Zonisamide add‐on for drug‐resistant partial epilepsy
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized double blind placebo controlled parallel group study. | |
| Participants | Multicentre study, 49 centres in Europe and 5 in South Africa | |
| Interventions | Placebo, 100 mg, 300 mg or 500 mg placebo, randomized in 2:1:1:2 ratio. | |
| Outcomes | Reduction in seizure frequency, proportion with a 50% or greater reduction in seizure frequency. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized double blind placebo controlled parallel group study. 2 treatment arms: 1 placebo and 1 zonisamide. | |
| Participants | Multi‐centre (20) US study. | |
| Interventions | Zonisamide 400 mg/day or placebo (weeks 8‐12). | |
| Outcomes | Primary: median percentage reduction from baseline of all partial seizures. | |
| Notes | Of the randomized participants 8 failed to complete week 5 in the placebo group, 15 in the zonisamide group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized double blind placebo controlled parallel group study. | |
| Participants | Participants from 10 European centres recruited between June 1984 and October 1986. | |
| Interventions | Zonisamide median dosage 400 mg/day (100 mg capsules) | |
| Outcomes | Primary: median percentage reduction in seizure frequency of all partial seizures from baseline. | |
| Notes | Because of the variable baseline periods, baseline seizure frequency was recalculated for the 8 weeks immediately before entry into the treatment period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized double blind placebo controlled parallel group study. | |
| Participants | Conducted at 4 US centres between August 1983 and July 1986. | |
| Interventions | Zonisamide median dosage 400 mg/day (100 mg capsules) | |
| Outcomes | Primary: median percentage reduction in seizure frequency of all partial seizures from baseline. | |
| Notes | Because of the variable baseline periods, baseline seizure frequency was recalculated for the 8 weeks immediately before entry into the treatment period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
AED: antiepileptic drug
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This trial did not compare zonisamide with placebo. The comparison was with valproate. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% responder rate ‐ post‐titration Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Zonisamide versus placebo, Outcome 1 50% responder rate ‐ post‐titration. | ||||
| 1.1 Any dose | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.51, 2.59] |
| 1.2 300 ‐ 500 mg zonisamide | 4 | 793 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.55, 2.67] |
| 2 50% responder rate ‐ whole treatment period Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Zonisamide versus placebo, Outcome 2 50% responder rate ‐ whole treatment period. | ||||
| 2.1 Any dose | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.74, 3.17] |
| 2.2 300 ‐ 500 mg zonisamide | 4 | 793 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.81, 3.30] |
| 3 50% responder rate ‐ best case Show forest plot | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.99, 3.31] |
| Analysis 1.3  Comparison 1 Zonisamide versus placebo, Outcome 3 50% responder rate ‐ best case. | ||||
| 4 50% responder rate ‐ worst case Show forest plot | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.14, 1.81] |
| Analysis 1.4  Comparison 1 Zonisamide versus placebo, Outcome 4 50% responder rate ‐ worst case. | ||||
| 5 50% responder rate ‐ dose effect Brodie 2005 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Zonisamide versus placebo, Outcome 5 50% responder rate ‐ dose effect Brodie 2005. | ||||
| 5.1 100 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 300 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 500 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 50% responder rate ‐ dose effect Faught 2001 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Zonisamide versus placebo, Outcome 6 50% responder rate ‐ dose effect Faught 2001. | ||||
| 6.1 100 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 200 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 400 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Withdrawal rates Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Zonisamide versus placebo, Outcome 7 Withdrawal rates. | ||||
| 7.1 Any dose | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.07, 2.02] |
| 7.2 300 ‐ 500 mg zonisamide | 4 | 793 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.20, 2.26] |
| 8 Adverse events Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Zonisamide versus placebo, Outcome 8 Adverse events. | ||||
| 8.1 Ataxia | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.50 [1.05, 19.22] |
| 8.2 Dizziness | 4 | 850 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.77 [1.00, 3.12] |
| 8.3 Nausea | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.50 [0.69, 3.28] |
| 8.4 Fatigue | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.32 [0.69, 2.50] |
| 8.5 Somnolence | 4 | 850 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.96 [1.12, 3.44] |
| 8.6 Agitation/irritability | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.37 [1.00, 5.64] |
| 8.7 Anorexia | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.00 [1.31, 6.88] |

Comparison 1 Zonisamide versus placebo, Outcome 1 50% responder rate ‐ post‐titration.

Comparison 1 Zonisamide versus placebo, Outcome 2 50% responder rate ‐ whole treatment period.
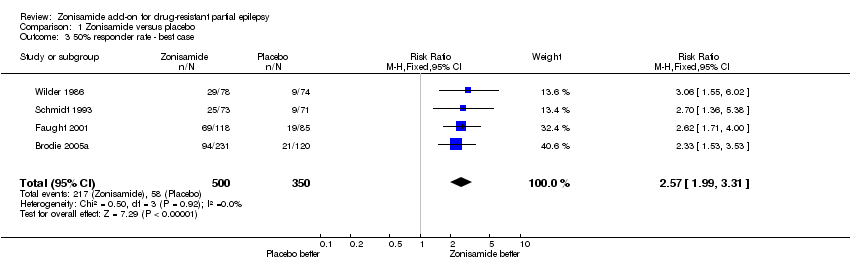
Comparison 1 Zonisamide versus placebo, Outcome 3 50% responder rate ‐ best case.
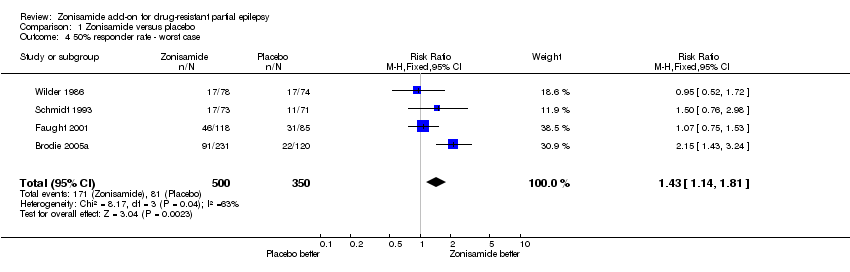
Comparison 1 Zonisamide versus placebo, Outcome 4 50% responder rate ‐ worst case.
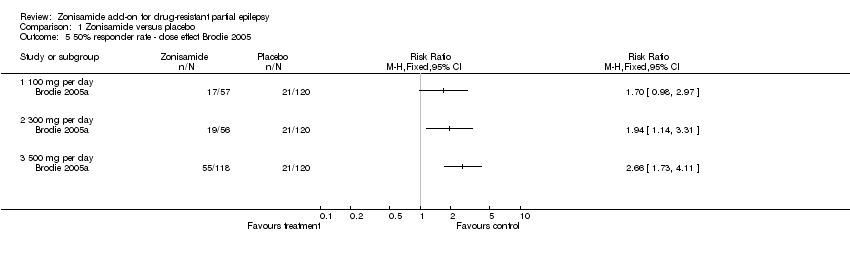
Comparison 1 Zonisamide versus placebo, Outcome 5 50% responder rate ‐ dose effect Brodie 2005.

Comparison 1 Zonisamide versus placebo, Outcome 6 50% responder rate ‐ dose effect Faught 2001.

Comparison 1 Zonisamide versus placebo, Outcome 7 Withdrawal rates.

Comparison 1 Zonisamide versus placebo, Outcome 8 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% responder rate ‐ post‐titration Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Any dose | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.51, 2.59] |
| 1.2 300 ‐ 500 mg zonisamide | 4 | 793 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.55, 2.67] |
| 2 50% responder rate ‐ whole treatment period Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any dose | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.74, 3.17] |
| 2.2 300 ‐ 500 mg zonisamide | 4 | 793 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.81, 3.30] |
| 3 50% responder rate ‐ best case Show forest plot | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.99, 3.31] |
| 4 50% responder rate ‐ worst case Show forest plot | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.14, 1.81] |
| 5 50% responder rate ‐ dose effect Brodie 2005 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 100 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 300 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 500 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 50% responder rate ‐ dose effect Faught 2001 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 100 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 200 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 400 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Withdrawal rates Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Any dose | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.07, 2.02] |
| 7.2 300 ‐ 500 mg zonisamide | 4 | 793 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.20, 2.26] |
| 8 Adverse events Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 8.1 Ataxia | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.50 [1.05, 19.22] |
| 8.2 Dizziness | 4 | 850 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.77 [1.00, 3.12] |
| 8.3 Nausea | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.50 [0.69, 3.28] |
| 8.4 Fatigue | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.32 [0.69, 2.50] |
| 8.5 Somnolence | 4 | 850 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.96 [1.12, 3.44] |
| 8.6 Agitation/irritability | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.37 [1.00, 5.64] |
| 8.7 Anorexia | 3 | 499 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.00 [1.31, 6.88] |

