Oestrogen therapy for urinary incontinence in post‐menopausal women
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT (double blind crossover) | |
| Participants | 29 women | |
| Interventions | group A (n=29): 4mg oestriol + 50mg PPA twice daily for 6/52 | |
| Outcomes | urodynamics, urinary diary, subjective assessment, assessment of atrophy, urethral + vaginal cytology, feeling of vaginal dryness, pad test | |
| Notes | no useable data; adverse events: group 1: 1 sweating, 1 constipation, 1 insomnia; group 2: 1 gastritis, 1 paraesthesia, 1 tachycardic attack, 1 dizziness, 2 nausea, 1 vomiting, 1 constipation; losses to follow up: group 1:1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomization was made in groups of 4" |
| Allocation concealment? | Unclear risk | no description |
| Blinding? | Low risk | double blind |
| Incomplete outcome data addressed? | Low risk | "all 29 completed, one woman withdrawn from analyses of second treatment period due to failure in medication" |
| Methods | randomised open comparative crossover trial; duration of study: 12/52; assessment after each 4/52 period of treatment | |
| Participants | 20 postmenopausal women | |
| Interventions | group A (n=20): 1mg oestriol vaginally daily for 4/52, then 50mg phenylpropanolamine (PPA) orally twice daily for 4/52, then combined for 4/52 | |
| Outcomes | subjective assessment of treatment result, urodynamics, atrophy evaluation by clinical picture, cytological investigation by urethral smear | |
| Notes | adverse events: 1 genital bleeding in period of combined treatment, 1 complete insomnia after 3 days with PPA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? | High risk | "randomised open comparative cross‐over trial" |
| Incomplete outcome data addressed? | Low risk | 18 out of 20 completed the study. |
| Methods | placebo controlled, single‐blind randomised crossover study | |
| Participants | 16 women, | |
| Interventions | crossover study | |
| Outcomes | urinary diary, cystometry | |
| Notes | no useable data; adverse events: skin reactions to transdermal system: mild erythema and itching, 2 patients had more severe cutaneous reactions, but continued with treatment, systemic adverse effects: mild breast tenderness, 1 patient with uterus experienced spotting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? | High risk | open‐label single blind (patients blinded?) |
| Incomplete outcome data addressed? | Low risk | 16 out of 19 completed. "Drop out not related to study medication" |
| Methods | multicentre study, 6 centres (10 participated, but 4 did not recruit any patients), countries: UK, NL, D, DK (London, Bristol, Maastricht, Munich, Aalborg, Cheltenham) | |
| Participants | 64 postmenopausal women with urodynamically confirmed urgency urinary incontinence | |
| Interventions | group A (n=34): oestriol orally 3 mg for 3/12 | |
| Outcomes | subjective assessment with doctor‐administered questionnaire concerning presence or absence of specific urinary symptoms (24 questions) and 4‐point severity score scale (9 questions), objective assessment with 3‐day urinary diary, filling and voiding cystometry, urethral pressure profile where available, vaginal smear for maturation index calculated by single pathologist; Number not cured: urgency urinary incontinence: A, 14/25; B, 16/23; stress incontinence: A, 5/11; B, 4/10 | |
| Notes | urgency incontinence stratified into motor and sensory urgency incontinence, motor urgency incontinence defined as uninhibited detrusor contractions exceeding 15 cm of water, sensory urgency incontinence defined as first desire to void during filling cystometry at less than 150 ml and a cystometric capacity of 400 ml in absence of detrusor activity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | A ‐ Adequate "Randomisation code was held centrally" |
| Blinding? | Low risk | "double blind" |
| Incomplete outcome data addressed? | Low risk | 8 out of 64 women did not complete study (3 reason unknown,1 loss of motivation, 1 lack of efficacy, 1 UTI, 1 exclusion criteria, 1 did not start. Equal dropouts between groups |
| Methods | double‐blind placebo‐controlled RCT | |
| Participants | 105 post‐menopausal women, | |
| Interventions | group A: 17B‐oestradiol vaginal pellet (Vagifem) 25 µgm at night for 12/52 | |
| Outcomes | questionnaire and frequency/volume chart, urethral brushing for cytology, compliance check regarding tablet usage, urodynamics, MSU, FSH, LH, oestradiol, endometrial biopsy, visual analogue score, symptom assessment sheet, cystometry data sheet | |
| Notes | no useable data; no numbers of women in groups; no adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear no description |
| Blinding? | Low risk | double blind |
| Incomplete outcome data addressed? | High risk | In both groups three women failed to commence treatment. Outcome data on 106 out of 110 randomised |
| Methods | RCT; duration of study: 8/52, F/U at 2, 4, 8/52 patients not blinded | |
| Participants | 40 post‐menopausal women | |
| Interventions | group A (n=20): combined contraceptive pill vaginally at night, one tablet per week (250ng levonorgestrel + 30mg ethinyl oestradiol) | |
| Outcomes | diary card to record use of drugs, side effects, urogenital symptoms; vaginal pH, cultures, smears, MSU urgency at 8 weeks A 3/20 B 3/20 | |
| Notes | not blinded as patients received either a tube of cream or tablets, before treatment. Nearly all women had bacteria in their urine, none of the patients had a symptomatic urinary tract infection during the 8 week trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? | Unclear risk | no mention |
| Incomplete outcome data addressed? | Low risk | no dropouts |
| Methods | RCT: placebo controlled, sealed opaque envelopes | |
| Participants | 88 post‐menopausal women | |
| Interventions | group A (44): intravaginal oestriol ovules, 1 mg daily for 2 weeks, 2 mg weekly for 6 months | |
| Outcomes | Incontinent at 6 months: group A: 37/44, group B; 44/44 | |
| Notes | participants and outcome assessors blinded to treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | A ‐ Adequate "sequenced, sealed, opaque envelopes" |
| Blinding? | Low risk | double blind "participants and investigators were blinded to the drug being dispensed and to the assigned treatment group" |
| Incomplete outcome data addressed? | Low risk | no dropouts |
| Methods | RCT (double‐blind crossover in 5th and 6th weeks of the study); duration of study: 8/52, F/U at 8/52 | |
| Participants | 16 post‐menopausal women with urodynamically proven GSI | |
| Interventions | all women (n=16): oestradiol valerate 2mg daily for 3/52, then 1mg for 1/52 | |
| Outcomes | residual urine, MSU, urodynamics, clinical stress test, periurethral vaginal biopsies, subjective symptoms | |
| Notes | no useable data; adverse events: "few and acceptable", very small changes in BP, no uterine bleeding; | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT, duration of study 4/52 | |
| Participants | 35 women | |
| Interventions | group A (n=15): 0.5 or 1 mg oestriol vaginally | |
| Outcomes | urinary diary, cystoscopy, urodynamics, urethral and vaginal smear | |
| Notes | no endometrial stimulation or breast tenderness noted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | placebo‐controlled RCT, randomisation following randomisation scheme, duration of study: 4/52 | |
| Participants | 40 post‐menopausal women | |
| Interventions | group A (n=15): 1 mg oestriol vaginally for 3/52 | |
| Outcomes | cystometry, urethral pressure profile, cystoscopy, urethral smear, urinary diary, MSU, FSH levels, E2 levels | |
| Notes | adverse outcomes: breast tenderness in 2 patients in group 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | as Enzelsberger A | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | multicentre trial, 2 centres, double‐blind placebo‐controlled RCT, patients stratified by baseline severity of symptoms, urodynamics and treatment site; blocked randomisation within each stratum at each site; sequenced sealed opaque envelopes | |
| Participants | 83 post‐menopausal women with UI inclusion criteria: all patients ambulatory, community‐dwelling residents, age 45 or older, involuntary loss of urine at least once a week, GSI was diagnosed when urine was objectively seen to be lost during exertion in absence of DI; DI defined as involuntary detrusor contractions during retrograde subtracted provocative cystometry; hypooestrogenism = plasma E2 levels 30 pg/ml or less exclusion criteria: institutionalisation, permanent catheterisation, impaired mental status, functional disability limiting use of toilet, neuropathic or uncontrolled metabolic conditions (e.g. diabetes mellitus), chronic UTI, reversible causes of urinary incontinence (e.g. faecal impaction), major contraindications for use of oestrogens (e.g. breast cancer) | |
| Interventions | group A (n=39): conjugated equine oestrogens 0.625 mg for 30 days + medroxyprogesterone 10 mg for 10 days of each cycle | |
| Outcomes | Data recorded in standardised urinary diaries Primary outcome: number of incontinent episode per week, N, mean (SD): A 39, 10 (10), B 44 13 (14) cure (definition not given), results provided as patient's perception of somewhat or much better: A 54% (21/39), B 45% (20/44), P= 0.435 secondary outcomes: | |
| Notes | loss to follow up: 2, and 8 only had diary or QoL data. Groups not given, unclear if these are extra to the 83 with outcome data reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | blocked randomisation |
| Allocation concealment? | Low risk | A ‐ Adequate sequenced,sealed, opaque envelopes, each containing the bottle number to be given to an individual patient |
| Blinding? | Low risk | Participants, clinicians and pharmacists blind to drug being dispensed |
| Incomplete outcome data addressed? | Low risk | no dropouts |
| Methods | RCT, randomisation stratified by clinical centre, within‐strata treatment randomised in fixed size blocks Follow up at 4 months then annually for 4 years | |
| Participants | 1525 participants age less than 80, post menopausal, with intact uterus, and at least 1 episode of UI per week | |
| Interventions | group A (n=768) conjugated oestrogen (Premarin) 0.625mg + medroxyprogesterone acetate (Cycrin) 2.5mg | |
| Outcomes | Worse or unchanged at 1 year: A 525/716, B 479/715 Number of incontinent episodes: A 5.5 per week (increase of 0.7), B 5.6 per week (decrease of 0.1) | |
| Notes | compliance at 1st year: A 82%, B 88% still taking the treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation codes prepared prepared with computer generated random numbers. Stratified by site and performed using randomly permuted blocks of 4 |
| Allocation concealment? | Low risk | A ‐ Adequate. Eligible participants assigned with equal probability to the two groups by tamper proof randomization |
| Blinding? | Low risk | Participants and investigators blind to treatment |
| Methods | RCT, duration of study: 3/12, follow up at 3/12 and 9/12 | |
| Participants | 100 women; | |
| Interventions | group A (n=24): conjugated equine oestrogen vaginal cream (Premarin) 1.25 mg at night for 12/52 | |
| Outcomes | pad test, urethral pressure studies at 3/12, symptom questionnaire at 9/12 | |
| Notes | numbers given as "cured or improved" and "unchanged"; recurrences of symptoms at 9/12 in women who had initially improved: group A: 3, group B: 1, group C: 3 (recurrence of symptoms immediately after discontinuing oestrogen treatment) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "allocated at random" |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? | High risk | not possible, drug compared to PFMT |
| Incomplete outcome data addressed? | Unclear risk | 100 out of 104 evaluated no info on dropouts |
| Methods | RCT, duration of study: 6/52 | |
| Participants | 26 postmenopausal women; inclusion criteria: GSI | |
| Interventions | group A (n=11): conjugated equine oestrogen vaginal cream (Premarin) 2 g at night for 6/52 | |
| Outcomes | vaginal pH, vaginal cell count from smear, pad test, electromyographic traces to assess urethral sphincter activity; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? | High risk | not possible |
| Methods | RCT, 25,597 women | |
| Participants | RCT of hormone replacement for prevention of coronary heart disease and hip fracture, with and without symptoms of stress, urgency urinary or mixed incontinence at baseline Incontinent (wet) women (Populations 1 and 2) Continent (dry) women (Populations 3 and 4) All women on HRT treatment at baseline had to have 3 month washout | |
| Interventions | Population 1 (wet at baseline, without uterus): | |
| Outcomes | Population 1 (wet at baseline, without uterus) Population 2 (wet at baseline, with uterus) Worsening was not related to one type of urinary incontinence alone Population 3 (dry at baseline) Population 4 (dry at baseline) | |
| Notes | No data suitable for meta‐analysis were provided in the original report but these have been requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "The study pill bottles had unique bar codes and computer based selection to enable double blinded dispensing" |
| Allocation concealment? | Low risk | A ‐ Adequate "Randomization was performed using a study database distributed by the WHI Clinical Coordinating Center to the local centers" |
| Blinding? | Low risk | double blind, clinic staff and participants |
| Incomplete outcome data addressed? | Unclear risk | At 1 year vital status was known for 99.9% of participants in the oestrogen + progesterone trial and 100% for those in oestrogen only trial?? At 1 year 9.7% of women taking oestrogen + progesterone and 6.6% taking placebo stopped taking the pills. Oestrogen alone 8.4% and placebo 8% stopped taking the pills |
| Methods | RCT | |
| Participants | Wet at baseline | |
| Interventions | Population 1 (wet at baseline, without uterus): | |
| Outcomes | Population 1 (wet at baseline, without uterus) Worsening was not related to one type of urinary incontinence alone | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT | |
| Participants | Wet at baseline | |
| Interventions | Population 2 (wet at baseline, with uterus): | |
| Outcomes | Population 2 (wet at baseline, with uterus) Worsening was not related to one type of urinary incontinence alone | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | double‐blind RCT, patients were randomised into 6 groups; duration of study 4/52, evaluation on day 28 of study | |
| Participants | n= 60 women | |
| Interventions | A (10): Intravaginal oestrogen (2 gr nocturnal) + PPA (50 mg twice daily) | |
| Outcomes | Subjective improvement: A: 9/10, B: 1/9, C: 4/10 D: 2/10, E: 0/9, F: 2/11 | |
| Notes | Data estimated from graphs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear no description |
| Blinding? | Low risk | double blind |
| Methods | randomised controlled trial duration of study 2 years monthly uterine ultrasound examination and 6‐monthly endometrial PAP smears Setting: Japan | |
| Participants | 66 post‐menopausal women with stress urinary incontinence, age 54 to 75 years, groups comparable at baseline Dropouts: a further 6 by choice, 1 for adverse drug reaction hepatopathy): A, 4; B, 3 inclusion criteria: stress urinary incontinence alone (based on questionnaires) exclusion criteria: urgency or mixed urinary incontinence 10% of women had a previous hysterectomy | |
| Interventions | A (32): PFMT + oestriol tablet (1 mg/day) B (34): PFMT alone PFMT was taught by a gynaecology specialist, supplemented by videotape. The aim was 15 minutes of exercise a day | |
| Outcomes | Persisting incontinence: A: 7/32; B, 11/34 (Mild UI: A, 0/12; B, 2/11: Moderate UI: A, 3/14; B, 5/18: Severe UI: A, 4/6; B, 4/5) Adverse effects: A: 1/36; B, 0/37 No report of effect on uterus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | randomly assigned |
| Blinding? | High risk | |
| Incomplete outcome data addressed? | Low risk | 4 out of 36 withdrew from exercise and oestrogen group 3 out of 37 from exercise only group |
| Methods | double‐blind placebo‐controlled RCT, duration of study 6/12; no definition of cure given, but results of no demonstrable stress incontinence on repeat cystometry (urodynamically 'cured') | |
| Participants | 67 post‐menopausal women with urodynamically proven GSI; inclusion criteria: >12/12 post‐menopausal, not taken HRT in previous 12/12 exclusion criteria: cancer of endometrium, liver, breast; endometrial thickness > 4mm Included women who had had hysterectomy | |
| Interventions | group A (n=33): oestradiol 2 mg orally for 6/12 | |
| Outcomes | urinary diary for 1 week, SF‐36 + B‐FLUTS questionnaires, 1 hour perineal pad test, urodynamics, repeat trans‐vaginal sampling + Pipelle biopsy if endometrial thickness>6mm, compliance, serum oestradiol levels | |
| Notes | losses to follow‐up: 3 in group A, 2 in group B; 1 patient in each group left at 3/12 for surgery, both were reassessed and data are included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation sequence generated by computer in block sizes of 10 (5 oestradiol, 5 placebo) |
| Allocation concealment? | Low risk | A ‐ Adequate, randomised by hospital pharmacy |
| Blinding? | Low risk | women participants and care providers |
| Incomplete outcome data addressed? | Low risk | Three out of 33 on oestradiol failed to complete the trial, two out of 34 on placebo. One woman in each arm left trial at three months opting for surgery (included in the analysis) |
| Methods | multicentre trial, 2 hospitals; placebo‐controlled double‐blind crossover RCT, duration of study 5 weeks | |
| Participants | 20 post‐menopausal women, patients in two hospitals in geriatric long‐stay beds for at least 1 month, 10 patients confused, 1 demented, 8 mentally normal; 14 had neurological disease | |
| Interventions | group A (n=18): quinestradol 0.25 mg qds for 1/12 | |
| Outcomes | observed numbers of incontinent episodes requiring bed change per week | |
| Notes | no useable data; unclear if groups were treated identically as patients had neurological disease or confusion and lived in different geriatric hospitals; losses to follow up: 2 (from initial 20); patients too confused for subjective assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | a set of random numbers was used |
| Allocation concealment? | Low risk | A ‐ Adequate, hospital pharmacist provided with active ingredient labelled capsule X and capsule Y along with an emergency sealed key for emergency use |
| Blinding? | Low risk | double blind |
| Methods | RCT (randomised double blind crossover) | |
| Participants | 36 post‐menopausal women | |
| Interventions | group A (n=36): oestriol 2mg orally + placebo twice daily | |
| Outcomes | Subjective improvement: A: 9/30, B: 16/30 | |
| Notes | no useable data; adverse events: dryness of the mouth + ? arrhythmia, itch, depression; but not clear on which treatment losses to follow up: 6 (3 intercurrent diseases, 3 possible drug effects: arrhythmia, itch, depression) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Blinding? | High risk | crossover design |
| Methods | placebo‐controlled double‐blind RCT, duration of study: 4/52 | |
| Participants | 42 post‐menopausal women | |
| Interventions | group A (n=21): 1 mg oestriol in 10 ml sesame‐oil intravesically (into the bladder) every other day for 3/52 | |
| Outcomes | urinary diary, MSU, first urge to void, max. bladder capacity, functional urethral length, maximum urethral closure pressure, depression coefficient, cystometry, cystoscopy to assess bladder mucosa, vaginal and urethral smears, hormone levels of LH, FSH, oestradiol, SHBG, urinary diary, subjective measurement with visual analogue scale, assessment of burning at micturition, pain at micturition, bladder spasms, nocturia, frequency | |
| Notes | no adverse outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | open, randomised, multicentre, parallel‐group controlled trial with an active control, 26 gynaecologists clinics + 1 outpatient clinic at Danish county hospital; duration of study: 24/52 | |
| Participants | 251 post‐menopausal women | |
| Interventions | group A (n=134): oestradiol releasing vaginal ring, which constantly releases 7.5 mg oestradiol/24 hours, ring staying in situ for 12/52, then changed, in total treatment for 24/52 | |
| Outcomes | questionnaire with symptom assessment using visual analogue scale, gynaecological examination including atrophy and pH assessment, assessment of form of administration using 5‐point scale questionnaire, primary outcomes: | |
| Notes | losses to follow up: group A: 5, group B: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | A ‐ Adequate, central office using numbers sequentially. No number was to be omitted |
| Blinding? | High risk | open |
| Incomplete outcome data addressed? | Unclear risk | 254 randomised, three did not receive treatment 251 eligible for intention‐to‐treat analysis |
| Methods | treatment assigned in random fashion; duration of study 3/12 | |
| Participants | 50 post‐menopausal women exclusion criteria: hormone treatment less than 6/12 ago, illness or malignancy that contraindicated oestrogen treatment, BP>140/80, positive MSU | |
| Interventions | group A (n=25): oestriol vaginally 0.5 mg / day for 14/7, then alternate days for 3/12 in total | |
| Outcomes | Biochemical markers: azotamine, glucose, GPT, GOT, g‐GT, bilirubin, total cholesterol, triglycerides, HDL‐cholesterol, FSH, oestriol levels Every 15 days women were asked about side effects of treatment and filled in diary with intensity of symptoms | |
| Notes | no adverse events, no losses to follow up; study designed to look at vaginal changes rather than at incontinence, incontinence forms part of menopausal symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | double‐blind placebo‐controlled RCT, randomisation by table of random numbers, odd and even number envelopes were kept in pharmacy | |
| Participants | 32 incontinent female nursing home residents | |
| Interventions | group A (n=15): oestrogen 0.625 mg + progesterone 2.5 mg, oral tablets, daily for 6/12 | |
| Outcomes | frequency (percentage of checks at which subjects were found to be wet by research staff during three 8‐hour days of prompted voiding) and volume (re‐weighing pre‐weighed pad tests) of urinary incontinence, appropriate toileting rate, bladder capacity, cough 'stress test' | |
| Notes | no useable data; adverse events: 2 women in group A had single episode of vaginal spotting, ˜10% of women had mild breast discomfort | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | used a table of random numbers |
| Allocation concealment? | Low risk | A ‐ Adequate, odd and even numbered envelopes kept in the pharmacy |
| Methods | Double‐blind RCT, placebo controlled | |
| Participants | 40 post‐menopausal women | |
| Interventions | group A (n=20): 17 beta oestradiol 25mg implant subcutaneously | |
| Outcomes | Outcomes measured by frequency/volume chart, King's Healthcare Quality of Life Questionnaire, urinary symptom questionnaire, visual analogue scale of symptom severity, uroflowmetry, video cystourethrography, serum oestradiol levels, endometrial thickness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | no description |
| Blinding? | Low risk | double blind |
| Incomplete outcome data addressed? | Unclear risk | 40 women randomised |
| Methods | RCT; duration of study: 90 days | |
| Participants | 34 post‐menopausal women | |
| Interventions | group A (n=17): oestrogen cream 0.5mg | |
| Outcomes | nocturia, frequency, dysuria, urgency urinary incontinence, pad changes, urodynamics, vaginal dryness, vaginal atrophy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear, no description |
| Blinding? | Low risk | outcome assessor and care giver |
| Incomplete outcome data addressed? | Low risk | no dropouts |
| Methods | double‐blind placebo‐controlled crossover RCT, sample of previous population study was randomly selected, of these 34 took part in study | |
| Participants | 34 post‐menopausal women, 11 with stress incontinence, 14 urgency urinary incontinence, 9 mixed incontinence; | |
| Interventions | oestriol 3 mg orally or placebo orally, not clear if each treatment has been given for 3/12 and then was crossed over or if the total duration of both treatments was 3/12 | |
| Outcomes | Papanicolau smear to assess percentage of surface cells, MSU, clinical classification of degree of vaginal atrophy, efficacy of bladder control | |
| Notes | no useable data; unclear how many patients had oestrogen first and then placebo or how many had placebo first and the oestrogen; no apparent assessment after each stage of study; methods of assessment not given; no mention of losses to follow up; adverse outcomes: mastodynia, metrorrhagia in 4 patients, subjective side effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | randomised controlled trial, openly randomised | |
| Participants | Post‐menopausal women diagnosed with SUI using clinical and urodynamic evaluation | |
| Interventions | 48 women randomised to 10mg promestriene as daily suppositories for 21 days before TVT procedure 50 randomised to TVT without preoperative pharmacological therapy | |
| Outcomes | postop clinical evaluation at 3, 6, and 12 months At 6 months subjective symptom questionnaire and Kings Health Questionnaire | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no mention |
| Allocation concealment? | High risk | openly randomised |
| Blinding? | High risk | |
| Methods | randomised comparative study computer‐generated randomization list | |
| Participants | Women with overactive bladder mean age 65.2; range 58‐73, mean parity 2.5; range 1‐5 | |
| Interventions | 40 women 2mg detrusitol; 40 women 2mg detrusitol and vaginal oestrogen 1gm twice a week for a thee month period | |
| Outcomes | clinical exam, bladder diary, UDI questionnaire assessed at 0,6,12, weeks after treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer‐generated randomisation list |
| Blinding? | High risk | |
| Methods | double‐blind RCT, duration of study 4/12 | |
| Participants | 29 post‐menopausal women with stress and mixed incontinence, inclusion criteria: postmenopausal, no detrusor hyperreflexia, | |
| Interventions | group A (n=15): oestradiol 2 mg + oestriol 1 mg orally for 20 days followed by 8 day break for 4/12; group B (n=14): placebo orally similar regime | |
| Outcomes | interview to differentiate between urgency and stress urinary incontinence, cystoscopy, cystometry, MSU, trigone biopsies during cystoscopy, urethra ‐, vagina ‐ and cervix smears, serum levels of oestradiol, cholesterol and triglyceride | |
| Notes | tables using different numbers of patients, unclear how they were classified and who had which sort of incontinence, no losses to follow up, no adverse outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear, no description |
| Blinding? | Low risk | double blind |
| Methods | double‐blind placebo‐controlled RCT, randomisation into two treatment groups by block randomisation using random numbers duration of study: 12/52; assessment at 4/52, 8/52, 12/52 | |
| Participants | 29 post‐menopausal women | |
| Interventions | group A (n=15): placebo for 4/52 (period 1), then phenylpropanolamine (PPA) 50mg twice daily + placebo for 4/52 (period 2), then PPA 50mg twice daily + oestriol 4mg daily for 4/52 (period 3) | |
| Outcomes | subjective drug preference, 3‐day urinary diary, incontinence, median voiding frequency, mean number of leakage episodes, pad test, vaginal cytology, urine cultures, side effects, heart rate, BP | |
| Notes | adverse events: 5 in placebo period, 6 in PPA period, 5 in oestriol period, 7 in PPA + oestriol period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | randomisation into two treatment groups by block randomisation using random numbers |
| Allocation concealment? | Low risk | randomisation into two treatment groups by block randomisation using random numbers |
| Blinding? | Low risk | women were blind to treatment |
| Methods | RCT using statistical tables for random allocation of 2 drugs | |
| Participants | 36 post‐menopausal women 6 had hysterectomy | |
| Interventions | group A (n=18): piperazine oestrone sulphate 3mg at night for 3/52 followed by one treatment‐free week | |
| Outcomes | 7 day bladder diary, 2 hour pad test (22 women only), number of micturitions, pad changes/24 hours, urethral pressure profile, vaginal cytology, oestrone, oestradiol, FSH, LH levels, subjective assessment: patients were asked if they were much improved, improved or no better | |
| Notes | losses to follow up: group A: 2 (same women as in adverse events) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | standard statistical tables for random allocation of two drugs |
| Blinding? | Low risk | double blind |
| Incomplete outcome data addressed? | Unclear risk | 18 oestrogen group two failed to complete (not included in analysis) 18 placebo group |
| Methods | RCT to determine if topical oestrogen therapy can help prevent overactive bladder symptoms after TVT | |
| Participants | 56 post‐menopausal women with SUI having a TVT + 3 who were not randomised | |
| Interventions | group A (n=28) intravaginal oestriol ovules (1mg) daily for one month, then 2mg once weekly for 5 months as maintenance therapy | |
| Outcomes | successful treatment (cure) of SUI was defined as no leakage of urine during the cough stress test and urodynamic test, and no leakage episodes reported in a 7 day voiding diary | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? | Low risk | outcome assessors blind to treatment |
| Incomplete outcome data addressed? | Low risk | 59 had TVT, three women who were eligible after TVT refused to take part. 28 oestrogen group, 28 placebo |
BMI = body mass index;
BP = blood pressure;
DO = detrusor overactivity (previously known as DI, detrusor instability);
FSH = follicle stimulating hormone;
HRT = hormone replacement therapy;
ITT = intention to treat;
IU/l = international units per litre;
LH = luteinising hormone;
MSU = midstream specimen of urine;
pH = measure of acidity/alkalinity;
PPA = phenylpropanolamine;
RCT = randomised controlled trial;
SUI = stress urinary incontinence (symptom diagnosis);
UI = urinary incontinence;
USI = urodynamic stress incontinence (previously known as GSI, genuine stress incontinence);
UTI = urinary tract infection;
UUI = urgency urinary incontinence.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Unclear if RCT, published in 1961 and in German | |
| Only a paragraph in a review, author contacted for more data, data have never been published, were collected from 1983‐1985, no data available any more | |
| Looking at vascular resistance index of periurethral vessels in patients with stress urinary incontinence treated with local oestrogens. No data though 'clinical improvement was found in 15 patients, most of whom received oestrogens' | |
| Not all women were incontinent at baseline | |
| cannot use data, does not state numbers randomised | |
| looking at urogenital symptoms and systemic postmenopausal complaints, incontinence only mentioned as part of "Urogenital Index" | |
| RCT using topical oestrogen cream and biofeedback combinations in four arms. Not all women incontinent at baseline | |
| Short abstract only, no results given | |
| All women treated with 2 mg oestradiol, dose finding study of dydrogesterone | |
| RCT but not all women incontinent at baseline | |
| Looking at long‐term effect of treatment with oestradiol 3/52 before vaginal repair, only 7 patients with incontinence which is not further specified | |
| No mention of incontinence, brief description of frequency and urgency only | |
| RCT of transdermal oestrogen versus placebo but for severe urogenital estrogen deficiency (not urinary incontinence) | |
| 67 women with symptoms of urinary urgency, but no comment on cure of urinary symptoms, only pH results given | |
| RCT of oestriol cream versus no cream prior to surgery, no outcomes on incontinence. Italian publication | |
| RCT with four arms, unclear allocation concealment, not enough usable information given, paper written in Polish | |
| Not all women were incontinent at baseline. Same study reported in 2005 by Goldstein et al | |
| RCT but included 6/38 continent women and 9/38 premenopausal women. Outcomes not reported separately | |
| Not RCT | |
| Excluded as trial looking at effect of treatment on continent women | |
| effect of HRT on calcium and collagen, no mention of urinary symptoms | |
| RCT but not all patients were incontinent at baseline | |
| RCT with four arms, two randomised tow patient choice. Excluded as only 70% of women incontinent at baseline | |
| Raloxifene not an oestrogen |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A comparative study between oestrogen replacement therapy, anticholinergic treatment and a combination of both in the management of detrusor instability in post‐menopausal women |
| Methods | |
| Participants | 80 participants, 4 random groups |
| Interventions | group A: tolterodine 2mg twice daily |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | tried to contact, no success |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence (women's observations) Show forest plot | 6 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.70, 0.90] |
| Analysis 1.1  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 1 Number with incontinence (women's observations). | ||||
| 1.1 Systemic administration | 4 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 1.2 Local administration | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.87] |
| 2 Number with incontinence not improved (women's observations) Show forest plot | 9 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.53, 0.72] |
| Analysis 1.2  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 2 Number with incontinence not improved (women's observations). | ||||
| 2.1 Systemic administration | 5 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.93] |
| 2.2 Local administration | 4 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.43, 0.65] |
| 3 Incontinence not improved (generic inverse variance) (women's observations) Show forest plot | 10 | RR (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 3 Incontinence not improved (generic inverse variance) (women's observations). | ||||
| 3.1 Systemic administration (any incontinence) | 6 | 6151 | RR (Fixed, 95% CI) | 1.32 [1.17, 1.48] |
| 3.2 Local administration (any incontinence) | 4 | 213 | RR (Fixed, 95% CI) | 0.74 [0.64, 0.86] |
| 4 Number with incontinence (objective observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 4 Number with incontinence (objective observations). | ||||
| 4.1 Local administration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number with incontinence not improved (objective observations) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 5 Number with incontinence not improved (objective observations). | ||||
| 5.1 Local administration | 2 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.40, 0.89] |
| 6 Number of pad changes over 24 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 6 Number of pad changes over 24 hours. | ||||
| 6.1 Systemic administration | 3 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.89, 0.66] |
| 7 Pad test weights Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 7 Pad test weights. | ||||
| 7.1 Systemic administration | 3 | 106 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [‐5.67, 9.16] |
| 8 Incontinent episodes over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 8 Incontinent episodes over 24 hours. | ||||
| 8.1 Systemic administration | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐0.50, 1.57] |
| 9 Number of voids over 24 hours Show forest plot | 7 | 237 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.81, ‐0.60] |
| Analysis 1.9  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 9 Number of voids over 24 hours. | ||||
| 9.1 Systemic administration | 3 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.22, 0.73] |
| 9.2 Local administration | 4 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.58, ‐1.03] |
| 10 Number of nocturnal voids Show forest plot | 5 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| Analysis 1.10  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 10 Number of nocturnal voids. | ||||
| 10.1 Systemic administration | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.40, 0.16] |
| 10.2 Local administration | 3 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.03 [‐2.82, ‐1.24] |
| 11 Number of women with frequency Show forest plot | 3 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.33] |
| Analysis 1.11  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 11 Number of women with frequency. | ||||
| 11.1 Systemic administration | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.89, 2.19] |
| 11.2 Local administration | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 12 Number of women with nocturia Show forest plot | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.42] |
| Analysis 1.12  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 12 Number of women with nocturia. | ||||
| 12.1 Systemic administration | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.62, 1.62] |
| 12.2 Local administration | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.11, 2.38] |
| 13 Number of women with urgency Show forest plot | 4 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.12] |
| Analysis 1.13  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 13 Number of women with urgency. | ||||
| 13.1 Systemic administration | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.83, 1.33] |
| 13.2 Local administration | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.99] |
| 14 Maximum urethral closure pressure (MUCP) Show forest plot | 7 | 291 | Mean Difference (IV, Fixed, 95% CI) | 3.61 [1.87, 5.35] |
| Analysis 1.14  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 14 Maximum urethral closure pressure (MUCP). | ||||
| 14.1 Systemic administration | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐6.24, 3.43] |
| 14.2 Local administration | 5 | 202 | Mean Difference (IV, Fixed, 95% CI) | 4.35 [2.49, 6.22] |
| 15 Volume at first urge to void Show forest plot | 7 | 269 | Mean Difference (IV, Fixed, 95% CI) | 18.80 [13.01, 24.60] |
| Analysis 1.15  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 15 Volume at first urge to void. | ||||
| 15.1 Systemic administration | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | 9.09 [‐25.45, 43.62] |
| 15.2 Local administration | 4 | 116 | Mean Difference (IV, Fixed, 95% CI) | 19.09 [13.21, 24.96] |
| 16 Maximum bladder capacity Show forest plot | 7 | 269 | Mean Difference (IV, Fixed, 95% CI) | 45.34 [31.86, 58.82] |
| Analysis 1.16  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 16 Maximum bladder capacity. | ||||
| 16.1 Systemic administration | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | 7.18 [‐33.26, 47.62] |
| 16.2 Local administration | 4 | 116 | Mean Difference (IV, Fixed, 95% CI) | 50.11 [35.81, 64.41] |
| 17 Number with adverse effects Show forest plot | 3 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [1.50, 12.03] |
| Analysis 1.17  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 17 Number with adverse effects. | ||||
| 17.1 Systemic administration | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [1.87, 90.21] |
| 17.2 Local administration | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.61] |
| 18 Number with bacteriuria Show forest plot | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.76] |
| Analysis 1.18  Comparison 1 Oestrogen versus placebo or no treatment, Outcome 18 Number with bacteriuria. | ||||
| 18.1 Systemic administration | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.37, 1.42] |
| 18.2 Local administration | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.13, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Number with incontinence (women's observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.1  Comparison 2 Oestrogen versus other treatments, Outcome 1 Number with incontinence (women's observations). | ||||||||||||||||
| 1.1 oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 2 Number with incontinence, crossover studies (women's observations) Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.2
Comparison 2 Oestrogen versus other treatments, Outcome 2 Number with incontinence, crossover studies (women's observations). | ||||||||||||||||
| 2.1 Oetrogens versus PPA | Other data | No numeric data | ||||||||||||||
| 3 Number with incontinence not improved (women's observations) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 2.3  Comparison 2 Oestrogen versus other treatments, Outcome 3 Number with incontinence not improved (women's observations). | ||||||||||||||||
| 3.1 oestrogen vs PPA | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.09] | ||||||||||||
| 4 Number with incontinence not improved, crossover studies (women's observations) Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.4
Comparison 2 Oestrogen versus other treatments, Outcome 4 Number with incontinence not improved, crossover studies (women's observations). | ||||||||||||||||
| 4.1 Oetrogens versus PPA | Other data | No numeric data | ||||||||||||||
| 5 Number with incontinence (objective observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.5  Comparison 2 Oestrogen versus other treatments, Outcome 5 Number with incontinence (objective observations). | ||||||||||||||||
| 5.1 oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 6 Number with incontinence not improved (objective observations) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 2.6  Comparison 2 Oestrogen versus other treatments, Outcome 6 Number with incontinence not improved (objective observations). | ||||||||||||||||
| 6.1 oestrogen vs PPA | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.90] | ||||||||||||
| 6.2 oestrogen vs PFMT | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.18, 2.23] | ||||||||||||
| 6.3 oestrogen vs electrostimulation | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.95, 1.75] | ||||||||||||
| 7 Number of pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.7  Comparison 2 Oestrogen versus other treatments, Outcome 7 Number of pad changes over 24 hours. | ||||||||||||||||
| 7.1 oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 8 Number of voids over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.8  Comparison 2 Oestrogen versus other treatments, Outcome 8 Number of voids over 24 hours. | ||||||||||||||||
| 8.1 oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 9 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.9  Comparison 2 Oestrogen versus other treatments, Outcome 9 Pad test weights. | ||||||||||||||||
| 9.1 oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 10 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.10  Comparison 2 Oestrogen versus other treatments, Outcome 10 Adverse effects. | ||||||||||||||||
| 10.1 oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 11 Maximum urethral closure pressure (MUCP) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||||||
| Analysis 2.11  Comparison 2 Oestrogen versus other treatments, Outcome 11 Maximum urethral closure pressure (MUCP). | ||||||||||||||||
| 11.1 oestrogen vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 11.2 oestrogen vs electrostimulation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 12 Maximum urethral closure pressure (MUCP), crossover studies Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 2.12
Comparison 2 Oestrogen versus other treatments, Outcome 12 Maximum urethral closure pressure (MUCP), crossover studies. | ||||||||||||||||
| 12.1 Oetrogens versus PPA | Other data | No numeric data | ||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence not improved (women's observations) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Oestrogen + other treatments versus placebo, Outcome 1 Number with incontinence not improved (women's observations). | ||||
| 1.1 oestrogen + progesterone vs placebo | 2 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.01, 1.16] |
| 2 Incontinence not improved (generic inverse variance) (women's observations) Show forest plot | 3 | Relative Risk (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Oestrogen + other treatments versus placebo, Outcome 2 Incontinence not improved (generic inverse variance) (women's observations). | ||||
| 2.1 Systemic administration of oestrogen + progesterone vs placebo | 3 | 10635 | Relative Risk (Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| 3 Incontinent episodes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Oestrogen + other treatments versus placebo, Outcome 3 Incontinent episodes over 24 hours. | ||||
| 3.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of diurnal voids per 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Oestrogen + other treatments versus placebo, Outcome 4 Number of diurnal voids per 24 hours. | ||||
| 4.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of nocturnal voids per 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 Oestrogen + other treatments versus placebo, Outcome 5 Number of nocturnal voids per 24 hours. | ||||
| 5.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3 Oestrogen + other treatments versus placebo, Outcome 6 Pad test weights. | ||||
| 6.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Use of drugs for incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 Oestrogen + other treatments versus placebo, Outcome 7 Use of drugs for incontinence. | ||||
| 7.1 oestrogen + progesterone vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of women having incontinence surgery Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.8  Comparison 3 Oestrogen + other treatments versus placebo, Outcome 8 Number of women having incontinence surgery. | ||||
| 8.1 oestrogen + progesterone vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||
| 1 Number with incontinence not improved (women's observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | ||||||||||||||||
| Analysis 4.1  Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 1 Number with incontinence not improved (women's observations). | |||||||||||||||||||
| 1.1 Oestrogen + PPA vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||
| 2 Number with incontinence not improved (women's observations) (cross‐over trials) Show forest plot | Other data | No numeric data | |||||||||||||||||
| Analysis 4.2
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 2 Number with incontinence not improved (women's observations) (cross‐over trials). | |||||||||||||||||||
| 2.1 oestrogen + PPA vs oestrogen | Other data | No numeric data | |||||||||||||||||
| 3 Number of pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | ||||||||||||||||
| Analysis 4.3  Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 3 Number of pad changes over 24 hours. | |||||||||||||||||||
| 3.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||
| 4 Number of voids over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | ||||||||||||||||
| Analysis 4.4  Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 4 Number of voids over 24 hours. | |||||||||||||||||||
| 4.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||
| 5 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | ||||||||||||||||
| Analysis 4.5  Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 5 Pad test weights. | |||||||||||||||||||
| 5.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||
| 6 Number of women with frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | ||||||||||||||||
| Analysis 4.6  Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 6 Number of women with frequency. | |||||||||||||||||||
| 6.1 oestrogen + progesterone vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||
| 6.2 oestriol + benzidamine vs oestriol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||
| 7 Number of women with nocturia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | ||||||||||||||||
| Analysis 4.7  Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 7 Number of women with nocturia. | |||||||||||||||||||
| 7.1 oestriol + benzidamine vs oestriol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||
| 8 Number with adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | ||||||||||||||||
| Analysis 4.8  Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 8 Number with adverse effects. | |||||||||||||||||||
| 8.1 Oestrogen + PPA vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||
| 9 Volume at first urge to void (cross‐over trials) Show forest plot | Other data | No numeric data | |||||||||||||||||
| Analysis 4.9
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 9 Volume at first urge to void (cross‐over trials). | |||||||||||||||||||
| 9.1 oestrogen + PPA vs oestrogen | Other data | No numeric data | |||||||||||||||||
| 10 Maximum bladder capacity (cross‐over trials) Show forest plot | Other data | No numeric data | |||||||||||||||||
| Analysis 4.10
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 10 Maximum bladder capacity (cross‐over trials). | |||||||||||||||||||
| 10.1 oestrogen +other vs oestrogen | Other data | No numeric data | |||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence (women's observations) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 1 Number with incontinence (women's observations). | ||||
| 1.1 oestrogen + PFMT vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 TVT vs TVT + vaginal oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number with incontinence not improved (women's observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 2 Number with incontinence not improved (women's observations). | ||||
| 2.1 oestrogen + PPA vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 3 Number of pad changes over 24 hours. | ||||
| 3.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of voids over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 4 Number of voids over 24 hours. | ||||
| 4.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 detrusitol + vaginal oestrogen vs detrusitol m | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.5  Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 5 Pad test weights. | ||||
| 5.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.6  Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 6 Adverse effects. | ||||
| 6.1 oestrogen + PPA vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 oestrogen + PFMT vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence not improved (women's observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Different types of oestrogen, Outcome 1 Number with incontinence not improved (women's observations). | ||||
| 1.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of women with nocturia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Different types of oestrogen, Outcome 2 Number of women with nocturia. | ||||
| 2.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of women with dysuria Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Different types of oestrogen, Outcome 3 Number of women with dysuria. | ||||
| 3.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of women with urgency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 Different types of oestrogen, Outcome 4 Number of women with urgency. | ||||
| 4.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 oestrogen cream vs vaginal oestrogen + progesterone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of women with frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.5  Comparison 6 Different types of oestrogen, Outcome 5 Number of women with frequency. | ||||
| 5.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 oestrogen cream vs vaginal oestrogen + progesterone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.6  Comparison 6 Different types of oestrogen, Outcome 6 Number of adverse events. | ||||
| 6.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Different routes of administration, Outcome 1 Number of pad changes over 24 hours. | ||||
| 1.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of voids over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Different routes of administration, Outcome 2 Number of voids over 24 hours. | ||||
| 2.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 Different routes of administration, Outcome 3 Pad test weights. | ||||
| 3.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of voids over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.1  Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 1 Number of voids over 24 hours. | ||||
| 1.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.87, ‐0.16] |
| 2 Number of nocturnal voids Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.2  Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 2 Number of nocturnal voids. | ||||
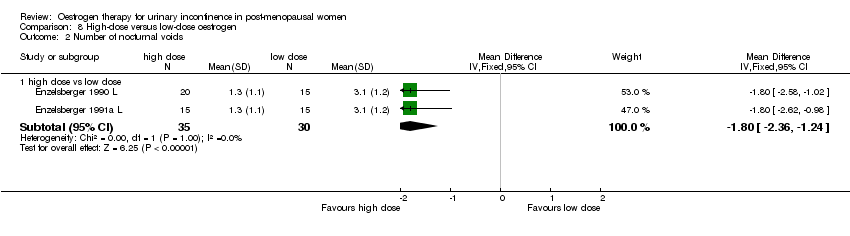
| 2.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.36, ‐1.24] |
| 3 Maximum urethral closure pressure (MUCP) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.3  Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 3 Maximum urethral closure pressure (MUCP). | ||||
| 3.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 3.84 [‐5.77, 13.46] |
| 4 Volume at first urge to void Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.4  Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 4 Volume at first urge to void. | ||||
| 4.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.56 [‐12.20, 9.08] |
| 5 Maximum bladder capacity Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.5  Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 5 Maximum bladder capacity. | ||||
| 5.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 34.90 [8.35, 61.45] |

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 1 Number with incontinence (women's observations).

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 2 Number with incontinence not improved (women's observations).

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 3 Incontinence not improved (generic inverse variance) (women's observations).
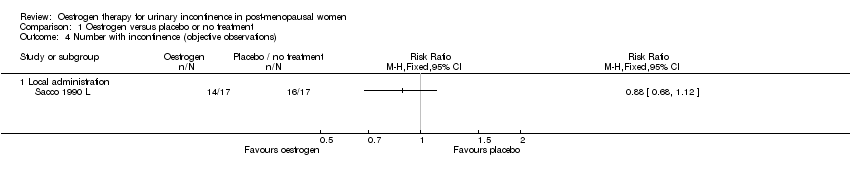
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 4 Number with incontinence (objective observations).
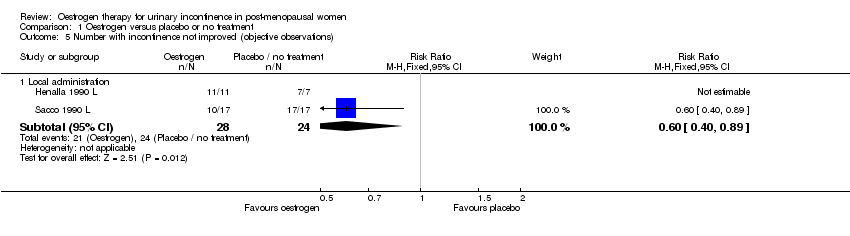
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 5 Number with incontinence not improved (objective observations).
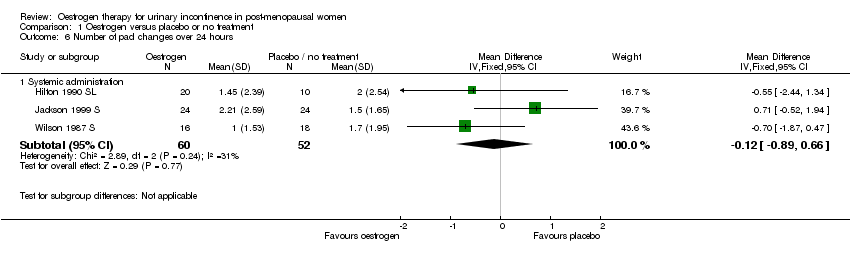
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 6 Number of pad changes over 24 hours.
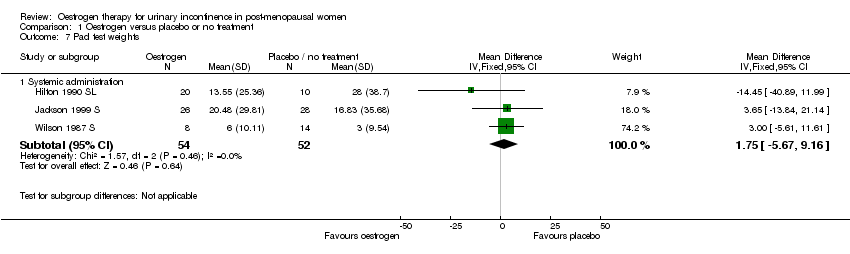
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 7 Pad test weights.
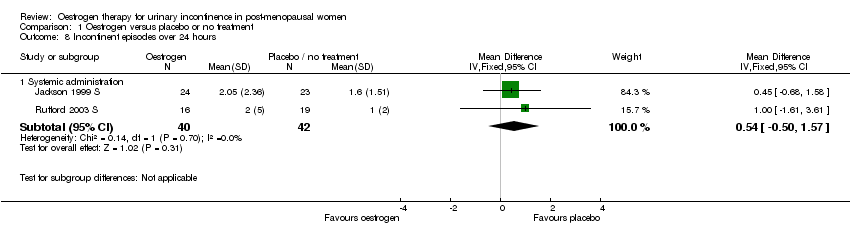
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 8 Incontinent episodes over 24 hours.

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 9 Number of voids over 24 hours.
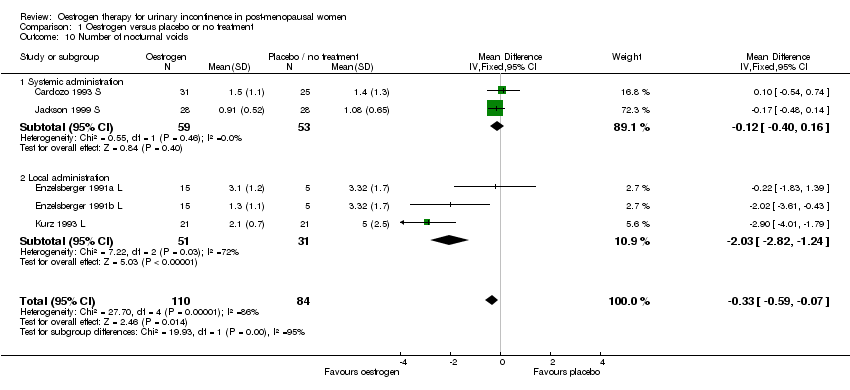
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 10 Number of nocturnal voids.

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 11 Number of women with frequency.
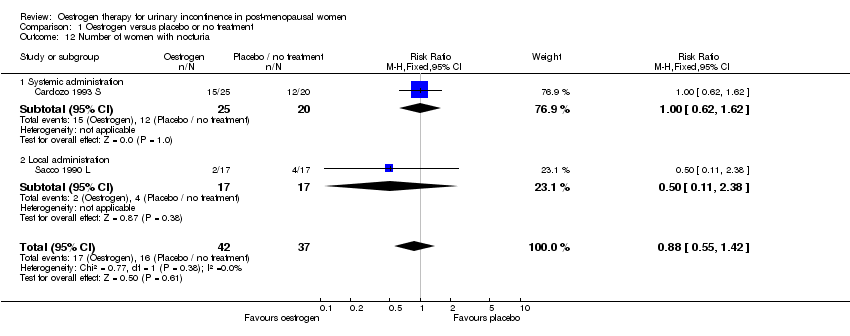
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 12 Number of women with nocturia.

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 13 Number of women with urgency.

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 14 Maximum urethral closure pressure (MUCP).

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 15 Volume at first urge to void.
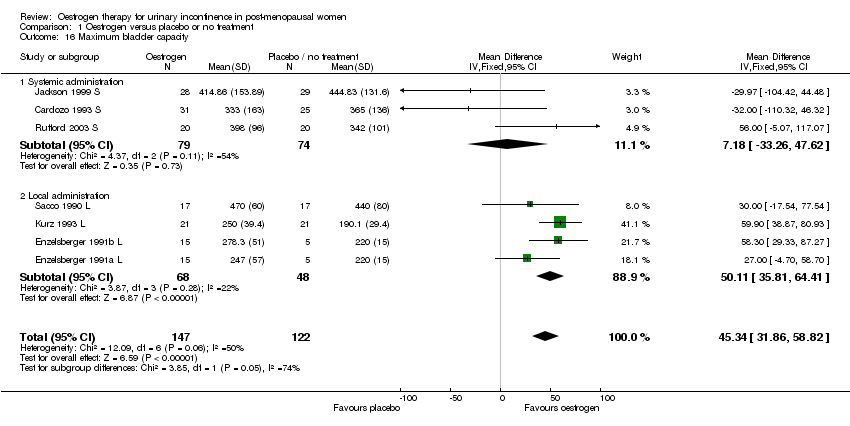
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 16 Maximum bladder capacity.
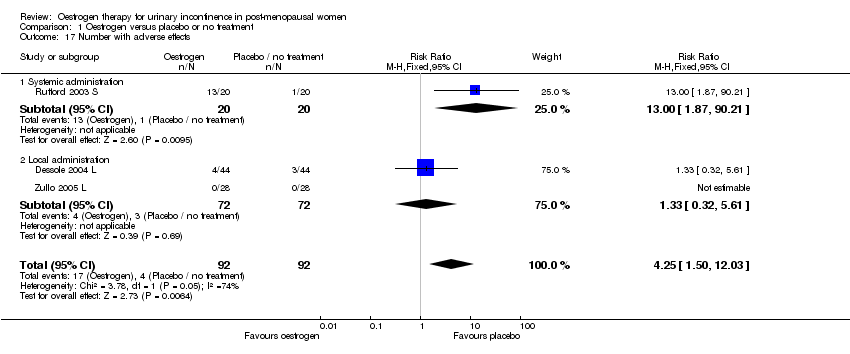
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 17 Number with adverse effects.

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 18 Number with bacteriuria.

Comparison 2 Oestrogen versus other treatments, Outcome 1 Number with incontinence (women's observations).
| Study | Oestrogen | PPA |
| Oetrogens versus PPA | ||
| Beisland 1984#L | 9/10 women | 10/10 women |
Comparison 2 Oestrogen versus other treatments, Outcome 2 Number with incontinence, crossover studies (women's observations).
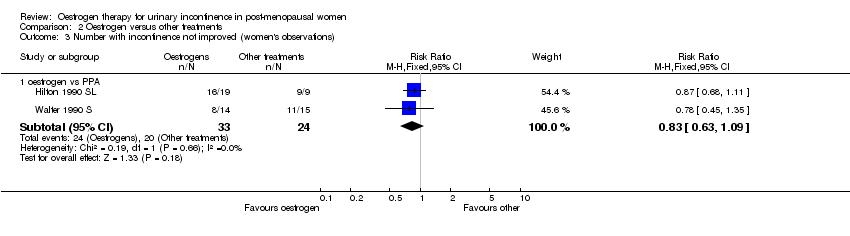
Comparison 2 Oestrogen versus other treatments, Outcome 3 Number with incontinence not improved (women's observations).
| Study | Oestrogen | PPA |
| Oetrogens versus PPA | ||
| Beisland 1984#L | 6/10 women | 2/10 women |
Comparison 2 Oestrogen versus other treatments, Outcome 4 Number with incontinence not improved, crossover studies (women's observations).

Comparison 2 Oestrogen versus other treatments, Outcome 5 Number with incontinence (objective observations).

Comparison 2 Oestrogen versus other treatments, Outcome 6 Number with incontinence not improved (objective observations).
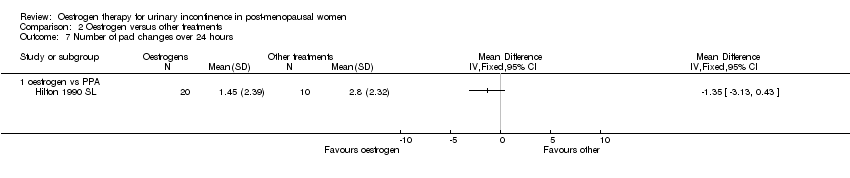
Comparison 2 Oestrogen versus other treatments, Outcome 7 Number of pad changes over 24 hours.

Comparison 2 Oestrogen versus other treatments, Outcome 8 Number of voids over 24 hours.
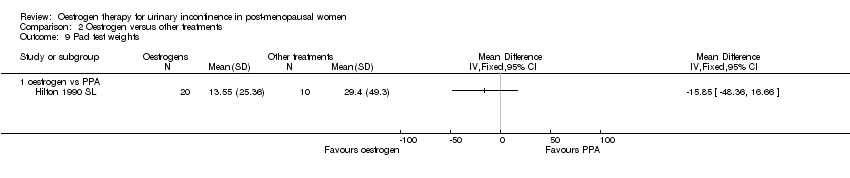
Comparison 2 Oestrogen versus other treatments, Outcome 9 Pad test weights.

Comparison 2 Oestrogen versus other treatments, Outcome 10 Adverse effects.

Comparison 2 Oestrogen versus other treatments, Outcome 11 Maximum urethral closure pressure (MUCP).
| Study | Oestrogen | PPA |
| Oetrogens versus PPA | ||
| Beisland 1984#L | Mean=41.6, N=10 | Mean=47.1, N=10 |
Comparison 2 Oestrogen versus other treatments, Outcome 12 Maximum urethral closure pressure (MUCP), crossover studies.
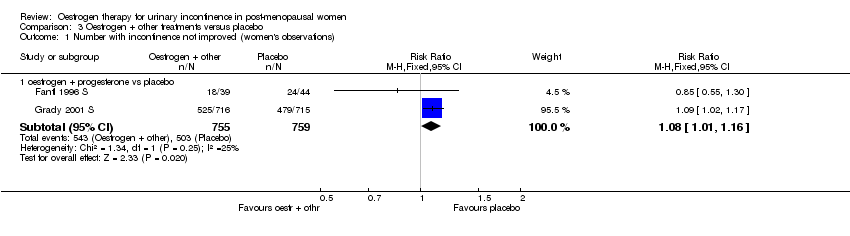
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 1 Number with incontinence not improved (women's observations).
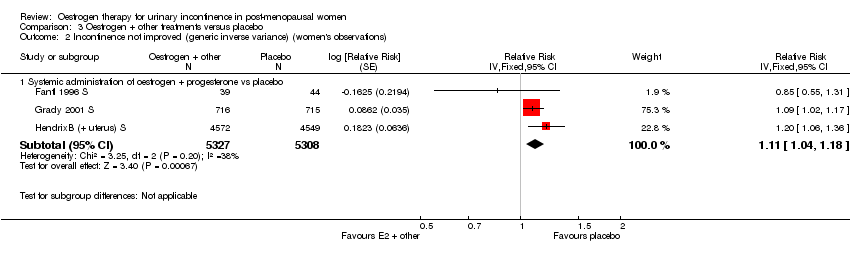
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 2 Incontinence not improved (generic inverse variance) (women's observations).

Comparison 3 Oestrogen + other treatments versus placebo, Outcome 3 Incontinent episodes over 24 hours.

Comparison 3 Oestrogen + other treatments versus placebo, Outcome 4 Number of diurnal voids per 24 hours.
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 5 Number of nocturnal voids per 24 hours.

Comparison 3 Oestrogen + other treatments versus placebo, Outcome 6 Pad test weights.
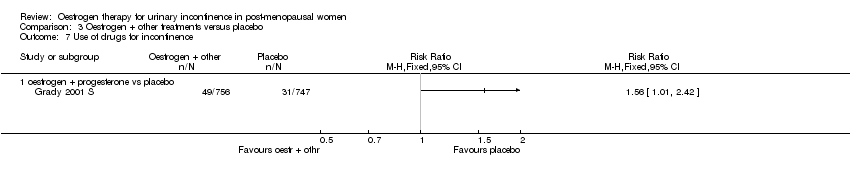
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 7 Use of drugs for incontinence.
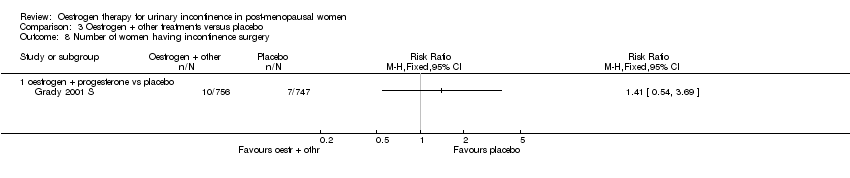
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 8 Number of women having incontinence surgery.

Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 1 Number with incontinence not improved (women's observations).
| Study | oestr + other drug | oestrogen |
| oestrogen + PPA vs oestrogen | ||
| Ahlstrom 1990#S | 17/27 | 21/26 |
| Kinn 1988#S | 14/30 | 21/30 |
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 2 Number with incontinence not improved (women's observations) (cross‐over trials).

Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 3 Number of pad changes over 24 hours.

Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 4 Number of voids over 24 hours.
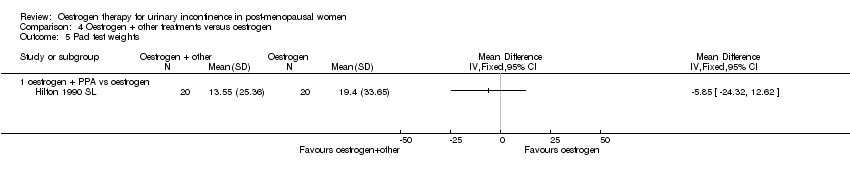
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 5 Pad test weights.

Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 6 Number of women with frequency.

Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 7 Number of women with nocturia.
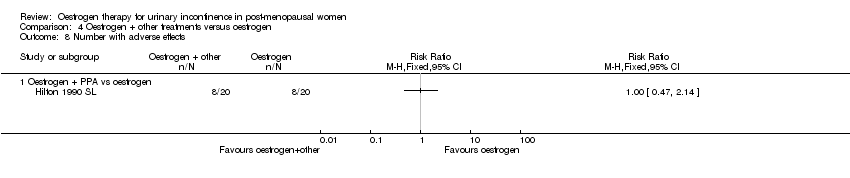
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 8 Number with adverse effects.
| Study | oestrogen + naproxen | oestrogen |
| oestrogen + PPA vs oestrogen | ||
| Blom 1995#S | 172 ml (SD 12) N=16 | 186 ml (SD 12) N=16 |
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 9 Volume at first urge to void (cross‐over trials).
| Study | oestr + naproxen | oestrogen |
| oestrogen +other vs oestrogen | ||
| Blom 1995#S | 316 ml (SD 17) N=16 | 318 ml (SD 20) N=16 |
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 10 Maximum bladder capacity (cross‐over trials).
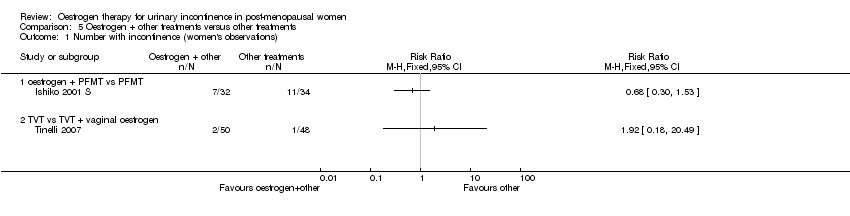
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 1 Number with incontinence (women's observations).
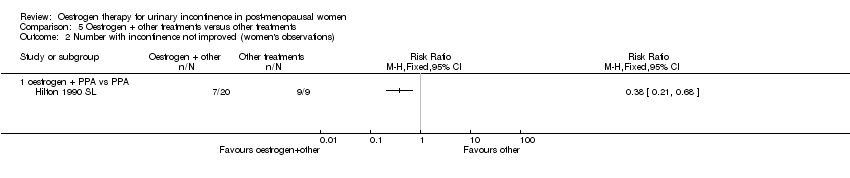
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 2 Number with incontinence not improved (women's observations).

Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 3 Number of pad changes over 24 hours.
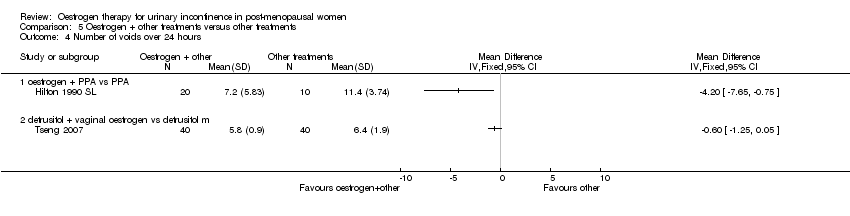
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 4 Number of voids over 24 hours.
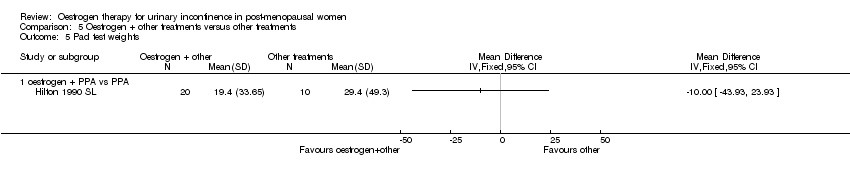
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 5 Pad test weights.

Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 6 Adverse effects.

Comparison 6 Different types of oestrogen, Outcome 1 Number with incontinence not improved (women's observations).
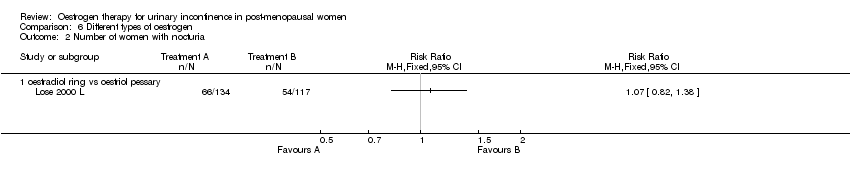
Comparison 6 Different types of oestrogen, Outcome 2 Number of women with nocturia.
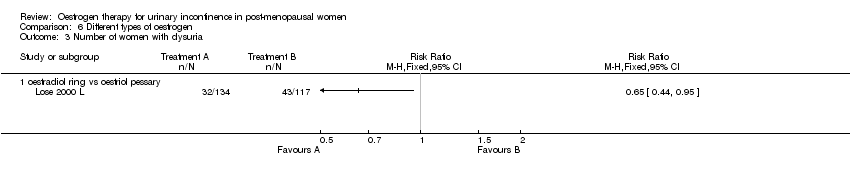
Comparison 6 Different types of oestrogen, Outcome 3 Number of women with dysuria.

Comparison 6 Different types of oestrogen, Outcome 4 Number of women with urgency.

Comparison 6 Different types of oestrogen, Outcome 5 Number of women with frequency.

Comparison 6 Different types of oestrogen, Outcome 6 Number of adverse events.
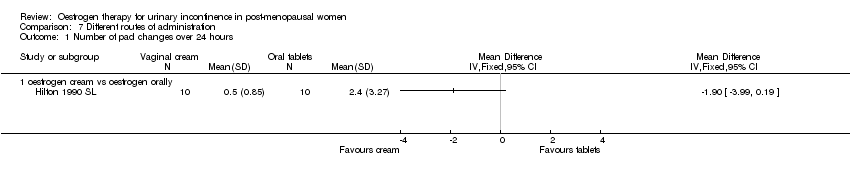
Comparison 7 Different routes of administration, Outcome 1 Number of pad changes over 24 hours.
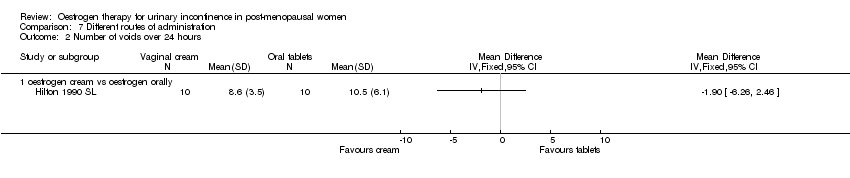
Comparison 7 Different routes of administration, Outcome 2 Number of voids over 24 hours.
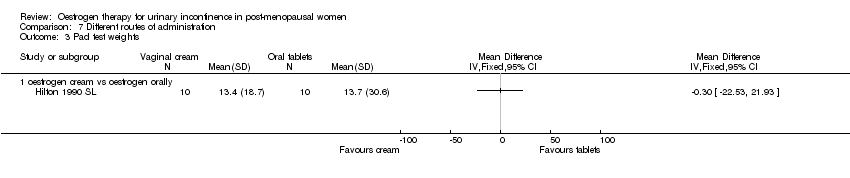
Comparison 7 Different routes of administration, Outcome 3 Pad test weights.

Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 1 Number of voids over 24 hours.
Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 2 Number of nocturnal voids.

Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 3 Maximum urethral closure pressure (MUCP).

Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 4 Volume at first urge to void.

Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 5 Maximum bladder capacity.
| Study ID | Type of Oestrogen | Route of administration | Dose | Length of treatment | Population |
| Oestriol | Systemic (oral) | 4mg | 6 weeks | USI (stress), postmenopausal | |
| Oestriol | Local (vaginal) | 1 mg | 8 weeks | UI (stress), postmenopausal | |
| Estradiol | Systemic (transdermal) | 0.05 mg | 8 weeks | UUI (OAB), elderly | |
| Oestriol | Systemic (oral) | 3 mg | 3 months | UUI (urge), postmenopausal | |
| Oestradiol | Vaginal (pessary, Vagifem) | 25 µgm | 3 months | OAB (urge), postmenopausal | |
| A: Ethinyl oestradiol + levonorgestrel B: conjugated equine oestrogen | A: Systemic (oral) B: Local (vaginal cream) | A: 30 mg /250 ng B: 0.625 mg | 2 months | UUI (urogenital symptoms), oestrogen deficiency, postmenopausal | |
| Oestriol | Local (intravaginal ovules) | 1‐2 mg | 6 months | UI (stress), postmenopausal | |
| Oestradiol | Systemic (oral) | 1 mg | 6 weeks | USI (stress), postmenopausal | |
| Oestriol | Local (vaginal) | 0.5, 1 or 2 mg | Not stated | UI (urge), OAB | |
| Oestriol | Local (vaginal) | 1 mg or 3 mg | 3 weeks | UI (urge), OAB, postmenopausal | |
| Conjugated equine oestrogens + medroxyprogesterone | Systemic (oral) | 0.625 mg / 10 mg | 3 months | UI (stress), postmenopausal | |
| Conjugated oestrogens (premarin) + medroxyprogesterone | Systemic (oral) | 0.625 mg / 2.5 mg | up to 4 years | UI (unspecified), postmenopausal, heart disease, age <80 years, with uterus | |
| Conjugated equine oestrogen | Local (vaginal cream) | 1.25 mg | 3 months | USI (stress) | |
| Conjugated equine oestrogen | Local (vaginal cream) | 2 gm | 6 weeks | USI (stress), postmenopausal | |
| Conjugated equine oestrogen | Systemic (oral) | 0.625 mg | 1 year | UI (SUI, UUI, MUI), postmenopausal, prevention of heart disease and hip fracture Without uterus | |
| Conjugated equine oestrogen + medroxyprogesterone | Systemic (oral) | 0.625 mg / 2.5 mg | 1 year | UI (SUI, UUI, MUI), postmenopausal, prevention of heart disease and hip fracture With uterus | |
| Oestrogen | Local (intravaginal) Systemic (oral) | 2 gm 1.25 mg | 1 month | USI (stress), postmenopausal | |
| Estriol | Systemic (oral) | 1 mg | 2 years | SUI (stress), postmenopausal | |
| Oestradiol | Systemic (oral) | 2 mg | 6 months | USI (stress), postmenopausal | |
| Quinestradiol | Not stated | 1 mg | 1 month | UI (unspecified), postmenopausal, geriatric inpatients | |
| Oestriol | Systemic (oral) | 4 mg | 1 month | USI (stress), postmenopausal | |
| Oestriol | Local (intravesical) | 1 mg | 3 weeks | UUI, OAB (urge), postmenopausal | |
| Oestradiol | Local (intravaginal ring, vaginal pessaries) | ring 7.5mg, pessary 0.5 mg | 6 months | UI (unspecified), LUT symptoms, postmenopausal | |
| Oestriol | Local (intravaginal) | 0.5 mg | 3 months | UI, menopausal symptoms | |
| Oestrogen + progesterone | Systemic (oral) | 0.625 mg / 2.5 mg | 6 months | UI (unspecified), nursing home residents | |
| 17‐beta oestradiol | Systemic (implant) | 25 mg | 6 months | UUI, OAB (urge), postmenopausal | |
| Oestrogen | Local (cream) | 0.5 mg | 3 months | USI (stress), postmenopausal | |
| Oestriol | Systemic (oral) | 3 mg | 3 months | MUI (mixture), postmenopausal | |
| Oestradiol + oestriol | Systemic (oral) | 2 mg / 1 mg | 4 months (with breaks) | SUI, MUI (stress and mixed), postmenopausal | |
| Oestriol | Systemic (oral) | 4 mg | 1‐2 months | SUI (stress), postmenopausal | |
| Piperazine oestrone sulphate | Systemic (oral) | 3 mg | 3 months (with breaks) | USI (stress), postmenopausal | |
| Estriol | Local (intravaginal ovules) | 1 mg | 6 months | SUI (stress), postmenopausal | |
| LUTs = lower urinary tract symptoms; MUI = mixed urinary incontinence; OAB = overactive bladder syndrome; SUI = stress urinary incontinence; UI = urinary incontinence; USI = urodynamic stress incontinence; UUI = urgency urinary incontinence. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence (women's observations) Show forest plot | 6 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.70, 0.90] |
| 1.1 Systemic administration | 4 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 1.2 Local administration | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.87] |
| 2 Number with incontinence not improved (women's observations) Show forest plot | 9 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.53, 0.72] |
| 2.1 Systemic administration | 5 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.93] |
| 2.2 Local administration | 4 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.43, 0.65] |
| 3 Incontinence not improved (generic inverse variance) (women's observations) Show forest plot | 10 | RR (Fixed, 95% CI) | Subtotals only | |
| 3.1 Systemic administration (any incontinence) | 6 | 6151 | RR (Fixed, 95% CI) | 1.32 [1.17, 1.48] |
| 3.2 Local administration (any incontinence) | 4 | 213 | RR (Fixed, 95% CI) | 0.74 [0.64, 0.86] |
| 4 Number with incontinence (objective observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Local administration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number with incontinence not improved (objective observations) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Local administration | 2 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.40, 0.89] |
| 6 Number of pad changes over 24 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Systemic administration | 3 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.89, 0.66] |
| 7 Pad test weights Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Systemic administration | 3 | 106 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [‐5.67, 9.16] |
| 8 Incontinent episodes over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Systemic administration | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐0.50, 1.57] |
| 9 Number of voids over 24 hours Show forest plot | 7 | 237 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.81, ‐0.60] |
| 9.1 Systemic administration | 3 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.22, 0.73] |
| 9.2 Local administration | 4 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.58, ‐1.03] |
| 10 Number of nocturnal voids Show forest plot | 5 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 10.1 Systemic administration | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.40, 0.16] |
| 10.2 Local administration | 3 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.03 [‐2.82, ‐1.24] |
| 11 Number of women with frequency Show forest plot | 3 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.33] |
| 11.1 Systemic administration | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.89, 2.19] |
| 11.2 Local administration | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 12 Number of women with nocturia Show forest plot | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.42] |
| 12.1 Systemic administration | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.62, 1.62] |
| 12.2 Local administration | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.11, 2.38] |
| 13 Number of women with urgency Show forest plot | 4 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.12] |
| 13.1 Systemic administration | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.83, 1.33] |
| 13.2 Local administration | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.99] |
| 14 Maximum urethral closure pressure (MUCP) Show forest plot | 7 | 291 | Mean Difference (IV, Fixed, 95% CI) | 3.61 [1.87, 5.35] |
| 14.1 Systemic administration | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐6.24, 3.43] |
| 14.2 Local administration | 5 | 202 | Mean Difference (IV, Fixed, 95% CI) | 4.35 [2.49, 6.22] |
| 15 Volume at first urge to void Show forest plot | 7 | 269 | Mean Difference (IV, Fixed, 95% CI) | 18.80 [13.01, 24.60] |
| 15.1 Systemic administration | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | 9.09 [‐25.45, 43.62] |
| 15.2 Local administration | 4 | 116 | Mean Difference (IV, Fixed, 95% CI) | 19.09 [13.21, 24.96] |
| 16 Maximum bladder capacity Show forest plot | 7 | 269 | Mean Difference (IV, Fixed, 95% CI) | 45.34 [31.86, 58.82] |
| 16.1 Systemic administration | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | 7.18 [‐33.26, 47.62] |
| 16.2 Local administration | 4 | 116 | Mean Difference (IV, Fixed, 95% CI) | 50.11 [35.81, 64.41] |
| 17 Number with adverse effects Show forest plot | 3 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [1.50, 12.03] |
| 17.1 Systemic administration | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [1.87, 90.21] |
| 17.2 Local administration | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.61] |
| 18 Number with bacteriuria Show forest plot | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.76] |
| 18.1 Systemic administration | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.37, 1.42] |
| 18.2 Local administration | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.13, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence (women's observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number with incontinence, crossover studies (women's observations) Show forest plot | Other data | No numeric data | ||
| 2.1 Oetrogens versus PPA | Other data | No numeric data | ||
| 3 Number with incontinence not improved (women's observations) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 oestrogen vs PPA | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.09] |
| 4 Number with incontinence not improved, crossover studies (women's observations) Show forest plot | Other data | No numeric data | ||
| 4.1 Oetrogens versus PPA | Other data | No numeric data | ||
| 5 Number with incontinence (objective observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number with incontinence not improved (objective observations) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 oestrogen vs PPA | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.90] |
| 6.2 oestrogen vs PFMT | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.18, 2.23] |
| 6.3 oestrogen vs electrostimulation | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.95, 1.75] |
| 7 Number of pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of voids over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Maximum urethral closure pressure (MUCP) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 oestrogen vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 oestrogen vs electrostimulation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Maximum urethral closure pressure (MUCP), crossover studies Show forest plot | Other data | No numeric data | ||
| 12.1 Oetrogens versus PPA | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence not improved (women's observations) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 oestrogen + progesterone vs placebo | 2 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.01, 1.16] |
| 2 Incontinence not improved (generic inverse variance) (women's observations) Show forest plot | 3 | Relative Risk (Fixed, 95% CI) | Subtotals only | |
| 2.1 Systemic administration of oestrogen + progesterone vs placebo | 3 | 10635 | Relative Risk (Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| 3 Incontinent episodes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of diurnal voids per 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of nocturnal voids per 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Use of drugs for incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 oestrogen + progesterone vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of women having incontinence surgery Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 oestrogen + progesterone vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence not improved (women's observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Oestrogen + PPA vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number with incontinence not improved (women's observations) (cross‐over trials) Show forest plot | Other data | No numeric data | ||
| 2.1 oestrogen + PPA vs oestrogen | Other data | No numeric data | ||
| 3 Number of pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of voids over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of women with frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 oestrogen + progesterone vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 oestriol + benzidamine vs oestriol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of women with nocturia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 oestriol + benzidamine vs oestriol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number with adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Oestrogen + PPA vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Volume at first urge to void (cross‐over trials) Show forest plot | Other data | No numeric data | ||
| 9.1 oestrogen + PPA vs oestrogen | Other data | No numeric data | ||
| 10 Maximum bladder capacity (cross‐over trials) Show forest plot | Other data | No numeric data | ||
| 10.1 oestrogen +other vs oestrogen | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence (women's observations) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 oestrogen + PFMT vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 TVT vs TVT + vaginal oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number with incontinence not improved (women's observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 oestrogen + PPA vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of voids over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 detrusitol + vaginal oestrogen vs detrusitol m | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 oestrogen + PPA vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 oestrogen + PFMT vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with incontinence not improved (women's observations) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of women with nocturia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of women with dysuria Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of women with urgency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 oestrogen cream vs vaginal oestrogen + progesterone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of women with frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 oestrogen cream vs vaginal oestrogen + progesterone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of voids over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pad test weights Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of voids over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.87, ‐0.16] |
| 2 Number of nocturnal voids Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.36, ‐1.24] |
| 3 Maximum urethral closure pressure (MUCP) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 3.84 [‐5.77, 13.46] |
| 4 Volume at first urge to void Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.56 [‐12.20, 9.08] |
| 5 Maximum bladder capacity Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 34.90 [8.35, 61.45] |

