | Phase III, double‐blind, randomised study |
| Participants | Healthy children aged 6 to 16 weeks |
| Interventions | Group A: pneumococcal conjugate vaccine GSK1024850A 4 doses, hepatitis A vaccine 2 doses, DTaP‐IPV/Hib vaccine 1 dose Group B: hepatitis A vaccine 3 doses, hepatitis B vaccine 3 doses, DTaP‐IPV/Hib vaccine 4 doses |
| Outcomes | Primary outcome: occurrence of likely bacterial community‐acquired pneumonia (CAP) cases |
| Starting date | June 2007; study complete date: June 2011 |
| Contact information | GSK Clinical Trials Call Centre |
| Notes | NCT00466947 |
| Trial name or title | 'Evaluation of Effectiveness of GSK Biologicals' Pneumococcal Conjugate Vaccine 1024850A Against Invasive Disease (FinIP)' |
| Methods | Cluster‐randomised, double‐blind trial |
| Participants | Healthy children aged younger than 19 months |
| Interventions | Infants aged younger than 7 months at the first vaccination received either a 3+1 or a 2+1 vaccination schedule, children aged 7 to 11 months received a 2+1 schedule and those 12 to 18 months of age received a 2‐dose schedule. Children received PD‐CV10 in 52 clusters or hepatitis vaccines as control in 26 clusters |
| Outcomes | Primary outcome: occurrence of culture‐confirmed pneumococcal invasive diseases due to any of the vaccine‐related pneumococcal serotypes |
| Starting date | March 2009; study complete date: January 2012 |
| Contact information | GSK Clinical Trials Call Centre |
| Notes | NCT00861380; published papers: effect PD‐CV10 on primary outcome (Palmu 2013a) and secondary outcome, i.e. outpatient antimicrobial purchases (Palmu 2013b) |
| Trial name or title | 'A Randomised Controlled Trial of Pneumococcal Conjugate Vaccines Synflorix and Prevenar13 in Sequence or Alone in High‐risk Indigenous Infants (PREV‐IX_COMBO): Immunogenicity, Carriage and Otitis Media Outcomes' |
| Methods | Open‐label, randomised study |
| Participants | Indigenous infants 4 to 6 weeks of age |
| Interventions | 3 doses of either PCV13 or PD‐CV10 versus an early schedule of a combination of 3 doses of PD‐CV10 and 1 dose of PCV13 |
| Outcomes | Primary outcome: immunogenicity |
| Starting date | August 2011 |
| Contact information | Amanda J Leach, PhD; Menzies School of Health Research, Darwin, Northern Territory, Australia, 0810 |
| Notes | NCT01174849 |
| Trial name or title | 'Evaluation of a Vaccine for Reducing Ear and Lung Infections in Children: Study to Determine Protective Efficacy Against Otitis Media and Assess Safety of an Investigational Pneumococcal Vaccine 2189242A in Healthy Infants' |
| Methods | Double‐blind, placebo‐controlled, randomised study |
| Participants | A healthy American Indian infant between and including 6 and 12 weeks (42 to 90 days) of age at the time of the first vaccination |
| Interventions | GSK2189242A vaccine versus placebo co‐administration of PCV13 and Hib‐CV. Hib‐CV will be given as study vaccine for infants of the immuno/reacto subgroup; for the other infants, this vaccine will be given as part of the routine vaccination schedule |
| Outcomes | Primary outcome: occurrence of any clinical AOM episodes diagnosed and verified against American Academy of Pediatrics (AAP) criteria |
| Starting date | May 2012 |
| Contact information | GSK Clinical Trials Call Center |
| Notes | NCT01545375 |
| Trial name or title | 'Pneumococcal Conjugate Vaccine (PCV) Schedules for the Northern Territory (NT): Randomised Controlled Trial of Booster Vaccines to Broaden and Strengthen Protection From Invasive and Mucosal Infections' |
| Methods | Single‐blind (outcomes assessor), randomised study |
| Participants | Australian indigenous infants who were participants in PREV‐IX_COMBO trial of primary course pneumococcal conjugate vaccines, age at least 2 months post final dose of primary course |
| Interventions | PCV13 versus PD‐CV10 |
| Outcomes | Primary outcome: immune response |
| Starting date | December 2012 |
| Contact information | Amanda J Leach, PhD; Menzies School of Health Research, Darwin, Northern Territory, Australia, 0810 |
| Notes | NCT01735084 |
AAP: American Academy of Pediatrics
AOM: acute otitis media
DTaP‐IPV/Hib: diphtheria‐tetanus toxoid‐acellular pertussis‐inactivated polio‐haemophilus influenzae type B vaccine
Hib‐CV: haemophilus influenzae type B conjugate vaccine

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
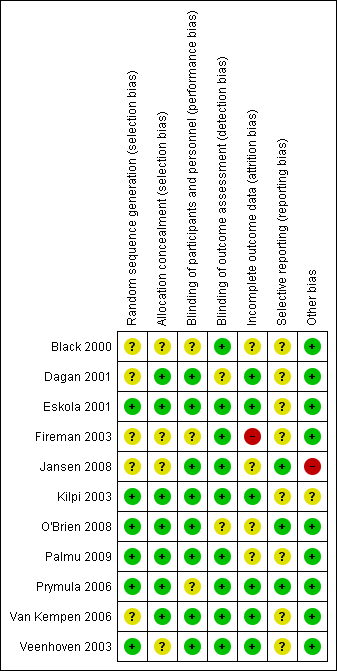
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Pneumococcal conjugate vaccine compared with control intervention for preventing acute otitis media | ||||
| Patient or population: children aged 12 years or younger and a follow‐up after vaccinations of at least 6 months Settings: open population Intervention: multivalent PCVs Comparison: control treatment | ||||
| Outcomes | VE ‐ relative effect (95% CI)* | No of participants | Quality of the evidence | Comments |
| Frequency of all‐cause AOM PCV7 administered in early infancy Follow‐up 6 to 42 months | RRR: ‐5% to 7% | 42,140 (4) | ⊕⊕⊕⊕ | Results are derived from 1 very large trial (Black 2000/Fireman 2003) and 3 trials of approximately equal size (944 to 1666 participants) (Eskola 2001; Kilpi 2003; O'Brien 2008) Lowest efficacy was found in high‐risk children (O'Brien 2008) |
| Frequency of all‐cause AOM PD‐PCV11 administered in early infancy Follow‐up 27 months | RRR 34% (21 to 44) | 4968 (1) | ⊕⊕⊕⊝ | Results derived from 1 high‐quality trial (Prymula 2006) Part of the effect may be related to the protein D to which the polysaccharides are conjugated in the vaccine PD‐PCV11, demonstrated to reduce non‐typeable H. influenzae by 35% (95% CI 2 to 57) AOM incidence rate in control group was low compared to the other studies on the effect on PCV7 in infants and the absolute risk difference was small (Table 1) |
| Frequency of all‐cause AOM CRM197‐PCV9 administered in healthy toddlers Follow‐up 24 months | RRR 17% (‐2 to 33) | 264 (1) | ⊕⊕⊕⊝ | Results derived from 1 trial of moderate methodological quality (Dagan 2001). Uncertainty about the effect size (statistically non‐significant effect) and outcome measure (parent‐reported OM) |
| Frequency of all‐cause AOM PCV7 administered in older children with a known history of AOM Follow‐up 6 to 26 months | RRR ‐29% to 57% | 1054 (3) | ⊕⊕⊕⊕ | Results are derived from 2 high‐quality trials (Veenhoven 2003; Van Kempen 2006) and 1 trial of moderate methodological quality (Jansen 2008). The 2 high‐quality trials found no beneficial effect of PCV in preventing AOM recurrences, while the other trial found PCV7/TIV not to be superior to TIV/placebo in preventing AOM during the influenza season |
| Frequency of pneumococcal AOM PCV7 administered in early infancy Follow‐up 6 to 42 months | RRR 20% to 34% | 1233 (2) | ⊕⊕⊕⊕ | Results are derived from 2 high‐quality trials (Eskola 2001/Palmu 2009; Kilpi 2003) |
| Frequency of pneumococcal AOM PD‐PCV11 administered in early infancy Follow‐up 27 months | RRR 52% (37 to 63) | 281 (1) | ⊕⊕⊕⊝ | Results derived from 1 high‐quality trial in which myringotomy was performed in all children (Prymula 2006) |
| GRADE Working Group grades of evidence *Results include both ITT and PP results; 95% CI lacking in case of multiple studies (range of effect estimates presented as we refrained from pooling). | ||||
| AOM: acute otitis media | ||||
| Intention‐to‐treat | Per‐protocol | ||||||
| Episodes/person year | VE expressed as relative reduction in risk (95% CI) | Episodes/person year | Incidence rate difference ‐ episodes per person year (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||
| Treatment | Control | Treatment | Control | ||||
| PCV administered in early infancy | |||||||
| ‐ ‐ | ‐ ‐ | 6% (4 to 9) 6% (4 to 8) | ‐ ‐ | ‐ ‐ | ‐ ‐ | 7% (4 to 10) 7% (4 to 9) | |
| ‐ | ‐ | ‐ | 1.16 | 1.24 | ‐0.08 ˜ | 6% (‐4 to 16) | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐1% (‐12 to 10) | |
| ‐ | ‐ | ‐ | 0.08 | 0.13 | ‐0.04 ˜ | 34% (21 to 44) | |
| 1.4 | 1.4 | ‐5% (‐25 to 12)# | 1.3 | 1.3 | 0.0 (‐0.13 to 0.14) | 0% (‐21 to 17) | |
| PCV administered at a later age | |||||||
| ‐ | ‐ | ‐ | 0.66 | 0.79 | ‐0.14 (‐0.29 to 0.02) | 17% (‐2 to 33) | |
| ‐ | ‐ | ‐25% (‐57 to 1) | 1.1 | 0.83 | 0.27 ˜ | ‐29% (‐62 to ‐2) | |
| ‐ | ‐ | ‐ | 0.78 | 0.67 | 0.11 ˜ | ‐16% (‐96 to 31) | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 57% (6 to 80)^ | |
| CI: confidence interval; HBV: hepatitis B vaccine; PCV: pneumococcal conjugate vaccine; TIV: trivalent influenza vaccine; VE: vaccine efficacy. *Cluster‐randomised trial. ˜ 95% CI could not be calculated as person‐time across treatment groups was not reported. Note: negative values for VE expressed as relative reduction in risk represent an increase in the risk for AOM. | |||||||
| Intention‐to‐treat | Per‐protocol | |||||||
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||||||
| Pneumococcal AOM | Vaccine‐type AOM | Cross‐reactive type AOM | Non‐vaccine‐type AOM | Pneumococcal AOM | Vaccine‐type AOM | Cross‐reactive type AOM | Non‐vaccine‐type AOM | |
| PCV administered in infancy | ||||||||
| ‐ ‐ | 65% P = 0.04 ‐ | ‐ ‐ | ‐ ‐ | ‐ ‐ | 67% P = 0.08 ‐ | ‐ ‐ | ‐ ‐ | |
| ‐ ‐ | 54% (41 to 64) ‐ | ‐ ‐ | ‐ ‐ | 34% (21 to 45) 20% (7 to 31) | 57% (44 to 67) ‐ | 51% (27 to 67) ‐ | ‐33% (‐80 to 1) ‐ | |
| ‐ | ‐ | ‐ | ‐ | 25% (11 to 37) | 56% (44 to 66) | ‐5% (‐47 to 25) | ‐27% (‐70 to 6) | |
| ‐ | ‐ | ‐ | ‐ | 52% (37 to 63) | 58% (41 to 69) | 66% (22 to 85) | 9% (‐64 to 49) | |
| O'Brien 2008* # | ‐ | 64% (‐34 to 90) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| PCV administered at a later age | ||||||||
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | 34% P = 0.22 | 52% P = 0.21 | ‐ | 21% P = 0.44 | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| VE: vaccine efficacy; PCV: pneumococcal conjugate vaccine; MEF: middle ear fluid. | ||||||||
| Intention‐to‐treat | Per‐protocol | |
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |
| PCV administered in infancy | ||
| 9% (4 to 14) 10% (7 to 13) | 9% (3 to 15) ‐ | |
| 9% (‐12 to 27) | 16% (‐6 to 35) | |
| ‐ | ‐ | |
| ‐ | 56% (‐2 to 81) | |
| ‐ | ‐ | |
| PCV administered at a later age | ||
| ‐ | ‐ | |
| ‐ | ‐ | |
| ‐ | ‐ | |
| ‐ | ‐ | |
| PCV: pneumococcal conjugate vaccine; VE: vaccine efficacy. | ||

