Цинк при простуде
Abstract
Background
The common cold is one of the most widespread illnesses and is a leading cause of visits to the doctor and absence from school and work. Trials conducted in high‐income countries since 1984 investigating the role of zinc for the common cold symptoms have had mixed results. Inadequate treatment masking and reduced bioavailability of zinc from some formulations have been cited as influencing results.
Objectives
To assess whether zinc (irrespective of the zinc salt or formulation used) is efficacious in reducing the incidence, severity and duration of common cold symptoms. In addition, we aimed to identify potential sources of heterogeneity in results obtained and to assess their clinical significance.
Search methods
In this updated review, we searched CENTRAL (2012, Issue 12), MEDLINE (1966 to January week 2, 2013), EMBASE (1974 to January 2013), CINAHL (1981 to January 2013), Web of Science (1985 to January 2013), LILACS (1982 to January 2013), WHO ICTRP and clinicaltrials.gov.
Selection criteria
Randomised, double‐blind, placebo‐controlled trials using zinc for at least five consecutive days to treat, or for at least five months to prevent the common cold.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality.
Main results
Five trials were identified in the updated searches in January 2013 and two of them did not meet our inclusion criteria. We included 16 therapeutic trials (1387 participants) and two preventive trials (394 participants). Intake of zinc was associated with a significant reduction in the duration (days) (mean difference (MD) ‐1.03, 95% confidence interval (CI) ‐1.72 to ‐0.34) (P = 0.003) (I2 statistic = 89%) but not the severity of common cold symptoms (MD ‐1.06, 95% CI ‐2.36 to 0.23) (P = 0.11) (I2 statistic = 84%). The proportion of participants who were symptomatic after seven days of treatment was significantly smaller (odds ratio (OR) 0.45, 95% CI 0.20 to 1.00) (P = 0.05) than those in the control, (I2 statistic = 75%). The incidence rate ratio (IRR) of developing a cold (IRR 0.64, 95% CI 0.47 to 0.88) (P = 0.006) (I2 statistic = 88%), school absence (P = 0.0003) and prescription of antibiotics (P < 0.00001) was lower in the zinc group. Overall adverse events (OR 1.58, 95% CI 1.19 to 2.09) (P = 0.002), bad taste (OR 2.31, 95% CI 1.71 to 3.11) (P < 0.00001) and nausea (OR 2.15, 95% CI 1.44 to 3.23) (P = 0.002) were higher in the zinc group. The very high heterogeneity means that the averaged estimates must be viewed with caution.
Authors' conclusions
Zinc administered within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people but some caution is needed due to the heterogeneity of the data. As the zinc lozenges formulation has been widely studied and there is a significant reduction in the duration of cold at a dose of ≥ 75 mg/day, for those considering using zinc it would be best to use it at this dose throughout the cold. Regarding prophylactic zinc supplementation, currently no firm recommendation can be made because of insufficient data. When using zinc lozenges (not as syrup or tablets) the likely benefit has to be balanced against side effects, notably a bad taste and nausea.
Резюме на простом языке
Цинк при простуде
Простуда часто вызывается риновирусом. Это одно из самых распространенных заболеваний, оно является одной из основных причин посещений врача и отсутствия в школе и на работе. Осложнения простуды включают инфекции уха, синусит и обострения реактивных заболеваний дыхательных путей. Нет доказанного лечения простуды. Однако, даже частично эффективные лечение и меры профилактики простуды могут заметно уменьшить проблемы со здоровьем и связанные с этим экономические потери.
Цинк подавляет репликацию вируса и был протестирован в испытаниях по лечению простуды. Этот обзор выявил 18 рандомизированных контролируемых испытаний, включивших 1781 участников всех возрастных групп, сравнивавших цинк с плацебо (без цинка). Мы обнаружили, что цинк (пастилки или сироп) снижает среднюю продолжительность простуды у здоровых людей при приёме в течение 24 часов с момента появления симптомов. Также, показана вероятность, что у людей, принимающих цинк, симптомы простуды не будут продолжаться долее семи дней лечения. Профилактическое использование цинка в виде добавокв течение не менее пяти месяцев снижает заболеваемость, пропуски школьных занятий и назначение антибиотиков детям с простудой, хотя антибиотики не требуются для лечения простуды. Лица, у которых обычные симптомы простуды могут быть проблемными (например, лица с хроническими заболеваниями, иммунодефицитом, астмой и т.д.) не были изучены. Поэтому использование цинка у этой категории лиц в настоящее время не может быть рекомендовано. Учитывая разнообразие доз, лекарственных форм и продолжительности использования цинка во включенных исследованиях, необходимы дополнительные исследования для решения этих вопросов, прежде, чем использование цинка при простуде может быть рекомендовано в общем случае. Однако, поскольку пастилки цинка были широко изучены, и показано значительное сокращение продолжительности простуды при использовании их в дозе ≥ 75 мг/сут, для тех, кто рассматривает использование цинка, наилучшим вариантом было бы использовать его при простуде именно в этой дозе. При использовании цинка в виде пастилок (не в виде сиропа или таблеток) потенциальная польза должна быть соотнесена с побочными эффектами, в частности, плохой вкус и тошнота. Что касается профилактического использования препаратов цинка, в настоящее время не могут быть даны четкие рекомендации в связи с недостаточными данными.
Authors' conclusions
Summary of findings
| Zinc compared with placebo for the common cold | ||||||
| Patient or population: patients with common cold Settings: outpatient Intervention: zinc lozenges or syrup Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc | |||||
| Duration of cold symptoms (days) | The mean duration of cold symptoms ranged across control groups from 5.1 to 9.38 days | The mean duration of cold symptoms ranged across control groups from 4 to 12.1 days | 1656 | ++O | ||
| Severity of symptom score | The mean severity of symptom score ranged across control groups from 0.4 to 5.61 | The mean severity of symptom score ranged across control groups from 0.2 to 3.45 | 513 | ++O | ||
| Incidence of common cold | 618 per 1000 | 382 per 1000 (354 to 431) | RR 0.64 (0.47 to 0.88) | 394 | +OO | |
| Number of participants symptomatic after 7 days of treatment | 563 per 1000 | 373 per 1000 (143 to 508) | OR 0.45 (0.2 to 1.0) | 476 | ++OO | |
| School absence (number of days) | The mean days of school absence ranged across control groups from 1.3 to 1.35 days | The mean days of school absence in the intervention groups was 0.37 lower (0.7 to 0.04 lower) | 394 | +OO | ||
| Antibiotic use | 330 per 1000 | 127 per 1000 (52 to 200) | OR 0.27 (0.16 to 0.46) | 394 | ++OO | |
| Any adverse event | 349 per 1000 | 424 per 1000 (132 to 898) | OR 1.58 (1.19 to 2.09) | 1217 | +++O | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious study limitations: all the studies had adequately concealed allocation and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear in Macknin 1998; Petrus 1998; Turner 2000a; Turner 2000b; Turner 2000c. Petrus 1998 did not adequately describe the sequence generation. Blinding was inadequate in Turner 2000a; Turner 2000b; Turner 2000c. 2Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 89%. The heterogeneity was due to differences in the nature of the different interventions (zinc gluconate versus acetate lozenges, zinc lozenges versus zinc syrup), wide dose ranges, varied duration of symptoms prior to administration of zinc (varying from 24 to 48 hours) and characteristics of the study population (children versus adults). 3No serious indirectness: studies both from low‐income and high‐income regions have assessed this comparison. Therefore, the result can be confidently generalised to all situations. 4No serious imprecision: though the 95% CI around the pooled effect is narrow, the lower limit does not suggest a clinically important reduction in the duration of cold (a decrease in duration of ≤ 1 day is not shown to be important to patients). 5Publication bias cannot be ruled out. 6No serious study limitation: all the studies had adequately concealed allocation and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear and adequate sequence was not generated in one study (Petrus 1998). 7Serious imprecision: the 95% CI around the pooled effect is wide, the lower limit is crossing the point of no effect. 8Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 84%. The heterogeneity may be due to differences in the nature of the different interventions (zinc gluconate or acetate lozenges, zinc sulphate syrup) and dose range (30 to 160 mg/day) as well as mean duration of symptoms prior to administration of zinc (varying from 24 to 48 hours), as well as the characteristics of the study population (children versus adults). However, subgroup analysis was not possible as there were not enough studies for each variable. 9Kurugol 2006b is a community‐based intervention including 200 healthy school children and studying the effect of daily administration of 15 mg zinc sulphate syrup over a period of seven months. Vakili 2009 is also a community‐based intervention including 200 healthy school children and studying the effect of daily administration of 10 mg zinc sulfate tablets over a period of seven months. 10Serious study limitation: though the study by Kurugol 2006b was of high quality, that by Vakili 2009 was of poor methodological quality. 11Serious inconsistency: there is substantial heterogeneity between the two trials: I2 statistic for heterogeneity = 88%. Both trials showed a benefit with zinc, however the size of this effect was much larger in Vakili 2009. The heterogeneity was due to differences in the trial methodology and the nature of the interventions. 12No serious imprecision: the 95% CI around the pooled effect is narrow. Even the lower limit suggests a clinically important reduction in the incidence rate ratio of cold which is shown to be important to patients. 13No serious study limitations: allocation concealment was unclear in two studies, i.e. Smith 1989 and Weismann 1990, though both the studies blinded both participants and study staff. 14Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 75%. The heterogeneity may be due to differences in the nature of the different interventions (zinc gluconate or acetate lozenges) and dose range (30 to 160 mg/day) as well as mean duration of symptoms prior to administration of zinc (varying from 24 to 48 hours, as well as the characteristics of the study population (children versus adults). However, subgroup analysis was not possible as there were not enough studies for each variable. 15Serious indirectness: only studies from high‐income regions have assessed this comparison. Therefore, the result cannot be generalised to all situations. 16No serious imprecision: both limits of the 95% CI suggest a clinically important reduction in proportion of participants given the intervention symptomatic after seven days of treatment. 17Serious inconsistency: there is substantial heterogeneity between the two trials: I2 statistic test for heterogeneity = 64%. Both trials showed reduced days of school absence with intervention, however, the size of this effect was much larger in Kurugol 2006b. The heterogeneity was due to differences in the trial methodology and the nature of the interventions. 18No serious imprecision: though the 95% CI around the pooled effect is narrow, the lower limit does not suggests a clinically important reduction in the duration of school absence (a decrease in duration of ≤ 1 day is not shown to be important to patients). 19No serious inconsistency: there was no statistical heterogeneity. I2 statistic = 0%. 20No serious imprecision: both limits of the 95% CI suggest a clinically important reduction in the rate of antibiotic use with intervention. 21No serious study limitations: all the studies had adequately concealed allocation (except Weismann 1990, in which allocation concealment is unclear) and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear in Macknin 1998 and Weismann 1990. Weismann 1990 did not adequately describe the sequence generation. 22No serious inconsistency: there is no statistical heterogeneity. I2 statistic = 21%. Both the lozenges and syrup preparation trials were pooled. 23No serious imprecision: the 95% CI around the pooled effect is narrow. The resulting adverse events from use of zinc are higher and this is significant. | ||||||
Background
Description of the condition
The common cold is one of the most widespread illnesses, with adults having two to four episodes annually (Garibaldi 1985). Children may have six to 10 colds a year (and up to 12 colds a year for school children) (Simasek 2007). In the United States, the common cold leads to 75 to 100 million physician visits annually at a conservative cost estimate of USD 7.7 billion per year. Americans spend USD 2.9 billion on over‐the‐counter drugs and another USD 400 million on prescription medicines for symptomatic relief (Garibaldi 1985; Simasek 2007). More than one‐third of patients who saw a doctor received an antibiotic prescription, which has implications for antibiotic resistance from overuse of such drugs (Fendrick 2003). An estimated 22 to 189 million school days are missed annually due to a cold. As a result, parents missed 126 million workdays to stay home to care for their children. When added to the 150 million work days missed by employees suffering from a cold, the total economic impact of cold‐related work loss exceeds USD 20 billion per year (Fendrick 2003; Garibaldi 1985). This accounts for 40% of time lost from work (Kirkpatrick 1996). The complications of the common cold include otitis media, sinusitis and exacerbations of reactive airway diseases (Couch 1984; Gwaltney 1966; Turner 2001). Rhinoviruses are the most frequent cause and may account for nearly 80% of common colds during autumn (Turner 2001). There is no proven treatment for the common cold. However, even a medication that is only partially effective in the treatment and prevention of the common cold could markedly reduce morbidity and economic losses due to this illness.
Description of the intervention
The effect of zinc lozenges on the incidence, duration or severity of common cold symptoms has been examined in different studies since 1984. In 1984, Eby et al (Eby 1984) reported for the first time on the efficacy of zinc gluconate lozenges for treatment of the common cold. However, later trials have given variable results. It has been hypothesised that there is a direct correlation between reductions in the duration of common cold symptoms and the daily dosage of all positively charged zinc species released from lozenges at physiologic pH (Eby 1995). The re‐analysis of 10 double‐blind, placebo‐controlled zinc trials by solution chemistry methods showed a significant correlation between total daily dosages of positively charged zinc species and a reduction in the mean duration of common colds (Eby 2004). Zinc gluconate and zinc acetate have very low chemical stability and mainly release positively charged zinc ions in aqueous solutions at physiologic pH, but stronger complexes do not (Eby 2004). Adding a strong zinc binding ligand, such as glycine or citric acid, to a solution containing a zinc complex that is weakly bonded results in the sequestration of zinc to the stronger ligand, reducing or eliminating the benefits of zinc lozenges (Eby 2004). In the review by Marshall it was concluded that zinc gluconate lozenges were effective in reducing the symptoms and duration of the common cold but the side effects and particularly bad taste might limit patient compliance (Marshall 1998). However, results from three trials (Kurugol 2006a; Kurugol 2006b; Kurugol 2007) using zinc sulfate syrup and one trial using zinc sulfate tablet (Vakili 2009) suggested that both the syrup and tablet form are well tolerated and an easy to administer therapy. Adverse effects were mild and had no significant association with the use of zinc sulfate syrup or tablet. The increased incidence of adverse effects noted in the zinc group in various trials may have been related to the use of different ligands (gluconate, acetate) rather than to zinc itself.
How the intervention might work
Interest in the use of zinc for the common cold grew following the results of a randomised controlled trial (RCT) conducted by Eby 1984. Results suggested that if treatment of a cold commenced within three days of the development of cold symptoms and consisted of one 23 mg zinc lozenge dissolved in the mouth every second waking hour, the average duration of cold symptoms was reduced by about seven days. This result was consistent with the earlier observation by Eby (Eby 1984) that a three‐year old girl diagnosed with acute lymphocytic leukaemia who had been treated with a 50 mg zinc tablet to improve her zinc status and to stimulate T‐cell lymphocyte responsiveness recovered from a cold within several hours of receiving treatment. In addition, this effect was claimed to be reproducible in other children and adults. Later trials gave inconclusive results (Turner 2001). Results of trials in which no effect of zinc was demonstrated were criticised for having inadequate sample sizes or formulations that reduced the release of zinc ions from the lozenge (Eby 1995).
In‐vitro assays indicate that zinc possesses antiviral properties (concentrations of 0.1 mM zinc inhibited growth of eight of nine strains of rhinoviruses) and although such activity suggests Eby's results are biologically plausible, only a handful of RCTs have been able to duplicate his findings. Of the 18 trials conducted since 1984, 11 trials have shown zinc may be useful in the treatment of the common cold and seven have shown no benefit. Most trials showing beneficial effects have been criticised for failing to mask treatment adequately due to the occurrence of side effects, while trials showing no benefit have been criticised for using formulations that reduced the bioavailability of zinc.
Although several possibilities have been suggested, the mechanisms of the efficacy of zinc on the common cold are still unexplained. One possibility is that the interaction of zinc with the host's immune function may have a beneficial effect on common cold symptoms (Macknin 1999). Besides this there is a local mechanism of action: the mouth–nose biologically closed electric circuit (BCEC) which appears to explain the rapid therapeutic response to zinc lozenges (Eby 2010). It moves electrons from the nose into the mouth and, in response to the electron flow, it moves positively charged metal ions, such as ionic zinc, from the mouth into the nose. Human rhinoviruses attaching to the nasal epithelium via the intracellular adhesion molecule‐1 (ICAM‐1) receptor cause most colds. The zinc ion, based on its electrical charge, has an affinity for ICAM‐1 receptor sites and may exert an antiviral effect by attaching to the ICAM‐1 receptors in the rhinovirus structure and nasal epithelial cells (Novick 1996). In addition, zinc inhibits viral replication by preventing the formation of viral capsid proteins (Geist 1987; Korant 1976). It has also been suggested that zinc stabilises cell membranes (Pasternak 1987), prevents histamine release (Harisch 1987) and inhibits prostaglandin metabolism (Kelly 1983).
Why it is important to do this review
There is no proven method of prevention or treatment for the common cold. However, any medication that is only partially effective in the treatment and prevention of the common cold could markedly reduce morbidity and economic losses due to this illness. There have been many clinical trials describing the effect of zinc (lozenges and syrup) on common cold symptoms; therefore it is important to know the effect of zinc on the common cold. The last review of all available RCTs of zinc for the common cold was published in 1999. Since then, several new studies (Eby 2006; Kartasurya 2012; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Macknin 1998; McElroy 2003; Petrus 1998; Prasad 2000; Prasad 2008; Turner 2000a; Turner 2000b; Turner 2000c; Vakili 2009; Veverka 2009) have been published. It is therefore important to update the information and include all new clinical trials. We undertook the review to assess the overall effectiveness of zinc (lozenges or syrup) in treating the common cold and to provide some guidance with respect to future research.
Objectives
To assess whether zinc (irrespective of the zinc salt or formulation used) is efficacious in reducing the incidence, severity and duration of common cold symptoms. In addition, we aimed to identify potential sources of heterogeneity in results obtained and to assess their clinical significance.
Methods
Criteria for considering studies for this review
Types of studies
Double‐blind, placebo‐controlled randomised controlled trials (RCTs).
Types of participants
Trial participants were of either gender and of any age.
Types of interventions
Therapeutic trials: interventions commenced within three days of participants developing common cold symptoms and consisted of 1.5 to 2‐hourly treatments with a zinc or placebo lozenge during waking hours, for more than six hours a day for a period of five or more consecutive days.
Prophylactic trials: intervention commenced and continued throughout the cold season for at least five months.
We considered all formulations of zinc (irrespective of the type of salt, formulation and concentration of zinc).
Types of outcome measures
Outcome measures frequently used to determine the clinical efficacy of any common cold treatment are the incidence, severity and duration of cold symptoms. Accordingly, for inclusion in this review, the incidence and severity of at least throat and nasal symptoms and cough needed to be assessed.
Primary outcomes
-
Duration of symptoms.
-
Severity of symptoms.
-
Incidence of the common cold.
Secondary outcomes
-
Proportion of participants symptomatic after three, five or seven days of treatment.
-
Time to resolution of individual symptoms: cough, nasal congestion, nasal drainage and sore throat.
-
Change in individual severity symptom scores: cough, nasal score.
-
School absence (days).
-
Antibiotic use.
-
Adverse events.
We defined duration as the number of days to cold resolution from start of treatment. We considered cold resolution to be the resolution of all cold symptoms or resolution of all but one cold symptom, or the participant believed they had recovered from the cold. Severity of cold symptoms needed to be graded: 0 ‐ no symptoms, 1 ‐ mild symptoms, 2 ‐ moderate symptoms and 3 ‐ severe symptoms. We defined incidence as number of colds per study participant during the study period. Adverse events included any or individual adverse events during or after taking the medications.
Search methods for identification of studies
Electronic searches
For this 2013 review update we updated the searches in CENTRAL, MEDLINE and EMBASE and in addition searched CINAHL, Web of Science and LILACS.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 12, part of The Cochrane Library, www.thecochranelibrary.com (accessed 18 January 2013), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (April 2010 to January week 2, 2013), EMBASE (1974 to January 2013), CINAHL (1981 to January 2013), Web of Science (1985 to January 2013) and LILACS (1982 to January 2013). See details of search strategy in Appendix 1. Details of the previous search strategy are in Appendix 2.
Searching other resources
We searched the US National Institutes of Health, Department of Health and Human Services trials registry www.clinicaltrials.gov and the WHO ICTRP trials registry http://www.who.int/ictrp/en/ (18 June 2012). We also searched bibliographies of published papers for unpublished trials. Two review authors (RRD, MS) assessed the studies to ensure appropriate trials were included in the review and to minimise the potential for selection bias.
Data collection and analysis
More information on the statistical methods used in this review can be found in the relevant section of the Cochrane Acute Respiratory Infections Review Group Module. Comparisons were zinc (lozenges or syrup or tablet) with placebo. We compared outcome measures before and after treatment, as well as after day three, five or seven to accommodate trials of different lengths.
Selection of studies
Two review authors (RRD, MS) independently reviewed the results for inclusion in the analysis. We resolved differences regarding study quality through discussion.
Data extraction and management
We recorded data on a pre‐structured data extraction form. The lead review author (MS) entered data directly into Review Manager (RevMan) (RevMan 2012). An independent coder verified accuracy of data entry. We made no attempt to contact investigators. Most trials were conducted over 10 years ago and in view of the information required to be provided by the investigators, we thought that they would be unable to comply.
Assessment of risk of bias in included studies
We assessed risk of bias in all included studies using The Cochrane Collaboration's 'Risk of bias' methodology (Higgins 2011). Two review authors (RRD, MS) assessed selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other biases, if any. Each item was assessed as high, low or unclear risk of bias along with relevant information reported in the RCT. When the methodological description was unambiguous, one review author entered the methodological description in the 'Risk of bias' table. When the description of methods was ambiguous, the same review author discussed the issue with the co‐author to reach a consensus. The methodological descriptions are summarised in Figure 1 and Figure 2.
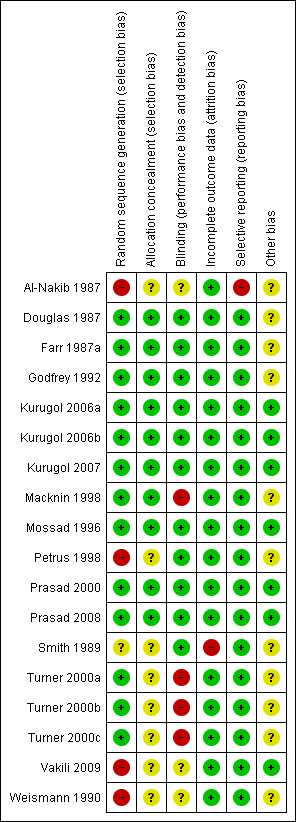
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
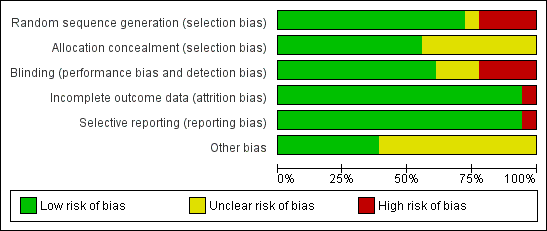
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Measures of treatment effect
We extracted outcome data and entered data into RevMan 2012 for statistical analysis. We used the standard methods of the Cochrane Acute Respiratory Infections (ARI) Review Group to synthesise the data. For dichotomous data, we calculated a pooled estimate of the treatment effect for each outcome across trials using the odds ratio (OR). But, for the measurement of incidence of common cold, the incident rate ratio (IRR) was calculated and expressed as RR to match it with the IRR. For continuous outcomes, we recorded both mean post‐treatment or post‐intervention values and standard deviation (SD). If standard errors (SE) had been reported (and if it were possible) we planned to convert these to standard deviations. We calculated a pooled estimate of treatment effect by calculating either the mean difference (MD) or standardised mean difference (SMD). For both dichotomous and continuous outcomes, we calculated the 95% confidence interval (95% CI) for individual studies. We used fixed‐effect models to obtain summary statistics of all types of outcome measures when there was low degree of heterogeneity. When significant heterogeneity was found, we calculated the overall efficacy using random‐effects models, which provided more realistic estimates of the CIs under these circumstances (Lau 1997). In this context, a P value < 0.05 indicated significant differences between studies and raised questions as to whether the results could be meaningfully combined. Where it was not possible to perform a meta‐analysis, we summarised the data for each trial.
Unit of analysis issues
We included only randomised, double‐blind, placebo‐controlled trials in this review. None of the trials were cross‐over or cluster‐randomised trials.
Dealing with missing data
As many trials were conducted 10 years ago, we thought that the investigators would be unable to compile the missing data, so we did not contact them. For all the outcomes, we considered that incomplete outcome data had been adequately addressed if 80% or more of the participants were included in the analysis, or if less than 80% were included but adequate steps were taken to ensure or demonstrate that this did not bias the results. We performed intention‐to‐treat (ITT) analysis where the above was not clear. In trials with missing statistics (such as SDs or correlation coefficients), we calculated the data from the available information.
Assessment of heterogeneity
We assessed the degree of heterogeneity by using the Chi2 test and the I2 statistic (Higgins 2003; Higgins 2011). The Chi2 test is known to be poor at detecting true heterogeneity among studies; while a statistically significant result indicates heterogeneity, a non‐significant result is not evidence of no heterogeneity. The I2 statistic describes the percentage of total variation across studies that is due to heterogeneity rather than chance. The values of the I2 statistic lie between 0% and 100%. For the current meta‐analysis, we used a simplified categorisation of the I2 statistic as follows: if significant heterogeneity (I2 statistic > 50%) was found, we used a random‐effects model and if low heterogeneity (I2 statistic < 50%) was found, we used a fixed‐effect model.
Assessment of reporting biases
We sought further information from trial authors, although this was not possible for the current meta‐analysis as many of the studies were very old. We looked hard for evidence of collection by study investigators of a small number of key outcomes that are routinely measured in the area in question, and reported which studies reported data on these and which did not. We also constructed a matrix indicating which outcomes were recorded in which studies (for example, with rows as studies and columns as outcomes). Complete and incomplete reporting was also indicated. This matrix showed us which studies did not report outcomes reported by most other studies. We assessed risk of bias due to selective reporting of outcomes for the study as a whole as well for each outcome. We also assessed the likelihood of small study effects, such as publication bias, by examining the funnel plot for asymmetry (Egger 1997).
Data synthesis
We analysed data using a fixed‐effect model in cases of low heterogeneity (I2 statistic value of < 50%) and a random‐effects model in cases of moderate to high heterogeneity between studies (I2 statistic value of > 50%).
Subgroup analysis and investigation of heterogeneity
We considered the following factors as possible explanations for the heterogeneity observed across the results of these studies: dosage and formulations of zinc used, age of participants (children and adults) and the mean duration of symptoms prior to administration of zinc. We plan to investigate these with subgroup analyses when there are sufficient included studies.
Sensitivity analysis
We had planned to perform sensitivity analyses based on methodological quality of the trials (with and without quasi‐randomised trials), but this was not possible as all were randomised, double‐blind, placebo‐controlled trials.
Results
Description of studies
Results of the search
We retrieved 77 additional studies (MEDLINE = 16, CENTRAL = 11, CINAHL = 41, Web of Science = 77, EMBASE = 15 and LILACS = 4) after removing duplicates in the new searches conducted between June 2010 and January 2013. Two review authors (RRD, MS) screened the new search results. Out of 77 new searches, one trial was screened for potential eligibility after reading the abstract. However, this trial (Kartasurya 2012) did not meet the inclusion criteria and was later excluded.
Included studies
We included three additional trials (Turner 2000a; Turner 2000b; Turner 2000c) that were previously excluded, based on unclear methodology. Besides this, no new trials contributed to the data synthesis in this updated review. We included 18 trials (1387 participants in the therapeutic trials and 394 in the preventive trials) in this review.
Location
Three trials were conducted in Turkey (Kurugol 2006a; Kurugol 2006b; Kurugol 2007); one trial each in Iran (Vakili 2009), Denmark (Weismann 1990), UK (Al‐Nakib 1987) and Australia (Douglas 1987); and 11 trials in the USA (Farr 1987a; Godfrey 1992; Macknin 1998; Mossad 1996; Petrus 1998; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c).
Participants
All the trial participants included in the analysis were both adults and children with age range varying from one to 65 years at the start of the trials. Five trials included children only and among these three trials included children aged one to 10 years (Kurugol 2006a; Kurugol 2006b; Kurugol 2007); one included children and adolescents aged six to 16 years (Macknin 1998) and another included children aged 6.5 to 10 years (Vakili 2009). Two trials recruited participants from volunteers experimentally inoculated with human rhinovirus (Al‐Nakib 1987; Farr 1987a). Given that not all participants had cold symptoms at the beginning of the intervention in one trial (Farr 1987a), this trial would be excluded from the statistical overview. In one trial neither the health status of the participants nor the exclusion criteria were stated, while in other trials only healthy subjects from the general population were included. In one trial, participants with cold durations of more than 24 hours (Macknin 1998) and in another trial participants with cold durations of more than 48 hours (Kurugol 2007) were excluded. The trials varied widely in size; two trials had fewer than 50 participants (Prasad 2000; Prasad 2008), four had more than 50 but fewer than 100 participants (Douglas 1987; Farr 1987a; Godfrey 1992; Mossad 1996), eight had more than 100 but fewer than 200 participants (Al‐Nakib 1987; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Petrus 1998; Smith 1989; Weismann 1990; Vakili 2009) and four had more than 200 participants (Macknin 1998; Turner 2000a; Turner 2000b; Turner 2000c).
Interventions
Zinc supplements were provided in the form of either syrup, lozenges or tablets. One trial used zinc sulfate tablet (Vakili 2009) and three trials used zinc sulphate syrup (Kurugol 2006a; Kurugol 2006b; Kurugol 2007). Among the trials using lozenge preparations, two different salts were used: zinc gluconate (Al‐Nakib 1987; Farr 1987a; Godfrey 1992; Macknin 1998; Mossad 1996; Smith 1989; Weismann 1990; Turner 2000a) and zinc acetate (Douglas 1987; Petrus 1998; Prasad 2000; Prasad 2008; Turner 2000b; Turner 2000c). The supplements were given for different periods of time in all the trials. In the therapeutic trials the duration of supplement was five days (Farr 1987a), six days (Al‐Nakib 1987; Douglas 1987), seven days (Farr 1987a; Godfrey 1992; Smith 1989), 10 days (Kurugol 2006a; Kurugol 2007; Weismann 1990), 14 days (Petrus 1998) and no duration mentioned (i.e. participants were given zinc as long as they were symptomatic) (Macknin 1998; Mossad 1996; Prasad 2000; Prasad 2008; Turner 2000a; Turner 2000b; Turner 2000c). In the three trials also studying the prophylactic role of zinc, the duration of supplement was 4.5 days (Al‐Nakib 1987), five months (Vakili 2009) and seven months (Kurugol 2006b).
Outcomes
Primary
Fourteen trials (Douglas 1987; Godfrey 1992; Kurugol 2006a; Kurugol 2007; Macknin 1998; Mossad 1996; Petrus 1998; Prasad 2000; Prasad 2008; Smith 1989; Weismann 1990; Turner 2000a; Turner 2000b; Turner 2000c) reported the duration of symptoms. Among these, five trials have provided the original data (Kurugol 2006a; Kurugol 2006b; Petrus 1998; Prasad 2000; Prasad 2008). The mean and SD were calculated either from the survival curves (Macknin 1998; Mossad 1996; Prasad 2000; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990) or from t/P value (Douglas 1987; Godfrey 1992) reported in other trials. Thirteen trials measured the total severity score of cold symptoms (Al‐Nakib 1987; Douglas 1987; Godfrey 1992; Kurugol 2006a; Kurugol 2007; Petrus 1998; Prasad 2000; Prasad 2008; Smith 1989; Weismann 1990; Turner 2000a; Turner 2000b; Turner 2000c) but results from only five trials (Kurugol 2006a; Kurugol 2007; Petrus 1998; Prasad 2000; Prasad 2008) could be pooled, as in eight trials (Al‐Nakib 1987; Douglas 1987; Godfrey 1992; Smith 1989; Weismann 1990; Turner 2000a; Turner 2000b; Turner 2000c) the results were not reported in a standard format. The incidence of cold symptoms was measured in two trials (Kurugol 2006b; Vakili 2009).
Secondary
The proportion of participants asymptomatic by day three or day five was reported in three trials (Mossad 1996; Smith 1989; Weismann 1990), whereas the proportion of participants asymptomatic by day seven was reported in five trials (Douglas 1987; Godfrey 1992; Mossad 1996; Smith 1989; Weismann 1990). In all these trials, ITT analysis was conducted. Time to resolution of individual cold symptoms was reported as follows: time to resolution of cough in four trials (Kurugol 2006a; Macknin 1998; Prasad 2000; Prasad 2008), time to resolution of nasal congestion in five trials (Kurugol 2006a; Macknin 1998; Petrus 1998; Prasad 2000; Prasad 2008), time to resolution of nasal drainage in five trials (Kurugol 2006a; Macknin 1998; Petrus 1998; Prasad 2000; Prasad 2008) and time to resolution of sore throat in four trials (Kurugol 2006a; Macknin 1998; Prasad 2000; Prasad 2008). Change in individual severity symptom score was reported as follows: change in cough symptom score in two trials (Douglas 1987; Petrus 1998), change in nasal symptom score in four trials (Douglas 1987; Kurugol 2006a; Kurugol 2007; Petrus 1998), change in throat symptom score in two trials (Douglas 1987; Petrus 1998). Standard error of mean (SEM) was not provided in one trial (Douglas 1987). Effect on school absence and antibiotic use were provided in two trials (Kurugol 2006b; Vakili 2009).
Adverse events
Fourteen trials (Douglas 1987; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Macknin 1998; Mossad 1996; Prasad 2000; Prasad 2008; Smith 1989; Weismann 1990; Vakili 2009; Turner 2000a; Turner 2000b; Turner 2000c) reported adverse events. Common adverse events included bad taste, nausea, constipation, diarrhoea, abdominal pain, dry mouth and oral irritation. We reported the adverse events separately for both lozenges and syrup formulations.
Other
Six trials using experimentally induced colds with rhinovirus also studied the number of participants shedding the virus, duration of viral shedding, number of virus‐positive days, as well as rise in antibody titre. These were not included in the outcome measures as we thought that it would not be of help in drawing conclusions. Three trials reported the effect of zinc supplementation on school absence. Among these, two (Kurugol 2006b; Vakili 2009) reported this outcome during a prophylactic trial, though another (Macknin 1998) was a therapeutic trial.
Excluded studies
We excluded five trials.
-
Inclusion criteria not defined, disproportionate number of drop‐outs from the zinc group (Eby 1984).
-
Two studies were not RCTs (McElroy 2003).
-
Measured upper respiratory tract infection as a whole (including common cold, seasonal influenza) (Veverka 2009).
-
Used both zinc gluconate nasal spray and zinc orotate lozenges simultaneously (Eby 2006).
-
Studies upper respiratory tract infection as a whole, used zinc supplementation for four months (Kartasurya 2012).
Risk of bias in included studies
Allocation
Allocation concealment was adequate in 10 studies (Douglas 1987; Farr 1987a; Godfrey 1992; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Macknin 1998; Mossad 1996; Prasad 2000; Prasad 2008). It was unclear in seven studies (Al‐Nakib 1987; Petrus 1998; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990) and not described in one (Vakili 2009).
Adequate sequence generation was described in seven studies (Douglas 1987; Godfrey 1992; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Macknin 1998; Mossad 1996). However, it was not clear in seven studies (Farr 1987a; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c) and not generated in four studies (Al‐Nakib 1987; Petrus 1998; Vakili 2009; Weismann 1990).
Blinding
All 18 studies were blinded but placebo blinding was adequately described in 10 trials (Douglas 1987; Godfrey 1992; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Macknin 1998; Mossad 1996; Prasad 2000; Prasad 2008; Smith 1989). Zinc‐treated participants also experienced higher incidences of side effects and/or complaints, and in 12 trials, zinc‐treated participants complained of altered, bad or unpalatable taste which suggests that the zinc lozenges were distinct from the placebo lozenges and, in this respect, blinding may have been compromised.
Incomplete outcome data
Data were fully detailed in 15 studies and in the remaining three studies (Al‐Nakib 1987; Turner 2000a; Turner 2000b; Turner 2000c) details of attrition and exclusions from the analysis were unavailable.
Selective reporting
Except two studies (Al‐Nakib 1987; Weismann 1990), 16 studies scored 'yes' for being free from selective reporting.
Other potential sources of bias
Eleven studies were funded by pharmaceutical companies (Al‐Nakib 1987; Douglas 1987; Godfrey 1992; Farr 1987a; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Macknin 1998; Petrus 1998; Smith 1989; Weismann 1990). Five studies were supported Medical Research Foundation (Godfrey 1992; Mossad 1996; Prasad 2000; Prasad 2008; Vakili 2009) and in addition by National Institute of Health (NIH) (Prasad 2008). Information on clearance by Ethics Committees or Institutional Review Boards was available for all except one study (Smith 1989). In three studies, other sources of bias were not clear (Turner 2000a; Turner 2000b; Turner 2000c).
Effects of interventions
See: Summary of findings for the main comparison
1. Primary outcomes
(i) Therapeutic effects of zinc
Duration of cold symptoms
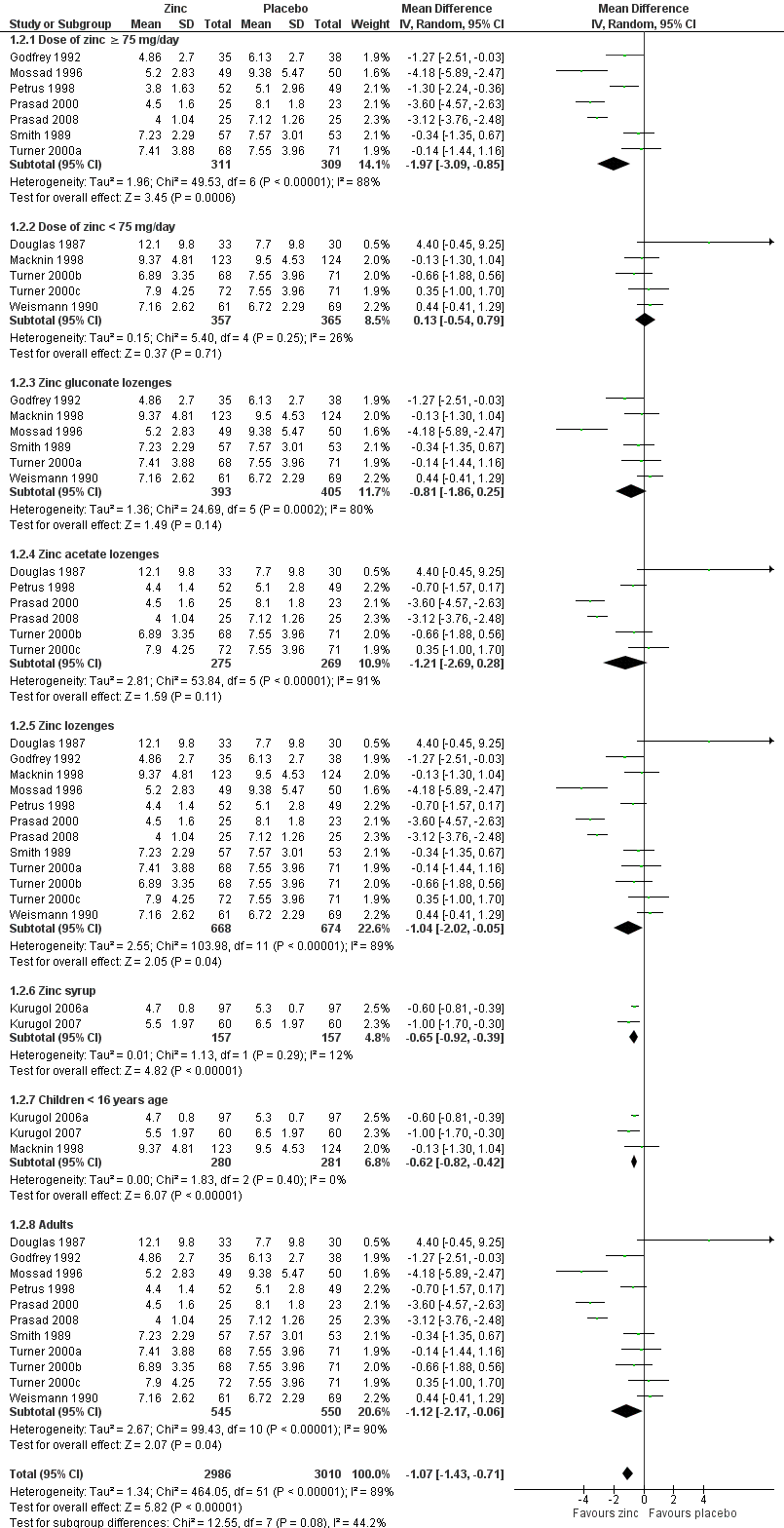
Fourteen studies (Godfrey 1992; Kurugol 2006a; Kurugol 2007; Macknin 1998; Mossad 1996; Petrus 1998; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990) reported this outcome. Results could be pooled from all the studies and there were 1656 participants including both children and adults (Figure 3; Analysis 1.1). The studies were heterogenous in terms of variable formulations (zinc gluconate or acetate lozenges, zinc sulphate syrup) and dose range (30 mg/day to 160 mg/day) as well as MD of symptoms prior to administration of zinc (varying from 24 to 48 hours). Intake of zinc lozenges or syrup was associated with a significant reduction in the duration (days) of common cold (mean difference (MD) ‐1.03, 95% confidence interval (CI) ‐1.72 to ‐0.34) (P = 0.003) when it was administered within 24 hours of the onset of symptoms. However, there was marked heterogeneity among the included trials. We did subgroup analysis for the following: dose (≥ 75 mg/day versus < 75 mg/day), types of lozenges (gluconate versus acetate), formulation (lozenges versus syrup) and age group (children < 16 years versus adults) (Figure 4; Analysis 1.2).
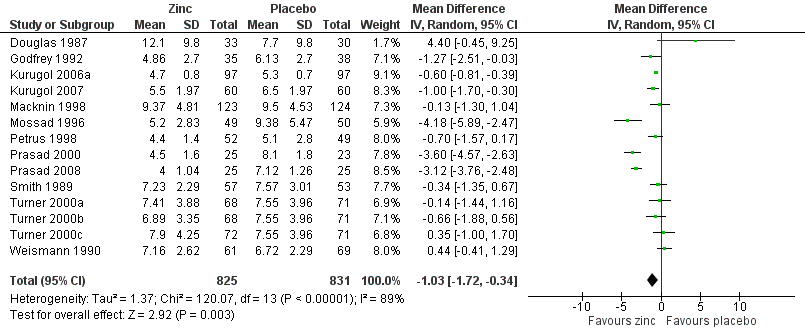
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.1 Duration of cold symptoms (in days).
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.1 Duration of cold symptoms (in days).
Seven trials (Godfrey 1992; Mossad 1996; Petrus 1998; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a) used zinc lozenges at a dose of ≥ 75 mg/day, and five trials (Douglas 1987; Macknin 1998; Turner 2000b; Turner 2000c; Weismann 1990) used a dose of < 75 mg/day. Pooled results from the trials using ≥ 75 mg/day of zinc showed a significant reduction in the duration (days) of common cold (MD ‐1.97, 95% CI ‐3.09 to ‐0.85) (P = 0.0006), whereas < 75 mg/day of zinc did not (MD 0.13, 95% CI ‐0.54 to 0.79) (P = 0.71). However, there was marked heterogeneity among the trials using ≥ 75 mg/day of zinc lozenges. Five trials (Godfrey 1992; Mossad 1996; Macknin 1998; Smith 1989; Turner 2000a) used zinc gluconate lozenges, and six trials (Douglas 1987; Petrus 1998; Prasad 2000; Prasad 2008; Turner 2000b; Turner 2000c) used zinc acetate lozenges. Pooled result from the trials using either zinc gluconate (MD ‐0.81, 95% CI ‐1.86 to 0.25) (P = 0.14) or acetate lozenges (MD ‐1.21, 95% CI ‐2.69 to 0.28) (P = 0.11) did not show a reduction in the duration (days) of common cold. Again, there was marked heterogeneity among the trials. Twelve trials (Douglas 1987; Godfrey 1992; Mossad 1996; Macknin 1998; Petrus 1998; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990) used zinc lozenges, and two trials (Kurugol 2006a; Kurugol 2007) used zinc syrup formulations. Pooled result from the trials using either zinc lozenges (MD ‐1.04, 95% CI ‐2.02 to ‐0.05) (P = 0.04) or syrup formulations (MD ‐0.65, 95% CI ‐0.92 to ‐0.39) (P < 0.00001) showed a significant reduction in the duration (days) of common cold. There was marked heterogeneity among the trials using lozenges formulations. Eleven trials (Douglas 1987; Godfrey 1992; Mossad 1996; Petrus 1998; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990) studied the effect in adults, and three trials (Kurugol 2006a; Kurugol 2007; Macknin 1998) in children < 16 years age. Pooled results from the trials on adults (MD ‐1.12, 95% CI ‐2.17 to ‐0.06) (P = 0.04) or children (MD ‐0.62, 95% CI ‐0.82 to ‐0.42) (P < 0.00001) showed a significant reduction in the duration (days) of common cold. However, there was marked heterogeneity among the trials involving adult participants.
Severity of cold symptoms
Thirteen studies measured the mean severity score of cold symptoms (Al‐Nakib 1987; Douglas 1987; Godfrey 1992; Kurugol 2006a; Kurugol 2007; Petrus 1998; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990). Results from five studies (Kurugol 2006a; Kurugol 2007; Petrus 1998; Prasad 2000; Prasad 2008) including a total of 513 participants (Analysis 1.3; Figure 5) could be pooled. The difference was not significant between the intervention and control groups for reduction in the severity of cold symptoms (MD ‐1.06, 95% CI ‐2.36 to 0.23) (P = 0.11). In all but two studies, the intervention started within 24 hours of onset of symptoms. In the studies by Douglas 1987 and Kurugol 2007 the intervention started within 24 to 48 hours after the onset of symptoms. In the study by Godfrey 1992 the authors found a significant decrease in the severity of symptoms when treatment was administered within 24 hours, compared to treatment administration within 48 hours. In the trial by Al‐Nakib 1987, the zinc group had a significantly lower mean daily clinical score than the placebo group; the difference in scores attaining statistical significance by day four and day five of treatment. However, in the study conducted by Douglas 1987, there were no significant differences between the two groups. Five studies (Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990) reported summed severity scores which could not be pooled. One study (Smith 1989) found a reduction in summed severity score in the zinc group, whereas others (Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990) did not. Again the dosages, formulations and time of administration of zinc differed among the studies. As insufficient data were available, we could only carry out subgroup analysis for lozenges versus syrup formulation (Analysis 1.4). Three trials (Petrus 1998; Prasad 2000; Prasad 2008) used lozenges and two trials (Kurugol 2006a; Kurugol 2007) used syrup formulations. Pooled results from the trials using either lozenges (MD ‐1.55, 95% CI ‐3.62 to 0.52) (P = 0.14) or syrup formulations (MD ‐0.27, 95% CI ‐1.51 to 0.97) (P = 0.67) did not show a reduction in the duration of the common cold. There was marked heterogeneity among the trials using the lozenge formulation.
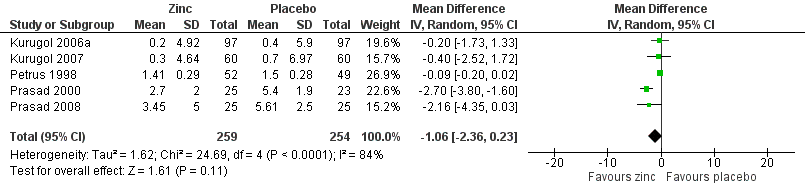
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.2 Severity of symptoms (score).
(ii) Prophylactic effects of zinc
Incidence of common cold
This was reported in two studies (Kurugol 2006b; Vakili 2009). The two studies used variable dose, formulation and duration of zinc. The follow‐up periods of the two studies were different, therefore we based the calculation of the incidence rates on person‐years. The person‐time incidence rate is an appropriate measure of incidence when follow‐up times are unequal (Rothman 1988). Incidence density is defined as the number of incident cases occurring in a susceptible population followed over a given time period; its units are therefore expressed as the number of cases per unit of person‐time. The incidence density ratio is defined as the ratio of incidence density of an exposed group to that of an unexposed group. For each study, we calculated the incident rate ratio (IRR) of catching a cold in treated participants compared to the risk in control participants (Analysis 1.5; Figure 6). The IRR of developing a cold in subjects who received the intervention was 0.64 (95% CI 0.47 to 0.88), compared to participants in the control group (P = 0.006).
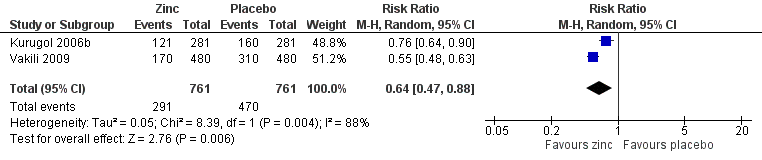
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.3 Incidence of common cold (IRR).
2. Secondary outcomes
(i) Therapeutic effects of zinc
Proportion of participants symptomatic after three, five or seven days of treatment
Proportion of participants symptomatic after three days of treatment
Three studies (Mossad 1996; Smith 1989; Weismann 1990) included a total of 340 participants. There was no significant difference between the intervention and control group for the proportion of participants symptomatic after day three of treatment (odds ratio (OR) 0.81, 95% CI 0.27 to 2.42) (P = 0.7) (Analysis 2.1).
Proportion of participants symptomatic after five days of treatment
Three studies (Mossad 1996; Smith 1989; Weismann 1990) included a total of 340 participants. There was no significant difference between the intervention and control group for proportion of participants symptomatic after day five of treatment (OR 0.78, 95% CI 0.32 to 1.95) (P = 0.6) (Analysis 2.2).
Proportion of participants symptomatic after seven days of treatment
Five studies (Douglas 1987; Godfrey 1992; Mossad 1996; Smith 1989; Weismann 1990) included a total of 476 participants. There was a significant difference between the intervention and control group for proportion of participants symptomatic after day seven of treatment (OR 0.45, 95% CI 0.20 to 1.00) (P = 0.05) (Analysis 2.3).
Time to resolution of individual cold symptoms
This was reported in days in five studies.
Time to resolution of cough
Four studies (Kurugol 2006a; Macknin 1998; Prasad 2000; Prasad 2008) included a total of 453 participants (intervention = 219, control = 234). The time taken for resolution of cough was significantly shorter in the intervention group (MD ‐1.73, 95% CI ‐3.49 to 0.03) (P = 0.05) (Analysis 2.4).
Time to resolution of nasal congestion
Five studies (Kurugol 2006a; Macknin 1998; Petrus 1998; Prasad 2000; Prasad 2008) included a total of 605 participants (intervention = 302, control = 303). The time taken for resolution of nasal congestion was significantly shorter in the intervention group (MD ‐0.7, 95% CI ‐1.39 to ‐0.01) (P = 0.02) (Analysis 2.5).
Time to resolution of nasal drainage
Five studies (Kurugol 2006a; Macknin 1998; Petrus 1998; Prasad 2000; Prasad 2008) included a total of 599 participants (intervention = 298, control = 301). The time taken for resolution of nasal drainage was significantly shorter in the intervention group (MD ‐1.01, 95% CI ‐2.01 to ‐0.01) (P = 0.05) (Analysis 2.6).
Time to resolution of sore throat
Four studies (Kurugol 2006a; Macknin 1998; Prasad 2000; Prasad 2008) included a total of 430 participants (intervention = 211, control = 219). The time taken for resolution of sore throat was significantly shorter in the intervention group (MD ‐0.46, 95% CI ‐0.82 to ‐0.09) (P = 0.02) (Analysis 2.7).
Change in individual severity symptom scores
Change in cough symptom score
This was reported in two studies (Douglas 1987; Petrus 1998). In the study by Douglas 1987, a total of 63 treatment courses were evaluated (intervention = 33, control = 30) and the mean cough score (standard error of mean (SEM) not provided) was lower in the control group (6.3) than in the intervention group (10.6), which was not statistically significant (P = 0.2). In the study by Petrus 1998, a total of 101 participants were included and there was a significant decrease in the mean cough score in the intervention group (MD ‐0.23, 95% CI ‐0.26 to ‐0.2) (P < 0.00001) (Analysis 2.8).
Change in nasal symptom score
This was reported in four studies (Douglas 1987; Kurugol 2006a; Kurugol 2007; Petrus 1998). In the study by Douglas 1987, a total of 63 treatment courses were evaluated and the mean nasal score (SEM not provided) was lower in the control group (9.8) than in the intervention group (11.7), which was not statistically significant (P = 0.5). In the study by Petrus 1998, a total of 101 participants were included and there was a decrease in the mean nasal score (not significant) in the intervention group (nasal congestion: placebo 1.43 ± 0.05, zinc 1.54 ± 0.08; nasal drainage: placebo 1.61 ± 0.07, zinc 1.45 ± 0.07). In the Kurugol 2006a and Kurugol 2007 studies a total of 314 participants were included and there was no difference between the two groups for the change in nasal symptom score (MD ‐0.2, 95% CI ‐1.34 to 0.94) (P = 0.73) (Analysis 2.9).
Change in throat symptom score
This was reported in two studies (Douglas 1987; Petrus 1998). In one study (Douglas 1987), a total of 63 treatment courses were evaluated and the mean throat score (SEM not provided) was lower in the intervention group (6.1) than in the control group (6.2), which was not statistically significant (P = 0.96). In another study (Petrus 1998), a total of 101 participants were included and there was a decrease in the mean throat score (not significant) in the intervention group (sore throat: placebo 1.34 ± 0.11, zinc 1.26 ± 0.06; scratchy throat: placebo 1.53 ± 0.08, zinc 1.38 ± 0.1).
(ii) Prophylactic effects of zinc
School absence (days)
Three trials reported this outcome. The pooled result from the two preventive trials (Kurugol 2006a; Vakili 2009) showed that zinc‐supplemented children were absent for fewer days from school (MD ‐0.66, 95% CI ‐0.99 to ‐0.33) (P < 0.0001) (Analysis 2.10). In one of the therapeutic trials (Macknin 1998), children taking zinc were less likely to be absent than children taking placebo (OR 0.60, 95% CI 0.32 to 1.13) (P = 0.12), but the result was not significant.
Antibiotics use
Two trials reported this outcome (Kurugol 2006b; Vakili 2009). The antibiotic prescription was more likely in placebo than in zinc‐supplemented children (OR 0.27, 95% CI 0.16 to 0.46) (P < 0.00001) (Analysis 2.11).
(iii) Adverse events
Thirteen trials (Douglas 1987; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Macknin 1998; Mossad 1996; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Weismann 1990) reported any or individual adverse events. The incidence of any adverse event was higher in the zinc group (OR 1.58, 95% CI 1.19 to 2.09) (P = 0.002) than in the placebo group. Among the zinc group, the lozenges formulation (OR 2.00, 95% CI 1.40 to 2.86) (P = 0.0001) was more like to produce any adverse events than the syrup formulation (OR 1.03, 95% CI 0.64 to 1.66) (P = 0.9) (Analysis 2.12). Among individual events, bad taste (OR 2.31, 95% CI 1.71 to 3.11) (P < 0.00001) (Analysis 2.13) and nausea (OR 2.15, 95% CI 1.44 to 3.23) (P = 0.002) (Analysis 2.14) had a higher incidence in the zinc group. Among the zinc group, the lozenges formulation (OR 2.66, 95% CI 1.91 to 3.69) (P < 0.00001) was more like to produce bad taste than the syrup formulation (OR 1.15, 95% CI 0.55 to 2.39) (P = 0.71). There was no significant difference between the two groups in the incidence of constipation (P = 0.17) (Analysis 2.15), diarrhoea (P = 0.08) (Analysis 2.16), abdominal pain (P = 0.25) (Analysis 2.17), dry mouth (P = 0.09) (Analysis 2.18) and oral irritation (P = 0.50) (Analysis 2.19).
(iv) Publication bias
To assess whether there was a bias in the published literature, we plotted the effect size of each trial versus variance for one of the primary outcome (duration of common cold). The funnel plot generated here shows that most of the precise studies (towards the top of the plots) have effect sizes which are either zero or very close to it (Analysis 1.1). One explanation for such asymmetry might be publication bias.
Discussion
The discussion is divided into two parts: the first will discuss important methodological issues that have emerged from research in this area, and the second part will discuss the results obtained and their clinical significance.
Part 1: methodology
Since Eby's trial in 1984 (Eby 1984), many trials have investigated whether zinc is efficacious in the treatment or prevention of the common cold. Among these 18 trials were included in this 2013 review. We rated the methodological quality of the included trials as good, with two trials excluded because of poor quality. Eby's trial realised a number of limitations which raised concerns regarding the validity of the results. Treatment blinding in the trial has been questioned as zinc lozenges were found to be unpalatable, distorted the taste of participants and caused a higher incidence of side effects. In addition, investigators relied solely on the subjective assessment of cold symptoms; laboratory confirmation of viral infection was not conducted and analyses were only conducted on a subgroup of those originally enrolled in the trial. Eby's trial was nevertheless instructive and highlighted a number of methodological issues.
Like Eby's trial, most trials have relied on community‐acquired infections. However, five trials recruited participants from volunteers experimentally inoculated with human rhinovirus (Al‐Nakib 1987; Farr 1987a; Turner 2000a; Turner 2000b; Turner 2000c). While high rates of infection with human rhinovirus were attained in the later trials and most participants experienced cold symptoms, in trials relying on community‐acquired infection, the infecting agent and the infection rates were generally not determined.
In trials relying on community‐acquired infection, investigators relied on trial participants or family members to assess the incidence and severity of cold symptoms. Though in most of the trials information was generally provided on how compliance with the recording of symptoms was assessed, objective periodic assessments of the clinical severity of respiratory symptoms were not conducted. In the trials conducted by Al‐Nakib 1987, Farr 1987a, Macknin 1998, Kurugol 2006a, Kurugol 2006b, Kurugol 2007 and Turner 2000a, Turner 2000b and Turner 2000c, symptoms were assessed by trial personnel thus providing some assurance as to the validity of clinical severity scores and estimates based on such scores. Assessment of response to treatment also depended on objective measurements such as nasal mucus weight or tissue counts, which was measured in one study (Al‐Nakib 1987) and the authors found that zinc gluconate reduced both of these parameters. However, as in most studies children were involved, this was not practical.
Research by Farr 1987b suggested that in most trials the size of the placebo‐blinding study used to determine whether zinc and placebo lozenges were indistinguishable was not sufficiently large to detect a significant difference. In their efforts to find a suitable matching placebo lozenge, Farr showed that a false negative result may occur if a small subject population (i.e. fewer than 20) is used. Given that placebo‐blinding studies were only conducted in six of the 18 trials, and with the exception of three trials (Farr 1987a; Prasad 2000; Prasad 2008) the size of the placebo‐matching studies in two of the remaining three trials (no information was provided on the size of placebo study conducted by Weismann 1990) ranged from eight to 20, the adequacy of blinding in most trials is questioned. Zinc‐treated participants also experienced higher incidences of side effects, complaints or both, and in seven trials zinc‐treated participants complained of altered, bad or unpalatable taste, which suggests that zinc lozenges were distinct from placebo lozenges and, in this respect, blinding may have been compromised. However, the increased incidence of bad taste and nausea found by Mossad 1996; constipation and mouth dryness found by Prasad 2000; and bad taste, nausea, mouth, tongue or throat discomfort and diarrhoea found by Macknin 1998, may have been related to the use of different ligands (gluconate, acetate) rather than to zinc itself.
Much of the controversy surrounding the use of zinc lozenges in the treatment of the common cold has concerned whether formulations used in trials showing no benefit failed to release sufficient zinc ions to be effective. It has been hypothesised that there is a direct correlation between reductions in the duration of common cold symptoms and the daily dosage of all positively charged zinc species released from lozenges at physiologic pH (Eby 1995). The reanalysis of 10 double‐blind, placebo‐controlled zinc trials by solution chemistry methods showed a significant correlation between total daily dosages of positively charged zinc species and a reduction in the mean duration of common colds (Eby 2004). Zinc gluconate and zinc acetate have very low chemical stability and mainly release positively charged zinc ions in aqueous solutions at physiologic pH, but stronger complexes do not (Eby 2004). Adding a strong zinc‐binding ligand, such as glycine, citric acid, tartaric acid, mannitol and sorbitol, to a solution containing a zinc complex that is weakly bonded results in the sequestration of zinc to the stronger ligand, reducing or eliminating the benefits of zinc lozenges (Eby 2004). The extent to which the zinc ion was released from formulations reporting no benefit is not known. However, experimental evidence suggests that in saliva the ionisation of zinc to free zinc for some formulations may have been completely (Zarembo 1992) or partially (Farr 1988) suppressed. A formulation developed by Godfrey (Godfrey 1992) that incorporates glycine has been shown to release more than 50% of the zinc from zinc gluconate as the zinc ion. Results from trials conducted by Godfrey (Godfrey 1992) and Mossad (Mossad 1996) suggest this formulation reduced the duration and severity of respiratory symptoms; whereas the trials conducted by Macknin (Macknin 1998) and Turner (Turner 2000a; Turner 2000b; Turner 2000c) suggest no effect of this formulation. The placebo‐matching was inadequate; consequently the adequacy of blinding in all these trials is questioned. Hatch et al (Hatch 1987) reported that zinc acetate releases essentially 100% of its zinc as Zn2+ ions at a physiological pH. Thus, use of zinc lozenges may be advantageous. Results from trials conducted by Petrus (Petrus 1998) and Prasad (Prasad 2000; Prasad 2008) suggest this formulation reduced the duration and severity of respiratory symptoms. These studies used compressed lozenges designed by George Eby that were identical in composition. In addition to zinc acetate, they contained directly compressible (agglomerated) dextrose as the tablet base, glycerol monostearate (2.5% of tablet weight) as the tablet lubricant, stevia for added sweetness, and peppermint oil for flavour, with the composition compressed to near maximal hardness for slowest dissolution. These ingredients were specifically chosen because they do not react with ionic zinc. The trials conducted by Douglas (Douglas 1987) and Turner (Turner 2000a; Turner 2000b; Turner 2000c) using zinc acetate suggest no effect of this formulation. Among these, the placebo‐matching and blinding were stated to be adequate in all except the trial by Turner. Three trials (Kurugol 2006a; Kurugol 2006b; Kurugol 2007) used syrup preparation of zinc (zinc sulphate) and found reduced duration and severity of respiratory symptoms without any increase in adverse effects in the zinc group. Placebo‐matching and adequacy of blinding was not stated in these trials. Another trial (Vakili 2009) used tablet preparation (zinc sulphate) and found decreased incidence, fewer school absences, less antibiotic administration and no adverse effects in zinc‐supplemented children. Again placebo‐matching and adequacy of blinding was not stated in this trial.
The toxicology of zinc has been well characterised. The potential for elevated blood levels of zinc to disrupt copper metabolism and other nutrients preclude its long‐term use in the treatment of the common cold (Pfeiffer 1980). Doses higher than 150 mg/day have also been associated with adverse effects (Chandra 1984). In addition, the higher incidence of side effects in zinc‐treated participants will most likely limit the usefulness of zinc in the treatment of common cold symptoms. In 14 trials (Douglas 1987; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Macknin 1998; Mossad 1996; Prasad 2000; Prasad 2008; Smith 1989; Turner 2000a; Turner 2000b; Turner 2000c; Vakili 2009; Weismann 1990) included for reporting of any or individual adverse events, the overall adverse events were higher in the intervention than in the control group, except in one trial (Vakili 2009).
Viral studies were performed in nine trials (Al‐Nakib 1987; Douglas 1987; Farr 1987a; Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Turner 2000a; Turner 2000b; Turner 2000c). While in‐vitro studies suggest zinc inhibits viral replication and the concentration of zinc in saliva should be sufficient to induce such an effect, six trials (Al‐Nakib 1987; Douglas 1987; Farr 1987a; Turner 2000a; Turner 2000b; Turner 2000c) found no effect of zinc on incidence or shedding of rhinovirus by study participants. In relation to this, trials by Farr and Douglas found no effect of zinc in the treatment of cold, while Al‐Nakib found reduction in the clinical symptom score. From the trial by Al‐Nakib, it might be suggested that medication may have had an effect on signs and symptoms of the colds rather than on virus replication. If this is the case, it would be interesting to know whether zinc would also have the same effect on corona virus colds or, indeed, on colds caused by other respiratory viruses. This may be the future area of research in zinc and common cold trials. Trials by Kurugol (Kurugol 2006a; Kurugol 2006b; Kurugol 2007) did not study the effect of zinc on rhinovirus cold; rather they excluded colds due to influenza viruses from analysis and found that zinc is effective in the treatment of the common cold.
Part 2: results
Although most investigators required participants to record the clinical severity of symptoms each day and used similar scales against which to rate symptom severity (symptoms were rated as none, mild, moderate or severe), there was little commonality in summary estimates used by investigators to describe the duration, incidence and severity of respiratory symptoms. Consequently for some outcomes it was not possible to pool the results from all the trials that reported the same outcome. In addition, there was insufficient detail provided in most published papers to determine whether trials used similar criteria for rating the severity of each symptom and therefore it was not possible to standardise clinical severity scores across all trials.
Among the trials reporting the duration of cold symptoms, results could be pooled from 14 trials. Intake of zinc lozenges or syrup was associated with a significant reduction in the duration of common cold, but there was marked heterogeneity among the included trials. Among these, two trials (Kurugol 2006a; Kurugol 2007) used syrup preparation at a daily dose of 30 mg, whereas other trials used lozenge preparation at a daily dose varying from 30 mg to 190 mg. In a subgroup analysis, the results were significant for the dose of zinc lozenges ≥ 75 mg/day, any formulation of zinc (lozenges or syrup) and any age group (children or adult). Results were not significant for any formulations of zinc lozenges (gluconate or acetate). However, the heterogeneity persisted for all these subgroups except syrup formulation of zinc. Among the two trials using syrup preparation, one trial (Kurugol 2007) found no significant effect, and the other found significant reduction of the duration of common cold. There has been speculation regarding how zinc cures common cold. The most biologically plausible hypothesis, as well as experimental, was by Eby (Eby 2010; Eby 2012). As described by him, the mouth–nose act as a biologically closed electric circuit (BCEC). It moves electrons from the nose into the mouth and, in response to the electron flow, it moves positively charged metal ions, such as ionic zinc, from the mouth into the nose. This BCEC does not transport neutrally or negatively charged zinc into the nose; so the lozenges made with non‐positively charged zinc are supposed not to work in common cold therapy. Considering these properties, the observation that zinc lozenges releasing positively charged ionic zinc shorten colds in a dose–response manner can be seen in a more plausible light. This is also supported by finding of usefulness of ≥ 75 mg/day of zinc lozenges in reducing the duration of common cold in the present review. The BCEC also explains why patients with colds and a history of allergy responded so much faster than patients with colds and no history of allergy, as shown by Petrus (Petrus 1998). Orally ingested forms such as tablets, liquids and syrups do not have these properties and therefore, according to Eby's research, are not effective therapy for the common cold (Eby 2012).
Among 13 trials measuring the severity score of cold symptoms, results from five trials (Kurugol 2006a; Kurugol 2007; Petrus 1998; Prasad 2000; Prasad 2008) could be pooled. There was a significant difference between the intervention and control group for reduction in the severity of cold symptoms. The trials using syrup preparation used a daily dose of 30 mg, whereas the trials using lozenge preparation used daily doses varying from 30 mg to 276 mg. Al‐Nakib (Al‐Nakib 1987) and Douglas (Douglas 1987) provided estimates of mean clinical scores. However, estimates were not directly comparable. In the trial by Al‐Nakib, the zinc group had a significantly lower mean daily clinical score than the placebo group, with the difference in scores attaining statistical significance on days four and five. However, in the trial conducted by Douglas, there were no statistically significant differences between zinc and placebo groups with respect to mean nasal, throat and cough scores. Results of the trial conducted by Godfrey (Godfrey 1992) suggested treatment with zinc reduced the frequency and severity of cold symptoms, which was noticeable by day five and significant by day seven. In the trial conducted by Turner (Turner 2000a; Turner 2000b; Turner 2000c) none of the zinc preparations (neither gluconate nor acetate) had any significant effect on the severity of common cold symptoms in the first three days of treatment, which might be related to the lower dose of zinc used in this trial. Among four trials (Douglas 1987; Kurugol 2006a; Kurugol 2007; Petrus 1998) measuring individual symptom scores there was a significant reduction in the cough score, with nasal and throat score being variably affected. In the trial conducted by Smith 1989, the zinc group had lower symptom severity scores on days four to seven of treatment which was statistically significant (P = 0.02); but in the trial conducted by Weismann 1990, no statistically significant differences between the two groups were found by day six of treatment (P = 0.14).
Among the two preventive trials measuring the incidence of the common cold (Kurugol 2006b; Vakili 2009), the incidence rate ratio (IRR) of developing a cold in participants who received the intervention was lower than in the placebo group. There was marked heterogeneity, therefore we used a random‐effects model for analysing this outcome. The second trial, though a randomised controlled trial (RCT), was not of good methodological quality, but this was included in the analysis as it included a large number of participants. Even after excluding this trial from analysis, the result still favoured zinc supplementation. The first trial used zinc sulfate syrup at a daily dose of 15 mg for seven months, whereas the second trial used zinc sulfate tablet at a daily dose of 10 mg for five months.
The proportion of participants who were asymptomatic after three, five and seven days of treatment was reported in the trials conducted by Mossad 1996, Weismann 1990 and Smith 1989, and the proportion asymptomatic after seven days of treatment was reported in all but the trials conducted by Farr 1987a and Al‐Nakib 1987. Analyses were conducted on an intention‐to‐treat (ITT) basis. In the trial conducted by Mossad (Mossad 1996) participants were less likely to have cold symptoms after three and five days of treatment in the zinc‐treated group. The odds ratios (ORs) for days three and five were 0.37 (95% confidence interval (CI) 0.14 to 0.92) and 0.35 (95% CI 0.16 to 0.76), respectively. In the trials conducted by Weismann 1990 and Smith 1989, the proportion of participants asymptomatic after three and five days in the zinc and placebo groups was similar. The test for heterogeneity attained statistical significance for day five, but not day three and consequently a combined OR for day five is not appropriate. The combined OR for day three was not significant 0.97 (95% CI 0.62 to 1.5). In three trials (Godfrey 1992; Mossad 1996; Weismann 1990), fewer participants in the zinc group had cold symptoms after seven days. The pooled result (OR 0.53, 95% CI 0.38 to 0.75) obtained from five trials (Douglas 1987; Godfrey 1992; Mossad 1996; Smith 1989; Weismann 1990) indicated fewer participants in the zinc group had cold symptoms after seven days of treatment. However, the test for heterogeneity was statistically significant.
In six trials (Godfrey 1992; Kurugol 2006a; Mossad 1996; Petrus 1998; Prasad 2000; Prasad 2008) with similar study designs, methodologies and efficacy assessments, zinc was found to be effective in reducing the duration and severity of common cold symptoms in healthy children and adults, when it was administered within 24 hours of the onset of symptoms. In another trial (Kurugol 2007) with similar study design, methodology and efficacy assessments, zinc was found to be effective in reducing the severity of common cold symptoms in healthy children (without any change in duration), when it was administered within 24 to 48 hours of the onset of symptoms. In the trial by Godfrey 1992, the authors found a significant decrease in the duration and severity of symptoms when treatment was administered within 24 hours, compared to treatment administration within 48 hours. So, if treatment with zinc is to be used for common cold, it should ideally commence within 24 hours of onset of symptoms.
There are a number of potential sources of heterogeneity in results obtained from trials included in this review. Most trials relied on community‐acquired infections in which the infecting agent was not identified and as such different agents may have been involved which may have differed in their sensitivity to zinc. The amount of zinc taken each day by participants varied largely across the trials, and given that some formulations released less zinc ion than others the effective dose of zinc across trials was variable. Blinding of treatment may not have been adequately controlled in some trials, thereby increasing the potential for performance and detection bias to occur. The time from onset of cold symptoms to commencement of treatment ranged from one to three days. Given the beneficial effects noted in trials commencing treatment with zinc within 24 hours, the results from all the trials may not be comparable. Last but not the least is the fact that the lifestyle of the study population in all the trials was different and the results might have been affected to some degree by this factor.
Summary of main results
Studies reporting duration and severity of cold symptoms suggest that the intake of zinc is associated with a significant reduction in the overall duration and severity of common cold symptoms. A higher proportion of participants became asymptomatic by day seven of treatment with zinc. Duration of individual cold symptoms was also significantly reduced in the zinc group, though the individual symptom severity scores were not significantly affected by the intake of zinc. Zinc supplementation led to reduction in the incidence of common cold, decreased school absence and decreased the risk of antibiotic use when used for at least five months. The incidence of adverse events was significantly higher in the zinc group with the syrup preparation being better tolerated than lozenges.
Overall completeness and applicability of evidence
The trials included in the analysis involved healthy children and adults of all ages (except infants) and both sexes. All except four trials (Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Vakili 2009) were conducted in upper‐middle and high‐income countries, where zinc deficiency is uncommon (including in young children). Out of four trials (Kurugol 2006a; Kurugol 2006b; Kurugol 2007; Vakili 2009), three were conducted in Turkey and one in Iran, which are low‐income countries. These later trials found beneficial effects of zinc when used either in syrup or tablet (not lozenges) form. So, any beneficial effect of zinc lozenges might not be directly extrapolated to children and adults of low‐income countries. In all the included trials, the main weakness of the data is that most trials, when presenting data, did not differentiate between cold due to rhinovirus and other viruses (as viral studies were not conducted in most of these trials). So it is unclear whether zinc helps those with rhinoviral cold or even cold due to other viruses. However, as rhinovirus is the most common aetiological agent of the common cold all over the world (in both low‐income and high‐income countries), it may be predicted that zinc lozenges prepared in the above mentioned formulations might also help people living in low‐income countries.
Quality of the evidence
The trial evidence included is generally of good quality, with a low risk of bias. All the studies were blinded, but placebo‐blinding was adequately described in only six trials. In 12 trials, zinc‐treated participants complained of altered, bad or unpalatable taste which suggests that zinc lozenges were distinct from placebo lozenges and, in this respect, blinding may have been compromised. Allocation concealment was adequate in nine studies and unclear in nine studies. Thirteen trials reported a low rate of loss to follow‐up, whereas three trials did not mention any loss to follow‐up. This suggests that the studies were of good quality. For all the outcomes, there was more than one study reporting the individual outcome. The majority were carefully conducted community trials, with active mechanisms to promote adherence to the intervention and both active and passive case finding. Last and most importantly, as there was a high degree of heterogeneity among the trials, the result should be interpreted with caution.
Potential biases in the review process
All the included trials, as expected, measured the effect of zinc on the common cold. There was therefore the potential to miss trials which may have measured common cold as upper respiratory tract infection in secondary outcomes which were less publicised or less well indexed within the electronic databases. We tried to avoid this by conducting a wide search and assessing the relevance of each paper identified in that search carefully. We extracted data from survival curves or t/P values given in studies that did not provide original data, which might have overestimated or underestimated the effect size. Lastly, publication bias cannot be ruled out as shown by funnel plot asymmetry.
Agreements and disagreements with other studies or reviews
The important changes in this updated review in comparison to the previous version (Marshall 1999) include the following.
-
Intake of zinc is associated with a significant reduction in the duration and severity of common cold symptoms.
-
Duration of individual cold symptoms was also significantly reduced in the zinc group.
-
The syrup and tablet preparation of zinc is better tolerated than lozenges.
-
Zinc supplementation reduces incidence, school absence and prescription of antibiotics in children with the common cold.
In the review conducted by Marshall (Marshall 1999) the included studies had missing information, due to which it was not possible to pool the results across the studies. For example, although most investigators required participants to record the clinical severity of symptoms each day and used similar scales against which to rate symptom severity (symptoms were rated as none, mild, moderate or severe), there was little commonality in the summary estimates used by investigators to describe the duration, incidence and severity of respiratory symptoms. It was therefore only possible to determine the proportion of participants who were asymptomatic after three and five days of treatment for three trials and after seven days of treatment for five trials. Except for one study (Godfrey 1992), duration of symptoms was not reported in the rest of the studies. Therefore, again it was not possible to pool the results for this outcome.
In the revisit of their previous meta‐analysis, Jackson (Jackson 2000) found statistically significant heterogeneity between the zinc trials. They calculated a pooled estimate of the zinc gluconate lozenges in colds using the random‐effects model of DerSimonian and Laird from eight trials. The summary OR for the presence of "any cold symptoms" at seven days was 0.52 (95% CI 0.25 to 1.2). They concluded that despite numerous randomised trials, the evidence for the effectiveness of zinc lozenges in reducing the duration of common colds is still lacking. Although, according to them, some of the negative results might be explained by low zinc ion availability, they did not examine the issue. Moreover, they have not looked for the effect of any other formulations, as well as high or low doses of zinc.
A review published in 2004 by Hulisz 2004, which was an overview of published articles through MEDLINE (1980 to 2003), concluded that zinc effectively reduces the duration and severity of common cold symptoms when administered within 24 hours of the onset of symptoms. The author also had some concerns regarding the clinical tests of zinc for the treatment of common colds being inconsistent, primarily because of study design, blinding and lozenge contents; early formulations of lozenges being unpalatable with a higher incidence of side effects.
In a systematic review of trials published between 1966 and 2006, Caruso 2007 used 11 features of experimental design affecting signal quality, chance, bias and blinding to evaluate 14 placebo‐controlled trials. They gave one point for each of the 11 quality items if it was satisfied, after which only four trials obtained the maximum of 11 points. Based on this, they suggested that the positive results in the zinc trials might be explained by methodological flaws. However, the use of scales for assessing quality or risk of bias is strongly discouraged in Cochrane systematic reviews. Moreover, such an approach, though simple, is not supported by evidence (Higgins 2011).
In another systematic review by Hemilä (Hemilä 2011), the author included 13 placebo‐controlled comparisons. Pooling of trials using a total daily zinc dose of less than 75 mg found no effect, but those using zinc acetate in daily doses of over 75 mg found a 42% reduction, and those using zinc salts other than acetate in daily doses of over 75 mg found a 20% reduction in the duration of colds. The author concluded that there is strong evidence that the zinc lozenge effect on common cold duration is heterogeneous so that benefit is observed with high doses of zinc but not with low doses. However, there were no descriptions of other formulations of zinc (syrup or tablet).
In the latest systematic review (Science 2012), the authors included 17 trials. Compared with patients given placebo, those receiving zinc had a shorter duration of cold symptoms (mean difference (MD) ‐1.65 days, 95% CI ‐2.50 to ‐0.81). However, heterogeneity was high. Zinc shortened the duration of cold symptoms in adults but no significant effect was seen among children. In contrast to this, we found zinc to be effective both in children (< 16 years age) and adults. The reduction in the duration of cold symptoms was greater with high doses of ionic zinc (MD ‐2.75, 95% CI ‐3.89 to ‐1.60) than with lower doses (MD ‐0.84, 95% CI ‐1.50 to ‐0.18). However, we found that only zinc lozenges containing high dose (≥ 75 mg/day) reduced the cold duration significantly.
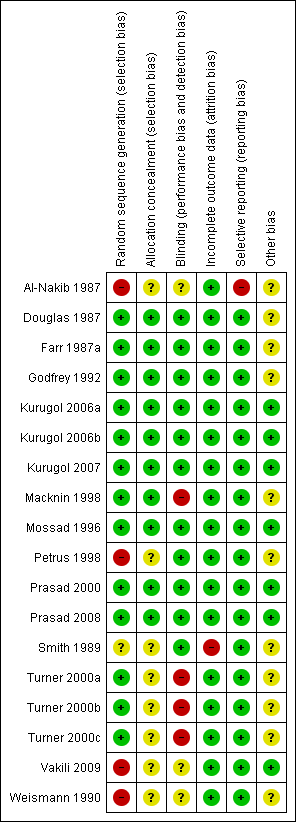
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
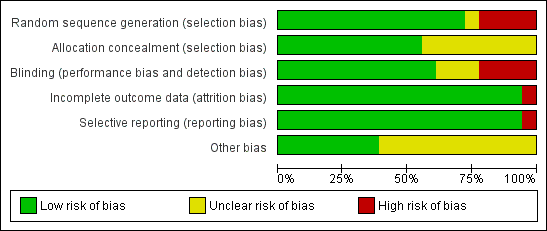
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
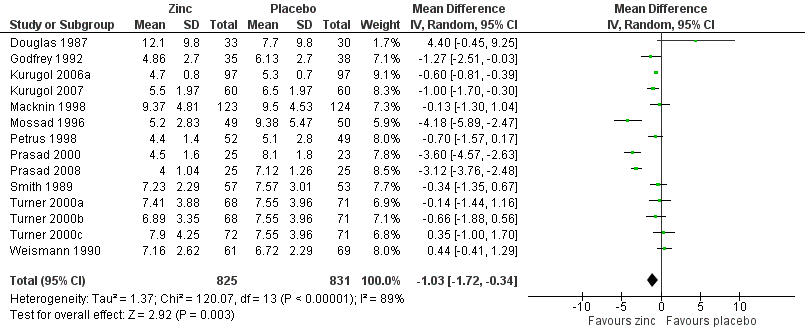
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.1 Duration of cold symptoms (in days).
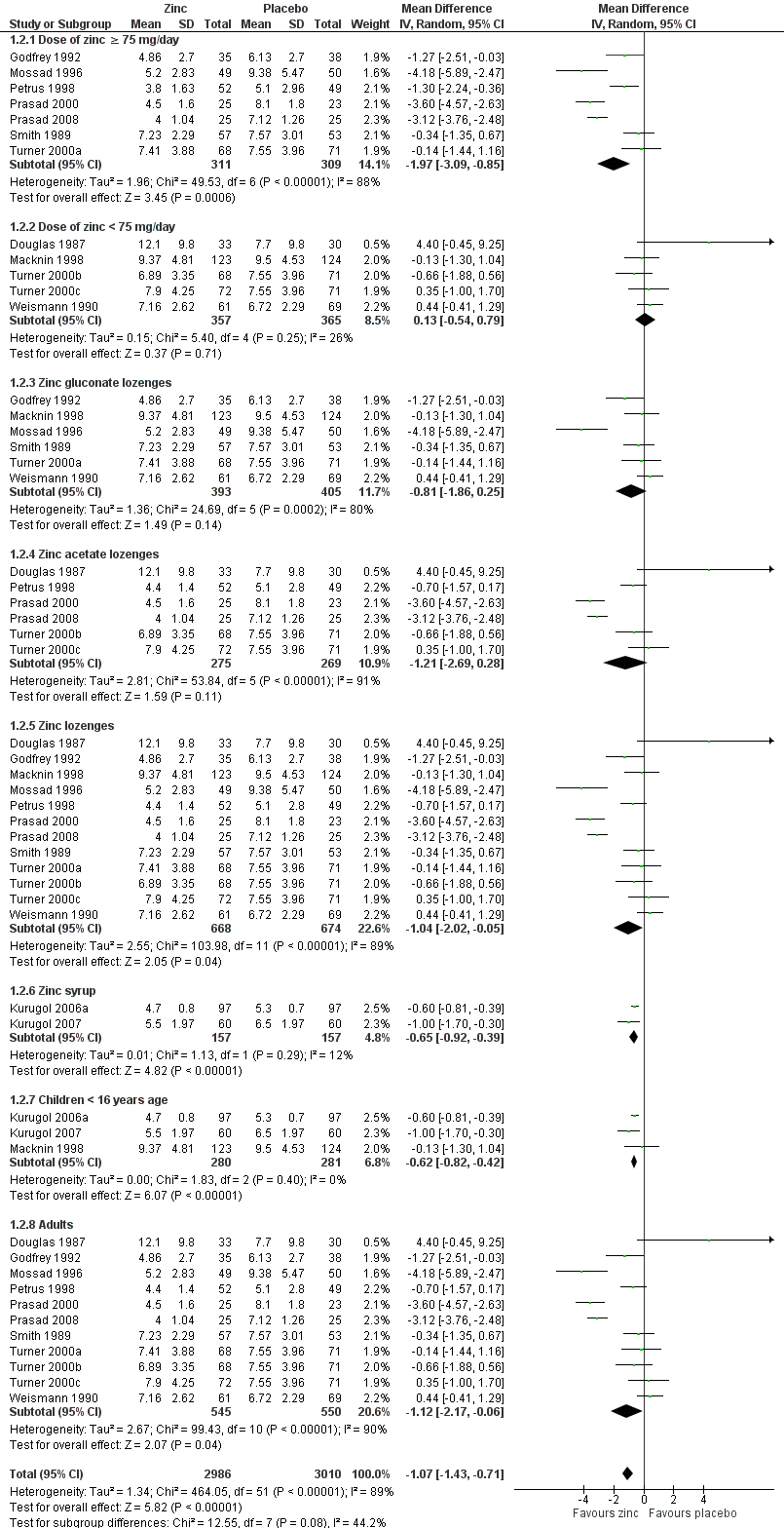
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.1 Duration of cold symptoms (in days).
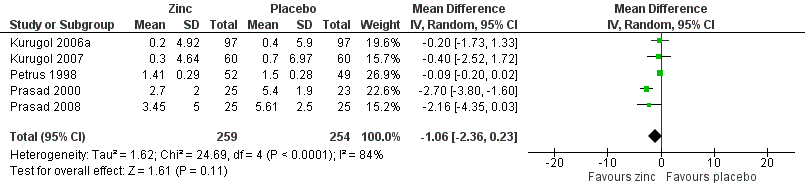
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.2 Severity of symptoms (score).
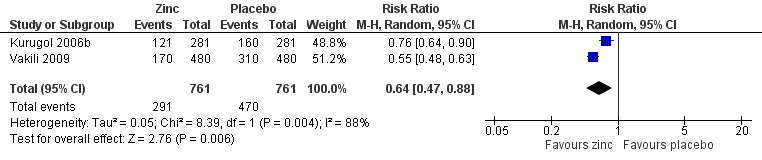
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.3 Incidence of common cold (IRR).
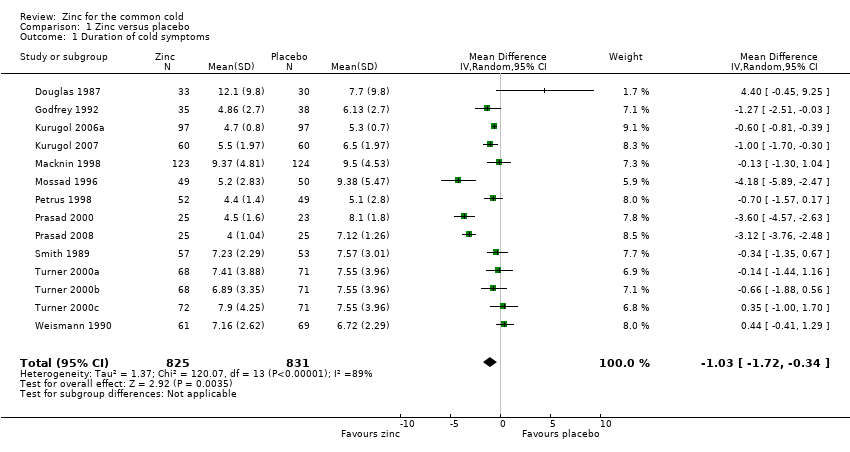
Comparison 1 Zinc versus placebo, Outcome 1 Duration of cold symptoms.
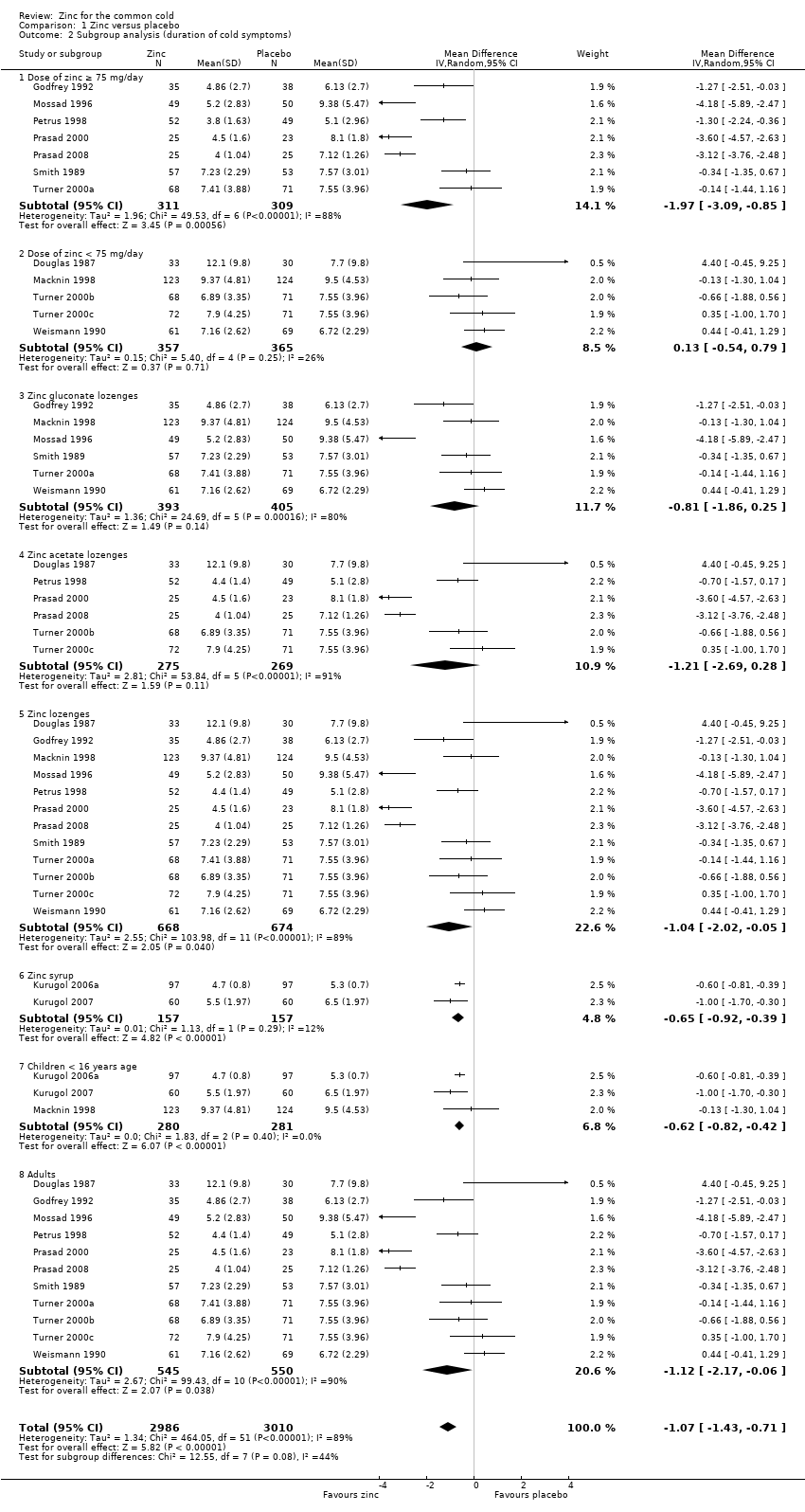
Comparison 1 Zinc versus placebo, Outcome 2 Subgroup analysis (duration of cold symptoms).
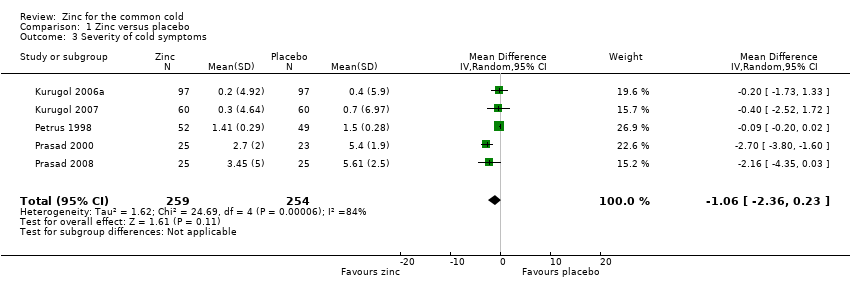
Comparison 1 Zinc versus placebo, Outcome 3 Severity of cold symptoms.
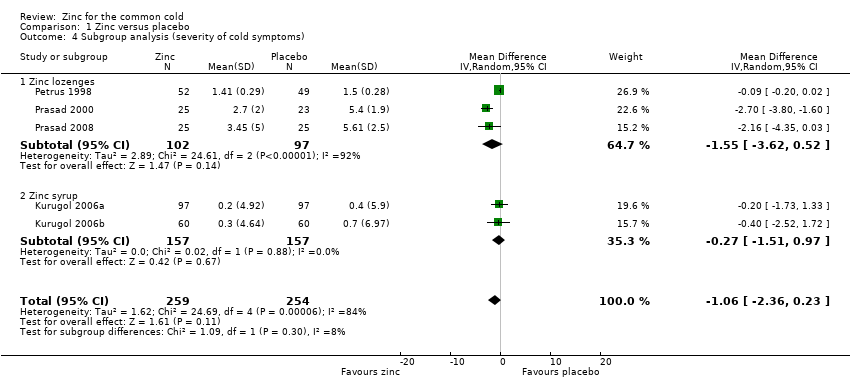
Comparison 1 Zinc versus placebo, Outcome 4 Subgroup analysis (severity of cold symptoms).
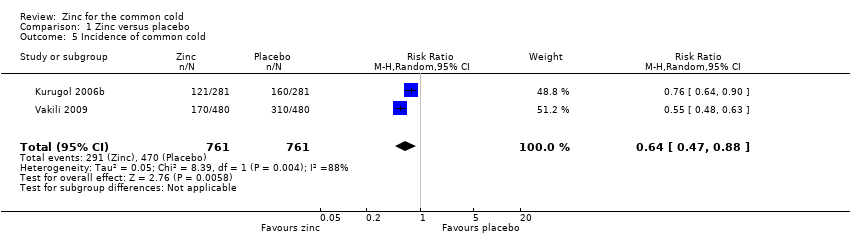
Comparison 1 Zinc versus placebo, Outcome 5 Incidence of common cold.
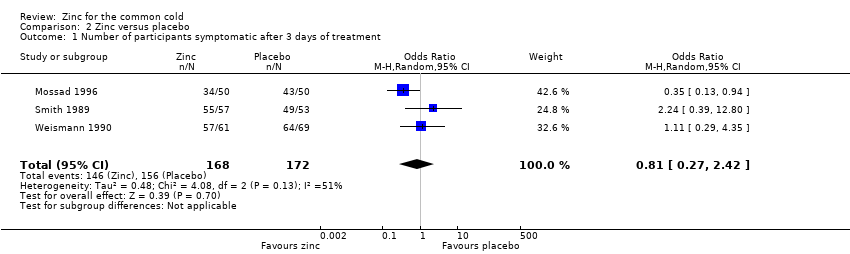
Comparison 2 Zinc versus placebo, Outcome 1 Number of participants symptomatic after 3 days of treatment.
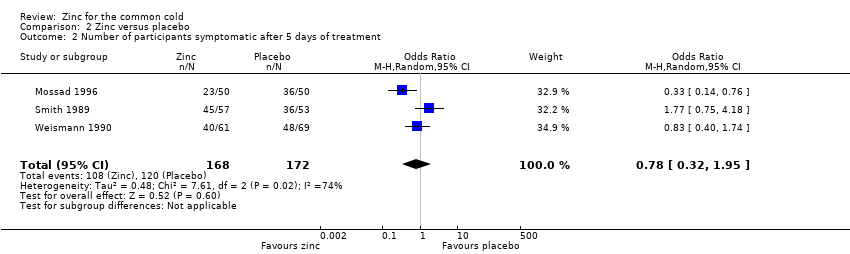
Comparison 2 Zinc versus placebo, Outcome 2 Number of participants symptomatic after 5 days of treatment.
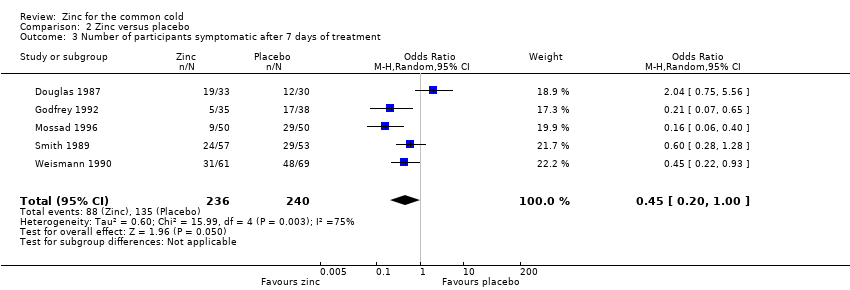
Comparison 2 Zinc versus placebo, Outcome 3 Number of participants symptomatic after 7 days of treatment.
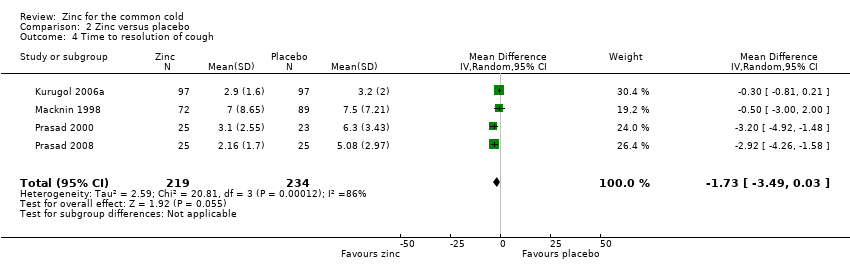
Comparison 2 Zinc versus placebo, Outcome 4 Time to resolution of cough.
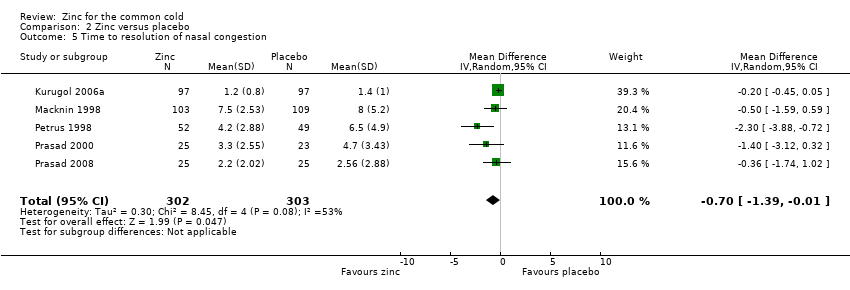
Comparison 2 Zinc versus placebo, Outcome 5 Time to resolution of nasal congestion.
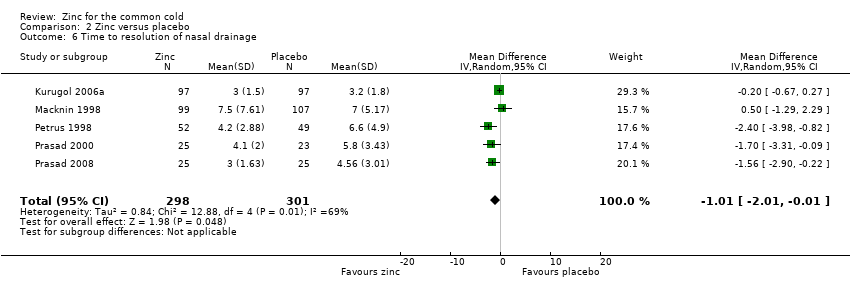
Comparison 2 Zinc versus placebo, Outcome 6 Time to resolution of nasal drainage.
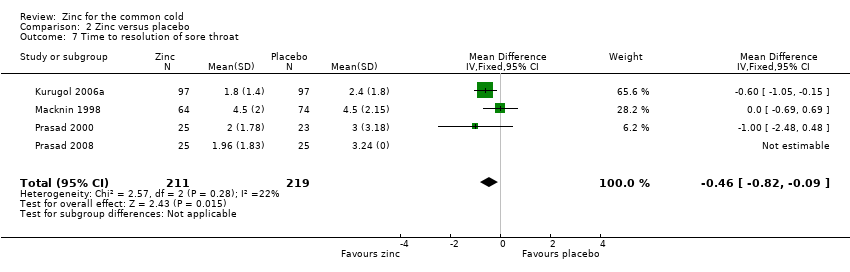
Comparison 2 Zinc versus placebo, Outcome 7 Time to resolution of sore throat.
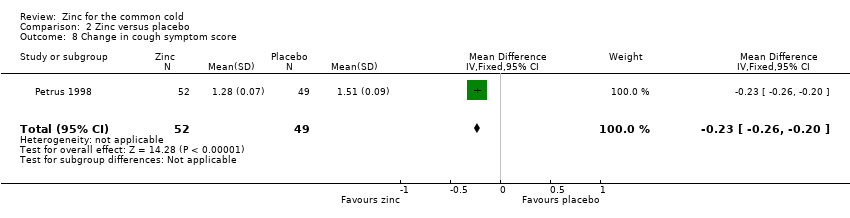
Comparison 2 Zinc versus placebo, Outcome 8 Change in cough symptom score.
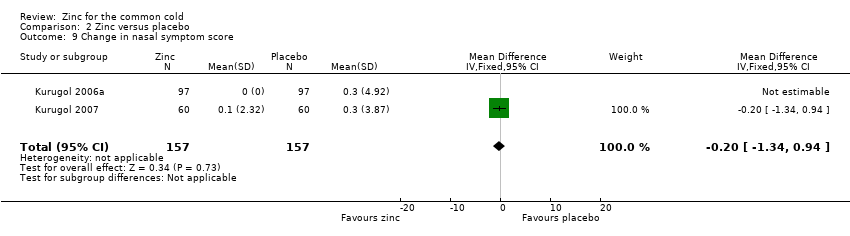
Comparison 2 Zinc versus placebo, Outcome 9 Change in nasal symptom score.
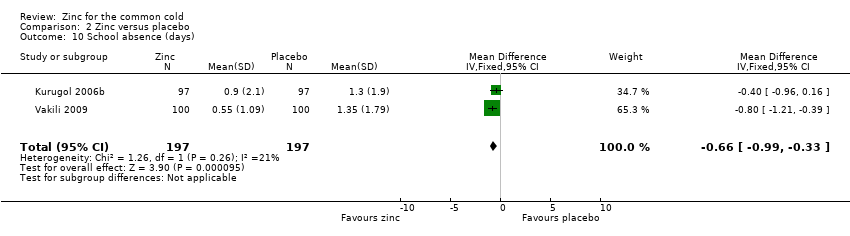
Comparison 2 Zinc versus placebo, Outcome 10 School absence (days).
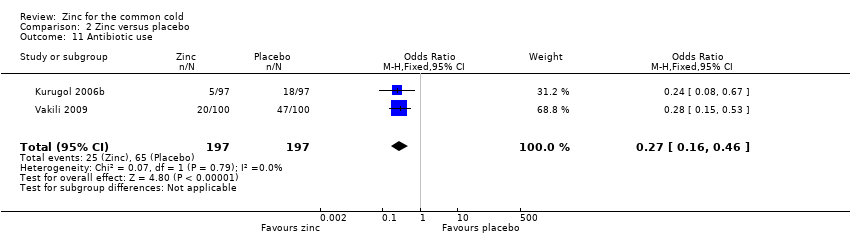
Comparison 2 Zinc versus placebo, Outcome 11 Antibiotic use.
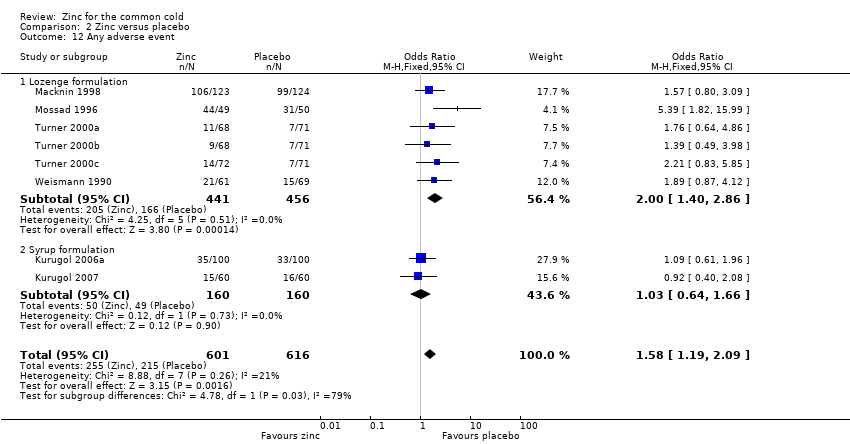
Comparison 2 Zinc versus placebo, Outcome 12 Any adverse event.
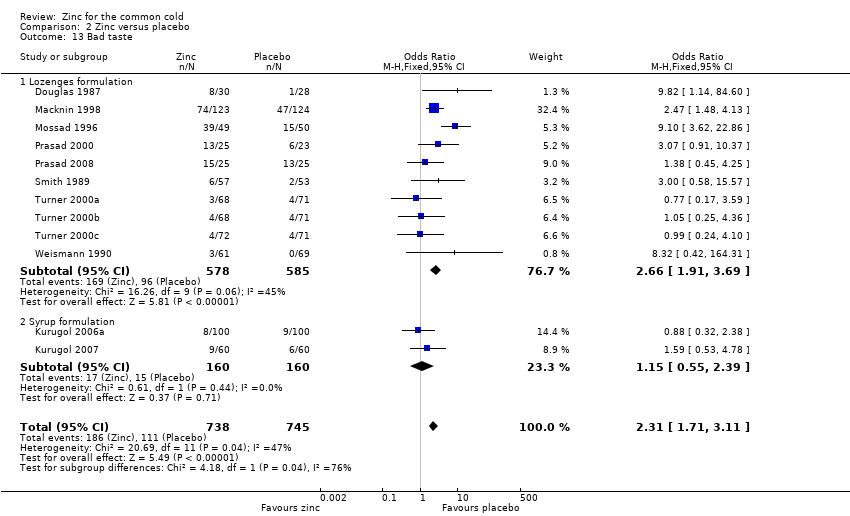
Comparison 2 Zinc versus placebo, Outcome 13 Bad taste.
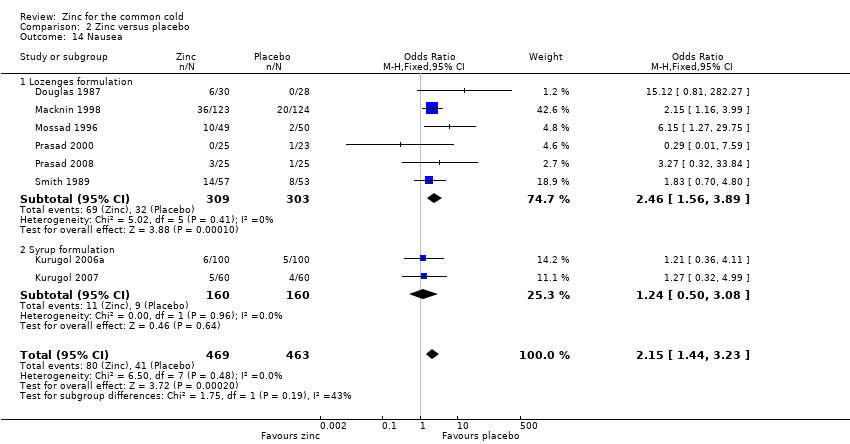
Comparison 2 Zinc versus placebo, Outcome 14 Nausea.
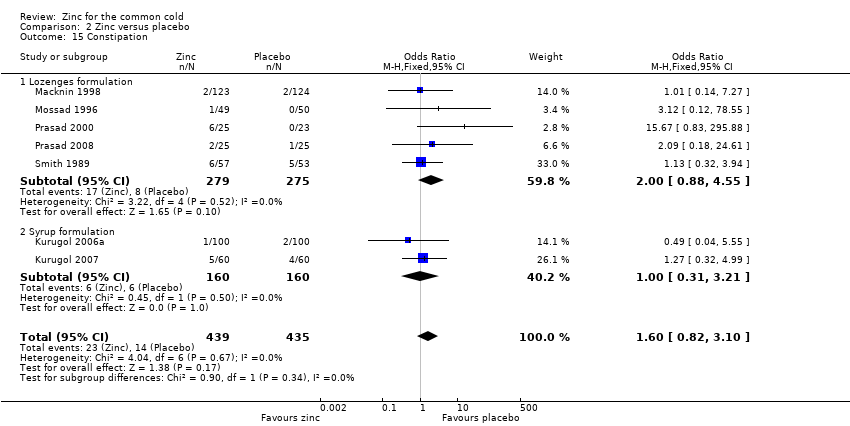
Comparison 2 Zinc versus placebo, Outcome 15 Constipation.
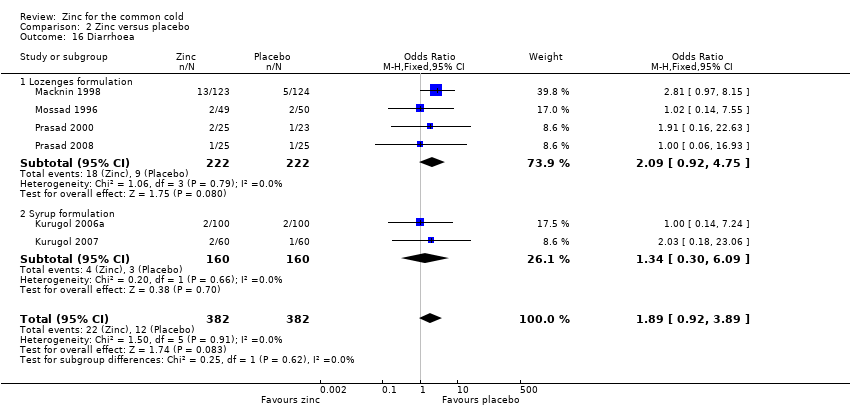
Comparison 2 Zinc versus placebo, Outcome 16 Diarrhoea.
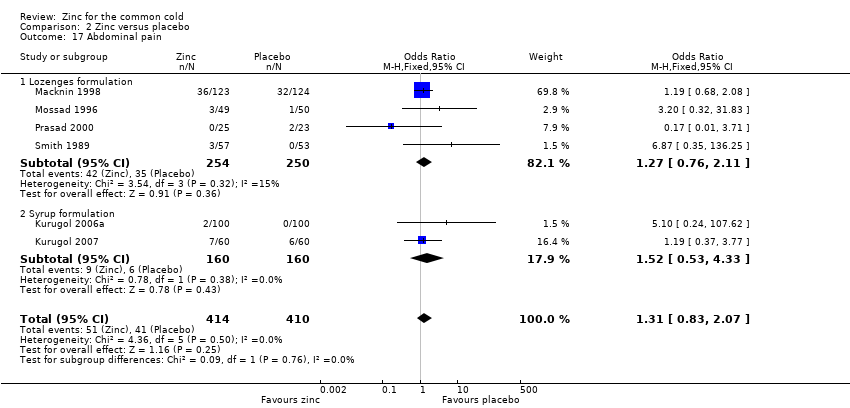
Comparison 2 Zinc versus placebo, Outcome 17 Abdominal pain.
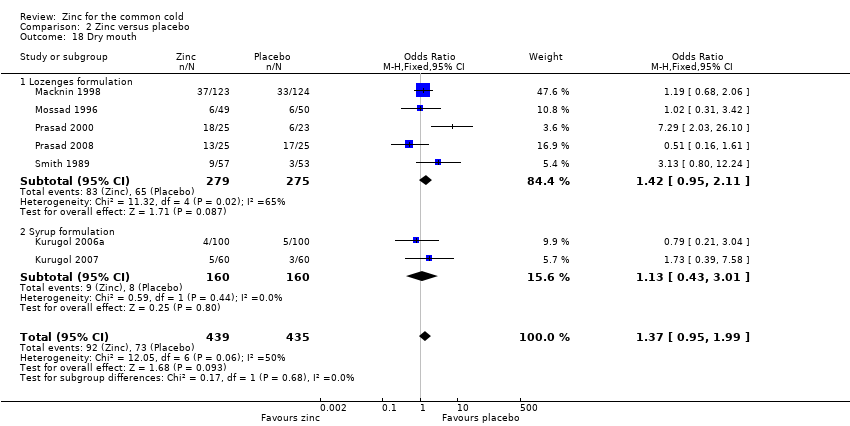
Comparison 2 Zinc versus placebo, Outcome 18 Dry mouth.
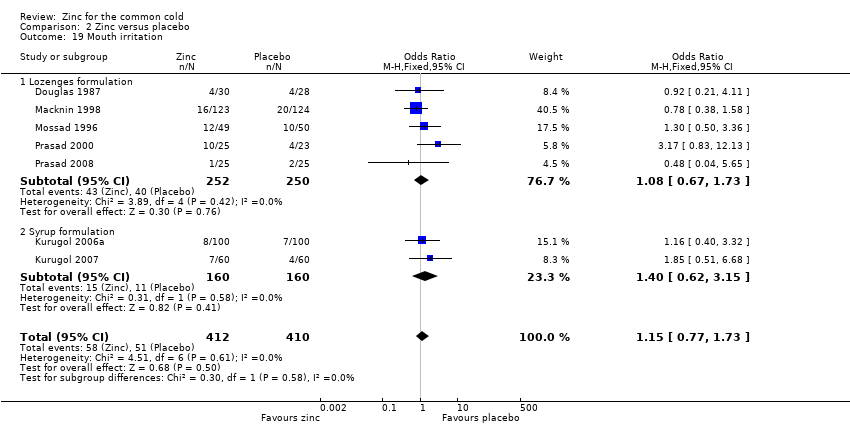
Comparison 2 Zinc versus placebo, Outcome 19 Mouth irritation.
| Zinc compared with placebo for the common cold | ||||||
| Patient or population: patients with common cold Settings: outpatient Intervention: zinc lozenges or syrup Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc | |||||
| Duration of cold symptoms (days) | The mean duration of cold symptoms ranged across control groups from 5.1 to 9.38 days | The mean duration of cold symptoms ranged across control groups from 4 to 12.1 days | 1656 | ++O | ||
| Severity of symptom score | The mean severity of symptom score ranged across control groups from 0.4 to 5.61 | The mean severity of symptom score ranged across control groups from 0.2 to 3.45 | 513 | ++O | ||
| Incidence of common cold | 618 per 1000 | 382 per 1000 (354 to 431) | RR 0.64 (0.47 to 0.88) | 394 | +OO | |
| Number of participants symptomatic after 7 days of treatment | 563 per 1000 | 373 per 1000 (143 to 508) | OR 0.45 (0.2 to 1.0) | 476 | ++OO | |
| School absence (number of days) | The mean days of school absence ranged across control groups from 1.3 to 1.35 days | The mean days of school absence in the intervention groups was 0.37 lower (0.7 to 0.04 lower) | 394 | +OO | ||
| Antibiotic use | 330 per 1000 | 127 per 1000 (52 to 200) | OR 0.27 (0.16 to 0.46) | 394 | ++OO | |
| Any adverse event | 349 per 1000 | 424 per 1000 (132 to 898) | OR 1.58 (1.19 to 2.09) | 1217 | +++O | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious study limitations: all the studies had adequately concealed allocation and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear in Macknin 1998; Petrus 1998; Turner 2000a; Turner 2000b; Turner 2000c. Petrus 1998 did not adequately describe the sequence generation. Blinding was inadequate in Turner 2000a; Turner 2000b; Turner 2000c. 2Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 89%. The heterogeneity was due to differences in the nature of the different interventions (zinc gluconate versus acetate lozenges, zinc lozenges versus zinc syrup), wide dose ranges, varied duration of symptoms prior to administration of zinc (varying from 24 to 48 hours) and characteristics of the study population (children versus adults). 3No serious indirectness: studies both from low‐income and high‐income regions have assessed this comparison. Therefore, the result can be confidently generalised to all situations. 4No serious imprecision: though the 95% CI around the pooled effect is narrow, the lower limit does not suggest a clinically important reduction in the duration of cold (a decrease in duration of ≤ 1 day is not shown to be important to patients). 5Publication bias cannot be ruled out. 6No serious study limitation: all the studies had adequately concealed allocation and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear and adequate sequence was not generated in one study (Petrus 1998). 7Serious imprecision: the 95% CI around the pooled effect is wide, the lower limit is crossing the point of no effect. 8Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 84%. The heterogeneity may be due to differences in the nature of the different interventions (zinc gluconate or acetate lozenges, zinc sulphate syrup) and dose range (30 to 160 mg/day) as well as mean duration of symptoms prior to administration of zinc (varying from 24 to 48 hours), as well as the characteristics of the study population (children versus adults). However, subgroup analysis was not possible as there were not enough studies for each variable. 9Kurugol 2006b is a community‐based intervention including 200 healthy school children and studying the effect of daily administration of 15 mg zinc sulphate syrup over a period of seven months. Vakili 2009 is also a community‐based intervention including 200 healthy school children and studying the effect of daily administration of 10 mg zinc sulfate tablets over a period of seven months. 10Serious study limitation: though the study by Kurugol 2006b was of high quality, that by Vakili 2009 was of poor methodological quality. 11Serious inconsistency: there is substantial heterogeneity between the two trials: I2 statistic for heterogeneity = 88%. Both trials showed a benefit with zinc, however the size of this effect was much larger in Vakili 2009. The heterogeneity was due to differences in the trial methodology and the nature of the interventions. 12No serious imprecision: the 95% CI around the pooled effect is narrow. Even the lower limit suggests a clinically important reduction in the incidence rate ratio of cold which is shown to be important to patients. 13No serious study limitations: allocation concealment was unclear in two studies, i.e. Smith 1989 and Weismann 1990, though both the studies blinded both participants and study staff. 14Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 75%. The heterogeneity may be due to differences in the nature of the different interventions (zinc gluconate or acetate lozenges) and dose range (30 to 160 mg/day) as well as mean duration of symptoms prior to administration of zinc (varying from 24 to 48 hours, as well as the characteristics of the study population (children versus adults). However, subgroup analysis was not possible as there were not enough studies for each variable. 15Serious indirectness: only studies from high‐income regions have assessed this comparison. Therefore, the result cannot be generalised to all situations. 16No serious imprecision: both limits of the 95% CI suggest a clinically important reduction in proportion of participants given the intervention symptomatic after seven days of treatment. 17Serious inconsistency: there is substantial heterogeneity between the two trials: I2 statistic test for heterogeneity = 64%. Both trials showed reduced days of school absence with intervention, however, the size of this effect was much larger in Kurugol 2006b. The heterogeneity was due to differences in the trial methodology and the nature of the interventions. 18No serious imprecision: though the 95% CI around the pooled effect is narrow, the lower limit does not suggests a clinically important reduction in the duration of school absence (a decrease in duration of ≤ 1 day is not shown to be important to patients). 19No serious inconsistency: there was no statistical heterogeneity. I2 statistic = 0%. 20No serious imprecision: both limits of the 95% CI suggest a clinically important reduction in the rate of antibiotic use with intervention. 21No serious study limitations: all the studies had adequately concealed allocation (except Weismann 1990, in which allocation concealment is unclear) and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear in Macknin 1998 and Weismann 1990. Weismann 1990 did not adequately describe the sequence generation. 22No serious inconsistency: there is no statistical heterogeneity. I2 statistic = 21%. Both the lozenges and syrup preparation trials were pooled. 23No serious imprecision: the 95% CI around the pooled effect is narrow. The resulting adverse events from use of zinc are higher and this is significant. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of cold symptoms Show forest plot | 14 | 1656 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.72, ‐0.34] |
| 2 Subgroup analysis (duration of cold symptoms) Show forest plot | 14 | 5996 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.43, ‐0.71] |
| 2.1 Dose of zinc ≥ 75 mg/day | 7 | 620 | Mean Difference (IV, Random, 95% CI) | ‐1.97 [‐3.09, ‐0.85] |
| 2.2 Dose of zinc < 75 mg/day | 5 | 722 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.54, 0.79] |
| 2.3 Zinc gluconate lozenges | 6 | 798 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.86, 0.25] |
| 2.4 Zinc acetate lozenges | 6 | 544 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐2.69, 0.28] |
| 2.5 Zinc lozenges | 12 | 1342 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐2.02, ‐0.05] |
| 2.6 Zinc syrup | 2 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.92, ‐0.39] |
| 2.7 Children < 16 years age | 3 | 561 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.82, ‐0.42] |
| 2.8 Adults | 11 | 1095 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.17, ‐0.06] |
| 3 Severity of cold symptoms Show forest plot | 5 | 513 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.36, 0.23] |
| 4 Subgroup analysis (severity of cold symptoms) Show forest plot | 5 | 513 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.36, 0.23] |
| 4.1 Zinc lozenges | 3 | 199 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐3.62, 0.52] |
| 4.2 Zinc syrup | 2 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.51, 0.97] |
| 5 Incidence of common cold Show forest plot | 2 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants symptomatic after 3 days of treatment Show forest plot | 3 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.27, 2.42] |
| 2 Number of participants symptomatic after 5 days of treatment Show forest plot | 3 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.32, 1.95] |
| 3 Number of participants symptomatic after 7 days of treatment Show forest plot | 5 | 476 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.20, 1.00] |
| 4 Time to resolution of cough Show forest plot | 4 | 453 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.49, 0.03] |
| 5 Time to resolution of nasal congestion Show forest plot | 5 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.39, ‐0.01] |
| 6 Time to resolution of nasal drainage Show forest plot | 5 | 599 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐2.01, ‐0.01] |
| 7 Time to resolution of sore throat Show forest plot | 4 | 430 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.82, ‐0.09] |
| 8 Change in cough symptom score Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.26, ‐0.20] |
| 9 Change in nasal symptom score Show forest plot | 2 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.34, 0.94] |
| 10 School absence (days) Show forest plot | 2 | 394 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.99, ‐0.33] |
| 11 Antibiotic use Show forest plot | 2 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.16, 0.46] |
| 12 Any adverse event Show forest plot | 8 | 1217 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.19, 2.09] |
| 12.1 Lozenge formulation | 6 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.40, 2.86] |
| 12.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.64, 1.66] |
| 13 Bad taste Show forest plot | 12 | 1483 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.71, 3.11] |
| 13.1 Lozenges formulation | 10 | 1163 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.91, 3.69] |
| 13.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.55, 2.39] |
| 14 Nausea Show forest plot | 8 | 932 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.44, 3.23] |
| 14.1 Lozenges formulation | 6 | 612 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.56, 3.89] |
| 14.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.08] |
| 15 Constipation Show forest plot | 7 | 874 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.82, 3.10] |
| 15.1 Lozenges formulation | 5 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.88, 4.55] |
| 15.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.21] |
| 16 Diarrhoea Show forest plot | 6 | 764 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.92, 3.89] |
| 16.1 Lozenges formulation | 4 | 444 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.92, 4.75] |
| 16.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.30, 6.09] |
| 17 Abdominal pain Show forest plot | 6 | 824 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.83, 2.07] |
| 17.1 Lozenges formulation | 4 | 504 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.76, 2.11] |
| 17.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.53, 4.33] |
| 18 Dry mouth Show forest plot | 7 | 874 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.95, 1.99] |
| 18.1 Lozenges formulation | 5 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.95, 2.11] |
| 18.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 3.01] |
| 19 Mouth irritation Show forest plot | 7 | 822 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.73] |
| 19.1 Lozenges formulation | 5 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.73] |
| 19.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.62, 3.15] |

