| Trial | No | Intervention | Comparison | Duration | Efficacy outcomes | Results(btwn grp cp) |
| SOY DIETARY SUPPLEMENTS | | | | | | |
| Albertazzi 1998 | 104 | 60 g soy powder (76 mg isoflavones) | Placebo (60 g casein) | 12 weeks | No flushes/day after treatment; %age decrease in number of flushes | At end of study, a significant difference between placebo and soy: ‐1.59 (‐1.95 to ‐1.2), p<0.01 representing a mean reduction of 1.6 flushes per day in soy group compared to placebo. 45% reduction in flushes with soy vs 30% reduction with placebo; p<0.01. |
| Balk 2002 | 27 | Soy and corn flour cereal (100 mg/d isoflavones) | Placebo (wheat cereal) | 24 weeks | Hot flush and night sweat symptom score after Rx (1‐4) | NS all outcomes |
| Brzezinski 1997 | 145 | Phytoestrogen enriched diet (individualized by dietician) (isoflavone amount not given) | Control ‐ regular diet (avoiding phytoestrogens) | 12 weeks | MSQ hot flush severity reduction sub score (0‐3) | Greater reduction with PE rich diet, p=0.004 (no CI given) |
| Burke 2003 | 241 | (1) soy drink 1 (42 mg/d isoflavones); (2) soy drink 2 (58 mg/d isoflavones) | Placebo (soy drink with isoflavones removed) | 2 years | No and severity of flushes/sweats per day after Rx (symptom diary); also subgroup analysis in women with 4+ symptoms/d at baseline | NS all outcomes |
| Dalais 1998 | 52 | (1) soy diet (53 mg/d isoflavones); (2) linseed diet (high in isoflavones ‐ amount not given) | Placebo (wheat diet (low isoflavones)) | 12 weeks + 12 weeks | %age decrease in no of hot flushes | NS. 22% reduction with soy; 41% reduction with linseed; 51% reduction with wheat. |
| Knight 2001 | 24 | Soy powder 60 g/d for beverage (134.4 mg/d isoflavones) | Placebo (casein powder for beverage) | 12 weeks | No flushes/wk after Rx | NS. 29 flushes/wk in soy group; 46 flushes/wk in placebo group (reduction in both from baseline) |
| Kotsopoulos 2000 | 94 | Soy powder for beverage (118 mg/d isoflavones) | Placebo (casein powder for beverage) | 12 weeks | Hot flush symptom score (severity) (0‐3) after Rx | NS. 0.77 score with soy; 0.83 score with placebo |
| Lewis 2006 | 99 | (1) Soy flour muffins (42 mg/d isoflavones); (2) flaxseed muffins (50 mg/d lignans) | Placebo (wheat flour muffins (low lignans and no isoflavones)) | 16 weeks | Menoquol vasomotor score; no flushes per day; severity of flushes (1‐7 scale) after Rx | NS all outcomes |
| St Germain 2001 | 69 | (1) Soy protein + (80.4 mg/d isoflavones); (2) soy protein ‐ (4.4 mg/d isoflavones) in muffins and powder for cooking | Placebo (whey protein) | 24 weeks | %age of participants perceiving a decrease in (1) frequency, (2) duration and (3) severity of flushes; no of flushes/wk after Rx; no of sweats/wk after Rx | NS |
| SOY EXTRACT | | | | | | |
| Bica 2004 | 75 | Standardized soy extract (33 mg/d isoflavones) | Placebo capsule | 25 weeks | Greene vasomotor sub scale (intensity); %age who experienced a decrease in frequency of flushes and sweats from baseline | NS Greene vasomotor scale; 74% with soy vs 43% with placebo had decrease in no of hot flushes; p=0.007; 68% with soy vs 46% with placebo had decrease in no of night sweats; p=0.049. NS: severity of symptoms. |
| Campagnoli 2005 | 36 | Standardized soy extract (Soy select) (60 mg/d isoflavones) | Placebo capsules | 12 weeks + 12 weeks | No flushes/week after Rx | NS |
| Duffy 2003 | 36 | Soy supplement capsules (Solgen) (60 mg/d isoflavones) | Placebo capsules | 12 weeks | Greene vasomotor symptom score (severity) | NS |
| Faure 2002 | 75 | Standardized soy extract capsules (70 mg/d isoflavones) | Placebo capsules | 16 weeks | %age decrease in flushes/day; %age of 'responders' (participants who had reduction of at least 50%) | 61% decrease with soy vs 21% reduction with placebo (p value not reported); 66% of soy group were responders vs 34% in placebo group; p<0.005. Repeated measures analysis confirmed the soy‐placebo treatment effect. |
| Han 2002 | 80 | Soy capsules (100 mg/d isoflavones) | Placebo capsules | 16 weeks | Kupperman vasomotor symptom score (severity) | Vasomotor score 8.2 in soy group vs 9.9 in placebo group; p<0.01 |
| Kaari 2006 | 79 | Soy extract capsules (S40/Ach‐1)(120 mg/d isoflavones) | Estrogen + placebo capsules | 24 weeks | %age of participants reporting reduction (subgroup) | NS |
| Khaodhiar 2007 | 207 | Soy extract capsule (isoflavone quantity not known) (1) 40 mg/d, (2) 60 mg/d | Placebo | 12 weeks | %age reduction in frequency of hot flushes (from daily diary), %age reduction in severity of hot flushes (daily diary) | 50% reduction in hot flush frequency in both soy groups at 12 weeks compared to 38% reduction with placebo (NS). When both treatment groups were combined, there was an average reduction of 1.2 more flushes per day in women on soy compared to women on placebo (p=0.016). NS for hot flush severity score (p=0.07) |
| Penotti 2003 | 62 | Soy tablets (72 mg/d isoflavones) | Placebo tablets | 24 weeks | No flushes per day after Rx | NS |
| Upmalis 2000 | 177 | Standardized soy extract tablets (50 mg/d of genistin and daidzin) | Placebo tablets | 12 weeks | %age change in flush severity/wk; %age change in flush frequency/wk; %age change in sweat frequency/wk | 34% change in severity with soy vs 21% change in severity with placebo: p=0.01); NS for %age change in frequency (p=0.078 repeated measures analysis); NS for change in reduction of night sweats. |
| RED CLOVER EXTRACTS | | | | | | |
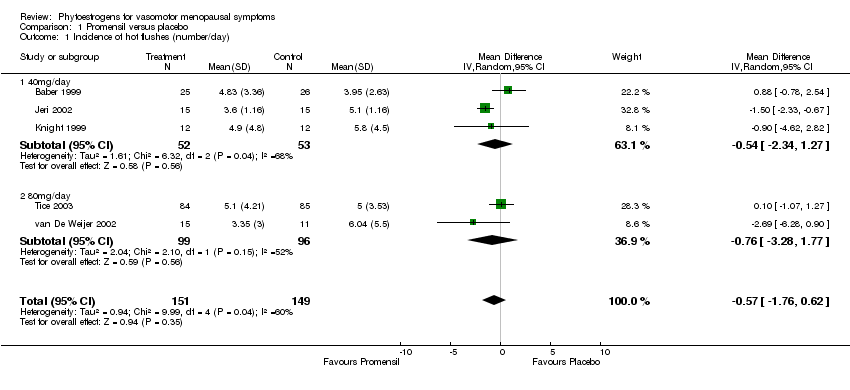
| Baber 1999 | 51 | Promensil (standardised red clover extract) (40 mg/d isoflavones) | Placebo tablet | 12 weeks + 12 weeks | No flushes per day after Rx; %age flush reduction | NS |
| Hidalgo 2005 | 60 | Red clover supplement capsules (80 mg/d isoflavones) | Placebo capsules | 12 weeks + 12 weeks | Kupperman Index score for hot flushes and sweats (severity) (expressed as percentage) | Hot flushes: 15% with red clover vs 98% with placebo; night sweats: 30% with red clover vs 93% with placebo; p value not given |
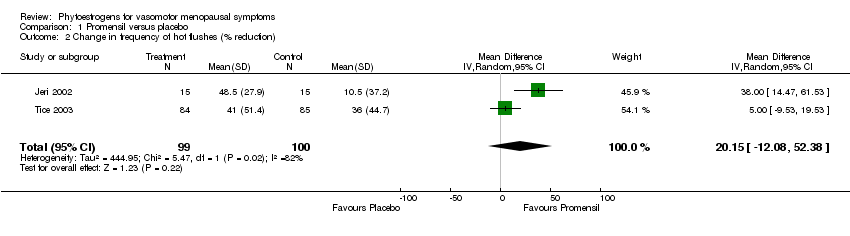
| Jeri 2002 | 30 | Promensil (standardized red clover extract) (40 mg/d isoflavones) | Placebo tablet | 16 weeks | %age reduction in no flushes per day; severity of flushes per day (scale) | Frequency: 49% reduction with red clover vs 11% reduction with placebo: p<0.001); severity: reduction from 2.53 to 1.33 with red clover vs no reduction with placebo: p<0.001. |
| Knight 1999 | 37 | Promensil (standardized red clover extract) (40 mg/d isoflavones) | Placebo tablet | 12 weeks | No flushes per day | NS |
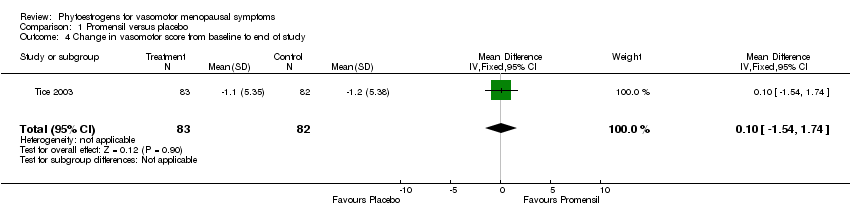
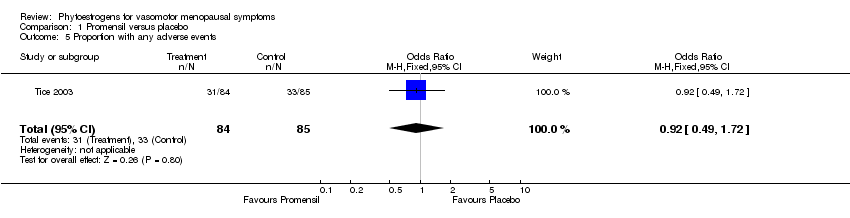
| Tice 2003 | 252 | (1) Promensil (standardized red clover extract) (82 mg/d isoflavones ‐ 2 tablets); (2) Rimostil (standardized red clover extract) (57 mg/d isoflavones ‐ 2 tablets) | Placebo tablets | 12 weeks | No flushes per day; %age reduction in flushes; rate of reduction over time | No flushes per day NS; %age reductions NS; Promensil had faster reduction over time vs placebo (p=0.03) |
| Van de Weijer 2002 | 30 | Promensil (standardized red clover extract) (80 mg/d isoflavones ‐ 2 tablets) | Placebo tablets | 12 weeks | No flushes per day; median %age change in no of flushes | 3.4 flushes per day with Promensil vs 6 flushes per day with placebo (p value not reported); 44% reduction with Promensil vs 0% reduction with placebo: p=0.01 (variation not reported) |
| OTHER PHYTOESTROGENS | | | | | | |
| Crisafulli 2004 | 90 | (1) Genistein extract (54 mg/d isoflavones); (2) continuous HRT (17B estradiol 1mg/d + norethisterone acetate) | Placebo tablets | 1 year | %age change in no of flushes per day | 24% reduction with genistein when compared with placebo: p<0.01; 30% reduction with HRT when compared with genistein: p<0.05 |
| Dodin 2005 | 112 | Flaxseed dietary supplement (in bread and ground grain) (21,071ug lignans) | Placebo (wheat germ) (196ug lignans) | 1 year | Menoquol hot flush and sweat scores (severity) | NS |
| Heyerick 2006 | 67 | (1) hop extract 1 (100ug 8‐prenylnaringenin); (2) hop extract 2 (250ug 8‐prenylnaringenin) (Menohop) | Placebo capsule | 12 weeks | Kupperman hot flush score (severity) | NS at the end of the study period |
| Woo 2003 | 136 | Sachets of Pueraria lobata (Chinese medicinal herb) (100 mg/d isoflavones) | (1) Sequential HRT (CEE 0.625 mg/d continuously + MPA 5mg/d for 14 days of cycle), (2) no treatment | 12 weeks | Vasomotor symptom score; mean change in score (menopause questionnaire) | NS |
| Dalais 1998 | | see above for results in the flaxseed arm | | | | |
| Lewis 2006 | | see above for results in the flaxseed arm | | | | |