Vacunas antineumocócicas conjugadas para la prevención de la otitis media
Información
- DOI:
- https://doi.org/10.1002/14651858.CD001480.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 02 abril 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
ACF, RPV co‐ordinated the review.
ACF, RPV, CWB were involved in data collection.
ACF and RPV performed 'Risk of bias' assessment and analysis of data.
All review authors (ACF, ES, EH, RPV, CWB, AS, RD) were involved in designing and writing the review and interpreting the data.
Sources of support
Internal sources
-
Department of Pediatric Immunology and Infectious Diseases, UMC Utrecht, Wilhelmina Children's Hospital Utrecht, Netherlands.
-
Julius Center for Health Sciences and Primary Care, UMC Utrecht, the Netherlands, Netherlands.
-
Department of Otorhinolaryngology, UMC Utrecht, Wilhelmina Children's Hospital Utrecht, Netherlands.
-
University Center for Pharmacy, PharmacoEpidemiology & PharmacoEconomics, University of Groningen, Netherlands.
External sources
-
No sources of support supplied
Declarations of interest
During the initial phase of the review ACF was employed by the Medical Affairs department of GlaxoSmithKline B.V., the Netherlands. Currently ACF is an employee of the Bacterial Vaccines Discovery and Early Development group at Crucell. Neither company was involved in any aspect of the submitted work.
AS: My team at UCL is supported by an NIHR Research Professorship award to establish an infrastructure and programme of clinical trials in ENT, Hearing and Balance. My institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
EH: I have authored a paper on the design of the CAPITA study (Neth J Med), but have not been involved in the actual conduct of that study, nor does it pose a conflict of interest to the current work.
ES: For research on pneumococcal vaccines, carriage and surveillance studies, I received money paid by the institution, by governmental agencies and by pharmaceutical companies GSK and Pfizer and paid to the institution or collaborating institutions. Furthermore, I participate in Independent Data Monitoring Committees and Advisory Boards for pharmaceutical companies for vaccine studies and/or respiratory tract infections with fees paid to the institution. In general, all fees are always paid to the institution and used for research purposes.
RPV, CWB and RD declare no conflicts of interest in the current work.
Acknowledgements
The authors are indebted to Reinier Veenhoven (in memoriam) for the invaluable collaboration and his contributions to prior versions of the review.
We would like to thank Angelique Jansen, Masja Straetemans and Gerhard Zielhuis for their contributions to prior versions of this review. We would also like to thank the following people for commenting on previous updates of this review: Barbara Loe Fisher, Morio Aihara, Mark Jones and Paul Glasziou.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Nov 24 | Pneumococcal conjugate vaccines for preventing acute otitis media in children | Review | Joline LH Sévaux, Roderick P Venekamp, Vittoria Lutje, Eelko Hak, Anne GM Schilder, Elisabeth AM Sanders, Roger AMJ Damoiseaux | |
| 2019 May 28 | Pneumococcal conjugate vaccines for preventing acute otitis media in children | Review | Alexandre C Fortanier, Roderick P Venekamp, Chantal WB Boonacker, Eelko Hak, Anne GM Schilder, Elisabeth AM Sanders, Roger AMJ Damoiseaux | |
| 2014 Apr 02 | Pneumococcal conjugate vaccines for preventing otitis media | Review | Alexandre C Fortanier, Roderick P Venekamp, Chantal WB Boonacker, Eelko Hak, Anne GM Schilder, Elisabeth AM Sanders, Roger AMJ Damoiseaux | |
| 2009 Apr 15 | Pneumococcal conjugate vaccines for preventing otitis media | Review | Angelique GSC Jansen, Eelko Hak, Reinier H Veenhoven, Roger AMJ Damoiseaux, Anne GM Schilder, Elisabeth AM Sanders | |
| 2004 Jan 26 | Pneumococcal vaccines for preventing otitis media | Review | Masja M Straetemans, Elisabeth AM Sanders, Reinier H Veenhoven, Anne GM Schilder, Roger AMJ Damoiseaux, Gerhard GA Zielhuis | |
| 2002 Feb 15 | Pneumococcal vaccines for preventing otitis media | Review | M Straetemans, E AM Sanders, Reinier RH Veenhoven, Anne AGM Schilder, Roger RAMJ Damoiseaux, Gerhard GZ Zielhuis, Masja M Straetemans MSc, Lieke EAM Sanders | |
Differences between protocol and review
None.
Notes
The focus in research has shifted from the use of pneumococcal polysaccharide vaccines (PPVs) to pneumococcal conjugate vaccines (PCVs) in children and the role of PPVs in the prevention of AOM in children has merely been assessed following PCVs and no longer as a primary intervention. Therefore, the focus of the current review has shifted from the effect of PPVs to the effect of PCVs on acute otitis media. No further attention will be paid to the effect of PPVs, which were described in prior versions of this review (Straetemans 2003).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Disease;
- Age Factors;
- Heptavalent Pneumococcal Conjugate Vaccine [adverse effects, therapeutic use];
- Otitis Media [microbiology, *prevention & control];
- Otitis Media with Effusion [drug therapy];
- *Pneumococcal Vaccines [adverse effects, therapeutic use];
- Randomized Controlled Trials as Topic;
- Vaccines, Conjugate [adverse effects, therapeutic use];
Medical Subject Headings Check Words
Child; Child, Preschool; Female; Humans; Infant; Male;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
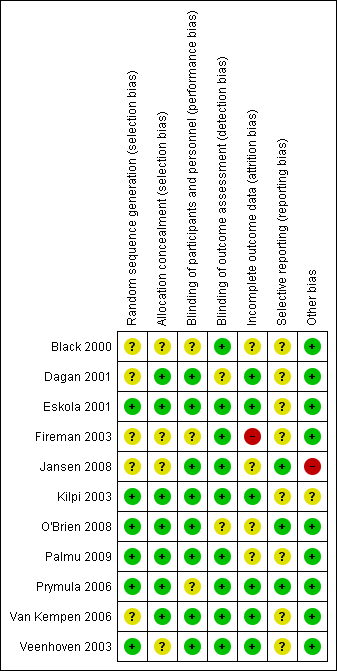
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Pneumococcal conjugate vaccine compared with control intervention for preventing acute otitis media | ||||
| Patient or population: children aged 12 years or younger and a follow‐up after vaccinations of at least 6 months Settings: open population Intervention: multivalent PCVs Comparison: control treatment | ||||
| Outcomes | VE ‐ relative effect (95% CI)* | No of participants | Quality of the evidence | Comments |
| Frequency of all‐cause AOM PCV7 administered in early infancy Follow‐up 6 to 42 months | RRR: ‐5% to 7% | 42,140 (4) | ⊕⊕⊕⊕ | Results are derived from 1 very large trial (Black 2000/Fireman 2003) and 3 trials of approximately equal size (944 to 1666 participants) (Eskola 2001; Kilpi 2003; O'Brien 2008) Lowest efficacy was found in high‐risk children (O'Brien 2008) |
| Frequency of all‐cause AOM PD‐PCV11 administered in early infancy Follow‐up 27 months | RRR 34% (21 to 44) | 4968 (1) | ⊕⊕⊕⊝ | Results derived from 1 high‐quality trial (Prymula 2006) Part of the effect may be related to the protein D to which the polysaccharides are conjugated in the vaccine PD‐PCV11, demonstrated to reduce non‐typeable H. influenzae by 35% (95% CI 2 to 57) AOM incidence rate in control group was low compared to the other studies on the effect on PCV7 in infants and the absolute risk difference was small (Table 1) |
| Frequency of all‐cause AOM CRM197‐PCV9 administered in healthy toddlers Follow‐up 24 months | RRR 17% (‐2 to 33) | 264 (1) | ⊕⊕⊕⊝ | Results derived from 1 trial of moderate methodological quality (Dagan 2001). Uncertainty about the effect size (statistically non‐significant effect) and outcome measure (parent‐reported OM) |
| Frequency of all‐cause AOM PCV7 administered in older children with a known history of AOM Follow‐up 6 to 26 months | RRR ‐29% to 57% | 1054 (3) | ⊕⊕⊕⊕ | Results are derived from 2 high‐quality trials (Veenhoven 2003; Van Kempen 2006) and 1 trial of moderate methodological quality (Jansen 2008). The 2 high‐quality trials found no beneficial effect of PCV in preventing AOM recurrences, while the other trial found PCV7/TIV not to be superior to TIV/placebo in preventing AOM during the influenza season |
| Frequency of pneumococcal AOM PCV7 administered in early infancy Follow‐up 6 to 42 months | RRR 20% to 34% | 1233 (2) | ⊕⊕⊕⊕ | Results are derived from 2 high‐quality trials (Eskola 2001/Palmu 2009; Kilpi 2003) |
| Frequency of pneumococcal AOM PD‐PCV11 administered in early infancy Follow‐up 27 months | RRR 52% (37 to 63) | 281 (1) | ⊕⊕⊕⊝ | Results derived from 1 high‐quality trial in which myringotomy was performed in all children (Prymula 2006) |
| GRADE Working Group grades of evidence *Results include both ITT and PP results; 95% CI lacking in case of multiple studies (range of effect estimates presented as we refrained from pooling). | ||||
| AOM: acute otitis media | ||||
| Intention‐to‐treat | Per‐protocol | ||||||
| Episodes/person year | VE expressed as relative reduction in risk (95% CI) | Episodes/person year | Incidence rate difference ‐ episodes per person year (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||
| Treatment | Control | Treatment | Control | ||||
| PCV administered in early infancy | |||||||
| ‐ ‐ | ‐ ‐ | 6% (4 to 9) 6% (4 to 8) | ‐ ‐ | ‐ ‐ | ‐ ‐ | 7% (4 to 10) 7% (4 to 9) | |
| ‐ | ‐ | ‐ | 1.16 | 1.24 | ‐0.08 ˜ | 6% (‐4 to 16) | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐1% (‐12 to 10) | |
| ‐ | ‐ | ‐ | 0.08 | 0.13 | ‐0.04 ˜ | 34% (21 to 44) | |
| 1.4 | 1.4 | ‐5% (‐25 to 12)# | 1.3 | 1.3 | 0.0 (‐0.13 to 0.14) | 0% (‐21 to 17) | |
| PCV administered at a later age | |||||||
| ‐ | ‐ | ‐ | 0.66 | 0.79 | ‐0.14 (‐0.29 to 0.02) | 17% (‐2 to 33) | |
| ‐ | ‐ | ‐25% (‐57 to 1) | 1.1 | 0.83 | 0.27 ˜ | ‐29% (‐62 to ‐2) | |
| ‐ | ‐ | ‐ | 0.78 | 0.67 | 0.11 ˜ | ‐16% (‐96 to 31) | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 57% (6 to 80)^ | |
| CI: confidence interval; HBV: hepatitis B vaccine; PCV: pneumococcal conjugate vaccine; TIV: trivalent influenza vaccine; VE: vaccine efficacy. *Cluster‐randomised trial. ˜ 95% CI could not be calculated as person‐time across treatment groups was not reported. Note: negative values for VE expressed as relative reduction in risk represent an increase in the risk for AOM. | |||||||
| Intention‐to‐treat | Per‐protocol | |||||||
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||||||
| Pneumococcal AOM | Vaccine‐type AOM | Cross‐reactive type AOM | Non‐vaccine‐type AOM | Pneumococcal AOM | Vaccine‐type AOM | Cross‐reactive type AOM | Non‐vaccine‐type AOM | |
| PCV administered in infancy | ||||||||
| ‐ ‐ | 65% P = 0.04 ‐ | ‐ ‐ | ‐ ‐ | ‐ ‐ | 67% P = 0.08 ‐ | ‐ ‐ | ‐ ‐ | |
| ‐ ‐ | 54% (41 to 64) ‐ | ‐ ‐ | ‐ ‐ | 34% (21 to 45) 20% (7 to 31) | 57% (44 to 67) ‐ | 51% (27 to 67) ‐ | ‐33% (‐80 to 1) ‐ | |
| ‐ | ‐ | ‐ | ‐ | 25% (11 to 37) | 56% (44 to 66) | ‐5% (‐47 to 25) | ‐27% (‐70 to 6) | |
| ‐ | ‐ | ‐ | ‐ | 52% (37 to 63) | 58% (41 to 69) | 66% (22 to 85) | 9% (‐64 to 49) | |
| O'Brien 2008* # | ‐ | 64% (‐34 to 90) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| PCV administered at a later age | ||||||||
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | 34% P = 0.22 | 52% P = 0.21 | ‐ | 21% P = 0.44 | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| VE: vaccine efficacy; PCV: pneumococcal conjugate vaccine; MEF: middle ear fluid. | ||||||||
| Intention‐to‐treat | Per‐protocol | |
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |
| PCV administered in infancy | ||
| 9% (4 to 14) 10% (7 to 13) | 9% (3 to 15) ‐ | |
| 9% (‐12 to 27) | 16% (‐6 to 35) | |
| ‐ | ‐ | |
| ‐ | 56% (‐2 to 81) | |
| ‐ | ‐ | |
| PCV administered at a later age | ||
| ‐ | ‐ | |
| ‐ | ‐ | |
| ‐ | ‐ | |
| ‐ | ‐ | |
| PCV: pneumococcal conjugate vaccine; VE: vaccine efficacy. | ||

