Цинк при простуде
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blind, placebo‐controlled randomised trial | |
| Participants | Healthy adults 18 to 50 years | |
| Interventions | Therapeutic trial: participants took 1 lozenge 2‐hourly for 6 days Prophylactic trial: participants took 1 lozenge/2 waking hours for a total of 12 lozenges/day for 4.5 days. On the second day they were challenged with HRV‐2 | |
| Outcomes | Severity of symptoms | |
| Notes | Although adults were stated to be healthy, no exclusion criteria were stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described. Participants divided into groups balanced by age and sex |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described in detail to allow a definite judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ of bias |
| Incomplete outcome data (attrition bias) | Low risk | There were no drop‐outs or withdrawals |
| Selective reporting (reporting bias) | High risk | 1 or more outcomes of interest in the review were insufficiently reported so that they could not be entered in a meta‐analysis |
| Other bias | Unclear risk | The zinc and placebo lozenges were gifted by RBS Pharma, Milan |
| Methods | Double‐blind, placebo‐controlled randomised trial | |
| Participants | Participants in the trial were healthy adults who had in the previous year participated in a study of interferon prophylaxis against rhinovirus infection | |
| Interventions | Participants took 6 to 8 lozenges/day at 2nd‐hourly intervals for a minimum of 3 days and maximum of 6 days if symptoms persisted. New course commenced after 2 weeks if symptoms persisted but type of treatment may differ. Consequently 33 zinc courses and 30 placebo courses | |
| Outcomes | Duration and severity of symptoms (nasal, throat or cough) | |
| Notes | The duration of the common cold was ≤ 2 days before starting treatment for 56 of the 58 participants. 2 zinc and 5 placebo treatment courses were excluded because lozenges had not been used for ≥ 3 days and at the rate of ≥ 4 per day. The SD value was not reported and was calculated from P value. The zinc lozenges contained high amount of tartrate; as a result zinc dissociates from acetate and binds instantly to tartrate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used sequentially numbered bottles |
| Allocation concealment (selection bias) | Low risk | Used blocked randomisation in blocks of 4. The code was broken twice (once in middle of study and then at the end of study) |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants and key study personnel ensured. It was unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Plausible effect size (difference in means) amongst missing outcomes was insufficient to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | The zinc and placebo lozenges were provided by Fauldings Ltd |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Healthy adults | |
| Interventions | Trial 1: treatment consisted of initial loading dose of 2 lozenges 36 hours following inoculation with HRV‐39, and thereafter 1 lozenge every 2 hours for a total of 8 lozenges/day for 5 days Trial 2: treatment consisted of initial loading dose of 2 lozenges 2 hours following inoculation with HRV‐13, and thereafter 1 lozenge every 2 hrs for a total of 8 lozenges/day for 7 days | |
| Outcomes | Severity and duration of symptoms | |
| Notes | Exclusion criteria were symptoms of any respiratory illness in the week before the study, a history of hay fever, any familiarity with the taste of either denatonium benzoate or zinc, a history of any chronic disease, pregnancy, lactation or an unacceptable contraceptive method in women of child‐bearing potential, and known abuse of habit‐forming drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Each lozenge was wrapped in cellophane and packaged in an opaque polyethylene bottle bearing the study number, the participants' number, the treatment day and dosing instructions |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Partly funded by Bristol Myers Products, Hillside, NJ |
| Methods | Double‐blind, placebo‐controlled randomised trial | |
| Participants | Participants were recruited from among Dartmouth College students and staff who spontaneously presented to the cold clinic at the College Health Service. Age ranged from 18 to 40 years. Inclusion required that the cold had lasted ≤ 2 days. Exclusion criteria were positive bacteriological throat culture, pregnancy, lactation and diabetes mellitus | |
| Interventions | Participants took 1 lozenge every 2 hours for up to 8 hours a day | |
| Outcomes | Frequency and severity of symptoms over 7 days | |
| Notes | The mean duration of the common cold was 1.3 days at entry. 8 zinc and 6 placebo participants withdrew from the trial. The SD value was not reported and was calculated from t‐value. The zinc lozenge contained glycine, which binds zinc tightly and therefore the free zinc ion level probably was much lower than suggested by the total zinc dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | A pharmacist, using a randomisation table provided by the study statistician, packaged containers for individual participants with lozenges according to the production run number and subject identification number |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Plausible effect size (difference in means) amongst missing outcomes was insufficient to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | The study was sponsored by Godfrey Science and Design, PA and by a grant from the Rorer Pharmaceutical corporation, PA, USA |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | The study was conducted at Ege University Nursery and Primary School including children aged 2 to 10 years. Children with chronic disease, immunodeficiency disorder, asthma and history of hypersensitivity were excluded | |
| Interventions | Therapeutic trial: children received syrup preparation of zinc twice daily for 10 days | |
| Outcomes | Duration and severity of cold symptoms | |
| Notes | Used zinc sulfate syrup. A total of 6 (3%) subjects discontinued, 4 for non‐compliance and 2 for adverse effects due to medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random numbers generator |
| Allocation concealment (selection bias) | Low risk | A statistical consultant programmed a computer‐generated randomisation code and prepared the packages of medication |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Berko Ilac Company, Turkey, supplied the active and placebo medications and digital thermometers. The company did not participate in designing the study, collecting and analysing the data, or in writing the report |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | The study was conducted at Ege University Nursery and Primary School including children aged 2 to 10 years. Children with chronic disease, immunodeficiency disorder, asthma and history of hypersensitivity were excluded | |
| Interventions | Prophylactic trial: children received syrup preparation of zinc once daily for 7 months. | |
| Outcomes | Number of colds per study child | |
| Notes | Used zinc sulfate syrup. A total of 6 (3%) participants discontinued, 4 for non‐compliance and 2 for adverse effects due to medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random numbers generator |
| Allocation concealment (selection bias) | Low risk | A statistical consultant programmed a computer‐generated randomisation code and prepared the packages of medication |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Berko Ilac Company, Turkey, supplied the active and placebo medications and digital thermometers. The company did not participate in designing the study, collecting and analysing the data, or in writing the report |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | The study was conducted at Ege University Nursery and Primary School including children aged 1 to 10 years. The children who developed symptoms of common cold within the first 24 to 48 hours were registered in the study. Exclusion criteria were common cold symptoms for > 48 hours, immunodeficiency disorder, chronic disease, recent acute respiratory disease (diagnosed by a physician in the previous 2 weeks), zinc allergy, allergic disease or non‐allergic rhinitis, positive culture for group A Streptococcus and a positive cell culture for influenza A or B viruses | |
| Interventions | Participants were asked to take 1 spoonful of syrup twice a day for 10 days | |
| Outcomes | Duration and severity of cold symptoms | |
| Notes | Used zinc sulfate syrup. 9 children (3 in the zinc group and 6 in the placebo group) dropped out during the study period because of using antibiotics, decongestants or cough medicine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random numbers generator |
| Allocation concealment (selection bias) | Low risk | A statistical consultant programmed a computer‐generated randomisation code and prepared the packages of medication. The packages were identical in appearance except for the randomisation numbers. The packages were randomly distributed by the study nurse |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | The effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Medications (active and placebo) and digital thermometers were supplied by Berko Ilaç, Turkey. The company did not participate in designing the study, collecting and analysing the data, or in writing the report |
| Methods | Double‐masked, placebo‐controlled trial | |
| Participants | Students were recruited from 2 school districts in Cleveland, Ohio. They were aged 6 to 16 years in grades 1 through to 12. Inclusion required that the cold had lasted for ≤ 24 hours. Participants were excluded if they had an oral temperature greater than 37.7ºC, had previously taken the zinc preparation, were pregnant, had a known immune deficiency, any acute illness other than common cold (e.g. pneumonia, gastroenteritis) or cold symptoms lasting more than 24 hours | |
| Interventions | Students asked to take zinc lozenges, 10 mg, orally dissolved, 5 times a day (in grades 1 to 6) or 6 times a day (in grades 7 to 12) until their cold symptoms had been completely resolved for 6 hours | |
| Outcomes | Duration of resolution and severity of symptoms | |
| Notes | The median percentage of prescribed lozenges taken was 82.5% in the zinc group. 2 students (1 in each group) provided false information at entry and were excluded from analysis. Mean and SD were not reported and were estimated from the figure. The zinc lozenge contained glycine, which binds zinc tightly and therefore the free zinc ion level probably was lower than suggested by the total zinc dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | A computer‐generated randomisation code was provided to the pharmacist, who held the code and prepared the packages of medication |
| Blinding (performance bias and detection bias) | High risk | Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | The study was supported by a grant from the Quigley Corporation, Doylestown, Pa |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Participants were recruited from among the Cleveland Clinic staff through announcements in internal clinic publications and by word of mouth. They were older than 18 years of age. Inclusion required that the cold had lasted for ≤ 24 hours. Exclusion criteria were pregnancy, immune deficiency or symptoms of the common cold for > 24 hours prior to interview | |
| Interventions | Participants took 1 lozenge 2‐hourly for every waking hour | |
| Outcomes | Duration and severity of cold symptoms | |
| Notes | Participants were assessed for non‐adherence to treatment; reasons for non‐adherence were: participants had taken antibiotics, condition diagnosed by physician to be other than the common cold, participants filled in diaries from memory, or insufficient lozenges were taken (i.e. fewer than 4 per day for the first 4 days). One participant in the zinc group withdrew from the study on the first day because she could not tolerate the lozenges. Mean and SD were not reported and were estimated from the figure. The zinc lozenge contained glycine, which binds zinc tightly and therefore the free zinc ion level probably was lower than suggested by the total zinc dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random numbers generator |
| Allocation concealment (selection bias) | Low risk | A statistical consultant prepared a computer‐generated randomisation code and the packages of medication |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | The effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | The study was supported by grants from the General Pediatrics Research Fund and the Departments of Infectious Diseases and General Pediatrics of the Cleveland Clinic Foundation |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Participants were recruited from the campus of the University of Texas through posted announcements. They were 18 to 54 years of age. Participants were excluded if they had a serious illnesses, organ transplants or disability (including HIV infection) | |
| Interventions | Participants were instructed to use a lozenge every 1.5 hours while awake during day 0, then 1 lozenge every 2 hours while awake on following days while symptoms were present for 14 days or 6 hours after disappearance of last symptoms | |
| Outcomes | Duration and severity of symptoms | |
| Notes | One subject was lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described or not described in sufficient detail to allow a definite judgement |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | The effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Funded by Weider Nutrition International, Salt Lake City, Utah |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Participants were students, staff and employees at Wayne State University, Michigan, who were ≥ 18 years. Inclusion required that the cold had lasted for ≤ 24 hours. Exclusion criteria were pregnancy, underlying immunodeficiency, chronic illness, symptoms of common cold for more than 24 hours, or had previously used zinc lozenges to treat common cold | |
| Interventions | Participants were asked to use 1 lozenge every 2 to 3 hours while awake for as long as they had symptoms | |
| Outcomes | Duration of symptoms | |
| Notes | Two participants in the placebo group dropped out on day 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used randomisation code |
| Allocation concealment (selection bias) | Low risk | A research consultant prepared the randomisation code and the packages of medication |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | The effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Funded partly by George and Patsy Eby Research Foundation. The research foundation had no role in the collection, analysis or interpretation of the data, or in the decision to publish the study |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Participants were students, staff and employees at Wayne State University, Michigan, who were ≥ 18 years. Inclusion required that the cold had lasted for ≤ 24 hours. Exclusion criteria were pregnancy, underlying immunodeficiency, chronic illness, symptoms of common cold for more than 24 hours, or had previously used zinc lozenges to treat common cold | |
| Interventions | Participants were asked to use 1 lozenge every 2 to 3 hours while awake for as long as they had symptoms | |
| Outcomes | Duration of symptoms | |
| Notes | No loss to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used randomisation code |
| Allocation concealment (selection bias) | Low risk | A research consultant prepared the randomisation code and the packages of medication |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | The effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Funded by National Institutes of Health; George and Patsy Eby Foundation, Austin, Texas (unrestricted research funds to Wayne State University for partial support) |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Participants were recruited from students from 3 colleges and from 1 family practice. They were older than 18 years. Participants were excluded if they had a serious acute or chronic medical condition, seasonal allergies, productive cough, required antibiotic therapy or had taken any treatment for symptoms within 8 hours of baseline assessment | |
| Interventions | Participants took a loading dose of 4 lozenges then took 2 every 2 hours for 7 days or 24 hours after disappearance of last symptoms | |
| Outcomes | Duration and severity of symptoms | |
| Notes | Sixty‐four subjects were excluded because of insufficient dose (< 10 lozenges on any day) or insufficient duration of therapy, and 2 were lost to follow‐up. Mean and SD were not reported and were estimated from the figure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’ |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding (performance bias and detection bias) | Low risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | High risk | The effect size among missing outcomes enough to induce clinically relevant bias in observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | The study was supported by a grant from McNeil Consumer Products Company |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Participants were recruited at 4 different study sites: IMTCI (Lenexa, KS), GFI Pharmaceutical Services (Evansville, IN), TKL Research (Paramus, NJ) and Research Across America (RAA; Dallas). They were 18 to 65 years of age | |
| Interventions | Participants took the lozenges every 2 to 3 hours (a total of 6 per day) while awake until cold symptoms resolved | |
| Outcomes | Duration and severity of symptoms | |
| Notes | Loss to follow‐up not described. Analysis was based on intention‐to‐treat principle. Mean and SD were not reported and were estimated from the figure. The zinc gluconate lozenge contained glycine, which effectively binds to zinc and therefore the free zinc ion level probably was lower than suggested by the total zinc dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used drug randomisation code |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up not described |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Participants were recruited from 4 different study sites: IMTCI (Lenexa, KS), GFI Pharmaceutical Services (Evansville, IN), TKL Research (Paramus, NJ) and Research Across America (RAA; Dallas). They were 18 to 65 years of age | |
| Interventions | Participants took the lozenges every 2 to 3 hours (a total of 6 per day) while awake until cold symptoms resolved | |
| Outcomes | Duration and severity of symptoms | |
| Notes | Loss to follow‐up not described. Analysis was based on intention‐to‐treat principle. Mean and SD were not reported and were estimated from the figure. The zinc gluconate lozenge contained glycine, which effectively binds to zinc and therefore the free zinc ion level probably was lower than suggested by the total zinc dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used drug randomisation code |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up not described |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Participants were recruited from 4 different study sites: IMTCI (Lenexa, KS), GFI Pharmaceutical Services (Evansville, IN), TKL Research (Paramus, NJ) and Research Across America (RAA; Dallas). They were 18 to 65 years of age | |
| Interventions | Participants took the lozenges every 2 to 3 hours (a total of 6 per day) while awake until cold symptoms resolved | |
| Outcomes | Duration and severity of symptoms | |
| Notes | Loss to follow‐up not described. Analysis was based on intention‐to‐treat principle. Mean and SD were not reported and were estimated from the figure. The zinc gluconate lozenge contained glycine, which effectively binds to zinc and therefore the free zinc ion level probably was lower than suggested by the total zinc dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used drug randomisation code |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants, key study personnel and outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up not described |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | School‐aged Iranian children in the suburb of Mashhad. The age range was 6.5 to 10 years. The participants were free from chronic diseases, such as sickle cell disease or protein‐energy malnutrition | |
| Interventions | Participants took tablet daily for 6 days a week for 5 months | |
| Outcomes | Occurrence and duration of common cold | |
| Notes | Used zinc sulfate tablets. No loss to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | This study was supported by grant of vice president for research, Mashhad University of Medical Sciences |
| Methods | Double‐blind, placebo‐controlled randomised trial | |
| Participants | 6 general practitioners residing in the suburban area of Copenhagen conducted the study. Participants were aged 18 to 65 years. Excluded were pregnant and lactating women, diabetics | |
| Interventions | Participants took 1 lozenge at 1 to 1.5‐hourly intervals | |
| Outcomes | Overall assessment of clinical condition assessed by participants using a visual analogue scale | |
| Notes | 14 participants were excluded because of a lack of records and 1 because of their young age. Mean and SD were not reported and were estimated from the figure. Consecutive allocation method was used in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) | Low risk | The effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | The lozenges were manufactured and supplied by a firm |
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The trial was rated of poor methodological quality. A higher incidence of side effects and complaints in the zinc group may have reduced compliance with treatment (no information was provided on whether compliance with treatment was assessed). Intention‐to‐treat analyses were not conducted; analyses were only conducted on a subset of those originally enrolled in the trial. Inclusion criteria were not adequately addressed and therefore there may have been potential for selection bias to occur. In addition, no information was provided on how allocation to treatment groups was concealed, and the power of the study was not stated. | |
| Used both zinc gluconate nasal spray and zinc orotate lozenges simultaneously | |
| Studied upper respiratory tract infection as a whole, used zinc supplementation for 4 months | |
| Poor methodological quality. Not a randomised trial | |
| Poor methodological quality. Not a randomised trial | |
| Poor methodological quality. Measured upper respiratory tract infection as a whole (common cold and seasonal flu) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of cold symptoms Show forest plot | 14 | 1656 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.72, ‐0.34] |
| Analysis 1.1  Comparison 1 Zinc versus placebo, Outcome 1 Duration of cold symptoms. | ||||
| 2 Subgroup analysis (duration of cold symptoms) Show forest plot | 14 | 5996 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.43, ‐0.71] |
| Analysis 1.2  Comparison 1 Zinc versus placebo, Outcome 2 Subgroup analysis (duration of cold symptoms). | ||||
| 2.1 Dose of zinc ≥ 75 mg/day | 7 | 620 | Mean Difference (IV, Random, 95% CI) | ‐1.97 [‐3.09, ‐0.85] |
| 2.2 Dose of zinc < 75 mg/day | 5 | 722 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.54, 0.79] |
| 2.3 Zinc gluconate lozenges | 6 | 798 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.86, 0.25] |
| 2.4 Zinc acetate lozenges | 6 | 544 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐2.69, 0.28] |
| 2.5 Zinc lozenges | 12 | 1342 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐2.02, ‐0.05] |
| 2.6 Zinc syrup | 2 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.92, ‐0.39] |
| 2.7 Children < 16 years age | 3 | 561 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.82, ‐0.42] |
| 2.8 Adults | 11 | 1095 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.17, ‐0.06] |
| 3 Severity of cold symptoms Show forest plot | 5 | 513 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.36, 0.23] |
| Analysis 1.3  Comparison 1 Zinc versus placebo, Outcome 3 Severity of cold symptoms. | ||||
| 4 Subgroup analysis (severity of cold symptoms) Show forest plot | 5 | 513 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.36, 0.23] |
| Analysis 1.4  Comparison 1 Zinc versus placebo, Outcome 4 Subgroup analysis (severity of cold symptoms). | ||||
| 4.1 Zinc lozenges | 3 | 199 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐3.62, 0.52] |
| 4.2 Zinc syrup | 2 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.51, 0.97] |
| 5 Incidence of common cold Show forest plot | 2 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.88] |
| Analysis 1.5  Comparison 1 Zinc versus placebo, Outcome 5 Incidence of common cold. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants symptomatic after 3 days of treatment Show forest plot | 3 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.27, 2.42] |
| Analysis 2.1  Comparison 2 Zinc versus placebo, Outcome 1 Number of participants symptomatic after 3 days of treatment. | ||||
| 2 Number of participants symptomatic after 5 days of treatment Show forest plot | 3 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.32, 1.95] |
| Analysis 2.2  Comparison 2 Zinc versus placebo, Outcome 2 Number of participants symptomatic after 5 days of treatment. | ||||
| 3 Number of participants symptomatic after 7 days of treatment Show forest plot | 5 | 476 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.20, 1.00] |
| Analysis 2.3  Comparison 2 Zinc versus placebo, Outcome 3 Number of participants symptomatic after 7 days of treatment. | ||||
| 4 Time to resolution of cough Show forest plot | 4 | 453 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.49, 0.03] |
| Analysis 2.4  Comparison 2 Zinc versus placebo, Outcome 4 Time to resolution of cough. | ||||
| 5 Time to resolution of nasal congestion Show forest plot | 5 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.39, ‐0.01] |
| Analysis 2.5  Comparison 2 Zinc versus placebo, Outcome 5 Time to resolution of nasal congestion. | ||||
| 6 Time to resolution of nasal drainage Show forest plot | 5 | 599 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐2.01, ‐0.01] |
| Analysis 2.6  Comparison 2 Zinc versus placebo, Outcome 6 Time to resolution of nasal drainage. | ||||
| 7 Time to resolution of sore throat Show forest plot | 4 | 430 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.82, ‐0.09] |
| Analysis 2.7  Comparison 2 Zinc versus placebo, Outcome 7 Time to resolution of sore throat. | ||||
| 8 Change in cough symptom score Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.26, ‐0.20] |
| Analysis 2.8  Comparison 2 Zinc versus placebo, Outcome 8 Change in cough symptom score. | ||||
| 9 Change in nasal symptom score Show forest plot | 2 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.34, 0.94] |
| Analysis 2.9  Comparison 2 Zinc versus placebo, Outcome 9 Change in nasal symptom score. | ||||
| 10 School absence (days) Show forest plot | 2 | 394 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.99, ‐0.33] |
| Analysis 2.10  Comparison 2 Zinc versus placebo, Outcome 10 School absence (days). | ||||
| 11 Antibiotic use Show forest plot | 2 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.16, 0.46] |
| Analysis 2.11  Comparison 2 Zinc versus placebo, Outcome 11 Antibiotic use. | ||||
| 12 Any adverse event Show forest plot | 8 | 1217 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.19, 2.09] |
| Analysis 2.12  Comparison 2 Zinc versus placebo, Outcome 12 Any adverse event. | ||||
| 12.1 Lozenge formulation | 6 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.40, 2.86] |
| 12.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.64, 1.66] |
| 13 Bad taste Show forest plot | 12 | 1483 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.71, 3.11] |
| Analysis 2.13  Comparison 2 Zinc versus placebo, Outcome 13 Bad taste. | ||||
| 13.1 Lozenges formulation | 10 | 1163 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.91, 3.69] |
| 13.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.55, 2.39] |
| 14 Nausea Show forest plot | 8 | 932 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.44, 3.23] |
| Analysis 2.14  Comparison 2 Zinc versus placebo, Outcome 14 Nausea. | ||||
| 14.1 Lozenges formulation | 6 | 612 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.56, 3.89] |
| 14.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.08] |
| 15 Constipation Show forest plot | 7 | 874 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.82, 3.10] |
| Analysis 2.15  Comparison 2 Zinc versus placebo, Outcome 15 Constipation. | ||||
| 15.1 Lozenges formulation | 5 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.88, 4.55] |
| 15.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.21] |
| 16 Diarrhoea Show forest plot | 6 | 764 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.92, 3.89] |
| Analysis 2.16  Comparison 2 Zinc versus placebo, Outcome 16 Diarrhoea. | ||||
| 16.1 Lozenges formulation | 4 | 444 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.92, 4.75] |
| 16.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.30, 6.09] |
| 17 Abdominal pain Show forest plot | 6 | 824 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.83, 2.07] |
| Analysis 2.17  Comparison 2 Zinc versus placebo, Outcome 17 Abdominal pain. | ||||
| 17.1 Lozenges formulation | 4 | 504 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.76, 2.11] |
| 17.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.53, 4.33] |
| 18 Dry mouth Show forest plot | 7 | 874 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.95, 1.99] |
| Analysis 2.18  Comparison 2 Zinc versus placebo, Outcome 18 Dry mouth. | ||||
| 18.1 Lozenges formulation | 5 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.95, 2.11] |
| 18.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 3.01] |
| 19 Mouth irritation Show forest plot | 7 | 822 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.73] |
| Analysis 2.19  Comparison 2 Zinc versus placebo, Outcome 19 Mouth irritation. | ||||
| 19.1 Lozenges formulation | 5 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.73] |
| 19.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.62, 3.15] |
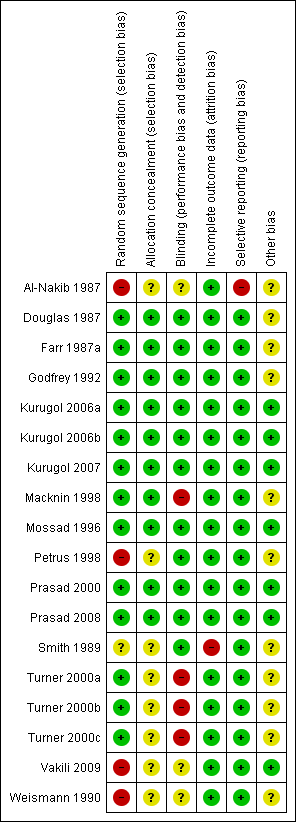
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
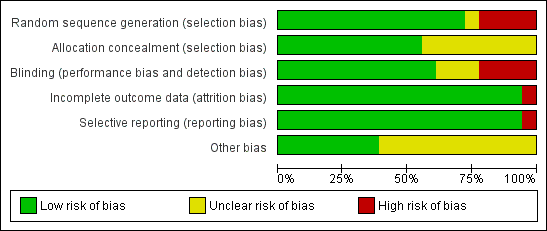
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
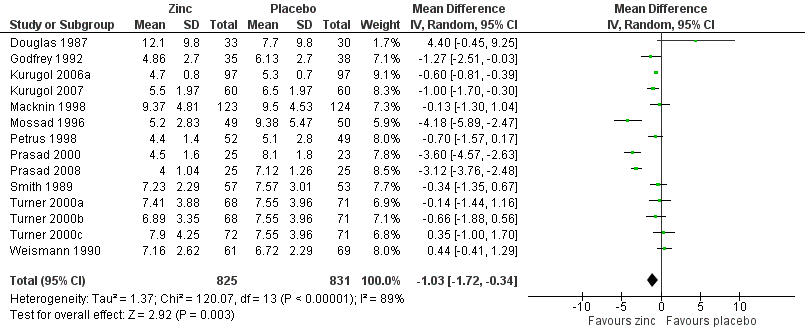
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.1 Duration of cold symptoms (in days).
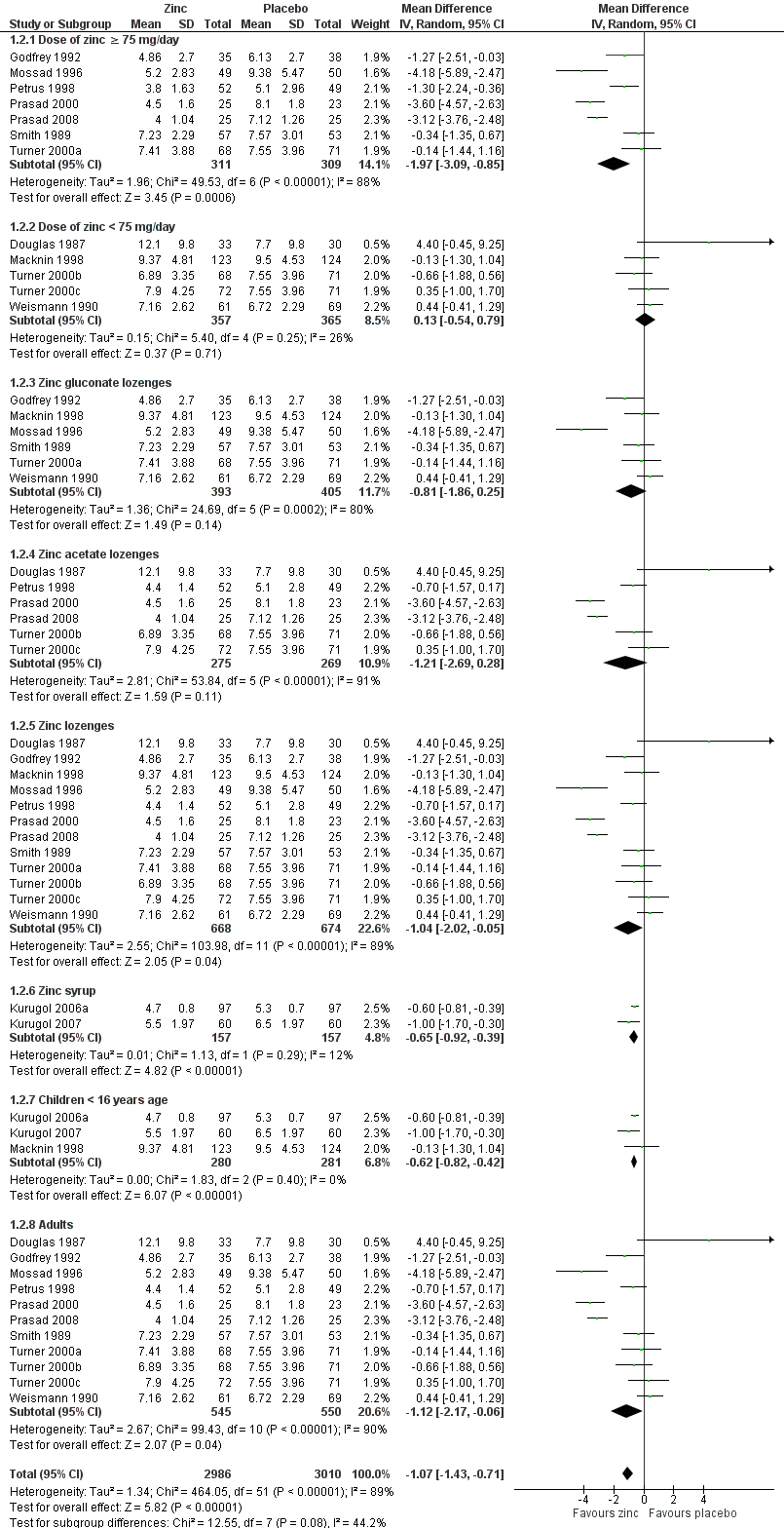
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.1 Duration of cold symptoms (in days).
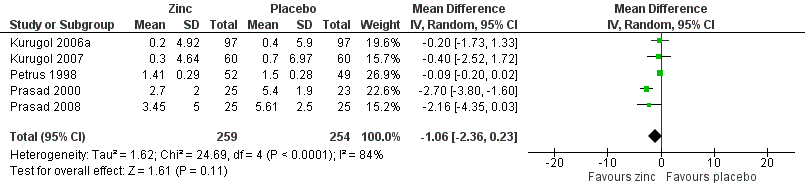
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.2 Severity of symptoms (score).
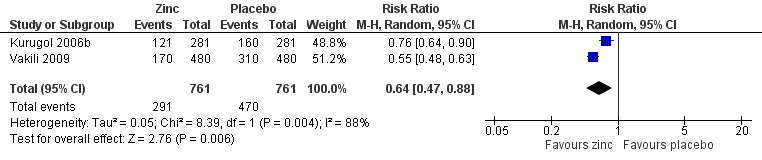
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.3 Incidence of common cold (IRR).
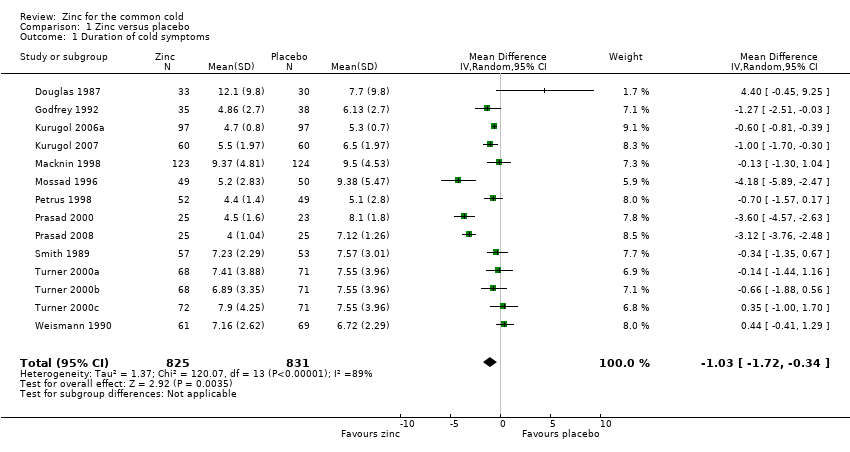
Comparison 1 Zinc versus placebo, Outcome 1 Duration of cold symptoms.
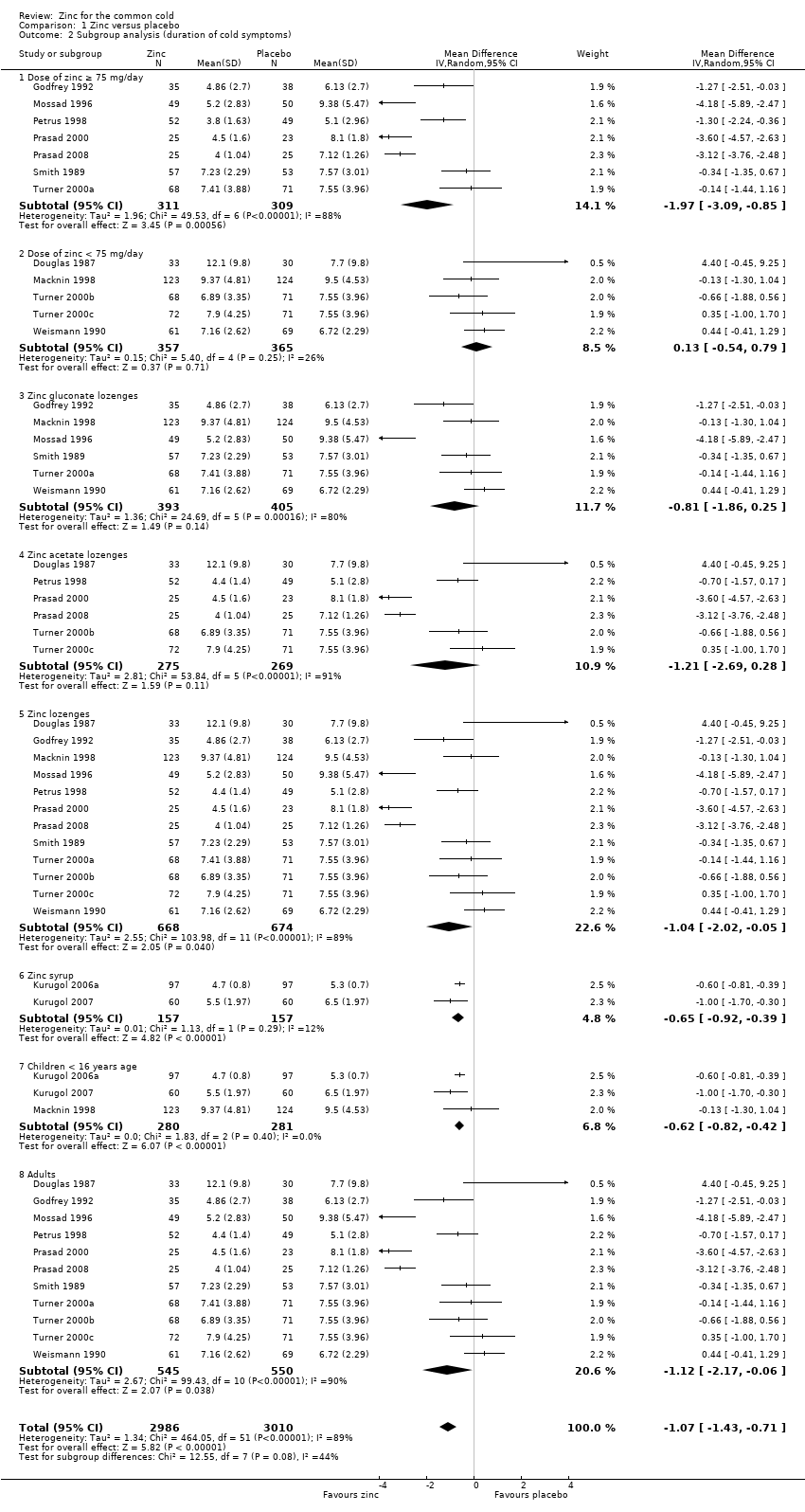
Comparison 1 Zinc versus placebo, Outcome 2 Subgroup analysis (duration of cold symptoms).
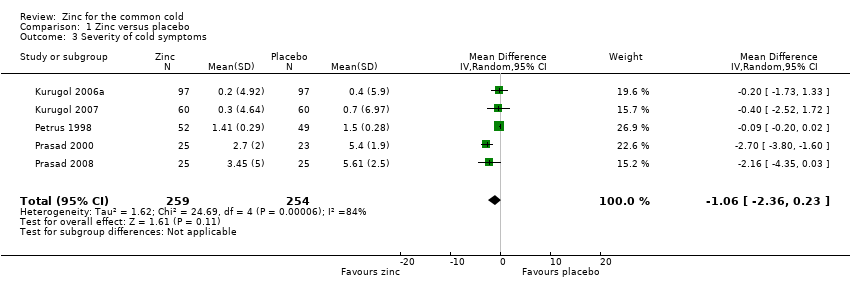
Comparison 1 Zinc versus placebo, Outcome 3 Severity of cold symptoms.
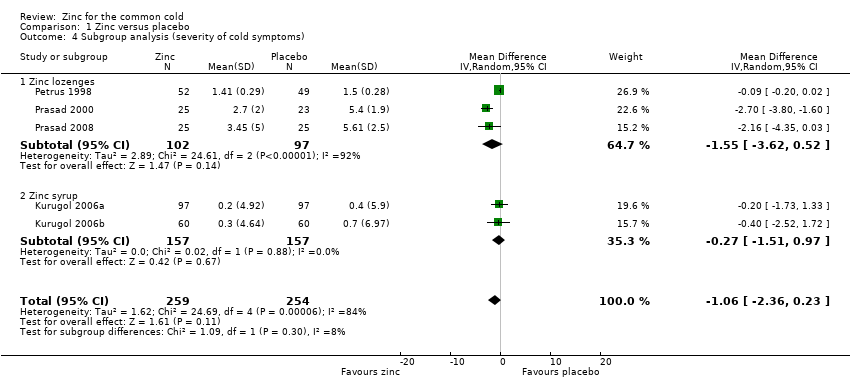
Comparison 1 Zinc versus placebo, Outcome 4 Subgroup analysis (severity of cold symptoms).
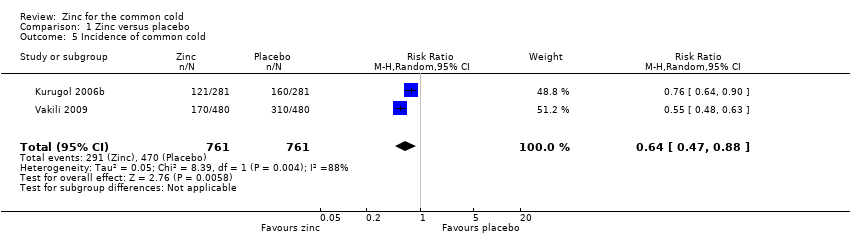
Comparison 1 Zinc versus placebo, Outcome 5 Incidence of common cold.
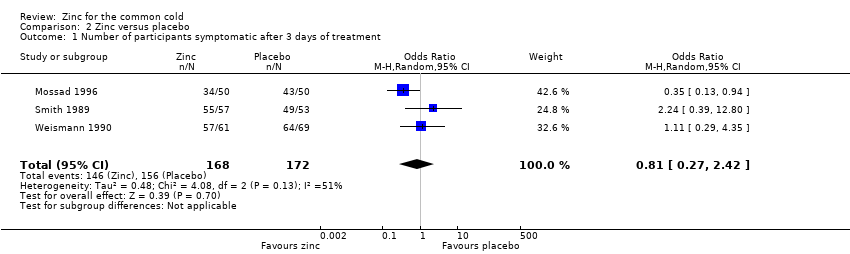
Comparison 2 Zinc versus placebo, Outcome 1 Number of participants symptomatic after 3 days of treatment.
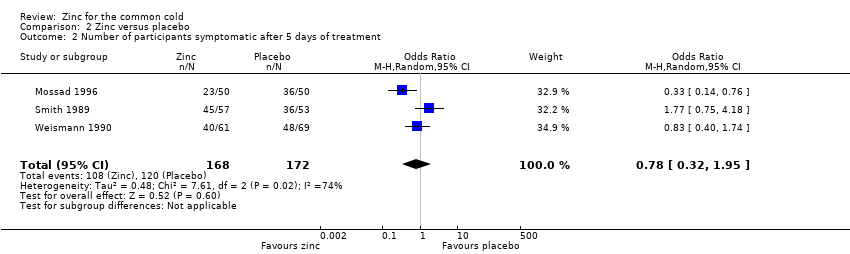
Comparison 2 Zinc versus placebo, Outcome 2 Number of participants symptomatic after 5 days of treatment.
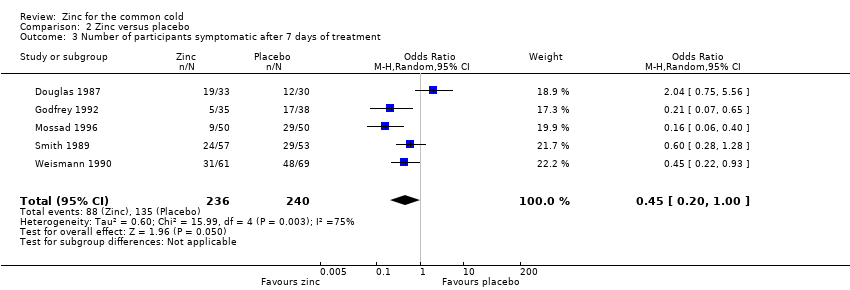
Comparison 2 Zinc versus placebo, Outcome 3 Number of participants symptomatic after 7 days of treatment.
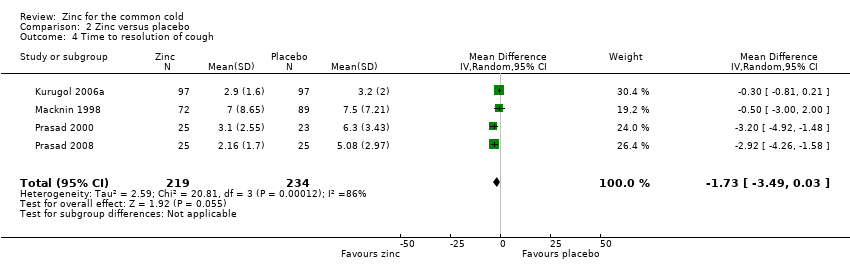
Comparison 2 Zinc versus placebo, Outcome 4 Time to resolution of cough.
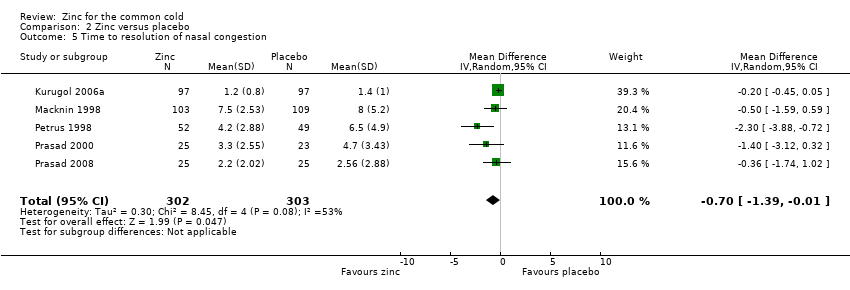
Comparison 2 Zinc versus placebo, Outcome 5 Time to resolution of nasal congestion.
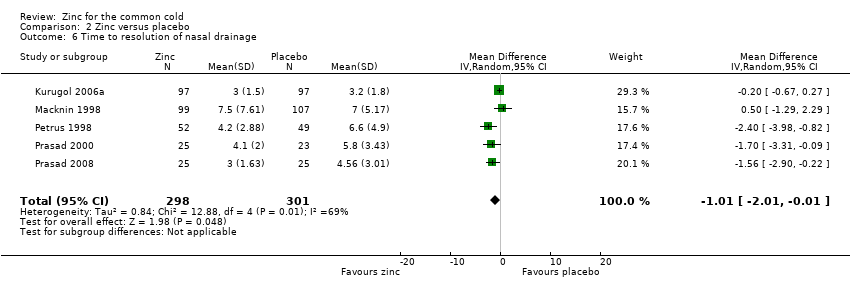
Comparison 2 Zinc versus placebo, Outcome 6 Time to resolution of nasal drainage.
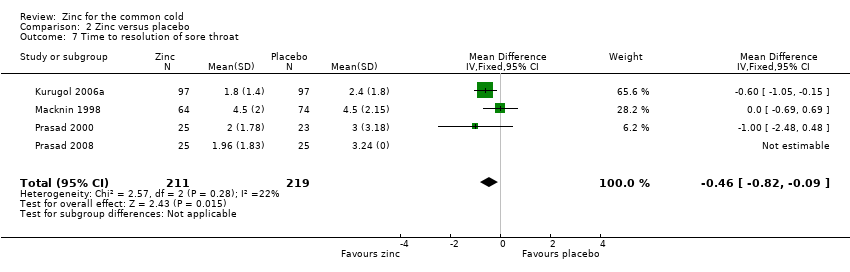
Comparison 2 Zinc versus placebo, Outcome 7 Time to resolution of sore throat.
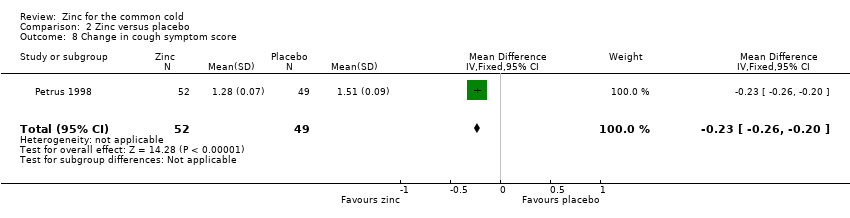
Comparison 2 Zinc versus placebo, Outcome 8 Change in cough symptom score.
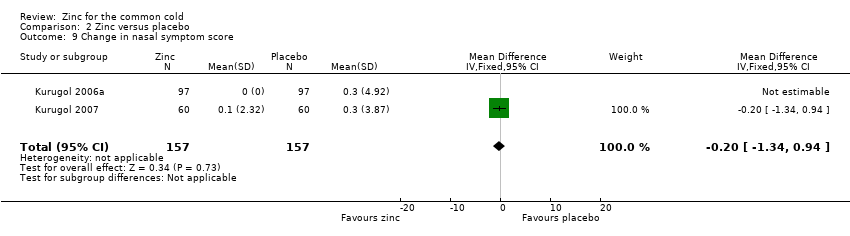
Comparison 2 Zinc versus placebo, Outcome 9 Change in nasal symptom score.
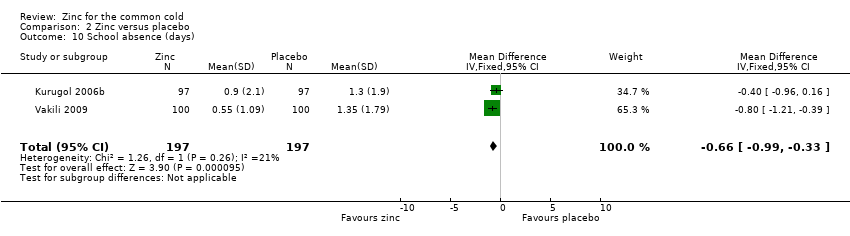
Comparison 2 Zinc versus placebo, Outcome 10 School absence (days).
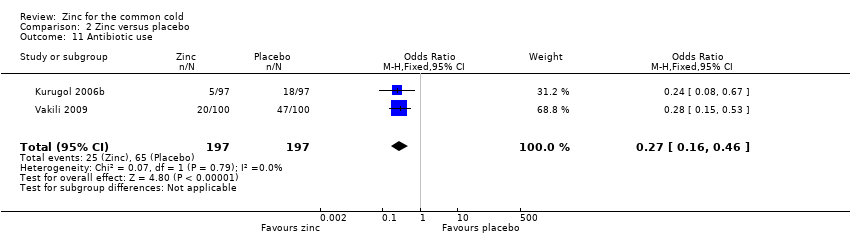
Comparison 2 Zinc versus placebo, Outcome 11 Antibiotic use.
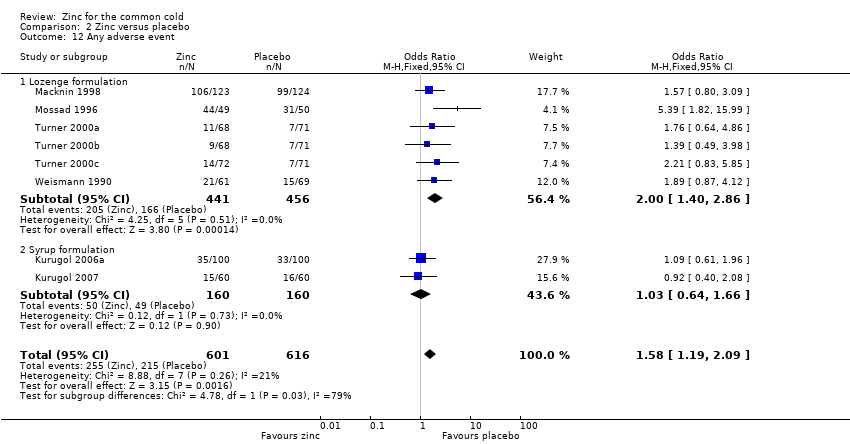
Comparison 2 Zinc versus placebo, Outcome 12 Any adverse event.
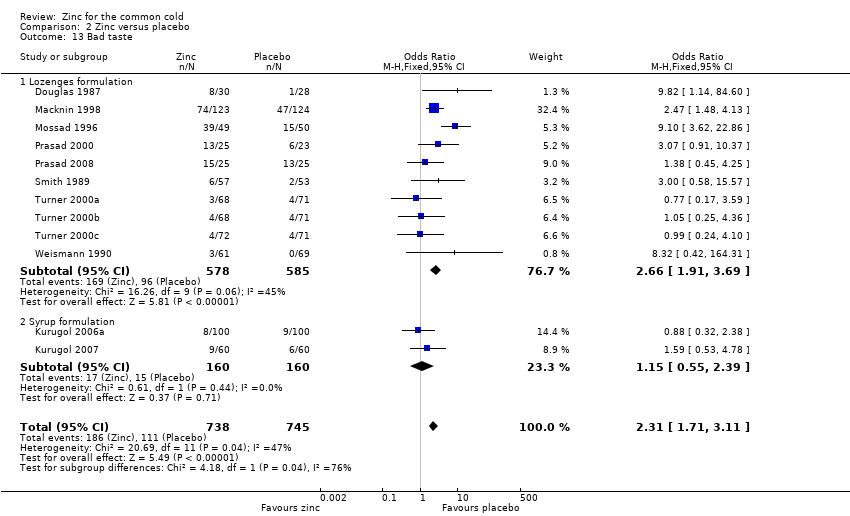
Comparison 2 Zinc versus placebo, Outcome 13 Bad taste.
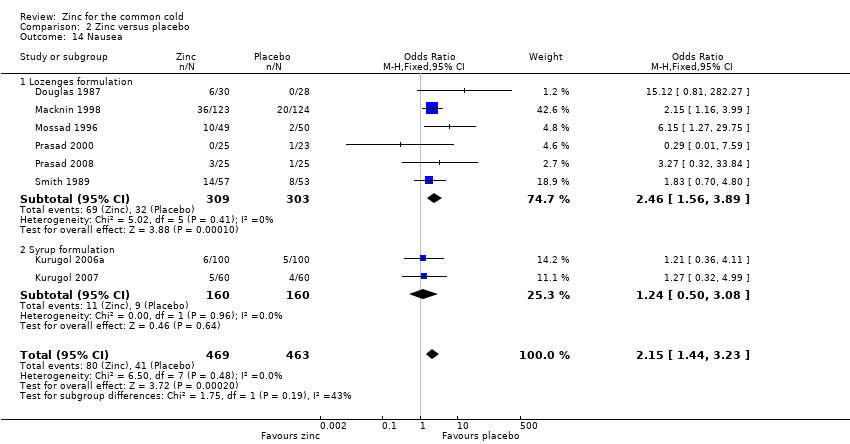
Comparison 2 Zinc versus placebo, Outcome 14 Nausea.
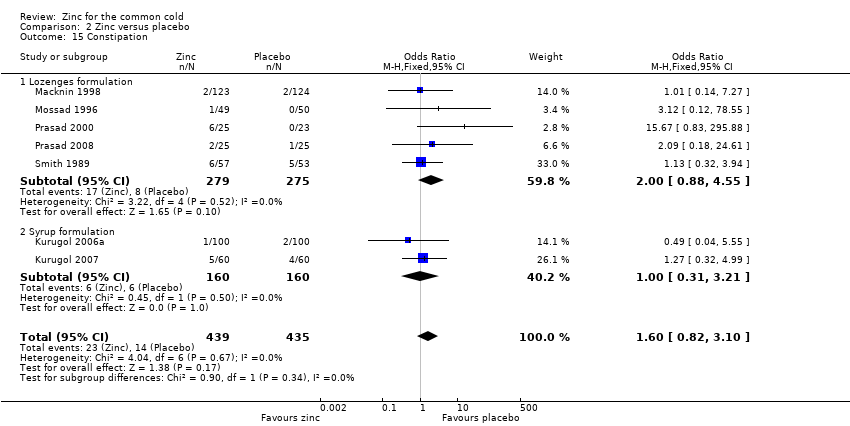
Comparison 2 Zinc versus placebo, Outcome 15 Constipation.
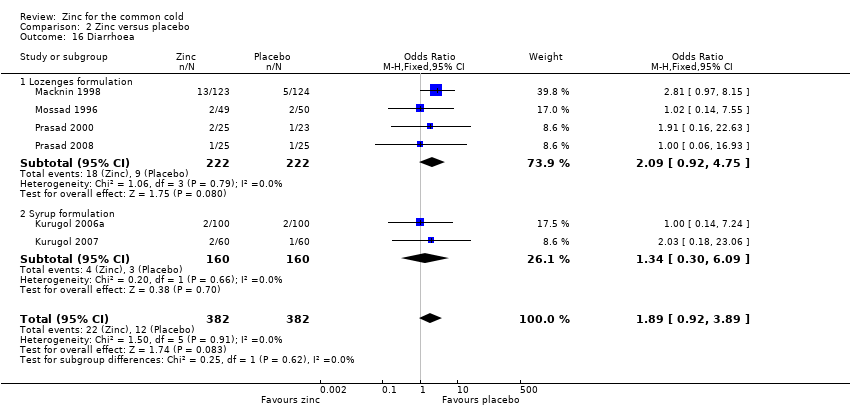
Comparison 2 Zinc versus placebo, Outcome 16 Diarrhoea.
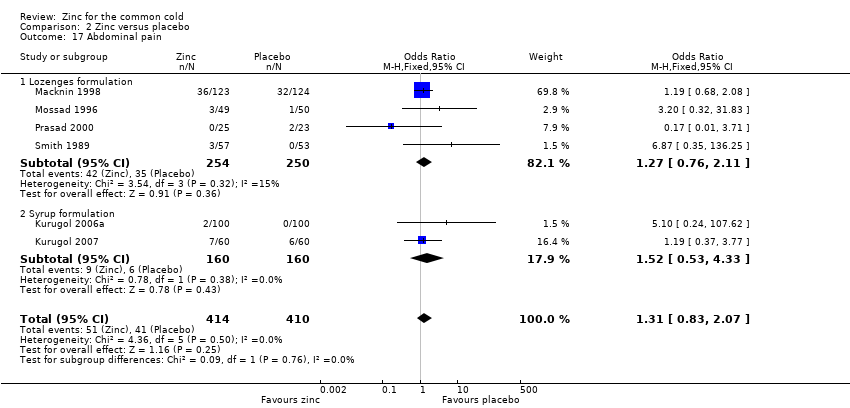
Comparison 2 Zinc versus placebo, Outcome 17 Abdominal pain.
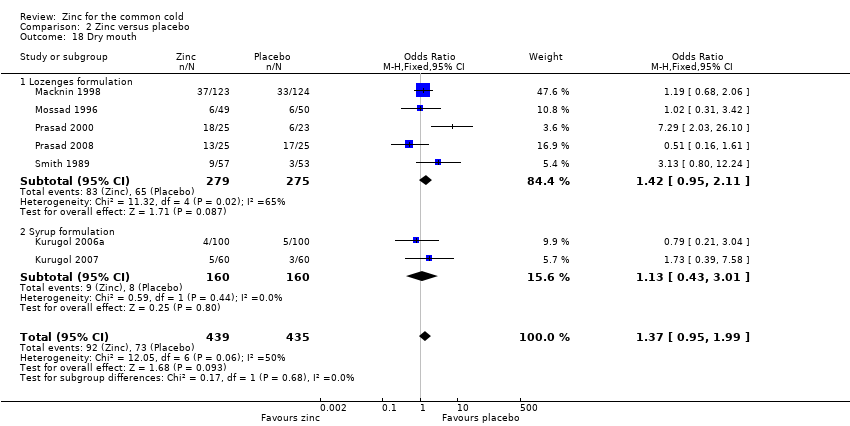
Comparison 2 Zinc versus placebo, Outcome 18 Dry mouth.
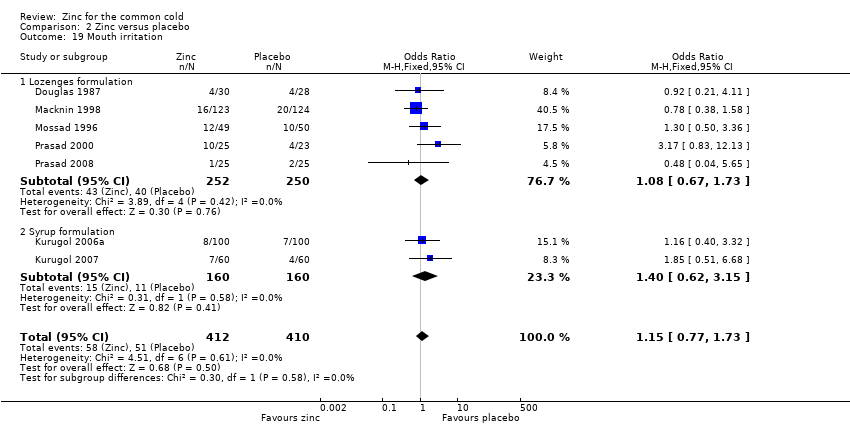
Comparison 2 Zinc versus placebo, Outcome 19 Mouth irritation.
| Zinc compared with placebo for the common cold | ||||||
| Patient or population: patients with common cold Settings: outpatient Intervention: zinc lozenges or syrup Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc | |||||
| Duration of cold symptoms (days) | The mean duration of cold symptoms ranged across control groups from 5.1 to 9.38 days | The mean duration of cold symptoms ranged across control groups from 4 to 12.1 days | 1656 | ++O | ||
| Severity of symptom score | The mean severity of symptom score ranged across control groups from 0.4 to 5.61 | The mean severity of symptom score ranged across control groups from 0.2 to 3.45 | 513 | ++O | ||
| Incidence of common cold | 618 per 1000 | 382 per 1000 (354 to 431) | RR 0.64 (0.47 to 0.88) | 394 | +OO | |
| Number of participants symptomatic after 7 days of treatment | 563 per 1000 | 373 per 1000 (143 to 508) | OR 0.45 (0.2 to 1.0) | 476 | ++OO | |
| School absence (number of days) | The mean days of school absence ranged across control groups from 1.3 to 1.35 days | The mean days of school absence in the intervention groups was 0.37 lower (0.7 to 0.04 lower) | 394 | +OO | ||
| Antibiotic use | 330 per 1000 | 127 per 1000 (52 to 200) | OR 0.27 (0.16 to 0.46) | 394 | ++OO | |
| Any adverse event | 349 per 1000 | 424 per 1000 (132 to 898) | OR 1.58 (1.19 to 2.09) | 1217 | +++O | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious study limitations: all the studies had adequately concealed allocation and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear in Macknin 1998; Petrus 1998; Turner 2000a; Turner 2000b; Turner 2000c. Petrus 1998 did not adequately describe the sequence generation. Blinding was inadequate in Turner 2000a; Turner 2000b; Turner 2000c. 2Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 89%. The heterogeneity was due to differences in the nature of the different interventions (zinc gluconate versus acetate lozenges, zinc lozenges versus zinc syrup), wide dose ranges, varied duration of symptoms prior to administration of zinc (varying from 24 to 48 hours) and characteristics of the study population (children versus adults). 3No serious indirectness: studies both from low‐income and high‐income regions have assessed this comparison. Therefore, the result can be confidently generalised to all situations. 4No serious imprecision: though the 95% CI around the pooled effect is narrow, the lower limit does not suggest a clinically important reduction in the duration of cold (a decrease in duration of ≤ 1 day is not shown to be important to patients). 5Publication bias cannot be ruled out. 6No serious study limitation: all the studies had adequately concealed allocation and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear and adequate sequence was not generated in one study (Petrus 1998). 7Serious imprecision: the 95% CI around the pooled effect is wide, the lower limit is crossing the point of no effect. 8Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 84%. The heterogeneity may be due to differences in the nature of the different interventions (zinc gluconate or acetate lozenges, zinc sulphate syrup) and dose range (30 to 160 mg/day) as well as mean duration of symptoms prior to administration of zinc (varying from 24 to 48 hours), as well as the characteristics of the study population (children versus adults). However, subgroup analysis was not possible as there were not enough studies for each variable. 9Kurugol 2006b is a community‐based intervention including 200 healthy school children and studying the effect of daily administration of 15 mg zinc sulphate syrup over a period of seven months. Vakili 2009 is also a community‐based intervention including 200 healthy school children and studying the effect of daily administration of 10 mg zinc sulfate tablets over a period of seven months. 10Serious study limitation: though the study by Kurugol 2006b was of high quality, that by Vakili 2009 was of poor methodological quality. 11Serious inconsistency: there is substantial heterogeneity between the two trials: I2 statistic for heterogeneity = 88%. Both trials showed a benefit with zinc, however the size of this effect was much larger in Vakili 2009. The heterogeneity was due to differences in the trial methodology and the nature of the interventions. 12No serious imprecision: the 95% CI around the pooled effect is narrow. Even the lower limit suggests a clinically important reduction in the incidence rate ratio of cold which is shown to be important to patients. 13No serious study limitations: allocation concealment was unclear in two studies, i.e. Smith 1989 and Weismann 1990, though both the studies blinded both participants and study staff. 14Serious inconsistency: there was high statistical heterogeneity. I2 statistic = 75%. The heterogeneity may be due to differences in the nature of the different interventions (zinc gluconate or acetate lozenges) and dose range (30 to 160 mg/day) as well as mean duration of symptoms prior to administration of zinc (varying from 24 to 48 hours, as well as the characteristics of the study population (children versus adults). However, subgroup analysis was not possible as there were not enough studies for each variable. 15Serious indirectness: only studies from high‐income regions have assessed this comparison. Therefore, the result cannot be generalised to all situations. 16No serious imprecision: both limits of the 95% CI suggest a clinically important reduction in proportion of participants given the intervention symptomatic after seven days of treatment. 17Serious inconsistency: there is substantial heterogeneity between the two trials: I2 statistic test for heterogeneity = 64%. Both trials showed reduced days of school absence with intervention, however, the size of this effect was much larger in Kurugol 2006b. The heterogeneity was due to differences in the trial methodology and the nature of the interventions. 18No serious imprecision: though the 95% CI around the pooled effect is narrow, the lower limit does not suggests a clinically important reduction in the duration of school absence (a decrease in duration of ≤ 1 day is not shown to be important to patients). 19No serious inconsistency: there was no statistical heterogeneity. I2 statistic = 0%. 20No serious imprecision: both limits of the 95% CI suggest a clinically important reduction in the rate of antibiotic use with intervention. 21No serious study limitations: all the studies had adequately concealed allocation (except Weismann 1990, in which allocation concealment is unclear) and blinded both participants and study staff to be considered at low risk of bias. Whether free of other bias was unclear in Macknin 1998 and Weismann 1990. Weismann 1990 did not adequately describe the sequence generation. 22No serious inconsistency: there is no statistical heterogeneity. I2 statistic = 21%. Both the lozenges and syrup preparation trials were pooled. 23No serious imprecision: the 95% CI around the pooled effect is narrow. The resulting adverse events from use of zinc are higher and this is significant. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of cold symptoms Show forest plot | 14 | 1656 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.72, ‐0.34] |
| 2 Subgroup analysis (duration of cold symptoms) Show forest plot | 14 | 5996 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.43, ‐0.71] |
| 2.1 Dose of zinc ≥ 75 mg/day | 7 | 620 | Mean Difference (IV, Random, 95% CI) | ‐1.97 [‐3.09, ‐0.85] |
| 2.2 Dose of zinc < 75 mg/day | 5 | 722 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.54, 0.79] |
| 2.3 Zinc gluconate lozenges | 6 | 798 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.86, 0.25] |
| 2.4 Zinc acetate lozenges | 6 | 544 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐2.69, 0.28] |
| 2.5 Zinc lozenges | 12 | 1342 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐2.02, ‐0.05] |
| 2.6 Zinc syrup | 2 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.92, ‐0.39] |
| 2.7 Children < 16 years age | 3 | 561 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.82, ‐0.42] |
| 2.8 Adults | 11 | 1095 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.17, ‐0.06] |
| 3 Severity of cold symptoms Show forest plot | 5 | 513 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.36, 0.23] |
| 4 Subgroup analysis (severity of cold symptoms) Show forest plot | 5 | 513 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.36, 0.23] |
| 4.1 Zinc lozenges | 3 | 199 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐3.62, 0.52] |
| 4.2 Zinc syrup | 2 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.51, 0.97] |
| 5 Incidence of common cold Show forest plot | 2 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants symptomatic after 3 days of treatment Show forest plot | 3 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.27, 2.42] |
| 2 Number of participants symptomatic after 5 days of treatment Show forest plot | 3 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.32, 1.95] |
| 3 Number of participants symptomatic after 7 days of treatment Show forest plot | 5 | 476 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.20, 1.00] |
| 4 Time to resolution of cough Show forest plot | 4 | 453 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.49, 0.03] |
| 5 Time to resolution of nasal congestion Show forest plot | 5 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.39, ‐0.01] |
| 6 Time to resolution of nasal drainage Show forest plot | 5 | 599 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐2.01, ‐0.01] |
| 7 Time to resolution of sore throat Show forest plot | 4 | 430 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.82, ‐0.09] |
| 8 Change in cough symptom score Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.26, ‐0.20] |
| 9 Change in nasal symptom score Show forest plot | 2 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.34, 0.94] |
| 10 School absence (days) Show forest plot | 2 | 394 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.99, ‐0.33] |
| 11 Antibiotic use Show forest plot | 2 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.16, 0.46] |
| 12 Any adverse event Show forest plot | 8 | 1217 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.19, 2.09] |
| 12.1 Lozenge formulation | 6 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.40, 2.86] |
| 12.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.64, 1.66] |
| 13 Bad taste Show forest plot | 12 | 1483 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.71, 3.11] |
| 13.1 Lozenges formulation | 10 | 1163 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.91, 3.69] |
| 13.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.55, 2.39] |
| 14 Nausea Show forest plot | 8 | 932 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.44, 3.23] |
| 14.1 Lozenges formulation | 6 | 612 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.56, 3.89] |
| 14.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.08] |
| 15 Constipation Show forest plot | 7 | 874 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.82, 3.10] |
| 15.1 Lozenges formulation | 5 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.88, 4.55] |
| 15.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.21] |
| 16 Diarrhoea Show forest plot | 6 | 764 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.92, 3.89] |
| 16.1 Lozenges formulation | 4 | 444 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.92, 4.75] |
| 16.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.30, 6.09] |
| 17 Abdominal pain Show forest plot | 6 | 824 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.83, 2.07] |
| 17.1 Lozenges formulation | 4 | 504 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.76, 2.11] |
| 17.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.53, 4.33] |
| 18 Dry mouth Show forest plot | 7 | 874 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.95, 1.99] |
| 18.1 Lozenges formulation | 5 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.95, 2.11] |
| 18.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 3.01] |
| 19 Mouth irritation Show forest plot | 7 | 822 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.73] |
| 19.1 Lozenges formulation | 5 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.73] |
| 19.2 Syrup formulation | 2 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.62, 3.15] |

